Abstract
A defined deletion in the Escherichia coli K-12 sodA gene (encoding manganese-superoxide dismutase) linked to a nontransposable selectable marker was generated by transposon Tn5 insertion in combination with in vitro mutagenesis. This mutant allele was used to replace the wild-type sodA gene in an E. coli clinical isolate of serotype O18ac:K1:H7 by bacteriophage P1 transduction. The O18ac:K1:H7 sodA mutant contained no manganese-superoxide dismutase and no hybrid manganese-iron-superoxide dismutase. The sodA mutant was more sensitive to paraquat toxicity than were the parental strain and an isogenic mutant bearing an analogously constructed sodA+ Tn5 insertion allele. In a suckling rat model for bacteremia following oral inoculation of E. coli K1, the sodA mutant was undiminished in its capabilities both to colonize the gastrointestinal tract and, surprisingly, to cause bacteremia. In conjunction with the rat model for E. coli K1 pathogenesis, the method for site-directed mutagenesis described in this paper permits determination of the role played in colonization and bacteremia by any K1 gene which either has a homolog in E. coli K-12 or can be cloned and manipulated therein.
Full text
PDF

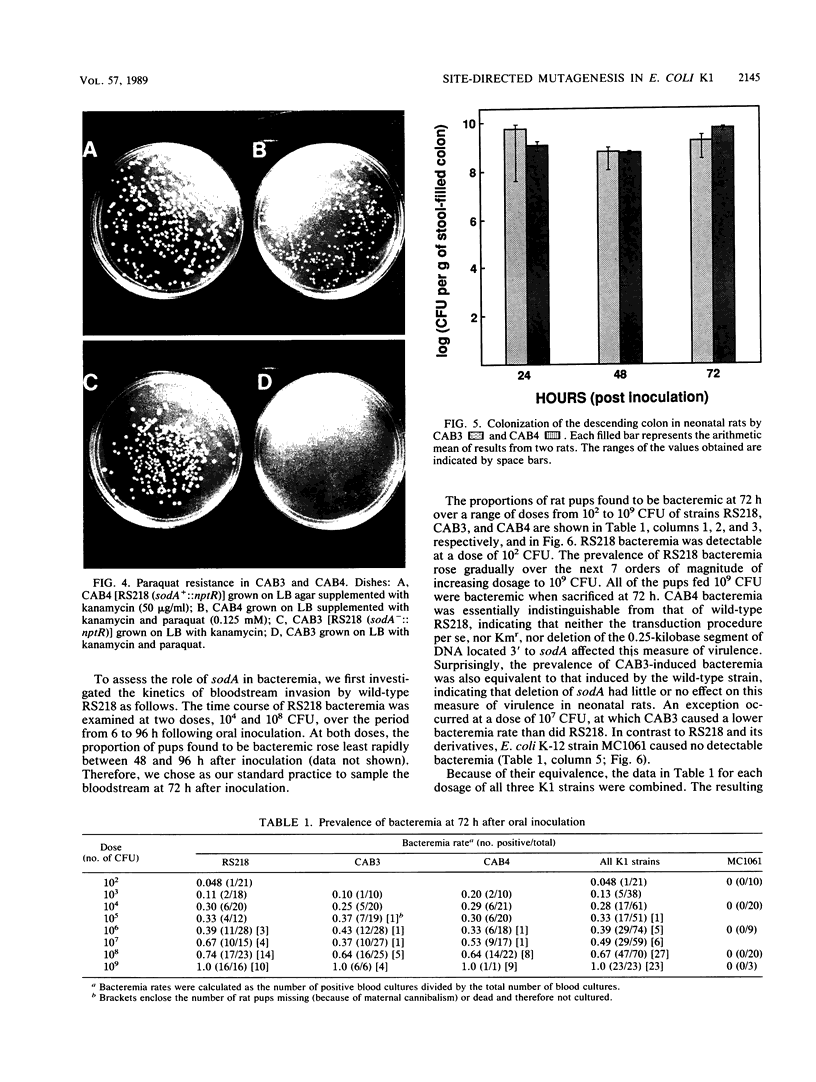
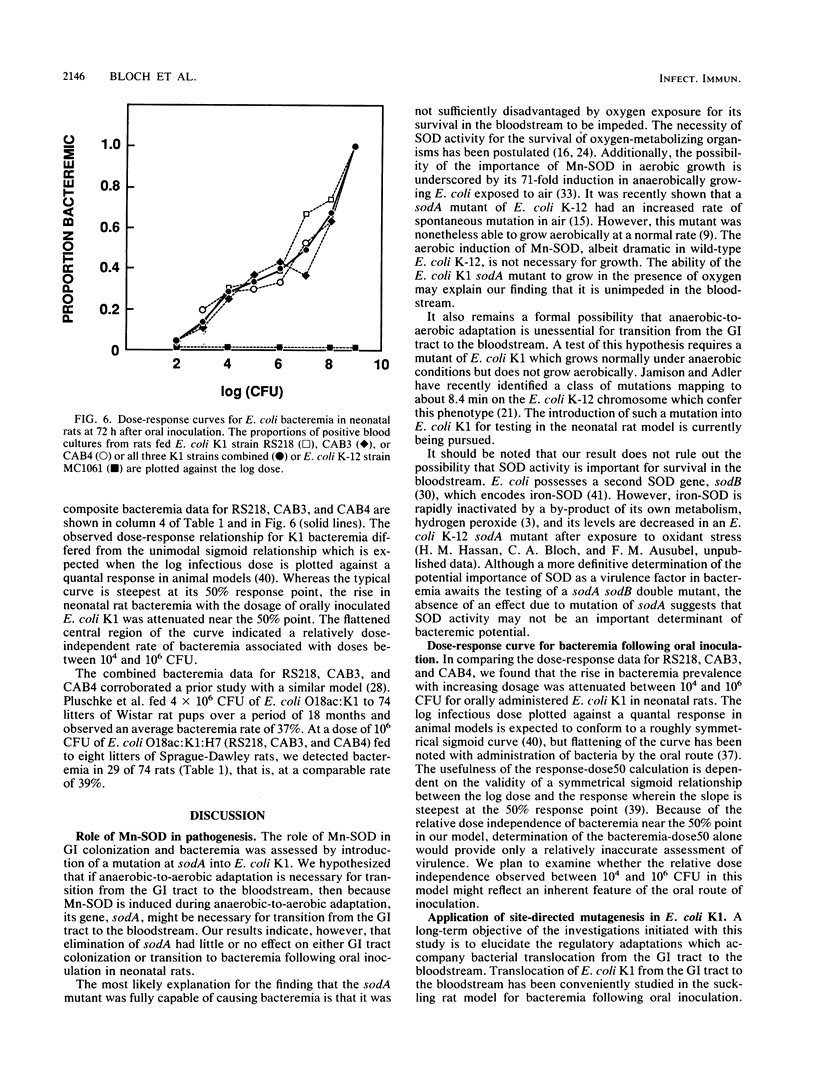


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder M., Aaronson W., Sutton A., Silver R. P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Pluschke G. Clonal analysis of descent and virulence among selected Escherichia coli. Annu Rev Microbiol. 1986;40:185–210. doi: 10.1146/annurev.mi.40.100186.001153. [DOI] [PubMed] [Google Scholar]
- Asada K., Yoshikawa K., Takahashi M., Maeda Y., Enmanji K. Superoxide dismutases from a blue-green alga, Plectonema boryanum. J Biol Chem. 1975 Apr 25;250(8):2801–2807. [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Berg C. M., Grullón C. A., Wang A., Whalen W. A., Berg D. E. Transductional instability of Tn5-induced mutations: generalized and specialized transduction of Tn5 by bacteriophage P1. Genetics. 1983 Oct;105(2):259–263. doi: 10.1093/genetics/105.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch C. A., Ausubel F. M. Paraquat-mediated selection for mutations in the manganese-superoxide dismutase gene sodA. J Bacteriol. 1986 Nov;168(2):795–798. doi: 10.1128/jb.168.2.795-798.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlioz A., Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986 Mar;5(3):623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Qasba P. K. Alkaline transfer of DNA to plastic membrane. Biochem Biophys Res Commun. 1984 Jul 18;122(1):340–344. doi: 10.1016/0006-291x(84)90480-7. [DOI] [PubMed] [Google Scholar]
- Cook L. N., Davis R. S., Stover B. H. Outbreak of amikacin-resistant Enterobacteriaceae in an intensive care nursery. Pediatrics. 1980 Feb;65(2):264–268. [PubMed] [Google Scholar]
- Cross A. S., Zollinger W., Mandrell R., Gemski P., Sadoff J. Evaluation of immunotherapeutic approaches for the potential treatment of infections caused by K1-positive Escherichia coli. J Infect Dis. 1983 Jan;147(1):68–76. doi: 10.1093/infdis/147.1.68. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr S. B., D'Ari R., Touati D. Oxygen-dependent mutagenesis in Escherichia coli lacking superoxide dismutase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8268–8272. doi: 10.1073/pnas.83.21.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983;23:239–257. doi: 10.1146/annurev.pa.23.040183.001323. [DOI] [PubMed] [Google Scholar]
- Glode M. P., Sutton A., Moxon E. R., Robbins J. B. Pathogenesis of neonatal Escherichia coli meningitis: induction of bacteremia and meningitis in infant rats fed E. coli K1. Infect Immun. 1977 Apr;16(1):75–80. doi: 10.1128/iai.16.1.75-80.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Enzymatic defenses against the toxicity of oxygen and of streptonigrin in Escherichia coli. J Bacteriol. 1977 Mar;129(3):1574–1583. doi: 10.1128/jb.129.3.1574-1583.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Regulation of the synthesis of superoxide dismutase in Escherichia coli. Induction by methyl viologen. J Biol Chem. 1977 Nov 10;252(21):7667–7672. [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Superoxide radical and the oxygen enhancement of the toxicity of paraquat in Escherichia coli. J Biol Chem. 1978 Nov 25;253(22):8143–8148. [PubMed] [Google Scholar]
- Jamison C. S., Adler H. I. Mutations in Escherichia coli that effect sensitivity to oxygen. J Bacteriol. 1987 Nov;169(11):5087–5094. doi: 10.1128/jb.169.11.5087-5094.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith B. R., Maurer L., Spears P. A., Orndorff P. E. Receptor-binding function of type 1 pili effects bladder colonization by a clinical isolate of Escherichia coli. Infect Immun. 1986 Sep;53(3):693–696. doi: 10.1128/iai.53.3.693-696.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light I. J., Walton R. L., Sutherland J. M., Shinefield H. R., Brackvogel V. Use of bacterial interference to control a staphylococcal nursery outbreak. Deliberate colonization of all infants with the 502A strain of Staphylococcus aureus. Am J Dis Child. 1967 Mar;113(3):291–300. doi: 10.1001/archpedi.1967.02090180051001. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983 Dec 8;306(5943):551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- Ochman H., Selander R. K. Evidence for clonal population structure in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jan;81(1):198–201. doi: 10.1073/pnas.81.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluschke G., Mercer A., Kusećek B., Pohl A., Achtman M. Induction of bacteremia in newborn rats by Escherichia coli K1 is correlated with only certain O (lipopolysaccharide) antigen types. Infect Immun. 1983 Feb;39(2):599–608. doi: 10.1128/iai.39.2.599-608.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- Sakamoto H., Touati D. Cloning of the iron superoxide dismutase gene (sodB) in Escherichia coli K-12. J Bacteriol. 1984 Jul;159(1):418–420. doi: 10.1128/jb.159.1.418-420.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimpff S. C., Young V. M., Greene W. H., Vermeulen G. D., Moody M. R., Wiernik P. H. Origin of infection in acute nonlymphocytic leukemia. Significance of hospital acquisition of potential pathogens. Ann Intern Med. 1972 Nov;77(5):707–714. doi: 10.7326/0003-4819-77-5-707. [DOI] [PubMed] [Google Scholar]
- Silver R. P., Aaronson W., Sutton A., Schneerson R. Comparative analysis of plasmids and some metabolic characteristics of Escherichia coli K1 from diseased and healthy individuals. Infect Immun. 1980 Jul;29(1):200–206. doi: 10.1128/iai.29.1.200-206.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. W., Neidhardt F. C. Proteins induced by aerobiosis in Escherichia coli. J Bacteriol. 1983 Apr;154(1):344–350. doi: 10.1128/jb.154.1.344-350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprunt K., Leidy G., Redman W. Abnormal colonization of neonates in an ICU: conversion to normal colonization by pharyngeal implantation of alpha hemolytic streptococcus strain 215. Pediatr Res. 1980 Apr;14(4 Pt 1):308–313. doi: 10.1203/00006450-198004000-00010. [DOI] [PubMed] [Google Scholar]
- Sprunt K., Leidy G., Redman W. Abnormal colonization of neonates in an intensive care unit: means of identifying neonates at risk of infection. Pediatr Res. 1978 Oct;12(10):998–1002. doi: 10.1203/00006450-197810000-00010. [DOI] [PubMed] [Google Scholar]
- Stibitz S., Black W., Falkow S. The construction of a cloning vector designed for gene replacement in Bordetella pertussis. Gene. 1986;50(1-3):133–140. doi: 10.1016/0378-1119(86)90318-5. [DOI] [PubMed] [Google Scholar]
- Touati D. Cloning and mapping of the manganese superoxide dismutase gene (sodA) of Escherichia coli K-12. J Bacteriol. 1983 Sep;155(3):1078–1087. doi: 10.1128/jb.155.3.1078-1087.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost F. J., Jr, Fridovich I. An iron-containing superoxide dismutase from Escherichia coli. J Biol Chem. 1973 Jul 25;248(14):4905–4908. [PubMed] [Google Scholar]