Abstract
Observation of self-produced hand movements through a mirror, creating an illusion of the opposite hand moving, was recently reported to induce ipsilateral motor cortex activation, that is, motor cortex activation for the hand in rest. The reported work goes far beyond earlier work on motor cortex activation induced by action observation, by implying a complete reversal of contralateral and ipsilateral motor cortex activation under mirror view conditions. Such a reversal would represent an unprecedented degree of neural plasticity. We considered such a reversal physiologically implausible and conducted a study with an improved design. The results refute the reversal of contralateral and ipsilateral motor cortex activation under mirrored viewing conditions as methodologically unsound. The investigation confirmed, however, more subtle expressions of motor cortical activity induced by self-produced movements observed through a mirror.
Keywords: electroencephalography, lateralized readiness potential, mirror visual feedback, motor cortex, movement-related potentials, visuomotor adaptation
Introduction
In 3 recent publications, Touzalin-Chretien and Dufour (2008) and Touzalin-Chretien et al. (2009, 2010) used the lateralized readiness potential (LRP) to investigate motor cortex activation induced by mirror visual feedback. Using a mirror placed in the midsagittal plane in front of the participant, the investigators had participants look at movements of their right hand as if it was their left hand. Based on analyses of the movement-related LRP, the authors inferred the presence of motor cortex activation contralateral to the resting left hand.
Motor cortex activation induced by movement observation is well established and existing evidence includes involvement of the contralateral primary motor cortex (Fadiga et al. 1995; Hari et al. 1998; Van Schie et al. 2008), the presumed origin of the LRP (Praamstra et al. 1999). However, the results reported by Touzalin-Chretien and coworkers imply a complete reversal of contralateral and ipsilateral motor cortex activation under mirrored viewing conditions. Such a reversal is incompatible with existing knowledge of sensorimotor physiology. Even after prolonged visuomotor adaptation with left–right reversing goggles, observed changes in cerebral activation patterns do not include the ipsilateral primary motor cortex (Sekiyama et al. 2000).
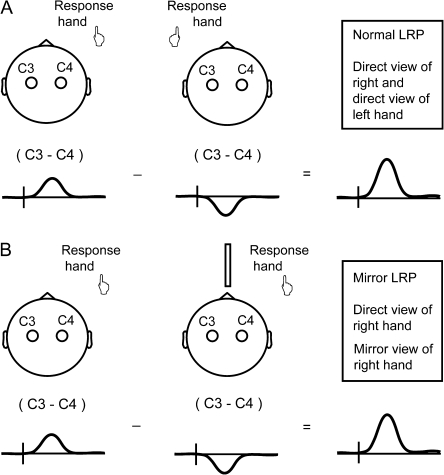
How do the reported results by Touzalin-Chretien and coworkers imply a reversal of contralateral and ipsilateral motor cortex activation under mirrored viewing conditions? The authors used the LRP, representing lateralized movement-related motor cortex activation derived by measuring the difference in voltage between electrodes overlying the motor cortex contralateral and ipsilateral to the side of movement. This difference potential between left and right motor cortex is normally measured separately for left and right hand movements and then combined. The authors derived the LRP for the direct view condition in a standard manner, illustrated in Figure 1A. The LRP for the mirror view condition was derived on the basis of data from right hand movements under direct view (same data as direct view LRP) and right hand movements viewed through a mirror (Fig. 1B). Since the authors obtained a mirror view LRP of identical amplitude as the direct view LRP, this implies that the balance of left and right motor cortex activation, during mirror view of the right hand moving, was identical to the balance in activation with left hand movements under direct view (Touzalin-Chretien and Dufour 2008; Touzalin-Chretien et al. 2010).
Figure 1.
(A) Touzalin-Chretien et al. derived a “normal LRP,” based on measurements during direct view movements of the right hand (first element of equation) and direct view movements of the left hand (second element of equation). (B) For the “mirror LRP,” they used the measurements obtained during direct view movements of the right hand (first element of equation) and subtracted measurements during right hand movements seen through a mirror (second element of equation). Since the normal and mirror LRPs were found to be of identical amplitude, the data imply that mirror viewed movements reverse the balance of ipsilateral and contralateral motor cortex activation.
The implied reversal in balance of ipsilateral and contralateral motor cortex activation, when viewing one's own hand moving in a mirror, would represent an unprecedented degree of neural plasticity. We suspected that the reported results are due to methodological problems, as explained in the Supplementary Material. In view of the promising role claimed for mirror visual feedback in restoring neurological function (Ramachandran and Altschuler 2009), we thought it is important to present a more realistic estimate of mirror view-induced motor cortex activation and conducted a similar experiment with improved design.
Materials and Methods
Participants
Participants were 9 right-handed adults (6 males; age 32 ± 11 years) with normal or corrected-to-normal vision. Data of one further participant were excluded because of excessive artifacts. All participants provided their informed consent, and the study had been approved by the local research ethics committee.
Procedure and Stimuli
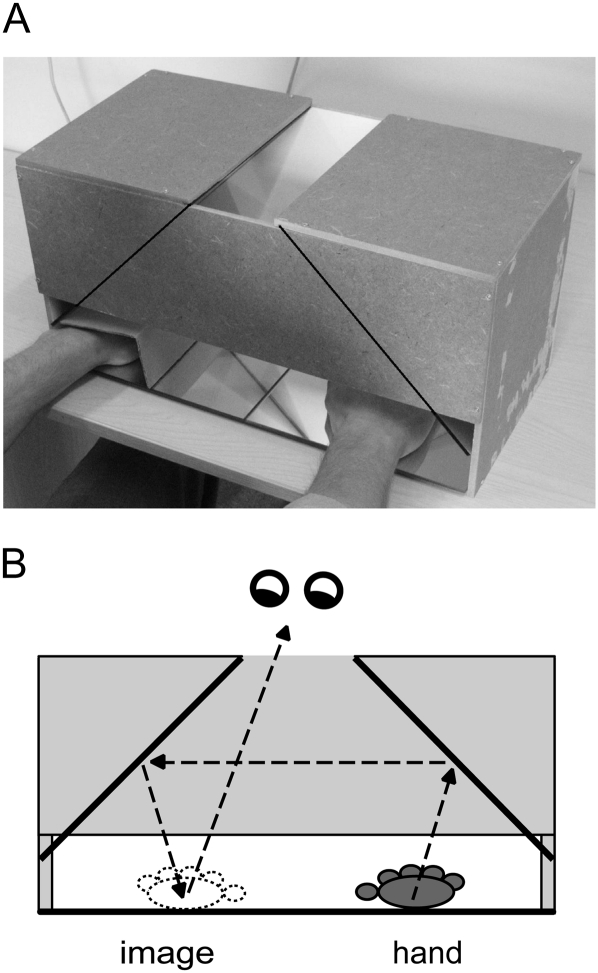
We constructed a mirror box pictured in Figure 2A. The box was designed to avoid the forced and asymmetric posture imposed by experimental setups with a mirror placed in the midsagittal plane. The box had an opening at the top. In the direct view condition, subjects were instructed to look through the opening to their moving hand, just left or right from the midline of the box. In the mirror view condition (Fig. 2B), they looked away from the moving hand to its reflection just opposite from the midline. The resting hand on that side was hidden from view by a cardboard cover, while the moving hand was also invisible, being positioned further away from the midline closer to the side of the box. It should be noted that there was a small remaining asymmetry in posture, since participants had to look left or right from the midline. Hence, gaze direction effects on motor cortical activity (Baker et al. 1999) cannot be ruled out entirely, although we have previously not been able to detect such effects in movement-related electroencephalography (EEG) potentials (Hesse et al. 2004).
Figure 2.
(A) Mirror box used for the present investigation. The hands are resting on a mirror surface, and mirrors are placed at an angle of 90°, as indicated by the lines on the box. Pictured is the positioning of hands for the mirror right condition, in which the right index finger is moved, viewed through the mirrors as the left hand index finger. The left hand is screened-off from view. (B) Looking through the opening at the top of the box, the mirror view (for this condition) requires the participant to look to the left of the midline.
Subjects made a brisk index finger extension followed by immediate flexion to return the finger to the surface on which the hand rested. The movement was made in response to an LED light mounted in the rear wall of the box. The LED was fitted in different positions for left and right hand movement conditions, so that it was always aligned to the moving finger at a distance of ∼2 to 3 cm. The LED lit up for 100 ms at random intervals between 2 and 3.5 s, in order to prevent anticipatory activation. Subjects were instructed to look at their finger while attending the LED signal.
The experiment comprised 8 blocks of 100 trials, that is, 2 blocks for each of 4 viewing conditions: direct right, direct left, mirror right, mirror left. Mirror right refers to the condition where the right hand moves but is seen through the mirror box as a left hand. Likewise, mirror left refers to the condition where the left hand moves but is seen through the mirror box as a right hand. The 2 blocks for the same condition were always run consecutively. All participants started with the right hand, but the order of mirror and direct view conditions was counterbalanced.
Data Acquisition
EEG was recorded continuously with Ag/AgCl electrodes from 130 scalp electrodes relative to common mode sense and driven right leg electrodes (http://www.biosemi.com/faq/cms&drl.htm) placed adjacent to the vertex electrode location Cz. The electrodes were placed according to the 10-5 extension of the International 10–20 electrode system using an elastic cap, carefully positioned relative to landmarks nasion, inion, and preauricular points. Vertical eye movements were monitored using electrodes Fp1 and Fp2 positioned above the left and right eye. Horizontal eye movements were monitored by inspection of electrodes FFT9h and FFT10h positioned close to the lateral canthus of the left and right eye. Electromyographic activity (EMG) was recorded from left and right musculus extensor indicis by 2 bipolar electrode pairs with electrodes placed at ∼2 cm distance from each other. EEG and EMG signals were amplified with a band-pass of 0–128 Hz by BioSemi ActiveTwo amplifiers and sampled at 512 Hz. While this data acquisition rate undersamples the EMG signal and required a low-pass filter setting that attenuates EMG amplitude, this was not critical to the analyses that we performed.
Data Processing and Analysis
For off-line analysis, the EEG data were recalculated to an average reference montage. To prevent confounds due to differences in response latency between conditions, lateralized movement-related EEG activity was analyzed relative to the movement instead of time locked to the reaction signal. To this purpose, the EMG data were high-pass filtered (10 Hz, 12 dB slope) and rectified. EMG time markers were then placed by a statistical algorithm, defining an EMG amplitude threshold of 4 times the standard deviation of the EMG signal in the time window −200 to 0 ms relative to the reaction signal (for review of EMG onset detection methods, see Van Boxtel et al. 1993). EMG onset detection was not set to detect the very earliest activity because this level of precision was not necessary and tended to disperse the EMG peak latency in our data. Based on the EMG markers, the EEG data were segmented in epochs from −300 to 500 ms relative to the EMG marker in order to create response-locked averages per subject and condition. Individual trials containing eye movements and other artifacts (on average 15 ± 7%) were removed before averaging, based on individually tailored artifact rejection thresholds and visual inspection. Rejection thresholds varied between ±40 and ±80 μV. Prior to the removal of artifact contaminated trials, an artifact correction based on principal component analysis (Ille et al. 2002) was applied to the data of 3 participants with frequent eyeblinks. In averaged data, the baseline was defined as the time period from −300 to −150 ms relative to the EMG marker. Grand-average waveforms displayed in the figures were low-pass filtered at 12 Hz. EMG was analyzed by computing the area-under-the-curve of the rectified EMG signal in subject averages. The area measure was computed in the window 0–200 ms for the moving hand EMG and a window of 0–100 ms for the resting hand EMG, as determined on the basis of the grand mean EMG signals.
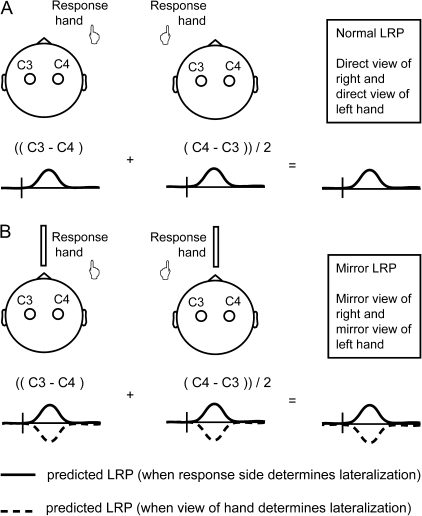
The direct view and mirror view LRPs were derived as described in Figure 3. The LRP quantifies motor cortical activity in terms of the voltage difference measured between electrodes located over the ipsilateral and contralateral motor cortex. Consequently, an amplitude difference of mirror view compared with direct view LRP can be due to an activation change in the motor cortex contralateral to the “viewed” or the “acting” hand (or both), making the LRP a less than ideal instrument for evaluating mirror-induced motor cortex activation. Against this background, LRP analyses were complemented with an analysis of the amplitude values at electrodes C3 and C4 that are used to compute the LRP, in order to evaluate the respective contributions of ipsilateral and contralateral motor cortex to the LRP. To simplify the presentation of this analysis, left and right hand conditions were collapsed, separately for direct view and mirror view conditions. This was done by transposing left and right hemisphere data in the left hand condition to permit averaging with the right hand condition.
Figure 3.
Computation of the direct view and mirror view LRPs in the current study. The method for computing the LRP differs from that in Figure 1, to clarify the logic of the derivation (see Supplementary Materials). Panel B depicts opposing predictions for the mirror view LRP: motor cortex activation uninfluenced by the view of the hand (continuous line) versus motor cortex activation fully determined by the view of the hand. The mirror in the midsagittal plane represents the mirror box.
Results
We hypothesized that viewing your own hand through a mirror would not or only minimally influence the lateralization of primary motor cortex activity, measured by the LRP. That is, the activation of the motor cortex contralateral to the moving hand was not expected to be subject to change. However, observation of the self-produced movement could induce additional activity contralateral to the mirror viewed hand. This would reduce the net difference in activation between left and right motor cortex and thus result in a lower amplitude of the mirror view LRP compared with the direct view LRP. Note that, in our design, results equivalent to those reported by Touzalin-Chretien and colleagues would mean not just a reduction in amplitude of the mirror view LRP but a reversal in polarity (see Fig. 3).
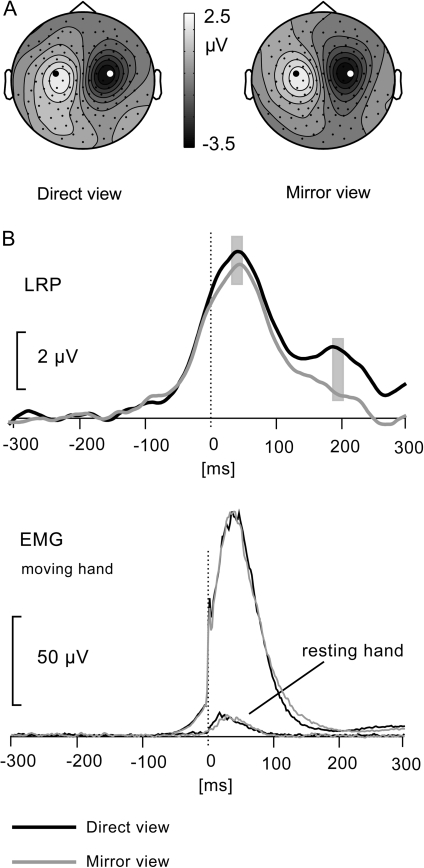
We first inspected the scalp distribution of movement-related lateralized cortical activity by subtracting left and right hand movement conditions, separately for the direct view and mirror view conditions. The resulting plots of the scalp voltage distributions (see Fig. 4A) thus represent left and right motor cortex activity of opposite polarity. The scalp distributions are helpful in demonstrating that the scalp maxima of left and right motor cortical activity are found around electrodes C3 and C4, respectively, for both the direct view and the mirror view conditions. These are the electrodes closest to the motor cortex and generally chosen for computation of the LRP. The scalp distributions also establish that there are no differential effects of residual eye movements distorting the scalp distribution.
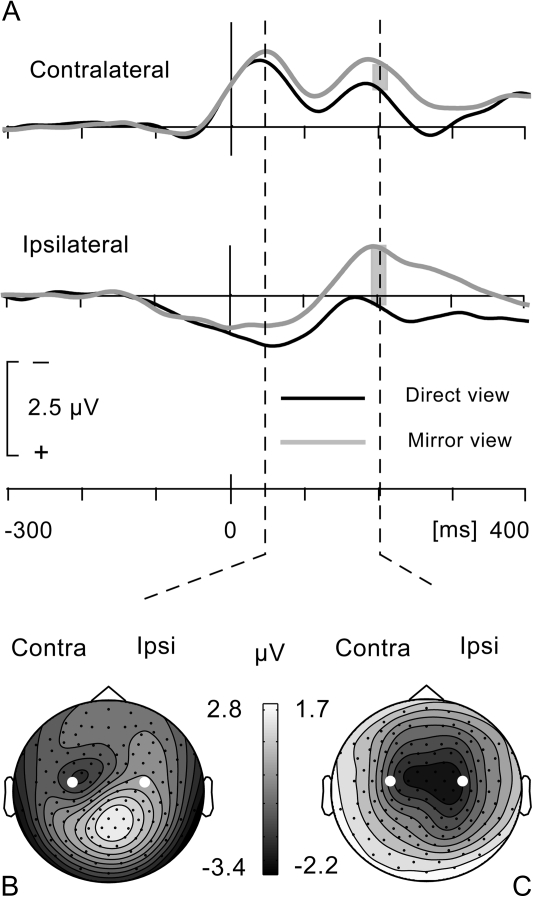
Figure 4.
(A) Scalp distribution of direct view and mirror view movement-related activity. The scalp distributions are based on a subtraction of left and right hand movement data (left minus right). (B) Direct view and mirror view LRPs measured at C3/C4. EMG activity of musculus extensor indicis, combined across left and right side movements, for the moving and the resting hand (scale bar for resting hand EMG is 5 μV). Time 0 is where the EMG signal crossed the defined amplitude threshold.
Figure 4B shows the response-locked LRP, as recorded from electrodes C3 and C4, in the top panel. The second panel shows the grand-average EMG signals of the moving hand and the resting hand. There was no difference between direct and mirror view conditions for either the moving hand (t8 = 0.42, P = 0.69) or the resting hand (t8 = 0.001, P = 0.99). The identical EMG signals for the moving hand, in the direct view and mirror view conditions, ensure that any difference in cerebral activation is not due to differences in force, amplitude, or duration of the movements. Identical EMG signals for the resting hand ensure that the comparison of the LRP in the direct view and in the mirror view conditions is not influenced by a greater tendency to synkinesia of the resting hand in the latter condition. Following inspection of the EMG signals, the LRP was statistically evaluated in terms of its mean amplitude around the peak latency (40–60 ms). Although a small difference in peak amplitude between conditions is visible in Figure 4B, this was not statistically significant (t8 = 0.76, P = 0.47).
Note that later in their time course, the direct view and mirror view LRPs diverge more robustly. We therefore also compared their amplitude in a time window of 190–210 ms coinciding with a second peak in the direct view LRP (see Fig. 4B). As supported by the scalp distribution (not shown), the second phase probably represents motor cortex activation related to the flexion of the index finger, returning it to a resting position following brisk extension. The lower amplitude of this second phase of the LRP may be explained by temporal jitter, by the movement being more automatic than intentional, and by the movement being less forceful than the preceding extension. That is, participants were instructed to elevate the index finger briskly and then let it drop back or at least avoid an active tapping movement. The amplitude difference between direct view and mirror view LRPs, during this second phase, was significant (t8 = 3.29, P < 0.05).
Whether the second phase of the mirror view LRP was attenuated compared with the direct view LRP as a result of mirror-induced motor cortex activation cannot be determined from the LRP data alone. We therefore reviewed the direct and mirror view conditions in the (combined) right and left hand data sets on which the LRP was based. As illustrated in Figure 5A, the waveforms recorded contralateral to the side of movement show 2 separate peaks, coincident with the 2 phases of the LRP and representing movement-related activity associated with the index finger extension and flexion movements, respectively. Comparing the mirror and direct view conditions reveals a sustained higher amplitude for the mirror view condition. In addition, at the latency of the second peak, the amplitudes at contralateral and ipsilateral electrode sites C3 and C4 are almost identical for the mirror view condition, while there is a marked asymmetry for the direct view condition. Amplitude values at these electrodes were analyzed with a 2-way analysis of variance with factors view (direct vs. mirror) and hemisphere (contralateral vs. ipsilateral). At the latency coinciding with the second LRP peak (190–210 ms), this analysis revealed a significant effect of view (F1,8 = 17.11, P = 0.01) due to the higher amplitude activity for the mirror view condition. At the same time, there was a significant interaction of view by hemisphere (F1,8 = 10.82, P = 0.011) due to the amplitude difference being larger over the ipsilateral than the contralateral hemisphere. This is illustrated in the scalp topography of the subtraction mirror view minus direct view data in Figure 5C. Note that the amplitude difference between direct and mirror view conditions already starts in the time window of the first LRP phase and accounts for the small (nonsignificant) amplitude difference found in this first LRP phase. Accordingly, the view by hemisphere interaction was not significant in this time window (40–60 ms) (F1,8 = 1.63, P = 0.273), while the effect of view only approached significance (F1,8 = 4.73, P = 0.061).
Figure 5.
(A) Data averaged across right and left hand movements, after left–right transposition of the data for the left hand condition. Contralateral waveforms show 2 phases corresponding to motor cortex activation for index finger extension and flexion, respectively. At the peak of the first phase, focal activation of the motor cortex can be distinguished in the scalp voltage distribution, shown for the mirror view condition (B). Contralateral and ipsilateral waveforms show higher amplitude movement-related activity for the mirror view condition. Subtracting mirror view and direct view conditions reveals the excess activity in the mirror view condition to be maximal over the ipsilateral hemisphere (C), indicating mirror-induced activation of ipsilateral motor cortex. Time 0 is where the EMG signal crossed the defined amplitude threshold. Electrodes marked in white are C3 (contralateral) and C4 (ipsilateral).
The above analysis complements the analysis of the LRP in 2 important ways. First, the distinct enhancement of sensorimotor cortex activity in the mirror view condition implies that any null effects in the analysis of the LRP cannot be attributed to the paradigm being ineffective. Secondly, the enhancement favored the ipsilateral hemisphere in the time window of the flexion movement, thus decreasing the amplitude difference between contralateral and ipsilateral motor cortex activation. It therefore explains the attenuated amplitude of the mirror view LRP in this time window, relative to the direct view LRP. The attenuation of the “late” mirror view LRP is thus revealed as likely being due to mirror-induced motor cortex activation.
Discussion
In recent publications, Touzalin-Chretien and coworkers reported movement-related EEG potential data suggesting a complete reversal of ipsilateral and contralateral motor cortex activation when self-produced hand movements are observed through a mirror. The results of the present investigation refute such a reversal. During the performance of brisk index finger extensions in response to a visual signal, the balance of ipsilateral and contralateral motor cortex activation was not significantly affected by whether the movement was viewed directly or through a mirror. We propose that the opposing results are due to the methodological improvements of our approach, but we will briefly consider whether differences in task may be responsible.
Touzalin-Chretien et al. used a choice response task, whereas we used a simple response task. Although the use of a choice response (between fingers of the same hand) was not explicitly motivated, it could be relevant that this task may involve stronger recruitment of nonprimary motor cortex than a simple response task. In combination with the fact that premotor rather than primary motor cortex is found activated in imaging studies of action observation (e.g., Buccino et al. 2001), one could argue that a choice response is more conducive to eliciting observation-induced ipsilateral motor cortex activation. In response to this argument, we should point out that a premotor cortex contribution to the LRP is hypothetical. Premotor cortex activation is also less strongly lateralized than primary motor cortex activity (Tanji et al. 1987; Horenstein et al. 2009), hence unlikely to dominate the lateralization direction and magnitude of the LRP. Accepting that the primary motor cortex is mainly responsible for the LRP, the premotor cortex could still influence the LRP through facilitatory and inhibitory ipsilaterally and contralaterally directed premotor–motor cortex interactions (Koch et al. 2006). Such interactions are typically invoked in between-hand choice, however, which does not apply here. Taken together, the use of a choice—instead of simple response task can at best have a small influence on the amplitude relation of direct view and mirror view LRPs; it is inconceivable that it would effect a change from identical to maximally different, that is, opposite polarity amplitudes.
The main analysis of the LRP, evaluating amplitudes at peak latency, contradicts findings reported by Touzalin-Chretien and coworkers and corroborates our view that the latter results are due to methodological problems (see Supplementary Material). However, this does not rule out the possibility of mirror view-induced motor cortex activation. The second phase of the LRP, concurrent with index finger flexion following initial extension, was of lower amplitude in the mirror view condition. We considered the possibility that this is due to suppression of reafferent input following the extension movement, as an adaptation to conflicting visual and proprioceptive signals (Bernier et al. 2009). However, the analyses reported above suggest that the attenuated movement-related lateralization in the mirror view condition is due to activation of the ipsilateral sensorimotor cortex, presumably observation induced. Two possible explanations spring to mind for this intriguing dissociation between the extension and flexion phases of the LRP. First, there might be a physiological difference between flexion and extension movements in the degree they depend on or are able to recruit ipsilateral corticospinal pathways. Second, the difference might be explained by the intentional nature of the extension movement contrasting with the more automatic flexion movement returning the finger to its rest position. That is, Schütz-Bosbach et al. (2009) inferred motor cortical inhibitory effects from a modulation of the EMG silent period during observation of movements that were illusorily interpreted as self-produced. Hence, in our experiment, a stronger sense of agency for the extension than for the flexion movement may have inhibited the ipsilateral motor cortex more effectively for extension than for flexion movements.
Against the background of established action observation effects on motor cortical activity, motor cortex activation induced by self-produced movements observed through a mirror is not lacking credibility. The phenomenon is interesting in its own right and has possible clinical application (Ramachandran and Altschuler 2009), albeit not unambiguously supported (Moseley et al. 2008; Ezendam et al. 2009). We have shown here, however, that recent neurophysiological demonstrations of mirror-induced motor cortex activation cannot be taken at face value. If not artifactual, the sheer magnitude of the contested effects implies extreme flexibility of motor cortical activity. While such effects would seem good news for neurological rehabilitation, the implied degree of flexibility might well be incompatible with stable function.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
RCM is supported by the Wellcome Trust.
Supplementary Material
Acknowledgments
We thank Dagmar Fraser and Michael Hanson for technical support. Conflict of Interest: None declared.
References
- Baker JT, Donoghue JP, Sanes JN. Gaze direction modulates finger movement activation patterns in human cerebral cortex. J Neurosci. 1999;19:10044–10052. doi: 10.1523/JNEUROSCI.19-22-10044.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier PM, Burle B, Vidal F, Hasbroucq T, Blouin J. Direct evidence for cortical suppression of somatosensory afferents during visuomotor adaptation. Cereb Cortex. 2009;19:2106–2113. doi: 10.1093/cercor/bhn233. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci. 2001;13:400–404. [PubMed] [Google Scholar]
- Ezendam D, Bongers RM, Jannink MJ. Systematic review of the effectiveness of mirror therapy in upper extremity function. Disabil Rehabil. 2009;31:2135–2149. doi: 10.3109/09638280902887768. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study. J Neurophysiol. 1995;73:2608–2611. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- Hari R, Forss N, Avikainen S, Kirveskari E, Salenius S, Rizzolatti G. Activation of human primary motor cortex during action observation: a neuromagnetic study. Proc Natl Acad Sci U S A. 1998;95:15061–15065. doi: 10.1073/pnas.95.25.15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse CW, Seiss E, Bracewell RM, Praamstra P. Absence of gaze direction effects on EEG measures of sensorimotor function. Clin Neurophysiol. 2004;115:29–38. doi: 10.1016/s1388-2457(03)00302-x. [DOI] [PubMed] [Google Scholar]
- Horenstein C, Lowe MJ, Koenig KA, Phillips MD. Comparison of unilateral and bilateral complex finger tapping-related activation in premotor and primary motor cortex. Hum Brain Mapp. 2009;30:1397–1412. doi: 10.1002/hbm.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ille N, Berg P, Scherg M. Artifact correction of the ongoing EEG using spatial filters based on artifact and brain signal topographies. J Clin Neurophysiol. 2002;19:113–124. doi: 10.1097/00004691-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Koch G, Franca M, Del Olmo MF, Cheeran B, Milton R, Alvarez Sauco M, Rothwell JC. Time course of functional connectivity between dorsal premotor and contralateral motor cortex during movement selection. J Neurosci. 2006;26:7452–7459. doi: 10.1523/JNEUROSCI.1158-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley GL, Gallace A, Spence C. Is mirror therapy all it is cracked up to be? Current evidence and future directions. Pain. 2008;138:7–10. doi: 10.1016/j.pain.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Schmitz F, Freund HJ, Schnitzler A. Magneto-encephalographic correlates of the lateralized readiness potential. Brain Res Cogn Brain Res. 1999;8:77–85. doi: 10.1016/s0926-6410(99)00008-7. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Altschuler EL. The use of visual feedback, in particular mirror visual feedback, in restoring brain function. Brain. 2009;132:1693–1710. doi: 10.1093/brain/awp135. [DOI] [PubMed] [Google Scholar]
- Schütz-Bosbach S, Avenanti A, Aglioti SM, Haggard P. Don't do it! Cortical inhibition and self-attribution during action observation. J Cogn Neurosci. 2009;21:1215–1227. doi: 10.1162/jocn.2009.21068. [DOI] [PubMed] [Google Scholar]
- Sekiyama K, Miyauchi S, Imaruoka T, Egusa H, Tashiro T. Body image as a visuomotor transformation device revealed in adaptation to reversed vision. Nature. 2000;403:192–195. doi: 10.1038/35030096. [DOI] [PubMed] [Google Scholar]
- Tanji J, Okano K, Sato KC. Relation of neurons in the nonprimary motor cortex to bilateral hand movement. Nature. 1987;327:618–620. doi: 10.1038/327618a0. [DOI] [PubMed] [Google Scholar]
- Touzalin-Chretien P, Dufour A. Motor cortex activation induced by a mirror: evidence from lateralized readiness potentials. J Neurophysiol. 2008;100:19–23. doi: 10.1152/jn.90260.2008. [DOI] [PubMed] [Google Scholar]
- Touzalin-Chretien P, Ehrler S, Dufour A. Behavioral and electrophysiological evidence of motor cortex activation related to an amputated limb: a multisensorial approach. J Cogn Neurosci. 2009;21:2207–2216. doi: 10.1162/jocn.2009.21218. [DOI] [PubMed] [Google Scholar]
- Touzalin-Chretien P, Ehrler S, Dufour A. Dominance of vision over proprioception on motor programming: evidence from ERP. Cereb Cortex. 2010;20:2007–2016. doi: 10.1093/cercor/bhp271. [DOI] [PubMed] [Google Scholar]
- Van Boxtel GJ, Geraats LH, Van den Berg-Lenssen MM, Brunia CH. Detection of EMG onset in ERP research. Psychophysiology. 1993;30:405–412. doi: 10.1111/j.1469-8986.1993.tb02062.x. [DOI] [PubMed] [Google Scholar]
- Van Schie HT, Koelewijn T, Jensen O, Oostenveld R, Maris E, Bekkering H. Evidence for fast, low-level motor resonance to action observation: an MEG study. Soc Neurosci. 2008;3:213–228. doi: 10.1080/17470910701414364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.