Abstract
Intrauterine growth restriction (IUGR) is associated with altered lung development in human and rat. The transcription factor PPARγ, is thought to contribute to lung development. PPARγ is activated by docosahexanoic acid (DHA). One contribution of PPARγ to lung development may be its direct regulation of chromatin modifying enzymes, such as Setd8. In this study, we hypothesized that IUGR would result in a gender-specific reduction in PPARγ, Setd8 and associated H4K20Me levels in the neonatal rat lung. Because DHA activates PPARγ, we also hypothesized that maternal DHA supplementation would normalize PPARγ, Setd8, and H4K20Me levels in the IUGR rat lung. We found that IUGR decreased PPARγ levels, with an associated decrease in Setd8 levels in both male and female rat lungs. Levels of the Setd8-dependent histone modification, H4K20Me, were reduced on the PPARγ gene in both males and females while whole lung H4K20Me was only reduced in male lung. Maternal DHA supplementation ameliorated these effects in offspring. We conclude that IUGR decreases lung PPARγ, Setd8 and PPARγ H4K20Me independent of gender, while decreasing whole lung H4K20Me in males only. These outcomes are offset by maternal DHA. We speculate that maintenance of the epigenetic milieu may be one role of PPARγ in the lung and suggest a novel benefit of maternal DHA supplementation in IUGR.
Introduction
Intrauterine growth restriction (IUGR) increases the risk of postnatal morbidities, with male IUGR infants more severely affected (1–5). In the United States, 5–12% of premature babies born annually are IUGR (6). IUGR, particularly when combined with prematurity, results in pulmonary morbidities, including bronchopulmonary dysplasia (BPD) (7–9). BPD is characterized histologically by impaired lung development (10–12). In a rat model of IUGR, we have demonstrated thickening of lung mesenchyme at birth, consistent with abnormal lung development (13).
Impaired lung development in IUGR is due to disruptions in epithelial-mesenchymal interactions and several lines of evidence suggest that the nuclear receptor transcription factor, peroxisome proliferator activated receptor gamma (PPARγ) is involved in these interactions. Evidence of PAPRγ’s involvement in epithelial-mesenchymal interactions in the lung is twofold. Firstly, a PPARγ-targeted lung epithelial knockout mouse has altered postnatal lung development, and changes in the expression of mesenchymal genes, resulting in enlarged airspaces, increased lung volumes, and decreased tissue resistance (14). Secondly, PPARγ is a critical component of the parathyroid hormone related protein (PTHrP)-driven epithelial-mesenchymal paracrine loop that also contributes to lung development (15).
A further role for PPARγ in lung development may be the transcriptional regulation of epigenetic modifying enzymes. Epigenetic modifying enzymes affect developmental processes by altering gene expression patterns of target genes. This is accomplished by the dynamic placement of epigenetic marks (such as methylation and acetylation) on histones associated with a particular gene. The marks then dictate interactions of the gene with transcription machinery. A number of chromatin modifying enzymes have PPAR response elements (PPRE) in their promoters and are bona fide transcriptional targets of PPARγ (16). One of these PPARγ responsive genes is the set domain containing histone methyltransferase, Setd8, which puts a monomethyl (Me) group on lysine (K) 20 of Histone (H) 4. Regulation of the placement of H4K20Me on the PPARγ gene may represent a feedback loop by which levels of PPARγ are regulated (16).
The PPARγ gene is well conserved between the human and rat gives rise to at least three mRNA variants, PPARγ1a, PPARγ1b and PPARγ2, and two protein isoforms, PPARγ1 and PPARγ2 (17–19) (detailed in Supplementary Data Figure 1). Expression of PPARγ isoforms is tissue-specific. PPARγ1 is ubiquitously expressed, while PPARγ2 is thought to be expressed exclusively in adipose tissue (17, 20–21). Levels and activity of PPARγ are increased by ligand activation. Ligand binding induces a conformational change affecting transcription of PPARγ and PPARγ target genes (reviewed in (22)) and the resulting effects are ligand specific. PPARγ ligands include the long chain fatty acid (FA) docosahexaenoic acid (DHA) (23–24). The effects of DHA on PPARγ expression and activity are well documented and include activation of ectopically expressed PPARγ in myoblasts; in differentiating monocytes; in endometrial tissue, and in tumor cells (25–30). A maternal plasma FA profile low in DHA has also recently been shown to be associated with small for gestational age (SGA) and preterm birth, and maternal supplementation with DHA during gestation is associated with longer gestation duration (31–33).
The effects of IUGR on PPARγ and Setd8 expression or associated levels of H4K20Me are unknown. The effects of maternal DHA supplementation on neonatal lung PPARγ are also unknown. In this study, we hypothesized that IUGR would result in a gender-specific reduction in PPARγ, Setd8 and associated H4K20Me levels in the neonatal rat lung. Because DHA activates PPARγ, we also hypothesized that maternal DHA supplementation would normalize PPARγ, Setd8, and H4K20Me levels in the IUGR rat lung. To test these hypotheses, we used a well-characterized model of uteroplacental insufficiency (UPI)-induced IUGR in the rat (34–38) and developed a model of maternal DHA supplementation in the context of the UPI-induced IUGR rat.
Materials and Methods
Animals
The rat uteroplacental insufficiency model of IUGR has been described in detail previously (39–41). All procedures were approved by the University of Utah Animal Care Committee and are in accordance with the American Physiological Society’s guiding principles (42). Body weights of IUGR pups are approximately 25% smaller than the control pups (43). The surgical procedures have been described previously (44–45). Briefly, on day 19 of gestation, pregnant Sprague-Dawley rats were anesthetized with intraperitonealxylazine (8 mg/kg) and ketamine (40 mg/kg), and both uterine arteries ligated giving rise to IUGR pups. Control dams underwent identical anesthetic procedures. Day 0 (d0) pups were delivered by caesarian section at term, 2.5 days after bilateral uterine artery ligation. Rat pups were killed by decapitation and blood collected for serum separation. Lungs were immediately removed from the same animals, flash-frozen in liquid nitrogen, and stored at −80°C. For each experiment, each group (control, IUGR and DHA-IUGR) had 6 male pups and 6 female pups, unless otherwise noted. Pups within each group were derived from different litters.
Maternal DHA Supplementation
DHA was administered via a custom diet. The diet, based on Harlen Teklad 8640 standard rodent diet (TD.8640, Harlan-Teklad, WI), substitutes 1% of the soybean oil in the standard chow with 1% purified DHA (cis-docosahexaenoic acid, # U-84-A, Nu-Chek Prep, MN). The resulting diet (here called 1% DHA diet) contains the same macronutrient content as standard rodent chow (21.8% protein, 40.8% carbohydrate and 5.4% fat – with a resulting caloric density of 3 Kcal/g). The pregnant rats were pair-fed regular diet or 1% DHA diet from E13 until term. However, the amount of food consumed by the pregnant rats was independent of diet.
Serum DHA quantification
Serum levels of DHA were measured in control and IUGR pups using gas chromatography. Samples were directly transesterified to fatty acid methyl esters (FAMEs) [42]. Rat serum (0.25μl) was mixed with 0.25ml of methanol:benzene 3:2 (v:v). This was followed by the addition of an equal amount of freshly prepared acetylchloride-methanol 5:100 (v:v) in glass tubes incubated at 100oC for 1 hour. FAME composition was determined by gas chromatography using an HP5890 GC equipped with an Omegawax 250 Capillary Column (Sigma-Aldrich, 30m x 0.25 mm x 0.25 μm film thickness). Gilson Unipoint software was used to measure the area under the curve (AUC) for each peak. Dividing each FAME AUC by the sum AUC of all peaks provided the wt:wt% for the fatty acids of interest.
Real-Time RT PCR
Real-time reverse transcriptase PCR was used to evaluate mRNA abundance of lung PPARγ variants as well as PPARγ responsive downstream gene, Setd8 as previously described, with GAPDH as an internal control (43). The following Assay-on-demand primer/probe sets were used: PPARγ1a- Rn01492275_m1, PPARγ1b - Rn01492273_m1, PPARγ2 - Rn00440940_m1, Setd8 - Rn01477383_g1 (Applied Biosystems). GAPDH primer and probe sequences; Forward: CAAGATGGTGAAGGTCGGTGT; Reverse: CAAGAGAAGGCAGCCCTGGT; Probe: GCGTCCGATACGGCCAAATCCG.
Protein Isolation, Immunoprecipitation and Mass Spectrometry
In order to confirm the specificity of the c-terminal PPARγ antibody for both isoforms of PPARγ, protein isolated from whole lungs was immunoprecipitated, electrophoresed, commassie stained and bands were then analyzed by mass spectrometry. Immunoprecipitation ws performed with antibody to c-terminal PPARγ (PPARγ H-100, sc-7196, Santa Cruz Biotechnology). Protein samples were Ziptip™ purified prior to digestion with TPCK-modified trypsin (Promega) or chymotrypsin (Princeton). Mass spectrometry analysis was performed using a LTQ-FT hybrid mass spectrometer (ThermoElectron Corp). Peptide molecular masses were measured by FT-ICR. Proteins were identified from peptides using the Mascot search engine (in-house licensed, ver. 2.2.1, Matrix Science, Inc.) and NCBI protein sequence files for PPARγ isoforms (NCBI accession numbers, PPARγ1 AAD40118, PPARγ2 NP-037256).
Immunoblot
Immunoblotting was used to determine relative abundance of lung PPARγ and Setd8 protein in IUGR and control rats as previously described (46–48) (antibodies, PPARγ H-100, sc-7196, Santa Cruz Biotechnology and Setd8 (sc-54998, Santa Cruz Biotechnology). Whole lung H4K20Me immunoblots were performed on acid-extracted histones, prepared as previously described (48). Levels of H4K20Me were quantified relative to total H4 (Histone H4 monomethyl Lys20 pAb, 39175, Active Motif).
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) was used to investigate the levels of histone modifications along the PPARγ gene. Positions along the PPARγ gene that were examined are marked with an * on Supplementary Data Figure 1 and include 5′ of P1, at P1, P2, Exon 4 and at the 3′ end of the PPARγ gene. Table 1 contains primers used for real-time PCR of ChIP DNA. ChIP was performed as as previously described (49–50) using anti- H4K20Me antibody (39175, Active Motif).
Table 1.
Primer/probe sets for ChIP analysis
| Transcript position relative to PPARγ gene | Sequence |
|---|---|
| 5′ of P1 | For: 5′ TCGACGGCTTTCTGAATGTG |
| Rev: 5′ CTTGCCCTCTTTCAGCTCTTTC | |
| Probe: 5′ ATCTTTAGGACAGATCATG | |
|
| |
| P1 | For: 5′ AAAAACAAACTTCTGCGTGACAGT |
| Rev: 5′ GGTCCCACGTTCCTCAGACA | |
| Probe: 5′ AGGGCACCAGCCGG | |
|
| |
| P2 | For: 5′ CCAAGTCTTGCCAAAGAAGCA |
| Rev: 5′ GATTGAGAGCCAGCTGTGACAA | |
| Probe: 5′ ACAGCATTATGACACACCAT | |
|
| |
| Exon 4 | For: 5′ CCATCAGGTTTGGGCGAAT |
| Rev: 5′ GATCTCCGCCAACAGCTTCT | |
| Probe: 5′ CCACAGGCCGAGAAG | |
|
| |
| 3′ End | For: 5′ CGCCAAGGTGCTCCAGAA |
| Rev: 5′ CTGCACGTGCTCTGTGACAA | |
| Probe: 5′ ATGACAGACCTCAGGCAG | |
Statistics
Data are presented as mean ± SEM. Statistical differences between control, IUGR and DHA-IUGR groups were determined using analysis of variance (ANOVA) followed by Fisher’s PLSD nonparametric test. We used Statistix 8 software package (Analytical Software, Tallahasse, FL). We accepted p ≤ 0.05 for statistical significance.
Results
IUGR decreases serum DHA in male neonatal IUGR pups
IUGR significantly decreased serum DHA from 4 ± 0.2 wt:wt% to 3 ± 0.3 wt:wt% (p=0.032) in male pups, with no significant difference in female pups (Figure 1). The addition of DHA to the maternal diet increased serum DHA levels in male and female DHA-IUGR pups to 5.9 ± 0.6 wt:wt% and 6.7 ± 0.3 wt:wt%, respectively.
Figure 1.
Maternal DHA supplementation increases serum DHA in deficient male neonatal IUGR pups. IUGR decreased serum DHA in male neonatal rat pups compared to control, with no significant difference seen in females. The addition of DHA to the maternal diet increased serum DHA levels in male and female DHA-IUGR relative to control. n=6, *p≤0.05, **p≤0.01.
IUGR decreases PPARγ variant mRNA levels at birth in neonatal rat lung; maternal DHA supplementation ameliorates this decrease
IUGR decreased PPARγ1a mRNA at birth in males (p=0.007) and females (p=0.004)) (Figure 2A). In male rat lungs, maternal DHA supplementation significantly increased PPARγ2 mRNA levels in DHA-IUGR compared to IUGR (p=0.05). IUGR also decreased PPARγ1b mRNA at birth in males (p=0.01) and females (p=0.003) (Figure 2B). Similarly, IUGR decreased PPARγ2 mRNA at birth in males (p=0.04) and females (p=0.02) (Figure 2C). Maternal DHA supplementation significantly increased PPARγ2 mRNA levels in male (p=0.04) and female (p=0.05) DHA-IUGR compared to IUGR (Figure 2). No significant difference to control was observed for DHA-IUGR PPARγ1a, -γ1b or γ2 mRNA (Figure 2).
Figure 2.

IUGR decreases PPARγ variant mRNA levels at birth in neonatal rat lung and maternal DHA supplementation ameliorates this decrease. A) PPARγ1a mRNA, B) PPARγ1b mRNA and C) PPARγ2 mRNA. Asterisks denote significant differences n=6, *p≤0.05, **p≤0.01.
IUGR decreases PPARγ2 protein abundance at birth in neonatal rat lung; maternal DHA supplementation ameliorates this decrease
Mass spectrometry used to confirm immunoblot band identity. The two bands resulting from immunoprecipitation with a c-terminal PPARγ antibody, each contained peptides for the common region of PPARγ and one band contained peptides for the unique PPARγ2 N-terminus (data not shown).
In accordance with mRNA data, IUGR significantly reduced PPARγ2 protein at birth in males (p=0.02) and females (p=0.03) (Figure 3B). Maternal DHA supplementation ameliorated this effect with abundance of PPARγ2 protein in DHA-IUGR lung not significantly different from gender-matched controls. In both male and female rat lungs, maternal DHA supplementation significantly increased PPARγ2 protein abundance in DHA-IUGR compared to IUGR in males (p=0.02) and females (p=0.03). (Figure 3B). Abundance of PPARγ1 protein was unchanged from control in IUGR and DHA-IUGR lungs at birth (Figure 3A).
Figure 3.
IUGR decreases PPARγ2 protein levels at birth in neonatal rat lung and maternal DHA supplementation ameliorates this decrease. A) PPARγ1 protein, B) PPARγ2 protein. Asterisks denote significant differences. n=6, *p≤0.05.
IUGR decreases Setd8 levels at birth in neonatal rat lung; maternal DHA supplementation ameliorates this decrease
Because Setd8 is a direct transcriptional target of PPARγ, the effect of IUGR and maternal DHA supplementation on Setd8 mRNA expression and protein abundance in rat lungs was evaluated. IUGR decreased Setd8 mRNA expression in males (p=0.01) and females (p=0.03) (Figure 4A). Maternal DHA supplementation ameliorated this decrease, with levels of Setd8 mRNA expression in DHA-IUGR lung not significantly different from gender-matched controls. In male rat lungs, maternal DHA supplementation significantly increased Setd8 mRNA levels in DHA-IUGR compared to IUGR (p=0.03) (Figure 4A).
Figure 4.
IUGR deceased mRNA (A) and protein levels (B) of the PPARγ target, Setd8, at birth in male and female rat lungs. Maternal DHA supplementation ameliorates the IUGR decreased in Setd8 mRNA and protein in rat lungs at birth. Asterisks denote significant differences. n=6, *p≤0.05, #p=0.07.
IUGR significantly reduced Setd8 protein abundance in males (p=0.02). In females, IUGR did not decrease Setd8 abundance (p=0.07). Maternal DHA supplementation ameliorated the decrease in male IUGR pups, with levels of Setd8 protein abundance in DHA-IUGR lung not significantly different from male controls. (Figure 4B).
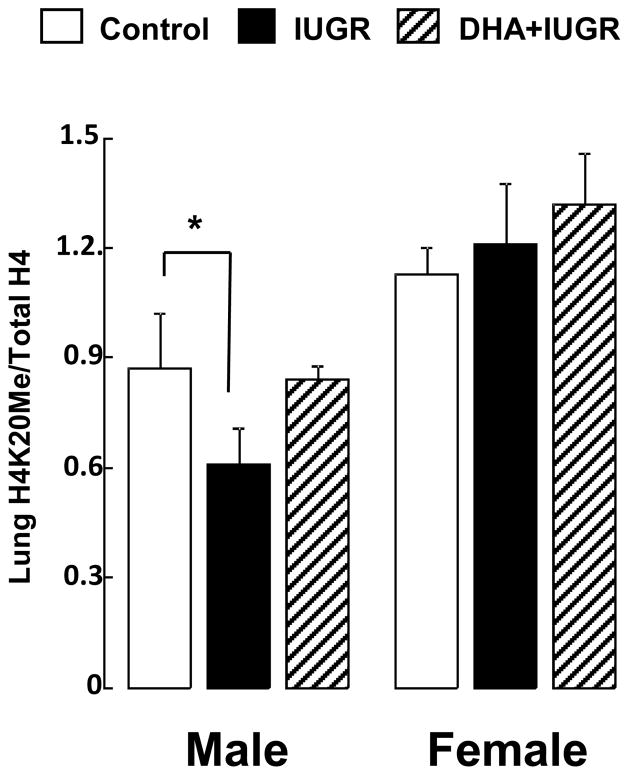
IUGR decreases whole lung H4K20Me in male neonatal rats; maternal DHA supplementation ameliorates this decrease
In light of reduced Setd8 mRNA expression and protein abundance in the IUGR lung, we examined levels of whole lung H4K20Me relative to total H4 in control, IUGR, and DHA-IUGR at birth. IUGR significantly reduced levels of H4K20Me at birth in lung of male IUGR pups (p=0.02) (Figure 5). Maternal DHA supplementation ameliorated this decrease, with levels of whole lung H4K20Me in lung of male DHA-IUGR pups not significantly different from male controls. No significant changes were observed in whole lung H4K20Me in lung of female pups.
Figure 5.
IUGR decreases whole lung H4K20Me, relative to total H4, in male rats at birth, with no change in females. Maternal DHA ameliorates this decrease in whole lung H4K20Me in male neonatal rats. Asterisks denote significant differences. n=6, *p≤0.05, **p≤0.01, #p=0.08.
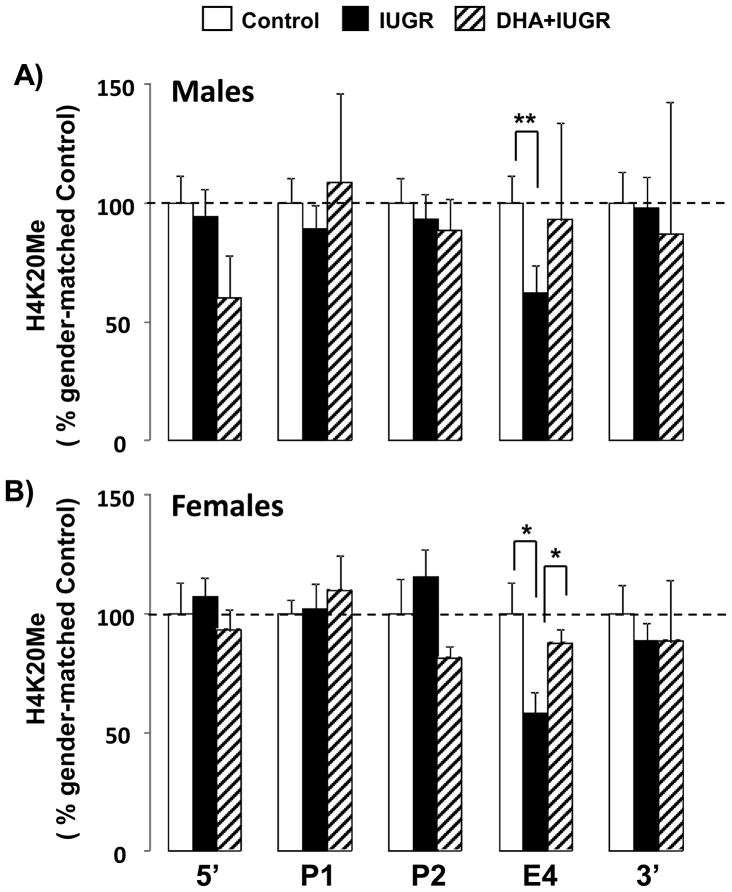
Maternal DHA ameliorates the IUGR decrease in H4K20Me at Exon 4 of the PPARγ gene in neonatal rat lung
Because Setd8 mRNA is known to specifically target the PPARγ gene, we examined levels of H4K20Me at 5 positions along the PPARγ gene at birth, in control, IUGR and DHA-IUGR rat lung. The abundance of H4K20Me was determined at the 5′ end, P1, P2, Exon 4, and the 5′end of the PPARγ gene. Levels of H4K20Me were quantified relative to a non-transcribed intergenic region that has previously been shown to contain low levels of all histone modifications (51). Data are presented as IUGR and DHA-IUGR relative to gender-matched normalized controls ± SEM in order to demonstrate the effect of IUGR and DHA-IUGR on H4K20Me levels along the length of the PPARγ gene. IUGR significantly reduced H4K20 levels at Exon 4 in both males (p=0.02) and females (p=0.05) (Figure 6). Maternal DHA supplementation ameliorated this decrease with levels of Exon 4 H4K20Me in DHA-IUGR lung not significantly different from control. In female rat lungs, maternal DHA supplementation significantly increased Exon 4 H4K20Me in DHA-IUGR compared to IUGR (p=0.05) (Figure 6).
Figure 6.
IUGR decreases levels of H4K20Me at Exon 4 of the PPARγ gene in male and female neonatal rat lung and maternal DHA ameliorates this decrease. Levels of H4K20Me, relative to a non-transcribing intergenic region, were quantified along the PPARγ gene in male and female lungs. Asterisks denote significant differences. n=5, *p≤0.05, **p≤0.01.
Discussion
Novel findings of this study are fourfold. First, the neonatal rat lung expresses all PPARγ mRNAs and protein iosforms. Second, in both male and female rats, IUGR decreases PPARγ mRNA and protein, with an associated decrease in a PPARγ downstream target: Setd8. Thirdly, this decrease in Setd8 is associated with a reduction in PPARγ specific H4K20Me in both males and females and a reduction in whole lung H4K20Me in males only. Finally, maternal DHA supplementation ameliorates these changes. Together, these data suggest both a role for PPARγ in IUGR induced epigenetic changes in the lung during development and a novel benefit of maternal DHA supplementation in IUGR.
While the importance of total PPARγ in the lung has been established (14, 52–53), the role of individual isoforms is unknown. In this study, we demonstrated that PPARγ2 is expressed in the neonatal rat lung. To the best of our knowledge, this is the first report of PPARγ2 expression in the lung. The PPARγ2 isoform has previously been associated predominantly with adipose tissue, where it acts as key regulator of adipogenesis (54–55). Only PPARγ1 expression has previously been reported in the lung. A possible explanation for the lack of detection of PPARγ2 mRNA in rabbit primary alveolar type II (ATII) cells (56) is that the first 7 nucleotides of the PPARγ2-specific primer used is not contained within any PPARγ2 sequences currently listed on the NCBI database. The additional nucleotides may have hampered detection. In a second study, using embryonic rat whole lung tissue and ATII cells, the primer sequence used to differentiate PPARγ1 from PPARγ2 was not reported (20). Western Blot analysis of cultured ATII cells was reported but a second band in the gels is not explained. In our study, we showed, by mass spectrometry that the whole rat lung expresses both PPARγ1 and PPARγ2.
PPARγ has roles in acute and chronic inflammatory responses in the lung (57–59), pulmonary fibrosis (57, 60), lung cancer (61) and pulmonary vascular disease (62); however, specific roles of PPARγ in lung development have yet to be determined. The discovery that PPARγ regulates the expression of chromatin modifying enzymes (16) suggests that PPARγ’s role in lung development may be via the regulation of epigenetic events. Epigenetics provides a way to selectively express information contained within the genome. This is particularly important in the context of developmentally specific gene expression. Methylation of histones by methyltransferase enzymes contributes to this regulation. The histone lysine methyltransferase, Setd8, places the H4K20Me mark. This mark is enriched in the promoter or coding regions of active genes and strongly correlated with gene activation when it appears in regions downstream of the transcriptional start site (63–64). Considering the potential importance of PPARγ and Setd8 in lung development, our findings of IUGR-induced reductions in PPARγ and Setd8 in the neonatal rat lung are consistent with the impaired lung development observed in IUGR rats. Our observation of decreased PPARγ2 and Setd8 in IUGR during the saccular stage of lung development, when the lung is primed to begin alveolar formation, is intriguing.
Whole lung H4K20Me and H4K20Me at Exon 4 of the PPARγ gene at the outset of alveolar formation may be an important determinant of appropriate expression of PPARγ and other genes governing alveolar formation. We have demonstrated that IUGR reduces levels of H4K20Me at PPARγ Exon4 in both males and females. This is likely an important determinant of the reduced PPARγ2 levels observed in our studies. Several lines of evidence suggest PPARγ2 levels are maintained by a feedback loop. Firstly, previous studies have demonstrated that reduced H4K20Me of the PPARγ gene is associated with reduced PPARγ transcription. Secondly, PPARγ regulates transcription of the H4K20Me placement enzyme Setd8. Finally, the PPARγ2 promoter binds PPARγ promoting the transcription of the PPARγ2 variant (16).
Our observation of a reduction in whole lung H4K20Me only in males, despite reduced Setd8 protein in both genders, suggests a gender-specific response in global Setd8 targets. This is important in the context of IUGR because gender-specific molecular changes are often observed (65–70). The mechanisms driving the gender-specific molecular responses remain unknown, and formulate an important and unanswered question in the field. Gender-specific responses to PPARγ polymorphisms have been demonstrated in human obesity (68, 71). These studies suggest that females may utilize other pathways to regulate PPARγ expression and that of down-stream targets, potentially those related to sex-steroids. Gender-specific vulnerability of Setd8 target genes may provide insight into mechanisms of gender-specific molecular responses and will warrant further investigation.
Maternal transfer of DHA to the fetus occurs primarily at the end of gestation, a process interrupted in the preterm infant, exacerbated by placental dysfunction (72–73). Our finding of reduced serum DHA in IUGR male rat pups is consistent with this observation. Again, it is interesting that, in our model, female rat pups do not appear to be as affected. We have also demonstrated that DHA administered to the rat dam via diet is transferred to the pups in utero. Activation of PPARγ by long chain fatty acids, particularly DHA, has received a great deal of attention over past years. This is particularly true in the context of maternal supplementation of pregnant women and has been the subject of extensive biochemical and clinical investigations (74–76). A number of these studies have addressed the role of DHA in brain development, visual acuity and child behavior (reviewed in (77)). Direct binding affinities for DHA and PPARγ remain to be determined, but molecular modeling studies suggest that the affinity of PPARγ for DHA is high and exceeds that of other long chain FA (78–79). In light of the improved levels of PPARγ, setd8 and H4K20Me observed in DHA-IUGR rat pups, maternal DHA supplementation may improve aspects of neonatal lung development in IUGR and warrants further investigation.
While we have shown that PPARγ isoforms can be detected in whole lung, we have not described the location of the mRNA variants or protein isoforms. Future studies will need to address the location of PPARγ1a, -γ1b and –γ2 mRNA expression and subsequent protein accumulation. This will be important in delineating isoform specific epithelial-mesenchymal signaling. A further limitation of this study is the lack of morphometric data describing the structure of the lung of DHA-IUGR rats in the context of our pharmacological dose of DHA. In future experiments, it will be important to include morphometric and functional studies to delineate clinically significant DHA doses that may improve the IUGR lung phenotype.
In conclusion, we have described the presence of all PPARγ mRNA variants and protein isoforms in the neonatal rat lung. We have also demonstrated that in male and female IUGR lungs, levels of PPARγ2 and its target, histone modifying enzyme setd8, are significantly reduced in association with decreased H4K20Me of the PPARγ gene. We have also demonstrated that maternal DHA supplementation ameliorates these effects. We speculate that in the rat, IUGR impairs lung development via alterations in the PPARγ regulated epigenetic milieu. We further speculate that the dietary activation of PPARγ via maternal DHA supplementation may favorably effect this epigenetic environment, with potential improvement of the pulmonary phenotype seen in IUGR.
Supplementary Material
Acknowledgments
We wish to acknowledge the support of J. Ross Milley and Ronald S. Bloom, Division of Neonatology.
Grants
This work was supported by the University of Utah’s Children’s Health Research Center, and the Primary Children’s Medical Center Foundation (LJM).
Footnotes
Conflict of Interest Statement
None of the authors have any conflicts of interest.
References
- 1.Frusca T, Soregaroli M, Valcamonico A, Guandalini F, Danti L. Doppler velocimetry of the uterine arteries in nulliparous women. Early Hum Dev. 1997 Apr 25;48(1–2):177–85. doi: 10.1016/s0378-3782(96)01854-3. [DOI] [PubMed] [Google Scholar]
- 2.Zimmermann P, Eirio V, Koskinen J, Kujansuu E, Ranta T. Doppler assessment of the uterine and uteroplacental circulation in the second trimester in pregnancies at high risk for pre-eclampsia and/or intrauterine growth retardation: comparison and correlation between different Doppler parameters. Ultrasound Obstet Gynecol. 1997 May;9(5):330–8. doi: 10.1046/j.1469-0705.1997.09050330.x. [DOI] [PubMed] [Google Scholar]
- 3.Melamed N, Yogev Y, Glezerman M. Effect of fetal sex on pregnancy outcome in twin pregnancies. Obstet Gynecol. 2009 Nov;114(5):1085–92. doi: 10.1097/AOG.0b013e3181bd8874. [DOI] [PubMed] [Google Scholar]
- 4.Spinillo A, Montanari L, Gardella B, Roccio M, Stronati M, Fazzi E. Infant sex, obstetric risk factors, and 2-year neurodevelopmental outcome among preterm infants. Dev Med Child Neurol. 2009 Jul;51(7):518–25. doi: 10.1111/j.1469-8749.2009.03273.x. [DOI] [PubMed] [Google Scholar]
- 5.Torrance HL, Bloemen MC, Mulder EJ, Nikkels PG, Derks JB, de Vries LS, et al. Predictors for outcome at two years of age after early intrauterine growth restriction. Ultrasound Obstet Gynecol. 2010 Mar 9; doi: 10.1002/uog.7627. [DOI] [PubMed] [Google Scholar]
- 6.Hoyert DL, Mathews TJ, Menacker F, Strobino DM, Guyer B. Annual summary of vital statistics: 2004. Pediatrics. 2006 Jan;117(1):168–83. doi: 10.1542/peds.2005-2587. [DOI] [PubMed] [Google Scholar]
- 7.Stein CE, Kumaran K, Fall CH, Shaheen SO, Osmond C, Barker DJ. Relation of fetal growth to adult lung function in south India. Thorax. 1997 Oct;52(10):895–9. doi: 10.1136/thx.52.10.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiss I, Landmann E, Heckmann M, Misselwitz B, Gortner L. Increased risk of bronchopulmonary dysplasia and increased mortality in very preterm infants being small for gestational age. Arch Gynecol Obstet. 2003 Nov;269(1):40–4. doi: 10.1007/s00404-003-0486-9. [DOI] [PubMed] [Google Scholar]
- 9.Regev RH, Lusky A, Dolfin T, Litmanovitz I, Arnon S, Reichman B. Excess mortality and morbidity among small-for-gestational-age premature infants: a population-based study. J Pediatr. 2003 Aug;143(2):186–91. doi: 10.1067/S0022-3476(03)00181-1. [DOI] [PubMed] [Google Scholar]
- 10.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001 Nov 15;164(10 Pt 1):1971–80. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- 11.Bhandari A, Bhandari V. Pathogenesis, pathology and pathophysiology of pulmonary sequelae of bronchopulmonary dysplasia in premature infants. Front Biosci. 2003 May 1;8:e370–80. doi: 10.2741/1060. [DOI] [PubMed] [Google Scholar]
- 12.Coalson JJ. Pathology of bronchopulmonary dysplasia. Semin Perinatol. 2006 Aug;30(4):179–84. doi: 10.1053/j.semperi.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 13.O'Brien EA, Barnes V, Zhao L, McKnight RA, Yu X, Callaway CW, et al. Uteroplacental insufficiency decreases p53 serine-15 phosphorylation in term IUGR rat lungs. Am J Physiol Regul Integr Comp Physiol. 2007 Jul;293(1):R314–22. doi: 10.1152/ajpregu.00265.2005. [DOI] [PubMed] [Google Scholar]
- 14.Simon DM, Arikan MC, Srisuma S, Bhattacharya S, Tsai LW, Ingenito EP, et al. Epithelial cell PPAR[gamma] contributes to normal lung maturation. Faseb J. 2006 Jul;20(9):1507–9. doi: 10.1096/fj.05-5410fje. [DOI] [PubMed] [Google Scholar]
- 15.Cerny L, Torday JS, Rehan VK. Prevention and treatment of bronchopulmonary dysplasia: contemporary status and future outlook. Lung. 2008 Mar–Apr;186(2):75–89. doi: 10.1007/s00408-007-9069-z. [DOI] [PubMed] [Google Scholar]
- 16.Wakabayashi K, Okamura M, Tsutsumi S, Nishikawa NS, Tanaka T, Sakakibara I, et al. The peroxisome proliferator-activated receptor gamma/retinoid X receptor alpha heterodimer targets the histone modification enzyme PR-Set7/Setd8 gene and regulates adipogenesis through a positive feedback loop. Mol Cell Biol. 2009 Jul;29(13):3544–55. doi: 10.1128/MCB.01856-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fajas L, Auboeuf D, Raspe E, Schoonjans K, Lefebvre AM, Saladin R, et al. The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem. 1997 Jul 25;272(30):18779–89. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y, Qi C, Korenberg JR, Chen XN, Noya D, Rao MS, et al. Structural organization of mouse peroxisome proliferator-activated receptor gamma (mPPAR gamma) gene: alternative promoter use and different splicing yield two mPPAR gamma isoforms. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7921–5. doi: 10.1073/pnas.92.17.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ershov AV, Bazan NG. Photoreceptor phagocytosis selectively activates PPARgamma expression in retinal pigment epithelial cells. J Neurosci Res. 2000 May 1;60(3):328–37. doi: 10.1002/(SICI)1097-4547(20000501)60:3<328::AID-JNR7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Barlier-Mur AM, Chailley-Heu B, Pinteur C, Henrion-Caude A, Delacourt C, Bourbon JR. Maturational factors modulate transcription factors CCAAT/enhancer-binding proteins alpha, beta, delta, and peroxisome proliferator-activated receptor-gamma in fetal rat lung epithelial cells. Am J Respir Cell Mol Biol. 2003 Nov;29(5):620–6. doi: 10.1165/rcmb.4912. [DOI] [PubMed] [Google Scholar]
- 21.Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996 Jan;137(1):354–66. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 22.Heikkinen S, Auwerx J, Argmann CA. PPARgamma in human and mouse physiology. Biochim Biophys Acta. 2007 Aug;1771(8):999–1013. doi: 10.1016/j.bbalip.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devchand PR, Ijpenberg A, Devesvergne B, Wahli W. PPARs: nuclear receptors for fatty acids, eicosanoids, and xenobiotics. Adv Exp Med Biol. 1999;469:231–6. doi: 10.1007/978-1-4615-4793-8_34. [DOI] [PubMed] [Google Scholar]
- 24.Krey G, Braissant O, L'Horset F, Kalkhoven E, Perroud M, Parker MG, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol. 1997 Jun;11(6):779–91. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- 25.Yu YH, Lin EC, Wu SC, Cheng WT, Mersmann HJ, Wang PH, et al. DHA regulates adipogenic genes in myoblasts via porcine PPAR{gamma} J Anim Sci. 2008 Aug 1; doi: 10.2527/jas.2008-1051. [DOI] [PubMed] [Google Scholar]
- 26.Zapata-Gonzalez F, Rueda F, Petriz J, Domingo P, Villarroya F, Diaz-Delfin J, et al. Human dendritic cell activities are modulated by the omega-3 fatty acid, docosahexaenoic acid, mainly through PPAR{gamma}:RXR heterodimers: comparison with other polyunsaturated fatty acids. J Leukoc Biol. 2008 Oct;84(4):1172–82. doi: 10.1189/jlb.1007688. [DOI] [PubMed] [Google Scholar]
- 27.Coyne GS, Kenny DA, Childs S, Sreenan JM, Waters SM. Dietary n-3 polyunsaturated fatty acids alter the expression of genes involved in prostaglandin biosynthesis in the bovine uterus. Theriogenology. 2008 Sep 15;70(5):772–82. doi: 10.1016/j.theriogenology.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 28.Zand H, Rahimipour A, Salimi S, Shafiee SM. Docosahexaenoic acid sensitizes Ramos cells to Gamma-irradiation-induced apoptosis through involvement of PPAR-gamma activation and NF-kappaB suppression. Mol Cell Biochem. 2008 Oct;317(1–2):113–20. doi: 10.1007/s11010-008-9838-x. [DOI] [PubMed] [Google Scholar]
- 29.Guillot N, Debard C, Calzada C, Vidal H, Lagarde M, Vericel E. Effects of docosahexaenoic acid on some megakaryocytic cell gene expression of some enzymes controlling prostanoid synthesis. Biochem Biophys Res Commun. 2008 Aug 8;372(4):924–8. doi: 10.1016/j.bbrc.2008.05.155. [DOI] [PubMed] [Google Scholar]
- 30.Sun H, Berquin IM, Owens RT, O'Flaherty JT, Edwards IJ. Peroxisome proliferator-activated receptor gamma-mediated up-regulation of syndecan-1 by n-3 fatty acids promotes apoptosis of human breast cancer cells. Cancer Res. 2008 Apr 15;68(8):2912–9. doi: 10.1158/0008-5472.CAN-07-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reece MS, McGregor JA, Allen KG, Harris MA. Maternal and perinatal long-chain fatty acids: possible roles in preterm birth. Am J Obstet Gynecol. 1997 Apr;176(4):907–14. doi: 10.1016/s0002-9378(97)70620-3. [DOI] [PubMed] [Google Scholar]
- 32.van Eijsden M, Hornstra G, van der Wal MF, Vrijkotte TG, Bonsel GJ. Maternal n-3, n-6, and trans fatty acid profile early in pregnancy and term birth weight: a prospective cohort study. Am J Clin Nutr. 2008 Apr;87(4):887–95. doi: 10.1093/ajcn/87.4.887. [DOI] [PubMed] [Google Scholar]
- 33.Olsen SF, Osterdal ML, Salvig JD, Weber T, Tabor A, Secher NJ. Duration of pregnancy in relation to fish oil supplementation and habitual fish intake: a randomised clinical trial with fish oil. Eur J Clin Nutr. 2007 Aug;61(8):976–85. doi: 10.1038/sj.ejcn.1602609. [DOI] [PubMed] [Google Scholar]
- 34.Baserga M, Hale MA, McKnight RA, Yu X, Callaway CW, Lane RH. Uteroplacental insufficiency alters hepatic expression, phosphorylation, and activity of the glucocorticoid receptor in fetal IUGR rats. Am J Physiol Regul Integr Comp Physiol. 2005 Nov;289(5):R1348–53. doi: 10.1152/ajpregu.00211.2005. [DOI] [PubMed] [Google Scholar]
- 35.Lane RH, Flozak AS, Ogata ES, Bell GI, Simmons RA. Altered hepatic gene expression of enzymes involved in energy metabolism in the growth-retarded fetal rat. Pediatr Res. 1996 Mar;39(3):390–4. doi: 10.1203/00006450-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Lane RH, MacLennan NK, Hsu JL, Janke SM, Pham TD. Increased hepatic peroxisome proliferator-activated receptor-gamma coactivator-1 gene expression in a rat model of intrauterine growth retardation and subsequent insulin resistance. Endocrinology. 2002 Jul;143(7):2486–90. doi: 10.1210/endo.143.7.8898. [DOI] [PubMed] [Google Scholar]
- 37.Lane RH, Tsirka AE, Gruetzmacher EM. Uteroplacental insufficiency alters cerebral mitochondrial gene expression and DNA in fetal and juvenile rats. Pediatr Res. 2000 Jun;47(6):792–7. doi: 10.1203/00006450-200006000-00019. [DOI] [PubMed] [Google Scholar]
- 38.Ogata ES, Bussey ME, Finley S. Altered gas exchange, limited glucose and branched chain amino acids, and hypoinsulinism retard fetal growth in the rat. Metabolism. 1986 Oct;35(10):970–7. doi: 10.1016/0026-0495(86)90064-8. [DOI] [PubMed] [Google Scholar]
- 39.Lane RH, Dvorak B, MacLennan NK, Dvorakova K, Halpern MD, Pham TD, et al. IGF alters jejunal glucose transporter expression and serum glucose levels in immature rats. Am J Physiol Regul Integr Comp Physiol. 2002 Dec;283(6):R1450–60. doi: 10.1152/ajpregu.00172.2002. [DOI] [PubMed] [Google Scholar]
- 40.Lane RH, Chandorkar AK, Flozak AS, Simmons RA. Intrauterine growth retardation alters mitochondrial gene expression and function in fetal and juvenile rat skeletal muscle. Pediatr Res. 1998 May;43(5):563–70. doi: 10.1203/00006450-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Unterman TG, Simmons RA, Glick RP, Ogata ES. Circulating levels of insulin, insulin-like growth factor-I (IGF-I), IGF-II, and IGF-binding proteins in the small for gestational age fetal rat. Endocrinology. 1993 Jan;132(1):327–36. doi: 10.1210/endo.132.1.7678218. [DOI] [PubMed] [Google Scholar]
- 42.Guiding principles for research involving animals and human beings 2002.
- 43.Joss-Moore LA, Wang Y, Campbell MS, Moore B, Yu X, Callaway CW, et al. Uteroplacental Insufficiency Increases Visceral Adiposity and Visceral Adipose PPARg2 Expression in Male Rat Offspring Prior to the Onset of Obesity. Early Hum Dev. 2010 doi: 10.1016/j.earlhumdev.2010.02.006. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kloesz JL, Serdikoff CM, Maclennan NK, Adibi SA, Lane RH. Uteroplacental insufficiency alters liver and skeletal muscle branched-chain amino acid metabolism in intrauterine growth-restricted fetal rats. Pediatr Res. 2001 Nov;50(5):604–10. doi: 10.1203/00006450-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Pham TD, MacLennan NK, Chiu CT, Laksana GS, Hsu JL, Lane RH. Uteroplacental insufficiency increases apoptosis and alters p53 gene methylation in the full-term IUGR rat kidney. Am J Physiol Regul Integr Comp Physiol. 2003 Nov;285(5):R962–70. doi: 10.1152/ajpregu.00201.2003. [DOI] [PubMed] [Google Scholar]
- 46.Baserga M, Hale MA, Ke X, Wang ZM, Yu X, Callaway CW, et al. Uteroplacental insufficiency increases p53 phosphorylation without triggering the p53-MDM2 functional circuit response in the IUGR rat kidney. Am J Physiol Regul Integr Comp Physiol. 2006 Aug;291(2):R412–8. doi: 10.1152/ajpregu.00880.2005. [DOI] [PubMed] [Google Scholar]
- 47.Baserga M, Hale MA, Wang ZM, Yu X, Callaway CW, McKnight RA, et al. Uteroplacental insufficiency alters nephrogenesis and downregulates cyclooxygenase-2 expression in a model of IUGR with adult-onset hypertension. Am J Physiol Regul Integr Comp Physiol. 2007 May;292(5):R1943–55. doi: 10.1152/ajpregu.00558.2006. [DOI] [PubMed] [Google Scholar]
- 48.Ke X, Lei Q, James SJ, Kelleher SL, Melnyk S, Jernigan S, et al. Uteroplacental insufficiency affects epigenetic determinants of chromatin structure in brains of neonatal and juvenile IUGR rats. Physiol Genomics. 2006 Mar 13;25(1):16–28. doi: 10.1152/physiolgenomics.00093.2005. [DOI] [PubMed] [Google Scholar]
- 49.Wells J, Farnham PJ. Characterizing transcription factor binding sites using formaldehyde crosslinking and immunoprecipitation. Methods. 2002 Jan;26(1):48–56. doi: 10.1016/S1046-2023(02)00007-5. [DOI] [PubMed] [Google Scholar]
- 50.Nelson JD, Denisenko O, Sova P, Bomsztyk K. Fast chromatin immunoprecipitation assay. Nucleic Acids Res. 2006;34(1):e2. doi: 10.1093/nar/gnj004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fu Q, Yu X, Callaway CW, Lane RH, McKnight RA. Epigenetics: intrauterine growth retardation (IUGR) modifies the histone code along the rat hepatic IGF-1 gene. Faseb J. 2009 Apr 15; doi: 10.1096/fj.08-124768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rehan VK, Dargan-Batra SK, Wang Y, Cerny L, Sakurai R, Santos J, et al. A paradoxical temporal response of the PTHrP/PPARgamma signaling pathway to lipopolysaccharide in an in vitro model of the developing rat lung. Am J Physiol Lung Cell Mol Physiol. 2007 Jul;293(1):L182–90. doi: 10.1152/ajplung.00319.2006. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Santos J, Sakurai R, Shin E, Cerny L, Torday JS, et al. Peroxisome proliferator-activated receptor gamma agonists enhance lung maturation in a neonatal rat model. Pediatr Res. 2009 Feb;65(2):150–5. doi: 10.1203/PDR.0b013e3181938c40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anghel SI, Bedu E, Vivier CD, Descombes P, Desvergne B, Wahli W. Adipose tissue integrity as a prerequisite for systemic energy balance: a critical role for peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007 Oct 12;282(41):29946–57. doi: 10.1074/jbc.M702490200. [DOI] [PubMed] [Google Scholar]
- 55.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006 Dec 14;444(7121):847–53. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Michael LF, Lazar MA, Mendelson CR. Peroxisome proliferator-activated receptor gamma1 expression is induced during cyclic adenosine monophosphate-stimulated differentiation of alveolar type II pneumonocytes. Endocrinology. 1997 Sep;138(9):3695–703. doi: 10.1210/endo.138.9.5373. [DOI] [PubMed] [Google Scholar]
- 57.Aoki Y, Maeno T, Aoyagi K, Ueno M, Aoki F, Aoki N, et al. Pioglitazone, a Peroxisome Proliferator-Activated Receptor Gamma Ligand, Suppresses Bleomycin-Induced Acute Lung Injury and Fibrosis. Respiration. 2008 Oct 31; doi: 10.1159/000168676. [DOI] [PubMed] [Google Scholar]
- 58.Wang AC, Dai X, Luu B, Conrad DJ. Peroxisome proliferator-activated receptor-gamma regulates airway epithelial cell activation. Am J Respir Cell Mol Biol. 2001 Jun;24(6):688–93. doi: 10.1165/ajrcmb.24.6.4376. [DOI] [PubMed] [Google Scholar]
- 59.Jaudszus A, Krokowski M, Mockel P, Darcan Y, Avagyan A, Matricardi P, et al. Cis-9, trans-11-conjugated linoleic acid inhibits allergic sensitization and airway inflammation via a PPARgamma-related mechanism in mice. J Nutr. 2008 Jul;138(7):1336–42. doi: 10.1093/jn/138.7.1336. [DOI] [PubMed] [Google Scholar]
- 60.Lakatos HF, Thatcher TH, Kottmann RM, Garcia TM, Phipps RP, Sime PJ. The Role of PPARs in Lung Fibrosis. PPAR Res. 2007;2007:71323. doi: 10.1155/2007/71323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reddy RC, Srirangam A, Reddy K, Chen J, Gangireddy S, Kalemkerian GP, et al. Chemotherapeutic drugs induce PPAR-gamma expression and show sequence-specific synergy with PPAR-gamma ligands in inhibition of non-small cell lung cancer. Neoplasia. 2008 Jun;10(6):597–603. doi: 10.1593/neo.08134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nisbet RE, Sutliff RL, Hart CM. The role of peroxisome proliferator-activated receptors in pulmonary vascular disease. PPAR Res. 2007;2007:18797. doi: 10.1155/2007/18797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007 May 18;129(4):823–37. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 64.Vakoc CR, Sachdeva MM, Wang H, Blobel GA. Profile of histone lysine methylation across transcribed mammalian chromatin. Mol Cell Biol. 2006 Dec;26(24):9185–95. doi: 10.1128/MCB.01529-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Campbell M, Joss-Moore LA, Wang Y, Callaway C, Yu X, McKnight R, et al. IUGR alters TNFα and NFκB mRNA levels in rat adipose tissue in gender specific manner. Journal of Investigative Medicine. 2009;57(1):214. [Google Scholar]
- 66.Choi GY, Tosh DN, Garg A, Mansano R, Ross MG, Desai M. Gender-specific programmed hepatic lipid dysregulation in intrauterine growth-restricted offspring. Am J Obstet Gynecol. 2007 May;196(5):477, e1–7. doi: 10.1016/j.ajog.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 67.Gayle DA, Desai M, Casillas E, Beloosesky R, Ross MG. Gender-specific orexigenic and anorexigenic mechanisms in rats. Life Sci. 2006 Sep 13;79(16):1531–6. doi: 10.1016/j.lfs.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 68.Morini E, Tassi V, Capponi D, Ludovico O, Dallapiccola B, Trischitta V, et al. Interaction between PPARgamma2 variants and gender on the modulation of body weight. Obesity (Silver Spring) 2008 Jun;16(6):1467–70. doi: 10.1038/oby.2008.225. [DOI] [PubMed] [Google Scholar]
- 69.Ozaki T, Nishina H, Hanson MA, Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol. 2001 Jan 1;530(Pt 1):141–52. doi: 10.1111/j.1469-7793.2001.0141m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sugden MC, Holness MJ. Gender-specific programming of insulin secretion and action. J Endocrinol. 2002 Dec;175(3):757–67. doi: 10.1677/joe.0.1750757. [DOI] [PubMed] [Google Scholar]
- 71.Mattevi VS, Zembrzuski VM, Hutz MH. Effects of a PPARG gene variant on obesity characteristics in Brazil. Braz J Med Biol Res. 2007 Jul;40(7):927–32. doi: 10.1590/s0100-879x2006005000114. [DOI] [PubMed] [Google Scholar]
- 72.Haggarty P, Page K, Abramovich DR, Ashton J, Brown D. Long-chain polyunsaturated fatty acid transport across the perfused human placenta. Placenta. 1997 Nov;18(8):635–42. doi: 10.1016/s0143-4004(97)90004-7. [DOI] [PubMed] [Google Scholar]
- 73.Lapillonne A, Jensen CL. Reevaluation of the DHA requirement for the premature infant. Prostaglandins Leukot Essent Fatty Acids. 2009 Aug–Sep;81(2–3):143–50. doi: 10.1016/j.plefa.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 74.Makrides M, Gibson RA. Long-chain polyunsaturated fatty acid requirements during pregnancy and lactation. Am J Clin Nutr. 2000 Jan;71(1 Suppl):307S–11S. doi: 10.1093/ajcn/71.1.307S. [DOI] [PubMed] [Google Scholar]
- 75.Innis SM. Perinatal biochemistry and physiology of long-chain polyunsaturated fatty acids. J Pediatr. 2003 Oct;143(4 Suppl):S1–8. doi: 10.1067/s0022-3476(03)00396-2. [DOI] [PubMed] [Google Scholar]
- 76.Koletzko B, Lien E, Agostoni C, Bohles H, Campoy C, Cetin I, et al. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. J Perinat Med. 2008;36(1):5–14. doi: 10.1515/JPM.2008.001. [DOI] [PubMed] [Google Scholar]
- 77.Carlson SE. Docosahexaenoic acid supplementation in pregnancy and lactation. Am J Clin Nutr. 2009 Feb;89(2):678S–84S. doi: 10.3945/ajcn.2008.26811E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gani OA, Sylte I. Molecular recognition of docosahexaenoic acid by peroxisome proliferator-activated receptors and retinoid-X receptor alpha. J Mol Graph Model. 2008 Sep;27(2):217–24. doi: 10.1016/j.jmgm.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 79.Olsen SF, Osterdal ML, Salvig JD, Mortensen LM, Rytter D, Secher NJ, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008 Jul;88(1):167–75. doi: 10.1093/ajcn/88.1.167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.