Abstract
Recently, we examined the neuronal substrate of predictive pursuit during memory-based smooth pursuit and found that supplementary eye fields (SEFs) contain signals coding assessment and memory of visual motion direction, decision not-to-pursue (“no-go”), and preparation for pursuit. To determine whether these signals were unique to the SEF, we examined the discharge of 185 task-related neurons in the caudal frontal eye fields (FEFs) in 2 macaques. Visual motion memory and no-go signals were also present in the caudal FEF but compared with those in the SEF, the percentage of neurons coding these signals was significantly lower. In particular, unlike SEF neurons, directional visual motion responses of caudal FEF neurons decayed exponentially. In contrast, the percentage of neurons coding directional pursuit eye movements was significantly higher in the caudal FEF than in the SEF. Unlike SEF inactivation, muscimol injection into the caudal FEF did not induce direction errors or no-go errors but decreased eye velocity during pursuit causing an inability to compensate for the response delays during sinusoidal pursuit. These results indicate significant differences between the 2 regions in the signals represented and in the effects of chemical inactivation suggesting that the caudal FEF is primarily involved in generating motor commands for smooth-pursuit eye movements.
Keywords: memory, movement preparation, muscimol, smooth pursuit, visual motion
Introduction
Smooth pursuit eye movements are essential to obtain accurate visual information about slowly moving objects. During smooth pursuit, target images are maintained on the foveae by predictive compensation for the inherent delays in the response to target movement (e.g., Becker and Fuchs 1985; Barnes and Asselman 1991), but the neural mechanisms of predictive pursuit are still poorly understood (for a review, see Leigh and Zee 2006). Prediction occurs not only in motor commands but also in the sensory and/or perception pathways. For example, visual responses anticipate the eventually renewed direction and speed of the target movement of a temporarily occluded visual input (cf., Barborica and Ferrera 2003). Such a mechanism may use memory (e.g., Newsome et al. 1988; Assad and Maunsell 1995; Bisley et al. 2004; cf., Umeno and Goldberg 1997); however, it is unknown where the memory of visual motion for predictive smooth pursuit is stored (e.g., Collins and Barnes 2005). To examine neuronal substrates for predictive pursuit, the discharge related to movement preparation must be distinguished from the discharge related to processing of target motion signals or their memory. Moreover, in daily life, there are many moving objects necessitating selection of a specific target, which includes the decision of whether to pursue or not.
To examine the neuronal substrates for these functions, we trained Japanese macaques to perform a memory-based smooth pursuit task (Shichinohe et al. 2009). In this task, we used 2 cues; cue 1 to indicate visual motion and cue 2 to instruct whether to prepare for pursuit (i.e., “go”) or not to pursue (i.e., “no-go”). Based on the memory of the visual motion direction presented at cue 1 and the go/no-go instruction presented at cue 2, monkeys had to select the correct pursuit direction or not pursue at all. We have shown that the supplementary eye fields (SEFs) contain separate signals coding assessment and memory of visual motion direction, the decision of whether or not to pursue during no-go trials, and movement preparation during go trials (Shichinohe et al. 2009). The next question was how these signals are generated in the SEF. It has been reported that neurons in the frontal eye fields (FEFs) exhibit visual latencies comparable with those in middle temporal (MT) area and medial superior temporal (MST) area and sometimes even as early as some neurons in V1 (Schmolesky et al. 1998). Since the SEF has reciprocal connections with the FEF (e.g., Huerta et al. 1987), it is possible that SEF signals, especially those reflecting memory of visual motion direction, come from the FEF.
The caudal part of the FEF in the fundus of the arcuate sulcus contains smooth pursuit-related neurons (i.e., pursuit neuron, e.g., MacAvoy et al. 1991; Gottlieb et al. 1993, 1994; Tanaka and Fukushima 1998; Akao et al. 2005, 2009; Kurkin, Akao et al. 2009), the majority of which carry visual signals about the direction and velocity of target motion (Fukushima et al. 2000, 2002). Studies have also shown that the discharge of these neurons is related to predictive target motion (for a review, see Fukushima et al. 2006), but these studies could not separate discharge related to visual motion-memory from predictive visual motion responses. If the SEF signals come from the FEF through the reciprocal connections between the 2 regions (Huerta et al. 1987), we should observe signals in the caudal FEF that resemble those in the SEF during memory-based smooth pursuit eye movements. To clarify whether the above SEF signals are unique to the SEF, we studied neuronal activity in the caudal FEF during memory-based smooth pursuit eye movements (Shichinohe et al. 2009). Some of our results have been published in preliminary form (Fukushima et al. 2008, Fukushima et al. 2009).
Materials and Methods
General Procedures
We used the same 2 monkeys (Macaca fuscata, Sh and J, 5–6 years old) as were used for recording in the previous SEF (Shichinohe et al. 2009) experiments and recorded from the caudal FEF during the same months that neuronal recordings were made in the SEF. All procedures complied with the guidelines for the Care and Use of Animals of the National Institutes of Health. The Animal Care and Use Committee of Hokkaido University School of Medicine approved our specific procedures. Our methods for animal preparation, training, recording, and data analysis are described elsewhere in detail (e.g., Fukushima et al. 2000; Shichinohe et al. 2009) and are briefly summarized here. Each monkey was sedated with ketamine hydrochloride (5 mg/kg, intramuscularly) and then anesthetized with sodium pentobarbital (25 mg/kg, intraperitoneal [i.p.]). Additional anesthesia (0.5–1.0% halothane mixed with 50% nitrous oxide and 50% oxygen) was administered as necessary. Under aseptic conditions, head holders were affixed to the skull. Vertical and horizontal components of eye movements were recorded using a scleral search coil (Fuchs and Robinson 1966).
Behavioral Paradigms and Recording Procedures
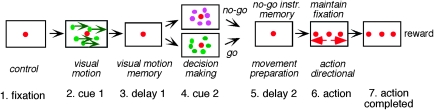
Each monkey was seated in a primate chair in darkness with the head firmly restrained facing a 22-inch computer display (Mitsubishi, RDF 221S, 120 Hz) placed 65 cm away from the eyes. Visual objects (spot and random dot pattern, see below) were presented in the central 10° by 10° of the visual field. The task conditions are schematically illustrated in Figure 1. A red stationary spot (0.5° diameter) appeared in the center, and the monkeys were required to fixate it (Fig. 1, 1. fixation). At cue 1, a random dot pattern was presented (0.5° spots occupied 40% of the 10° × 10° area, ∼150 dots) and was moved along one of 8 directions at 10°/s for 0.5 s (Fig. 1, 2. cue 1). Directions were separated by 45° and either horizontal (right or left), vertical (up or down), or one of the 4 diagonal directions. Each dot in the pattern moved in the same direction (i.e., 100% correlation, Newsome and Pare 1988). In successive trials, the direction of the moving pattern (e.g., right or left) was random but of equal frequency for each direction. The monkeys were required to remember the color of the pattern and the movement direction. After a delay (Fig. 1, 3. delay 1 of 1–4 s, typically 1 s), a stationary pattern was presented as the second cue for 0.5 s (Fig. 1, 4. cue 2) (0.5° spots presented across 40% of the 10° × 10° area, ∼150 dots) for go/no-go selection. If the color of cue 2 was the same as the cue 1 color, it instructed the monkeys to prepare to pursue a spot that would move in the direction instructed by cue 1 (i.e., go). If the color of cue 2 was different from cue 1, it instructed the monkeys not to pursue (i.e., no-go) but to maintain fixation of a stationary spot by remembering the no-go instruction. After the second delay (Fig. 1, 5. delay 2, typically 2 s), the monkeys were required to perform the pursuit eye movement by selecting the correct spot or to maintain fixation (i.e., no-go, Fig. 1, 6. action). For this, the stationary spot remained but spawned 2 identical spots; one moved in the direction instructed by cue 1 and the other moved in the opposite direction at 10°/s. The monkeys were required to respond correctly, either to pursue the correct spot (go) or not to pursue (no-go) by maintaining fixation of the stationary spot. The frequency of occurrence of fixation (i.e., no-go) trials was set at 24%, and in the remaining 76% of the trials, the monkeys were required to pursue one of the 2 moving spots (i.e., go) as described above.
Figure 1.
The task conditions. For further explanation, see text.
Reward circuits compared the monkeys’ eye position signals with the position signals of the stationary spot during the initial fixation, cue 1, cue 2, and the 2 delay periods and with the correct target spot during the action period (Fig. 1). If the monkeys’ gaze was within the error window of ±2°, apple juice was automatically delivered to the animal at the end of each trial (Fig. 1, reward). If the monkeys’ gaze was outside the error window, the trial was aborted and restarted. Typically, we prepared 3 sets of different-colored dots for cue 1 and cue 2, and each set was presented as a block. The monkeys were trained to perform this task over several months to a year. By the time we started FEF recordings, the error rate was less than 10%.
A recording chamber was stereotaxically implanted (center aimed at anterior 24 mm and lateral 16 mm) on the skull to allow single-unit recording in the caudal FEF (e.g., Akao et al. 2005). Analgesics (pentazocine, 0.2 mg/kg) and antibiotics (flomoxef sodium, 50 mg/kg) were administered postsurgically.
We recorded extracellularly in the caudal FEF as described previously (Tanaka and Fukushima 1998; Fukushima et al. 2000, 2002; Akao et al. 2005, 2009; Kurkin, Akao et al. 2009). Once task-related neurons were isolated (see Data Analysis), we determined preferred directions for their responses by moving cue 1 along different directions. Similarly, to previous SEF studies (Shichinohe et al. 2009), we searched for neurons that carried the direction- and/or instruction-specific signals during delay 1 and/or delay 2. For neurons that showed such responses, we presented cue 1 visual motion either in the preferred direction or antipreferred direction.
To inactivate the caudal FEF where task-related neurons were recorded, we used a microrecording needle (Crist Instrument) that was attached to a Hamilton syringe, and 1.0 μL of γ-aminobutyric acid agonist muscimol dissolved in physiological saline (10 μg/μL) was infused into the identified sites. Unilateral injection was tested 3 times, 2 times in one monkey (Sh), and once in the other monkey (J). In addition, we performed bilateral injections once in the first monkey (Sh). The effects of muscimol injection on the monkeys’ performance of the task (Fig. 1) were examined. For this, we prepared 5 sets of different-colored dots for cue 1 and cue 2, and each set was presented randomly within a block before and after infusion as we did previously for SEF infusion (Shichinohe et al. 2009). Typically, 100 trials were tested after muscimol infusion followed by simple pursuit using a single spot (0.5° diameter) and moving it sinusoidally at different frequencies (0.3–1.5 Hz, ±10°).
Data Analysis
To analyze the discharge of each neuron, traces were aligned on the onset of cue 1. Eye position, target position, and neuronal discharge were sorted by correct direction as instructed by cue 1 and cue 2. Trials for go and no-go were sorted separately. Mean discharge rates of individual neurons during each period (e.g., Fig. 2A, periods 1–7) were measured and compared as the mean (±standard deviation [SD]) rate of each period versus the mean discharge rate (±SD) during the initial fixation (period 1), which acted as a control for each condition for each neuron (Fig. 2A. period 1). We defined significant differences as those having a P value < 0.05 using Student’s t test with the Bonferroni correction for multiple comparisons (Shichinohe et al. 2009). Neurons that exhibited significant modulation were defined as task-related neurons. A total of 197 neurons exhibited modulation. Of these, 12 neurons (6%) exhibited gradually increasing activity during the control period as though the discharge of these neurons reflected anticipation of the occurrence of cue 1 (e.g., Chen and Wise 1995; Shichinohe et al. 2009). Since they responded before any cue, we were unable to estimate control discharge rate of these neurons accurately during the fixation period, so we did not include these neurons so that further analysis was done on 185 neurons.
Figure 2.
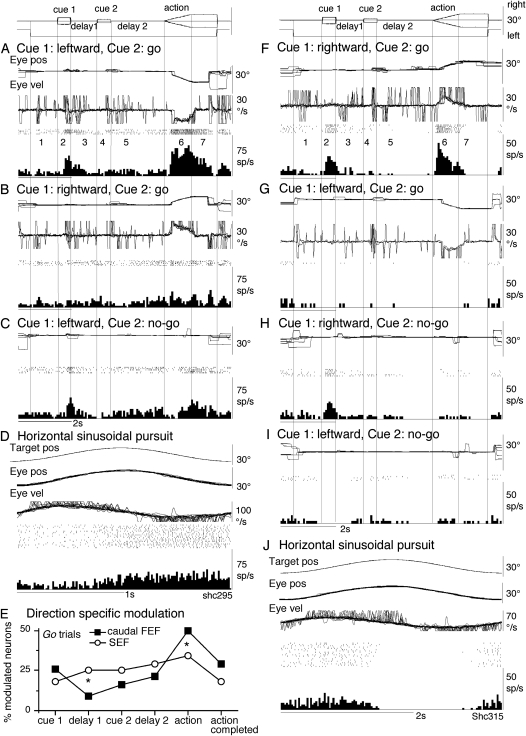
Discharge of representative caudal FEF neurons and comparison of direction-specific modulation during go trials in the caudal FEF and SEF. A–D show discharge of a visual memory neuron recorded in the left caudal FEF. (E) Comparison of percentage of modulated neurons that exhibited direction-specific modulation (of the total number of task-related neurons) during different task periods for go trials in the caudal FEF and SEF. F–J show discharge of another caudal FEF neuron recorded in the right caudal FEF that exhibited a visual motion response to cue 1 but that had no directional or instruction-specific discharge during delay 1 and delay 2. (A, B) and (F,G) go trials as indicated. (C,H and I) no-go trials as indicated. (D and J) Sinusoidal pursuit of a single spot. Traces from top to bottom in A,B and F–G are superimposed eye position (eye pos), eye velocity (Eye vel), spike rasters, and histograms of neuron discharge. Eye velocity during saccades were clipped. (C and H,I) Similar presentation without eye velocity. Traces in D and J are target position (target pos), eye position, eye velocity, spike rasters, and histogram of neuron discharge. For further explanation, see text.
Similarly, to our previous study (Shichinohe et al. 2009), neurons that showed direction- and/or instruction-specific responses during delay 1 and/or delay 2 were classified as one of 4 groups based on their responses during go trials and no-go trials (Table 1, neuron groups 1–4). The monkeys occasionally made small eye movements during the delay periods (e.g., Fig. 2A, eye vel). Some were blinks. These eye movements did not contribute to the observed neuronal responses.
Table 1.
Classification of delay 1/delay 2 direction/instruction-specific neurons and comparison of number and percentage of task-related neurons in the caudal FEF and SEF
| Go trials | No-go trials | Caudal FEF | SEF | |||||
| Neuron groups | Delay 1 | Delay 2 | Delay 1 | Delay 2 | n | % | n | % |
| 1. Visual memory | Yes | No | Yes | No | 6 | 3 | 14 | 7 |
| 2. Visual memory + movement preparation | Yes | Yes | Yes | No | 10 | 5a | 25 | 12b |
| 3. Movement preparation | No | Yes | No | No | 28 | 15 | 20 | 10 |
| 4. No-go | No | No | No | Yes | 16 | 9c | 50 | 24d |
| Total delay 1/delay 2 direction/instruction specific neurons | — | 60 | 32 | 109 | 52 | |||
| Other task-related neurons | 125 | 68 | 99 | 48 | ||||
| Total task-related neurons | 185 | 100 | 208 | 100 | ||||
Note: Superscripts in neuron groups 2 (a vs. b) and 4 (c vs. d) indicate that differences in the percentages (of total task-related neurons) between caudal FEF and SEF neurons were significant (P < 0.05). Data for SEF neurons were taken from Shichinohe et al. (2009). Other task-related neurons include all task-related neurons that did not exhibit direction- or instruction-specific discharge during delay 1 or delay 2. For further explanation, see text.
Latencies of visual motion responses to cue 1 were examined for neurons that exhibited directional discharge modulation during cue 1. For this, 10 or more trials were aligned to obtain mean responses for each neuron. Onset of the neuronal responses to cue 1 was determined as the time at which the mean discharge rate exceeded 2 SD of the control value during the initial fixation (e.g., Akao et al. 2005). Similarly, latencies of no-go responses relative to the onset of cue 2 were examined for no-go neurons. Onset of the neuronal responses to cue 2 was determined as the time at which the mean discharge rate exceeded 2 SD of the control value calculated for the 500 ms during delay 1 before cue 2 onset.
To analyze the effects of muscimol injection, 80–100 trials were aligned with the onset of cue 1 before and after injection. To examine the effects of muscimol on pursuit eye movements, eye position and velocity traces were examined for correct performance. Desaccaded eye velocity was averaged to compare mean eye velocity. For sinusoidal target motion, phase and gain of desaccaded and averaged eye velocities were calculated relative to peak target velocity before and after muscimol infusion (e.g., Fukushima et al. 2000).
Histological Procedures
Near the conclusion of recordings in the 2 monkeys, some of the recording sites were marked by passing current (50 μA for 30 s) through the tip of an iron-tipped tungsten electrode. After recording was completed, the monkeys were deeply anesthetized with pentobarbital sodium (50 mg/kg, i.p.) and perfused with physiological saline followed by 3.5% formalin. After histological fixation, coronal sections were cut at 100-μm thickness on a freezing microtome. These sections were stained using the Nissl method, and the recording sites were verified microscopically as previously described (e.g., Tanaka and Fukushima 1998; Fukushima et al. 2000; Akao et al. 2005).
Results
Discharge of Task-Related Neurons in the Caudal FEF
We examined the activity of a total of 185 task-related neurons (see Data Analysis) in the caudal FEF in the 2 monkeys (134 from monkey Sh, 51 from monkey J) during memory-based smooth pursuit. Discharge characteristics of neurons recorded in the 2 monkeys were similar. Of the 185, 60 neurons exhibited direction- and/or instruction-specific discharge during delay 1 and/or delay 2, and these neurons were classified into one of the 4 groups as described in our previous SEF study (see Data Analysis, Shichinohe et al. 2009); these groups were visual memory neurons (n = 6), visual memory + movement preparation neurons (n = 10), movement preparation neurons (n = 28), and no-go neurons (n = 16). Table 1 (neuron groups 1–4) summarizes the number of neurons in each group and their percentages of the total number of task-related neurons in the caudal FEF. The responses of the remaining 125 task-related FEF neurons did not exhibit direction-specific or instruction-specific discharge during delay 1 or delay 2 (Table 1, other task-related neurons). Some of these neurons correspond to previously described FEF neuron types from other studies (e.g., visual or movement neurons), as described below. Similarly to SEF neurons (Shichinohe et al. 2009), the great majority of the 185 neurons (>80%) exhibited excitation as illustrated in Figure 2A. In the following sections, we performed quantitative analyses of the excitatory responses. We first show discharge of the first 3 groups of neurons in the caudal FEF (Table 1, groups 1–3).
Visual Memory Neurons
Discharge of a representative visual memory neuron recorded in the left caudal FEF is illustrated in Figure 2A–D. This neuron increased discharge rate during cue 1 when cue 1 motion was leftward (but not rightward, Fig. 2A vs. B, go trials, period 2), and the increased discharge rate was maintained during the initial phase of delay 1 but declined thereafter (Fig. 2A, period 3). Increased discharge rate to cue 1 and during delay 1 were basically similar during no-go trials when cue 1 motion was leftward (Fig. 2C, periods 2 and 3) but not rightward (not shown). During cue 2 and delay 2, discharge modulation was not significantly different compared with the control during go trials (Fig. 2A,B, periods 4 and 5) and no-go trials (Fig. 2C, periods 4 and 5). During the action period of go trials, this neuron clearly increased discharge during leftward (but not rightward) pursuit (Fig. 2A vs. B, periods 6 and 7), indicating that this was a pursuit neuron (e.g., MacAvoy et al. 1991).
All 6 visual memory neurons recorded (Table 1, group 1) exhibited directional discharge modulation during cue 1 that was maintained during delay 1 (e.g., Fig. 2A, period 2), and 4 of the 6 also exhibited directional modulation during the action period with the same preferred direction as the cue 1 response (e.g., Fig. 2A, period 6). The remaining 2 neurons did not show significant modulation during the action period. Preferred directions of visual motion direction for 5 visual memory neurons were ipsilateral to the recoding side and that of the remaining neuron was contralateral to the recording side.
Sinusoidal pursuit was tested for 2 visual memory neurons that exhibited directional eye movement-related discharge during the action period. Consistent with their activity during the action period of go trials (e.g., Fig. 2A, period 6), the discharge of both neurons was modulated during sinusoidal pursuit as illustrated in Figure 2D.
Previous studies showed that nearly half of pursuit neurons in the caudal FEF exhibit visual motion responses to a moving spot with preferred directions similar to the pursuit-preferred direction when they were tested while monkeys fixated another stationary spot (Fukushima et al. 2000, 2002). In the present study, we recorded 32 neurons that exhibited directional visual motion responses to cue 1 but that did not exhibit direction-specific or instruction-specific discharge during delay 1 or delay 2. These neurons were included in “other task-related neurons” in Table 1 (see below). The majority of them (21/32 = 66%) also exhibited directional discharge modulation during the action period with the same preferred directions as the cue 1 visual motion responses. Discharge of a representative neuron recorded in the right caudal FEF is shown in Figure 2F–J. This neuron exhibited a visual motion response when cue 1 motion was rightward (but not leftward) during both go trials (Fig. 2F vs. G) and no-go trials (Fig. 2H vs. I). During the action period of go trials (but not no-go trials), it discharged during rightward (but not leftward) pursuit (Fig. 3F vs. G), indicating that this was a pursuit neuron (e.g., MacAvoy et al. 1991; Fukushima et al. 2000). Discharge during the action period of the remaining 10 neurons (of the 32) was not directional.
Figure 3.
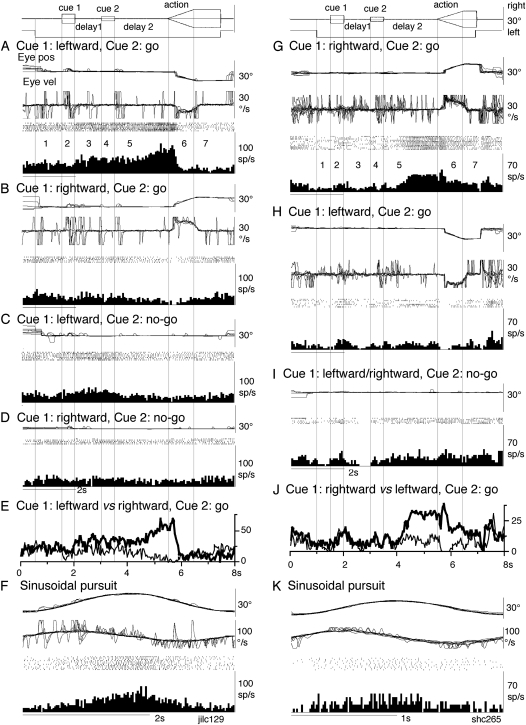
Discharge of representative visual memory + movement preparation neuron and movement preparation neuron in the caudal FEF. (A–F) A visual memory + movement preparation neuron recorded in the left caudal FEF. (G–K) A movement preparation neuron recorded in the right caudal FEF. (A,B and G,H) Go trials as indicated. (C,D and I) No-go trials as indicated. (E) Comparison of mean discharge rates of go trials during leftward pursuit (thick) versus rightward pursuit (thin). (J) Comparison of mean discharge rates of go trials during rightward pursuit (thick) versus leftward pursuit (thin). (F and K) Sinusoidal pursuit of a single spot. Traces from top to bottom in A–B and G–H are superimposed eye position (eye pos), eye velocity (Eye vel), spike rasters, and histograms of neuron discharge. Eye velocity during saccades was clipped. (C,D, and I) Similar presentation without eye velocity. Traces in F and K are eye position, eye velocity, spike rasters, and histogram of neuron discharge during sinusoidal pursuit of a single spot. For further explanation, see text.
Sinusoidal pursuit was tested in 13 of the 21 neurons that exhibited directional discharge modulation during the action period in addition to cue 1 responses. All of them were modulated during sinusoidal pursuit with preferred directions that were consistent with the preferred direction during the action period of memory-based pursuit (e.g., Fig. 2J vs. F). Sinusoidal pursuit was also tested in 4 of the remaining 11 (of the 32) neurons that exhibited directional cue 1 responses but their discharge during the action period was not directional. Only one of the 4 was modulated during sinusoidal pursuit. Thus, discharge modulation of these neurons during sinusoidal pursuit primarily reflected their modulation related to smooth pursuit eye movements per se.
Visual Memory + Movement Preparation Neurons
Discharge of a visual memory + movement preparation neuron recorded in the right caudal FEF is illustrated in Figure 3A–F. This neuron did not respond to cue 1 but increased discharge rate during delay 1 when cue 1 motion was leftward (but not rightward) in both go trials (Fig. 3A vs. B, period 3) and no-go trials (Fig. 3C vs. D, period 3). The difference is clear in Figure 3E that plots mean discharge rates of go trials when cue 1 motion was leftward versus rightward (thick and thin lines, respectively). The difference in discharge rate during delay 1 was maintained during cue 2 and delay 2 of go trials (Fig. 3A vs. B, also 3E, periods 4 and 5), and the higher discharge rate further increased shortly before the action period when the monkey prepared for leftward pursuit (Fig. 3A). During the action period, this neuron exhibited directional responses (i.e., increased discharge during leftward pursuit but decreased activity during rightward pursuit, Fig. 3A vs. B, period 6). However, the increased discharge was seen only during the initial phase of pursuit eye movements (Fig. 3A, period 6), different from the discharge of typical FEF pursuit neurons (e.g., Fig. 2A,F, period 6). During no-go trials, discharge was not significant during delay 2 and the action periods (Fig. 3C,D, periods 5–7).
All the 10 visual memory + movement preparation neurons (Table 1, group 2) increased the discharge rate difference further during the action period (e.g., Fig. 3E); 7 exhibited discharge only during the initial phase of the action period, similar to Figure 3A, whereas the remaining 3 discharged during the whole action period. Preferred directions for the discharge during the action period was contralateral to the recording side (n = 8), ipsilateral (n = 1), and downward (n = 1).
Similar to visual memory + movement preparations in the SEF (Shichinohe et al. 2009), the preferred direction of the visual memory response during delay 1 and that of movement preparation response during delay 2 were the same in all visual memory + movement preparation neurons recorded in the caudal FEF (Table 1).
Of the 10 visual memory + movement preparation neurons (Table 1), 7 were also tested during sinusoidal pursuit using a single spot; 4 were modulated with the same preferred directions, whereas 3 exhibited the opposite preferred direction, as that displayed during delay periods in the memory-based pursuit task. An example of the latter response is shown in Figure 3F; the preferred direction of this neuron during memory-based pursuit was leftward, while during sinusoidal pursuit, the peak activity occurred during rightward peak eye position (Fig. 3F), suggesting that discharge modulation of this neuron during sinusoidal pursuit may have reflected a movement preparation component for leftward pursuit.
Movement Preparation Neurons
Discharge of a movement preparation neuron is shown in Figure 3G–K. This neuron was recorded in the right caudal FEF and exhibited directional modulation during delay 2 and the action period of go trials before and during rightward pursuit (Fig. 3G vs. H) but not during no-go trials (Fig. 3I). The differential activity is clear in Figure 3J which plots mean discharge rates of go trials when cue 1 motion was rightward versus leftward (thick and thin lines, respectively). The clear difference was observed only during delay 2 and the action period.
Preferred direction of delay 2 modulation and that of the action period was the same and was ipsilateral to the recording side (n = 11), contralateral (n = 15), and downward (n = 2).
Of the 28 movement preparation neurons (Table 1, group 3), 15 exhibited discharge modulation during the whole action period (e.g., Fig. 3G), whereas the remaining 13 neurons discharged only during the initial phase of the action period, similar to the visual memory + movement preparation neuron illustrated in Figure 3A (period 6). In addition, 3 movement preparation neurons exhibited direction-specific modulation during due 1 (not shown).
Sinusoidal pursuit was tested for 14 movement preparation neurons; the majority of them (11/14 = 79%) were modulated during sinusoidal pursuit with the same preferred direction as during the action period of go trials (e.g., Fig. 3G vs. K). Responding neurons during sinusoidal pursuit included 7 neurons that exhibited discharge modulation during whole action period of memory-based pursuit and 4 neurons that discharged only during the initial phase of the action period of memory-based pursuit.
Comparison of Task-Related Neuron Groups between the Caudal FEF and SEF
Table 1 compares numbers and percentages of caudal FEF with SEF task-related neurons from our previous study (Shichinohe et al. 2009). Both the caudal FEF and SEF contained all the 4 groups of neurons (Table 1, neuron groups 1–4), indicating that the 2 regions carried qualitatively similar signals. However, there were quantitative differences in the signals represented in the 2 regions; the percentages of visual memory + movement preparation neurons (10/185 = 5%a vs. 25/208 = 12%b) and no-go neurons (16/185 = 9%c vs. 50/208 = 24%d) were significantly lower in the caudal FEF than those in the SEF (Table 1, Chi-square test, P < 0.05).
Table 2 further compares the percentages of visual memory coding neurons (Table 1, neuron groups 1 + 2) and movement preparation coding neurons (Table 1, neuron groups 2 + 3) in the 2 regions. Although there was no significant difference in the percentages of neurons coding movement preparation in the 2 regions (Table 2), there was a significant difference in the percentages of neurons coding directional visual memory (caudal FEFa < SEFb, Chi-square test, P < 0.05).
Table 2.
Comparison of caudal FEF and SEF neurons that exhibited visual motion memory responses and movement preparation responses
| % Visual memory coding neurons | % Movement preparation coding neurons | |
| Caudal FEF | 8 (16/185)a | 20 (38/185) |
| SEF | 19 (39/208)b | 22 (45/208) |
Note: Superscripts in % of visual memory coding neurons indicate that differences in the percentages (of total task-related neurons) between caudal FEF and SEF neurons (a vs. b) were significant (P < 0.05). Data for SEF neurons were taken from Shichinohe et al. (2009). For further explanation, see text.
We also compared the latencies of neurons that exhibited directional visual motion responses with cue 1 in the caudal FEF and SEF. In the caudal FEF, 46 neurons exhibited such responses. Figure 4 plots latencies of 44 neurons that exhibited excitation; these neurons included 6 visual memory neurons, 4 visual memory + movement preparation neurons, 3 movement preparation neurons, and 31 neurons that were classified as other task-related neurons (Table 1). Latencies of SEF neurons were taken from previous study (Shichinohe et al. 2009). The 2 distributions were significantly different (Mann–Whitney U test, P < 0.01, 2 tailed). Neurons with shorter visual motion latencies were observed more frequently in the caudal FEF than SEF (Fig. 4A vs. B, see Discussion).
Figure 4.
Comparison of latencies of visual motion responses of caudal FEF and SEF neurons to cue 1. Latencies of individual neurons in the 2 regions that exhibited a directional visual motion response to cue 1 are summarized in A and B. In the caudal FEF (A), latencies were plotted for 6 visual memory neurons, 4 visual memory + movement preparation neurons, 3 movement preparation neurons, and 31 neurons that exhibited directional visual motion response to cue 1. For SEF, data were reanalyzed from Shichinohe et al. (2009).
To further compare direction-specific discharge modulation during go trials in the caudal FEF and SEF, Figure 2E plots percentages of modulated neurons (of the total task-related neurons) that showed direction-specific modulation in each period (Fig. 1, 2–7) for the caudal FEF and SEF. There were significant differences (Chi-square test, P < 0.05) in percentages of modulated neurons between the 2 regions during delay 1 and the action period (Fig. 2E, *); the percentage of modulated neurons in the caudal FEF was significantly lower than that of SEF during delay 1 but higher than that of SEF during the action period. No significant differences between the 2 regions were detected in other periods (Fig. 2E).
Comparison of Delay 1 and Delay 2 Discharge between the Caudal FEF and SEF
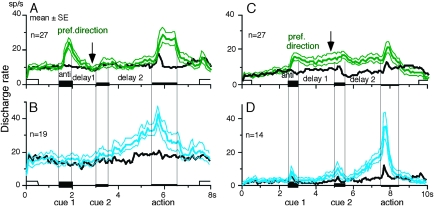
To examine how the difference in the visual motion memory coding responses (Table 2) was reflected in the time course of mean discharge of neurons responding to cue 1 in the caudal FEF and SEF, Figure 5A plots mean ± standard error (SE) discharge of caudal FEF neurons that exhibited directional responses to cue 1 in their preferred (green) and antipreferred directions (black) during go trials. Included in this figure were 6 visual memory neurons, 4 visual memory + movement preparation neurons, and 17 other task-related neurons that exhibited directional visual motion response during cue 1 that were tested in the same task condition (i.e., the durations of delay 1 and delay 2 were set at 1 and 2 s, respectively). Although caudal FEF neurons exhibited a residual visual motion response to cue 1 at the beginning of delay 1, the responses returned to the control level near the end of the delay 1 before cue 2 onset (Fig. 5A, arrow). This contrasts with SEF neurons that exhibited directional responses to cue 1 visual motion as illustrated in Figure 5C (from our previous study, Shichinohe et al. 2009); cue 1 discharge was maintained during the whole delay 1 period (2 s, Fig. 5C arrow).
Figure 5.
Comparison of the time course of mean (±SE, thin lines) discharge rates of caudal FEF and SEF neurons during go trials. In A, caudal FEF neurons (n = 27) that exhibited a directional responses to cue 1 visual motion were selected to show the time course of the discharge modulation in the preferred direction for each neuron (green) and antipreferred direction (black). Included in A were 6 visual memory neurons, 4 visual memory + movement preparation neurons, and 17 neurons that exhibited directional visual motion response during cue 1 that were tested in the same task condition (i.e., the durations of delay 1 and delay 2 were set at 1 and 2 s, respectively). In C, SEF neurons (n = 27) that exhibited directional responses to cue 1 visual motion were selected to show the time course of the discharge modulation in the preferred direction for each neuron (green) and antipreferred direction (black). B and D are the time course of discharge modulation of movement preparation neurons in FEF (B, n = 19) and SEF (D, n = 14) in the preferred direction for each neuron (light blue) and antipreferred direction (black). C and D are taken from Shichinohe et al. (2009). For further explanation, see text.
Figure 5B illustrates mean (±SE) discharge of movement preparation neurons of the caudal FEF (n = 19) in their preferred direction (light blue) and antipreferred direction (black) in the same task condition (i.e., the durations of delay 1 and delay 2 were set at 1 and 2 s, respectively). For comparison, Figure 5D shows mean (±SE) discharge of SEF movement preparation neurons (n = 14) in their preferred direction (light blue) and antipreferred direction (black) from previous study (Shichinohe et al. 2009). The time course of the mean discharge of movement preparation neurons in the 2 regions was similar.
No-go Neurons
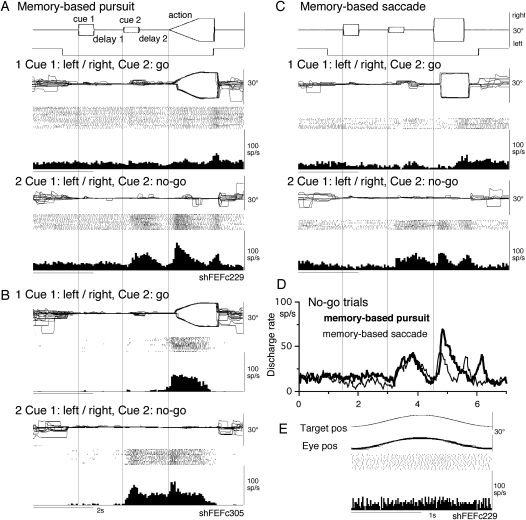
Figure 6A illustrates the discharge of a representative no-go neuron in the left caudal FEF. This neuron did not exhibit significant modulation during smooth pursuit of a single spot moving sinusoidally (Fig. 6E), indicating that this was not a pursuit neuron (e.g., MacAvoy et al. 1991; Fukushima et al. 2000). During go trials of our memory-based pursuit task, discharge modulation of this neuron during the action period was not significant except for a weak discharge during recentering saccades at the beginning of the reward period (Fig. 6A1). However, during no-go trials, it clearly increased its discharge during cue 2 and delay 2 in addition to the modulation during the action period (Fig. 6A2). SEF no-go neurons discharged during the action period of go trials but without directional selectivity (Shichinohe et al. 2009). Six of the 16 no-go neurons (Table 1) also discharged during the action period of go trials as illustrated in another neuron in Figure 6B recorded in the right caudal FEF, but the discharge was nondirectional (Fig. 6B1). During no-go trials (Fig. 6B2), this neuron discharged clearly after cue 2 and during delay 2 in addition to the discharge during the action period. Discharge of the remaining 10 neurons during the action period of go trials was not significant (e.g., Fig. 6A1).
Figure 6.
Discharge of no-go neurons in the caudal FEF. A, C, D, and E were taken from a single neuron recorded in the left caudal FEF. (B) From another neuron recorded in the right caudal FEF. (A and B) Memory-based smooth pursuit task. (C) Memory-based saccade task. (A1, B1, and C1) Go trials. Trials with leftward and rightward cue 1 motion were combined. (A2, B2, and C2) no-go trials. Trials with leftward and rightward cue 1 motion were combined. (D) Comparison of discharge modulation during no-go trials during memory-based smooth pursuit (thick) and memory-based saccades (thin). (E) Simple pursuit of a single spot that moved sinusoidally at 0.5 Hz. Neither neuron responded during simple pursuit of a single spot.
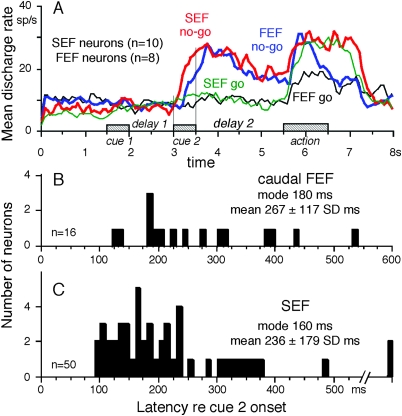
Figure 7A compares the time course of mean discharge rates of no-go neurons in the caudal FEF (n = 8) and SEF (n = 10) during no-go trials (blue vs. red) and go trials (black vs. green) that were tested in the same task condition (i.e., when the durations of delay 1 and delay 2 were set at 1 and 2 s, respectively). During no-go trials, the time courses of discharge modulation of the 2 groups of neurons during cue 2 and delay 2 were similar and the discharge was clearly higher than that of go trials during cue 2 and delay 2. Higher discharge modulation was also observed during the action period of no-go trials (Fig. 7A, blue and red). The mean latency of the averaged activity after the onset of cue 2 was shorter for SEF neurons than that of caudal FEF neurons (Fig. 7A, red vs. blue). The mean latency difference was ∼100 ms.
Figure 7.
Comparison of no-go discharge in the caudal FEF and SEF. (A) The time course of mean discharge of no-go neurons in the caudal FEF (n = 8) and SEF (n = 10) during no-go trials (blue vs. red) and go trials (black vs. green) in the same task conditions when the durations of delay 1 and delay 2 were set at 1 and 2 s, respectively. (B and C) Latency histograms (relative to cue 2 onset) of no-go discharge for caudal FEF no-go neurons (B) and SEF no-go neurons (C). Modal and mean (±SD) values are indicated (insets).
Figure 7B,C compares the distribution of latencies of no-go discharge relative to the onset of cue 2 for caudal FEF neurons (B) and SEF neurons (C). Although the percentage of no-go neurons (among the total task-related neurons) in the caudal FEF was significantly lower than that in the SEF as described above (Table 1, neuron group 4), latencies of no-go discharge were similarly distributed in the 2 regions (Mann–Whitney U test, P = 0.07, 2 tailed, Fig. 7C vs. B). In particular, the modal value of caudal FEF no-go neurons was 180 ms and was similar to the modal value of SEF no-go neurons of 160 ms. However, only 2 of the 16 (13%) caudal FEF no-go neurons exhibited latencies shorter than 160 ms, whereas 40% (20/50) of SEF no-go neurons exhibited latencies shorter than 160 ms, indicating that neurons with shorter latencies were observed more frequently in the SEF than the caudal FEF.
Our previous study showed that SEF no-go neuron discharge during memory-based saccades as well (Shichinohe et al. 2009). The representative neuron shown in Figure 6A also discharged during saccadic no-go trials as shown in Figure 6C (C2 vs. C1). The time course of no-go-related discharge was similar during memory-based smooth pursuit and memory-based saccades as shown in Figure 6D (thick vs. thin), suggesting that no-go-related discharge was common for the 2 tasks as observed in SEF no-go neurons (Shichinohe et al. 2009).
Other Task-Related Neurons
In the present study, the majority of task-related neurons in the caudal FEF (125/185 = 68%) did not exhibit direction-specific or instruction-specific discharge during delay 1 or delay 2 (Table 1, other task-related neurons). These neurons exhibited virtually any combinations of responses from cue 1 to the action period discharge (Fig. 1). Here, we briefly describe their discharge characteristics. Of the 125, 32 neurons exhibited directional responses during cue 1 visual motion and 21 of the 32 also exhibited directional discharge modulation during the action period as described above (e.g., Fig. 2F). Sixteen exhibited only directional eye movement related discharge during the action period of go trials. Thirteen were modulated during the action period with directional selectivity but were also modulated during delay 1 and/or delay 2 without direction specificity or instruction specificity. Twelve neurons responded to cue 1 but without directional specificity; some were modulated during delay 1 and/or delay 2 without directional specificity. Ten were modulated during the action period only without directional specificity. The remaining 42 neurons (of the 125) exhibited significant discharge modulation during delay 1 and/or delay 2 but their modulation was nondirectional and instruction nonspecific; for example, they discharged during trials with both leftward and rightward cue 1 motion. Many of them also exhibited discharge modulation during delay 2 of both go and no-go trials. Thus, 50 (=21 + 16 + 13) of the 125 neurons that exhibited direction-specific discharge during the action period were pursuit neurons, and their preferred directions were widely distributed; nearly one-third were primarily ipsilateral (n = 19), another one-third primarily contralateral (n = 19), and the remaining neurons were purely vertical (i.e., 7 downward and 5 upward).
Sinusoidal pursuit was tested using a single spot in 17 neurons that exhibited directional eye movement modulation during the action period. Of these, 13 neurons also exhibited directional responses during cue 1 visual motion as described above (Fig. 2J), and the remaining 4 showed directional-specific modulation only during the action period. All of them were modulated during sinusoidal pursuit. In contrast, only about one third of the other neurons tested were modulated during sinusoidal pursuit (11/37); these included 1/4 of neurons that exhibited directional visual motion responses to cue 1 but their discharge during other periods varied, 1/4 of the neurons that responded to cue 1 but without directional specificity, 0/3 of the neurons that exhibited modulation during the action period without directional selectivity, and 9/26 of the other neurons that exhibited nondirectional and instruction nonspecific discharge modulation during delay 1 or delay 2. These results suggest that, although sinusoidal pursuit consistently activated pursuit neurons, it also modulated other task-related neurons less frequently.
Chemical Inactivation of the Caudal FEF
Lesion or chemical inactivation of the caudal FEF pursuit area in monkeys impairs smooth pursuit eye movements as first reported by Lynch (1987) and later by others (e.g., MacAvoy et al. 1991; Keating 1991, 1993; Shi et al. 1998; Fukushima et al. 1999). To examine what effects chemical inactivation of the caudal FEF exert on memory-based smooth pursuit eye movements, we injected muscimol into the caudal FEF at the locations where we recorded responsive neurons. Unilateral injection was tested 3 times and bilateral injection was tested once (see Materials and Methods). Results were consistent for each injection in the 2 monkeys.
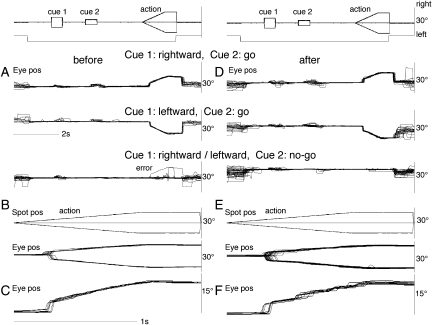
Representative results are shown in Figure 8 before (A–C) and after (D–F) infusion into the left caudal FEF (Fig. 11A, *) for either rightward or leftward cue 1 motion. Before infusion (Fig. 8A–C), our monkeys performed the task well with few errors (Fig. 8A, error) in both go- and no-go trials. Unlike SEF injection (Shichinohe et al. 2009), muscimol injection (1 μL = 10 μg) into the caudal FEF pursuit area did not result in a significant change in error rates in pursuit direction or in go/no-go selection. After injection (Fig. 11A, *), the monkey performed the task without any errors (Fig. 8D). The mean error rate before infusion in 3 unilateral injections was 5% (range 0–9%). After infusion, mean error rates did not change significantly (5%, range 0–7%) from the control.
Figure 8.
Effects of chemical inactivation of the unilateral caudal FEF on memory-based smooth pursuit eye movements. Eye position traces (A, D) were aligned with the onset of cue 1 before (A) and after muscimol infusion (1 μL = 10 μg) into the left caudal FEF (D). (B and E) Spot position and eye position traces were aligned with the onset of action period before (B) and after (E) muscimol infusion. (C and F) Typical eye position traces aligned with the onset of action period before (C) and after (F) muscimol infusion to show smooth (C) and saccadic (F) nature of tracking eye movements.
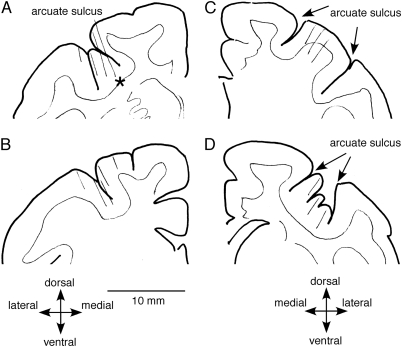
Figure 11.
Recording locations. Schematic coronal sections of monkey Sh are shown for left frontal cortex (A, B) and right frontal cortex (C, D). Thin oblique lines indicate recording tracks. Responsive neurons were recorded mostly in the fundus of the arcuate sulcus (A, D) and in the close vicinity including the posterior bank (B) of the arcuate sulcus. Sections A and B were 1.0 mm apart. Similarly, sections C and D were 1.0 mm apart. A muscimol infusion site was estimated by asterisk in A.
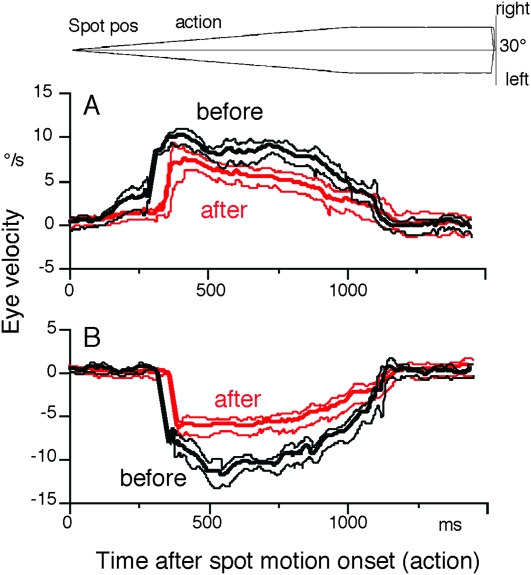
A clear difference after muscimol infusion was observed during the action period. As shown in Figure 8E–F, smooth pursuit eye movements became saccadic compared with the control (see Fig. 8C vs. F for typical eye position traces before vs. after injection). Figure 9A illustrates mean ± SD desaccaded eye velocity during contraversive (i.e., rightward) pursuit before and after infusion (black vs. red). After unilateral (left) muscimol infusion (Fig. 11A, *), both initial pursuit eye velocity before catch-up saccades and steady-state eye velocity after catch-up saccades were significantly reduced (Fig. 9A). Also, the latencies of catch-up saccades were delayed (Fig. 9A). Although during ipsiversive (i.e., leftward) pursuit (Fig. 9B), initial pursuit eye velocity before catch-up saccades was not clearly observed before infusion, the latencies of catch-up saccades were clearly delayed and pursuit eye velocity immediately after the catch-up saccades decreased together with the decrease in steady-state eye velocity (Fig. 9B, black vs. red). Mean peak eye velocity gain immediately after catch-up saccades (eye velocity/spot velocity) after unilateral injection decreased by 0.5 (from 1.15 to 0.65) for ipsiversive pursuit and by 0.4 for contraversive pursuit (from 1.1 to 0.74). Steady-state eye velocity during 0.5–1 s after the onset of spot motion also decreased by 0.5 for both ipsiversive and contraversive pursuit. The monkeys compensated for low eye velocity by catch-up saccades during ocular tracking (e.g., Fig. 8F).
Figure 9.
Impairment of pursuit eye velocity after chemical inactivation of the unilateral caudal FEF. (A) Mean ± SD desaccaded averaged eye velocities aligned with the onset of action period during rightward pursuit before (black) and after (red) muscimol. (B) Mean ± SD desaccaded averaged eye velocities aligned with the onset of action period during leftward pursuit before (black) and after (red) muscimol. In A and B, desaccaded portions during initial catch-up saccades were connected by straight lines.
In one monkey (Sh), we injected muscimol into the caudal FEF bilaterally and the results were similar; the monkey did not exhibit direction errors or go/no-go selection errors. Similar to the results shown in Figures 8 and 9, impairment was confined to pursuit eye movements.
Figure 10A,B illustrates the effects of the same unilateral injection (Fig. 11A, *) on sinusoidal pursuit of a single spot at 1 Hz (±10°) before (Fig. 10A) and after muscimol injection into the caudal FEF (Fig. 10B). Clearly after injection, pursuit eye velocity decreased resulting in catch-up saccades (Fig. 10B). Figure 10C compares desaccaded and averaged eye velocity before and after muscimol injection. Peak eye velocity lagged after injection (Fig. 10C, *). Figure 10D,E summarizes phase and gain (retarget velocity) of desaccaded and averaged eye velocity before and after infusion (open vs. filled squares, respectively). Chemical inactivation of the caudal FEF not only decreased eye velocity gain (Fig. 10E) as reported by many researchers (e.g., Lynch 1987; MacAvoy et al. 1991; Keating 1991, 1993; Shi et al. 1998) but also impaired delay compensation of pursuit eye movements during sinusoidal pursuit at higher target frequencies (∼1 Hz, Fig. 10D). These results suggest that the caudal FEF is necessary for response delay compensation during sinusoidal pursuit (see Discussion).
Figure 10.
Effects of chemical inactivation of the unilateral caudal FEF on sinusoidal pursuit of a single spot. (A and B) Superimposed eye position (pos) and eye velocity (vel) before (A) and after (B) muscimol infusion (1 μL) into the left caudal FEF. Eye velocity traces during saccades (B) are clipped. C compares desaccaded and averaged eye velocity before and after muscimol infusion into the caudal FEF. Arrow indicates that peak eye velocity lagged after muscimol infusion. D and E plot phase and gain of eye velocity (relative to target velocity) before (open) and after (filled) muscimol infusion into the left caudal FEF.
Recording Locations
Figure 11 illustrates representative recording tracks in monkey Sh. Recording tracks for responsive neurons were found in the fundus of the arcuate sulcus and in the surrounding vicinity, including the posterior bank of the arcuate sulcus bilaterally (Fig. 11A–D). Recording tracks were found in similar locations in monkey J (not shown).
Discussion
Using the same monkeys employed in our analysis of SEF neuron discharge during memory-based smooth pursuit (Shichinohe et al. 2009), the present results indicate significant differences between the caudal FEF and SEF in the signals represented and in the effects of chemical inactivation of the 2 regions as discussed below.
Differences in the Signals Represented in the Caudal FEF and SEF
Direction- and Instruction-Specific Neurons in Delay 1 and/or Delay 2
Since anatomical studies demonstrate reciprocal connections between the SEF and FEF (Huerta et al. 1987), and since neurons in the FEF have been shown to exhibit visual latencies comparable with those in MT and MST and sometimes even as early as some neurons in V1 (Schmolesky et al. 1998), the possibility exists that SEF signals reflecting memory of visual motion direction come from the FEF (see Introduction). Latency comparison of neurons in the 2 regions that exhibited direction-specific visual motion response to cue 1 indeed showed significant differences (Fig. 4A vs. B), consistent with previous results (Schmolesky et al. 1998). Further comparison of direction- and instruction-specific neurons during delay 1 and/or delay 2 indicate that qualitatively similar signals were represented in the 2 regions including visual memory neurons and visual memory + movement preparation neurons (Table 1). However, our results indicate significant differences between the 2 regions in the percentages of modulated neurons that coded visual memory signals (Fig. 2E, delay 1, SEF > FEF), action-related signals (Fig. 2E, action, FEF > SEF), and no-go signals (Table 1, neuron group 4, SEF > FEF). The presence of more frequent action-related signals in the caudal FEF (Fig. 2E) is also reflected in the discharge of visual memory neurons and visual memory + movement preparation neurons; most of these neurons also carried direction-specific action-related signals (e.g., Figs 2A and 3A; see below).
Although we did not find a significant difference in the percentage of visual memory neurons in the 2 regions (Table 1, neuron group 1), this may be due to the small numbers of responsive neurons in the caudal FEF. An actual difference might appear if the sample was larger and the following argue in support of such a difference. First, as summarized in Table 1, a significant difference was observed in the percentages of visual memory + movement preparation neurons (but not movement preparation neurons) between the 2 regions; and second, by comparing the percentages of visual memory coding neurons (Table 1, neuron groups 1 + 2) and movement preparation coding neurons (Table 1, neuron groups 2 + 3) in the 2 regions (Table 2), a significant difference was observed in the former but not in the latter (Table 2).
The differences in signals represented in the caudal FEF and SEF, especially in visual memory responses (Table 2), were reflected in the mean responses of the neuron population in the 2 regions that showed directional visual motion responses to cue 1 (Fig. 5A vs. C). Although visual motion responses of caudal FEF were observed at the beginning of delay 1, they were not maintained but decayed exponentially before the onset of cue 2 (Fig. 5A, arrow). In contrast, cue 1 discharge was maintained during the whole delay 1 period in the SEF, reflecting the importance of visual memory signals in the SEF (Fig. 5C, arrow, Shichinohe et al. 2009). These results do not support the possibility that the SEF signals reflecting memory of visual motion direction come from the FEF (see Introduction, cf., Fukushima et al. 2002).
We still do not know exactly where the SEF visual memory signals are generated (Shichinohe et al. 2009; see Introduction). The dorsolateral prefrontal cortex contains neurons that respond to visual motion (Kim and Shadlen 1999; Zaksas and Pasternak 2006). This region has been linked to temporal storage of sensory signals (Goldman-Rakic 1995). Kim and Shadlen (1999) have demonstrated that visual motion responses can be maintained during a delay period in prefrontal cortex neurons. However, in their studies, discharge related to the memory of visual motion could not be separated from discharge related to movement preparation (also Zaksas and Pasternak 2006).
Another potential site is MST, since this region, especially the dorsomedial MST (MSTd) (Desimone and Ungerleider 1986; for a review, see Leigh and Zee 2006), sends direct projections to the SEF (see Fig. 13P,Q, and R of Huerta and Kaas 1990), and the MSTd has been suggested to be involved in perception and/or memory of visual motion (e.g., Celebrini and Newsome 1994; Britten and van Wezel 2002; Gu et al. 2007; Liu and Angelaki 2009). However, representative signals in the MSTd clearly differed from those in the SEF during memory-based smooth pursuit eye movements; none of the 108 MSTd neurons that showed directional visual motion response to cue 1 exhibited direction- and/or instruction-specific discharge during the delay periods (Kurkin, Shichinohe et al. 2009). Although we do not exclude the possibility that there may be another type of MSTd neurons coding assessment and memory of visual motion direction (Ferrera and Lisberger 1997), it seems more likely that visual motion direction information that is sent from MST and caudal FEF to the SEF is further processed to create assessment and the memory of visual motion direction within the SEF.
Our study shows the existence of no-go neurons in the caudal FEF during memory-based smooth pursuit, although the percentage of no-go neurons among task-related neurons was significantly lower in the caudal FEF than that in the SEF (Table 1, Shichinohe et al. 2009). No-go neurons were reported earlier in a saccadic go/no-go task in the SEF region (Mann et al. 1988) and prefrontal cortex and FEFs (Hasegawa et al. 2004). Our results also show that, like SEF no-go neurons, no-go signals in the caudal FEF discharged similarly for both saccadic and smooth pursuit eye movements in our task conditions (Fig. 6D).
We still do not know the function of no-go neurons in the caudal FEF. However, depending on whether or not they discharged during the action period of go trials, there were 2 types; the majority (10/16 = 63%) did not exhibit significant modulation during the action period of go trials (Fig. 6A1). But like SEF no-go neurons, the remaining neurons (6/16) discharged without directional selectivity (Fig. 6B1; also Shichinohe et al. 2009). Because both types of no-go neurons did not respond during simple pursuit or to cue 1 visual motion (Fig. 6E), it is unlikely that their discharge reflected either a motor command or a visual response to spot motion. The 2 types of no-go neurons may have different functions. It is possible that no-go neurons that did not discharge during the action period of go trials may be involved in inhibiting a possible motor command (e.g., Mann et al. 1988; Hasegawa et al. 2004), although we do not exclude the possibility that they may participate in memory of no-go instruction during the action period as well (Fig. 1). The second type of no-go neurons may also participate in memory of no-go instruction during the action period. It is also possible that they may partly contribute to performance monitoring as we suggested earlier for SEF no-go neurons (see Discussion of Emeric et al. 2008).
Other Task-Related Neurons
In the present study, task-related neurons that did not exhibit direction- or instruction-specific discharge during delay 1 or delay 2 were observed more frequently in the caudal FEF than SEF (Table 1, other task-related neurons, 125/185 = 68% vs. 99/208 = 48%, Chi-square test, P < 0.05). These neurons included those that exhibited direction-specific discharge during cue 1 and/or the action period of go trials. More specifically, of 50 neurons that exhibited direction-specific discharge during pursuit eye movements of go trials (i.e., pursuit neurons), nearly half (21/50) exhibited visual motion response to cue 1 with the same preferred directions (e.g., Fig. 2F–J). These results are consistent with those of previous studies in which visual responses of pursuit neurons to a moving spot were tested during fixation of another stationary spot (Fukushima et al. 2000, 2002).
The majority of other task-related neurons exhibited significant discharge modulation during delay 1 or delay 2 but their discharge was nondirectional and/or instruction nonspecific. It has been shown that some FEF neurons carry attention-related signals (e.g., Gregoriou et al. 2009; Zhou and Thompson 2009). It is possible that nondirectional/instruction nonspecific discharge may contribute to attention. Contribution of attention is suggested in the present study by the difference in discharge modulation during cue 2 of go trials; the identical cue 2 stimulus resulted in responses with different magnitude depending on whether cue 1 visual motion was applied in the preferred direction or antipreferred direction of these neurons (e.g., new Fig. 3E, period 4). However, this difference in response to identical cue 2 was not observed in the population responses of FEF neurons tested (Fig. 5A) but clearly seen in SEF neurons (Fig. 5C).
Differences in the Effects of Chemical Inactivation of the Caudal FEF and SEF
The differences in direction- and/or instruction-specific signals during delay 1 and/or delay 2 represented in the caudal FEF and SEF discussed above are consistent with the differences in the effects of chemical inactivation of the 2 regions on memory-based smooth pursuit eye movements. Lesion or chemical inactivation of the caudal FEF impairs smooth pursuit eye movements as first demonstrated by Lynch (1987), whereas SEF lesions do not induce clear effects on pursuit eye movements per se (see a review by Tehovnik et al. 2000). The present results and our previous study (Shichinohe et al. 2009) indicate that chemical inactivation of the caudal FEF impaired pursuit eye movements, consistent with previous observations (for a review, see Leigh and Zee 2006) but did not induce errors in pursuit eye movement direction or go/no-go selection in our task (e.g., Fig. 8D). On the other hand, SEF (but not caudal FEF) inactivation results in direction errors for pursuit eye movements and go/no-go selection errors as reported previously (Shichinohe et al. 2009). Thus, the muscimol effects could be interpreted as the loss of major signals represented in the 2 areas.
Of note, our unilateral inactivation of the caudal FEF impaired pursuit eye movements bidirectionally (Fig. 9A,B). The bidirectional deficit of pursuit after unilateral inactivation or surgical lesion of the simian caudal FEF has been reported earlier (e.g., Lynch 1987; Keating 1993; Shi et al. 1998; for a review, see Sharpe 2008) and is consistent with representation of preferred direction of pursuit neurons; unlike FEF saccade neurons that primarily prefer contraversive saccades, preferred directions of pursuit neurons in the caudal FEF of one hemisphere are distributed in all directions (for a review, see Leigh and Zee 2006).
We interpret the impaired response delay compensation after caudal FEF inactivation results (Fig. 10C,D) as reflecting primarily impaired generation of motor commands (Fig. 2E, action).
Different Roles of the Caudal FEF and SEF in Memory-Based Smooth Pursuit Eye Movements
The primate frontal cortex contains 2 pursuit-related areas: the caudal FEF and SEF. Many previous studies have examined signals represented in the 2 regions during smooth pursuit eye movements (for a review, see Leigh and Zee 2006). Although some differences in signals from the 2 regions have been reported (for a review, see Fukushima et al. 2006), the roles of the 2 regions in predictive pursuit remain poorly understood since lesions or chemical inactivation of the SEF do not cause clear impairment of smooth pursuit eye movements per se, as described above (see also Tehovnik et al. 2000; Introduction of Shichinohe et al. 2009).
Our memory-based smooth pursuit task distinguishes neuronal discharge related to movement preparation from the discharge related to the processing of target motion signals or their memory. Using this task, the present results and our previous study (Shichinohe et al. 2009) have revealed differences in the signals represented in the caudal FEF and SEF and in the effects of chemical inactivation of the 2 regions. These differences suggest distinct functions for the 2 regions in that the SEF is primarily involved in planning smooth pursuit by coding signals for assessment and memory of visual motion direction, the decision not-to-pursue, and preparation for pursuit (also Mann et al. 1988; Kim and Shadlen 1999; Kim et al. 2005; de Hemptinne et al. 2008), whereas the caudal FEF is primarily involved in generating motor commands for pursuit eye movements by coding signals for preparation and execution of smooth pursuit (e.g., Fig. 2E, Tables 1 and 2). Taken together, these results suggest that both the SEF and caudal FEF are necessary for planning and generating motor commands for required eye movements appropriate for the task conditions and that the 2 regions have different but complimentary roles.
Funding
Grant-in-Aid for Scientific Research on Priority Areas (System study on higher order brain functions) (17022001, 18023004, 2002300409, 21119501) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; National Institutes of Health Grants EY-06558 (NEI) and RR-00166.
Acknowledgments
We thank Dr Peter M. Olley of Sappporo Medical University for his valuable comments on the manuscript and Mr Motoyuki Kanashima for technical assistance. Conflict of Interest: None declared.
References
- Akao T, Kurkin S, Fukushima J, Fukushima K. Visual and vergence eye movement related responses of pursuit neurons in the caudal frontal eye fields to motion-in-depth stimuli. Exp Brain Res. 2005;164:92–108. doi: 10.1007/s00221-004-2213-6. [DOI] [PubMed] [Google Scholar]
- Akao T, Kurkin S, Fukushima J, Fukushima K. Otolith inputs to pursuit neurons in the frontal eye fields of alert monkeys. Exp Brain Res. 2009;193:455–466. doi: 10.1007/s00221-008-1644-x. [DOI] [PubMed] [Google Scholar]
- Assad JA, Maunsell JHR. Neuronal correlates of inferred motion in primate posterior parietal cortex. Nature. 1995;37:518–521. doi: 10.1038/373518a0. [DOI] [PubMed] [Google Scholar]
- Barborica A, Ferrera VP. Estimating invisible target speed from neuronal activity in monkey frontal eye field. Nat Neurosci. 2003;6:66–74. doi: 10.1038/nn990. [DOI] [PubMed] [Google Scholar]
- Barnes GR, Asselman PT. The mechanism of prediction in human smooth pursuit eye movements. J Physiol (Lond) 1991;439:439–461. doi: 10.1113/jphysiol.1991.sp018675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker W, Fuchs AF. Prediction in the oculomotor system: smooth pursuit during transient disappearance of a visual target. Exp Brain Res. 1985;57:562–575. doi: 10.1007/BF00237843. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Zalsas D, Droll JA, Pasternak T. Activity of neurons in cortical area MT during a memory for motion task. J Neurophysiol. 2004;91:286–300. doi: 10.1152/jn.00870.2003. [DOI] [PubMed] [Google Scholar]
- Britten KH, van Wezel RJ. Area MST and heading perception in Macaque monkeys. Cereb Cortex. 2002;12:692–701. doi: 10.1093/cercor/12.7.692. [DOI] [PubMed] [Google Scholar]
- Celebrini S, Newsome WT. Neuronal and psychophysical sensitivity to motion signals in extrastriate area MST of the macaque monkey. J Neurosci. 1994;14:4109–4124. doi: 10.1523/JNEUROSCI.14-07-04109.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL, Wise SP. Neuronal activity in the supplementary eye field during acquisition of conditional oculomotor associations. J Neurophysiol. 1995;73:1101–1121. doi: 10.1152/jn.1995.73.3.1101. [DOI] [PubMed] [Google Scholar]
- Collins CJ, Barnes GR. Scaling of smooth anticipatory eye velocity in response to sequences of discrete target movements in humans. Exp Brain Res. 2005;167:404–413. doi: 10.1007/s00221-005-0044-8. [DOI] [PubMed] [Google Scholar]
- de Hemptinne C, Lefevre P, Missal M. Neuronal basis of directional expectation and anticipatory pursuit. J Neurosci. 2008;28:4298–4310. doi: 10.1523/JNEUROSCI.5678-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Ungerleider LG. Multiple visual areas in the superior temporal sulcus of the macaque. J Comp Neurol. 1986;248:164–189. doi: 10.1002/cne.902480203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeric EE, Brown JW, Leslie M, Pouget P, Stuphorn V, Schall JD. Performance monitoring local field potentials in the medial frontal cortex of primates: anterior cingulate cortex. J Neurophysiol. 2008;99:759–772. doi: 10.1152/jn.00896.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrera VP, Lisberger SG. Neuronal responses in visual areas MT and MST during smooth pursuit target selection. J Neurophysiol. 1997;78:1433–1446. doi: 10.1152/jn.1997.78.3.1433. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movements chronically in the monkey. J Appl Physiol. 1966;21:1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Akao T, Kurkin S, Kaneko CRS, Fukushima K. The vestibular-related frontal cortex and its role in smooth-pursuit eye movements and vestibular-pursuit interactions. J Vestib Res. 2006;16:1–22. [PMC free article] [PubMed] [Google Scholar]
- Fukushima J, Akao T, Shichinohe N, Kurkin S, Kaneko CRS, Fukushima K. Neuronal activity in the caudal frontal eye fields (FEF): comparison with the supplementary eye fields (SEF) and cerebellar dorsal vermis during memory-based smooth pursuit eye movements. Program No. 559.7. Abstract Viewer/Itinerary Planner. Chicago (IL): Society for Neuroscience, Washington, DC; 2009. [Google Scholar]
- Fukushima K, Akao T, Shichinohe N, Nitta T, Kurkin S, Fukushima J. Predictive signals in the pursuit area of the monkey frontal eye fields. Prog Brain Res. 2008;171:433–440. doi: 10.1016/S0079-6123(08)00664-X. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Fukushima J, Sato T. Vestibular-pursuit interactions: gaze velocity and target velocity signals in the monkey frontal eye fields. Ann N Y Acad Sci. 1999;871:248–259. doi: 10.1111/j.1749-6632.1999.tb09189.x. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Sato T, Fukushima J, Shinmei Y, Kaneko CRS. Activity of smooth pursuit-related neurons in the monkey periarcuate cortex during pursuit and passive whole-body rotation. J Neurophysiol. 2000;83:563–587. doi: 10.1152/jn.2000.83.1.563. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Yamanobe T, Shinmei Y, Fukushima J. Predictive responses of periarcuate pursuit neurons to visual target motion. Exp Brain Res. 2002;145:104–120. doi: 10.1007/s00221-002-1088-7. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Gottlieb JP, Bruce CJ, MacAvoy MG. Smooth eye movements elicited by microstimulation in the primate frontal eye field. J Neurophysiol. 1993;69:786–799. doi: 10.1152/jn.1993.69.3.786. [DOI] [PubMed] [Google Scholar]
- Gottlieb JP, MacAvoy MG, Bruce CJ. Neural responses related to smooth pursuit eye movements and their correspondence with electrically elicited slow eye movements in the primate frontal eye field. J Neurophysiol. 1994;72:1634–1653. doi: 10.1152/jn.1994.72.4.1634. [DOI] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324:1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, DeAngelis GC, Angelaki DE. A functional link between area MSTd and heading perception based on vestibular signals. Nat Neurosci. 2007;10:1038–1047. doi: 10.1038/nn1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa RP, Peterson BW, Goldberg ME. Prefrontal neurons coding suppression of specific saccades. Neuron. 2004;42:415–425. doi: 10.1016/j.neuron.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Kaas JH. Supplementary eye field as defined by intracortical microstimulation: connections in macaques. J Comp Neurol. 1990;293:299–330. doi: 10.1002/cne.902930211. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys. II. Cortical connections. J Comp Neurol. 1987;265:332–361. doi: 10.1002/cne.902650304. [DOI] [PubMed] [Google Scholar]
- Keating EG. Frontal eye field lesions impair predictive and visually-guided pursuit eye movements. Exp Brain Res. 1991;86:311–323. doi: 10.1007/BF00228954. [DOI] [PubMed] [Google Scholar]
- Keating EG. Lesions of the frontal eye field impair pursuit eye movements, but preserve the predictions driving them. Behav Brain Res. 1993;53:91–104. doi: 10.1016/s0166-4328(05)80268-2. [DOI] [PubMed] [Google Scholar]
- Kim J-M, Shadlen MN. Neural correlates of decision in the dorsolateral prefrontal cortex of the macaque. Nat Neurosci. 1999;2:176–185. doi: 10.1038/5739. [DOI] [PubMed] [Google Scholar]
- Kim Y-G, Badler JB, Heinen SJ. Trajectory interpretation by supplementary eye field neurons during ocular baseball. J Neurophysiol. 2005;94:1385–1391. doi: 10.1152/jn.00109.2005. [DOI] [PubMed] [Google Scholar]
- Kurkin S, Akao T, Fukushima J, Fukushima K. Discharge of pursuit-related neurons in the caudal part of the frontal eye fields in juvenile monkeys with up-down pursuit asymmetry. Exp Brain Res. 2009;193:181–188. doi: 10.1007/s00221-008-1606-3. [DOI] [PubMed] [Google Scholar]
- Kurkin S, Shichinohe N, Akao T, Fukushima J, Fukushima K. MST activity during memory-based smooth pursuit eye movements: comparison with the supplementary eye fields (SEF). Program No. 559.8. Abstract Viewer/Itinerary Planner. Chicago (IL): Society for Neuroscience, Washington, DC; 2009. [Google Scholar]
- Leigh RJ, Zee DS. The neurology of eye movements. 4th ed. New York: Oxford University Press; 2006. [Google Scholar]
- Liu S, Angelaki DE. Vestibular signals in macaque extrastriate visual cortex are functionally appropriate for heading perception. J Neurosci. 2009;29:8936–8945. doi: 10.1523/JNEUROSCI.1607-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JC. Frontal eye field lesions in monkeys disrupt visual pursuit. Exp Brain Res. 1987;68:437–441. doi: 10.1007/BF00248811. [DOI] [PubMed] [Google Scholar]
- MacAvoy MG, Gottlieb JP, Bruce CJ. Smooth pursuit eye movement representation in the primate frontal eye field. Cereb Cortex. 1991;1:95–102. doi: 10.1093/cercor/1.1.95. [DOI] [PubMed] [Google Scholar]
- Mann SE, Thau R, Schiller PH. Conditional task-related responses in monkey dorsomedial frontal cortex. Exp Brain Res. 1988;69:460–468. doi: 10.1007/BF00247300. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Pare EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT) J Neurosci. 1988;8:2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome WT, Wurtz RH, Komatsu H. Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. J Neurophysiol. 1988;60:604–620. doi: 10.1152/jn.1988.60.2.604. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Hanes DP, Thompson KG, Leutgeb S, Schall JD, Leventhal AG. Signal timing across the macaque visual system. J Neurophysiol. 1998;79:3272–3278. doi: 10.1152/jn.1998.79.6.3272. [DOI] [PubMed] [Google Scholar]
- Sharpe JA. Neurophysiology and neuroanatomy of smooth pursuit: lesion studies. Brain Cogn. 2008;68:241–254. doi: 10.1016/j.bandc.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Shi D, Friedman HR, Bruce CJ. Deficits in smooth pursuit eye movements after muscimol inactivation within the primate frontal eye field. J Neurophysiol. 1998;80:458–464. doi: 10.1152/jn.1998.80.1.458. [DOI] [PubMed] [Google Scholar]
- Shichinohe N, Akao T, Kurkin S, Fukushima J, Kaneko CRS, Fukushima K. Memory and decision-making in the frontal cortex during visual motion-processing for smooth pursuit eye movements. Neuron. 2009;62:717–732. doi: 10.1016/j.neuron.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Fukushima K. Neuronal responses related to smooth pursuit eye movements in the periarcuate cortical area of monkeys. J Neurophysiol. 1998;80:28–47. doi: 10.1152/jn.1998.80.1.28. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA, Chou HI, Slocum WM, Schiller PH. Eye fields in the frontal lobes of primates. Brain Res Brain Res Rev. 2000;32:413–448. doi: 10.1016/s0165-0173(99)00092-2. [DOI] [PubMed] [Google Scholar]
- Umeno MM, Goldberg ME. Spatial processing in the monkey frontal eye field. I. Predictive visual responses. J Neurophysiol. 1997;78:1373–1383. doi: 10.1152/jn.1997.78.3.1373. [DOI] [PubMed] [Google Scholar]
- Zaksas D, Pasternak T. Directional signals in the prefrontal cortex and in area MT during a working memory for visual motion task. J Neurosci. 2006;26:11726–11742. doi: 10.1523/JNEUROSCI.3420-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HH, Thompson KG. Cognitively directed spatial selection in the frontal eye field in anticipation of visual stimuli to be discriminated. Vision Res. 2009;49:1205–1215. doi: 10.1016/j.visres.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]