TO THE EDITOR
Being amenable to genetic modification, mice are critical in vivo tools to test the role of gene mutations in malignant melanoma (MM) development. They are the animal model of choice for studying MM growth and treatments either as autochthonous tumors or as xenografts. However, adult mice do not have epidermal melanocytes (MCs), and primary murine MMs are usually dermal, reminiscent of human malignant blue nevi (see, e.g., Dhomen et al., 2009), animal type, or nodular MM. In contrast, most human MMs appear to originate in the epidermis, although they can progress to a vertical growth phase and invade the dermis. Although most murine MMs are dermal, lesions from the hepatocyte growth factor model often exhibit epidermal “pagetoid” spread on the albino (FVB) but not C57BL6 background (Noonan et al., 2001; Florell et al., 2007). Focal pagetoid spread is also seen in PtenF/F::Tyr-CreERT2::LSL-BrafCA/wt mice (Dankort et al., 2009).
One factor that differentiates mouse from human skin is the expression of KIT receptor ligand (KITL), highly expressed in the human but not murine epidermis (Kunisada et al., 1998; Longley and Carter, 1999). It is expressed in 76% of human MMs, not only in adjacent keratinocytes, but also sometimes in the tumor cells (Giehl et al., 2007). Mice with engineered keratinocyte Kitl expression (K14-Kitl) have epidermal MCs throughout life (Kunisada et al., 1998). Remarkably, they do not develop MMs even after chronic UVR exposures (Yamazaki et al., 2004). When crossed onto a DNA repair defective (Xpa-null) background, they developed lentigo-like lesions (Yamazaki et al., 2005) after a harsh chronic UVR regimen. Although the physiological relevance of this study is questionable, as many animals died from severe sunburn, epidermal MMs did develop. Recently, K14-Kitl mice were crossed with animals carrying activation of the metabotropic glutamate receptor-1 (Abdel-Daim et al., 2010), but only dermal MMs were reported.
We previously reported the development of dermal MMs in Arf−/−::Tyr-NrasQ61K mice (Ferguson et al., 2010). These mice develop MMs with early age of onset (average of 114 days) after neonatal UVR. We hypothesized that the same mutations (in Arf and Nras) should lead to the development of epidermal MMs in mice with epidermal MCs. Arf−/−::Tyr-NrasQ61K::K14-Kitl mice were exposed to a single neonatal UVB treatment and followed for the development of MMs. Experiments were undertaken with institute animal ethics approval. As with K14-Kitl mice, we observed MCs along the basal layer of the compound mutants (Figure 1a). The hyperpigmented skin was bleached, and histopathological analysis revealed nests of MCs in the epidermis and scattered MCs in the dermis (Figure 1b). We confirmed the epidermal localization of MCs by staining with anti-S100 or anti-Tyrp1 antibodies and anti-keratin 14 to stain keratinocytes (Figure 1c). The presence of the increased numbers of MCs singly and in small nests in all levels of the epidermis, some of which stained for Ki-67 (Figure 1d), is suggestive of early epidermal MM. As MCs do not normally remain in the adult murine epidermis, we wanted to determine how long they survive there when supported by constitutive Kitl expression. We performed label retention experiments beginning with injection of K14-Kitl neonates with BrdU. When skin was examined 8 weeks later, many epidermal MCs still carried label (Supplementary Figure S1a and b online), indicating that they are slow cycling and can remain for long periods in the epidermis. This is consistent with the MC nests emanating from expansion of these epidermal MCs rather than invasion of dermal MCs.
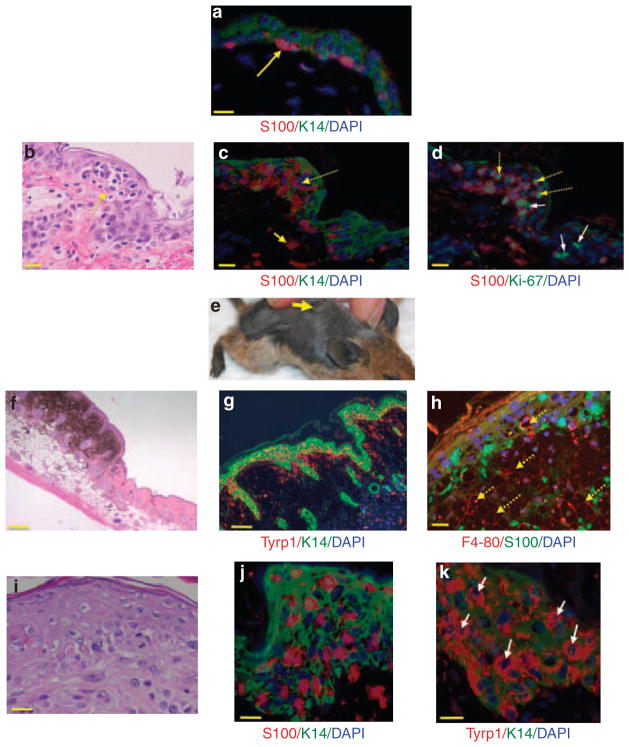
Figure 1. Histopathology of superficial spreading melanoma equivalents in Arf−/−::Tyr-Nras::K14-Kitl mice.
(a) Double-label immunofluorescence (IF) staining for S100 and K14 on “normal” Arf−/−::Tyr-Nras::K14-Kitl mouse skin (methods fully described in Supplementary Material online). Briefly, sections of formalin-fixed and paraffin-embedded skin were stained with anti-S100 (Dako, Eli, UK) primary antibody, followed by an AlexaFluor 555-labeled donkey anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). After several washes, we stained keratinocytes using anti-K14 (Dako) followed with AlexaFluor 488-labeled donkey anti-rabbit (Jackson ImmunoResearch Laboratories). The yellow arrow points to red S100 staining MCs sitting at the dermoepidermal junction. Scale bar = 20 μM. (b) Hematoxylin and eosin (H&E) staining of bleached formalin-fixed, paraffin-embedded sections of Arf−/−::Tyr-Nras::K14-Kitl mouse skin. Bleaching is described in Supplementary Methods online. Note cluster (nest) of atypical MC cells in the epidermis, indicated by a dotted yellow arrow. Scale bar = 20 μM. (c) IF staining of a similar portion of epidermis with a dense cluster of S100-positive epidermal MCs (indicated by dotted arrows). Solid yellow arrow points to a dermal MC. Scale bar = 20 μM. (d) Epidermal MC cluster stained for S100 and Ki-67. Dotted yellow arrow shows MCs in the suprabasal epidermis positive for Ki-67. Solid white arrows denote keratinocytes (S100-negative cells) staining for Ki-67. Scale bar = 20 μM. (e) Arf−/−::Tyr-Nras::K14-Kitl mouse with a slightly elevated pigmented plaque on its back. Note that because of the heavy pigmentation of the whole skin, the melanoma is not easily discernible in this photograph. Mouse background: FVB, two generations down C57BL6. (f) H&E-stained section showing raised plaque. Scale bar = 200 μM. (g) IF staining of a Arf−/−::Tyr-Nras::K14-Kitl plaque for Tyrp1 (red) and K14 (green). Scale bar = 100 μM. (h) Double-layer IF image of a pigmented plaque staining for F4/80 (red) and S100 (green). Dotted yellow arrows denote macrophage lineage cells (melanophages) in a “cobblestone” pattern. The pigmented part of the tumor consists of many melanophages interspersed with some melanocytes/melanoma cells. Scale bar = 20 μM. (i) Higher-power H&E image of MC nesting in the thickened epidermis within a plaque. Scale bar = 20 μM. (j) Higher-power image of IF staining of S100-positive epidermal MCs within an epidermal melanoma. K14-positive keratinocytes are green. Scale bar = 20 μM. (k) Double-label IF staining for Tyrp1 using the PEP1 antibody, a gift from Dr Vince Hearing. K14-positive keratinocytes are green. Whereas S100 IF staining somewhat obscures the 4,6-diamidino-2-phenylindole (DAPI)-stained nuclei, Tyrp1 staining allows better visualization of MC nuclei of varying sizes and shapes (indicated by white arrows). Scale bar = 20 μM.
By eye, it was not possible to discern the MC nests from “normal” skin because of its hyperpigmented nature, but slightly elevated plaques were also observed (Figure 1e–k), developing at an average age of onset of 100 days. Although this is earlier than Arf−/−::Tyr-NrasQ61K lesions (Supplementary Figure S2 online), the difference may be because of strain differences between the cohorts. Critically, whereas Arf−/− ::Tyr-NrasQ61K mice developed deep dermal lesions clearly separated from the epidermis by layers of collagen (Figure 2a), the plaques developing in Arf−/−::Tyr-NrasQ61K::K14-Kitl animals exhibited an epidermal pagetoid growth pattern with epidermal hyperplasia (Figure 1g–k), as well as spread within the adjacent dermis (Figure 2b). The deeper highly pigmented dermal portion of the plaques contains mostly melanophages interspersed with scattered MCs (Figure 1h). As atypical MCs are present within all levels of the epidermis, and to some extent beneath the epidermis in the papillary dermis, there are clear similarities between these murine lesions and human superficial spreading MMs (Figure 2c).
Figure 2. Comparative histopathology of murine and human epidermal melanomas.
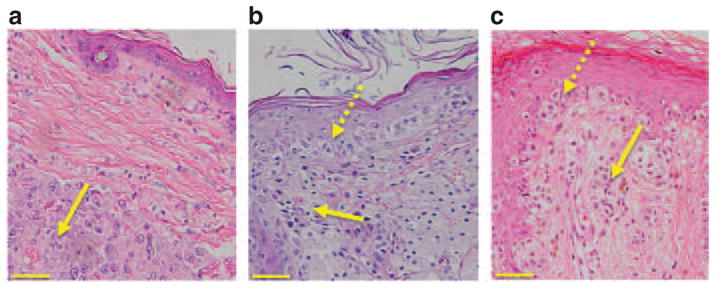
(a) Hematoxylin and eosin (H&E)-stained section showing a deep dermal malignant melanoma (MM) in an Arf−/−::Tyr-Nras mouse. The yellow arrow denotes a nodular melanoma composed of sheets of pleomorphic melanocytes (MCs). This nodular lesion is clearly separated from the epidermis by layers of collagen. (b) MM from an Arf−/−::Tyr-Nras::K14-Kitl mouse. Dotted yellow arrow denotes atypical MCs in the epidermis, and filled yellow arrow atypical MCs in the dermis. There is an increased number of MCs, varying in size and shape, not only at the dermoepidermal junction but also at all levels of the epidermis. (c) Human superficial spreading MM. Dotted yellow arrow denotes atypical MCs in the epidermis, and filled yellow arrow atypical MCs in the dermis. The morphological similarities between Figure 2b and c are striking. Scale bars = 100 μM.
We also examined skin from other anatomical locations (Supplementary Figure S3 a–i online). In the ears and tail, we saw slightly atypical MCs along the entire dermoepidermal junction and to some degree in the dermis. The effect of keratinocyte Kitl expression was especially noticeable in the footpads (Supplementary Figure S3 g–i online). In Arf−/−::Tyr-NrasQ61K mice, the footpads are white with no MCs present (data not shown). However, Arf−/−::Tyr- NrasQ61K::K14-Kitl mice have black footpads, and histopathology shows MCs singly, and as nests, throughout the epidermis. We did not observe visible plaques on any anatomical location other than the dorsal skin.
A common criticism of mice as MM models is the dermal localization of lesions. Using a dermal murine MM model, we show that overexpression of just one cytokine in keratinocytes—Kitl—essentially translocates MMs into the epidermis, where they exhibit nesting and pagetoid spread. Hence, it is likely that many aspects of transformation of murine MCs are similar to those occurring during human MM progression. It is noteworthy that there are no good mutation signatures that differentiate superficial spreading and nodular MMs. At least in our experimental model, the same mutations (a complement of mutations commonly present in human melanomas that together with neonatal UVR are sufficient for MC transformation) can induce both MM subtypes. The growth pattern/localization of the lesions is totally controlled by a keratinocyte cytokine. The development of better methods of specifically targeting mutations to epidermal MCs in K14-Kitl mice should engage the full power of the mouse as a preclinical model to study the mechanisms of epidermal MM growth. Such models, along with the one we report in this study, will facilitate improved in vivo modeling of gene–environment interactions in MM carcinogenesis in adult mice.
Supplementary Material
Acknowledgments
We thank Dr Vince Hearing for the anti-Tyrp1 antibody. This work was funded by the National Health & Medical Research Council (NH&MRC) of Australia and the Cancer Council of Queensland. GJW is the recipient of a Senior Research Fellowship from the Cancer Council of Queensland.
Abbreviations
- MC
melanocyte
- MM
malignant melanoma
Footnotes
CONFLICT OF INTEREST
HPS is a shareholder and consultant for Molemap, Australia. The other authors state no conflict of interest.
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
References
- Abdel-Daim M, Funasaka Y, Komoto M, et al. Pharmacogenomics of metabotropic glutamate receptor subtype 1 and in vivo malignant melanoma formation. J Dermatol. 2010;37:635–46. doi: 10.1111/j.1346-8138.2010.00833.x. [DOI] [PubMed] [Google Scholar]
- Dankort D, Curley DP, Cartlidge RA, et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–52. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhomen N, Reis-Filho JS, da Rocha Dias S, et al. Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer Cell. 2009;15:294–303. doi: 10.1016/j.ccr.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Ferguson B, Muller HK, Handoko HY, et al. Differential roles of the pRb and Arf/p53 pathways in murine naevus and melanoma genesis. Pigment Cell Melanoma Res. 2010;23:771–80. doi: 10.1111/j.1755-148X.2010.00752.x. [DOI] [PubMed] [Google Scholar]
- Florell SR, Thomas J, Grossman D. Predominant formation of heavily pigmented dermal melanocytomas resembling ‘animal-type’ melanomas in hepatocyte growth factor (C57BL/6 × C3H)F1 mice following neonatal UV irradiation. J Cutan Pathol. 2007;34:667–74. doi: 10.1111/j.1600-0560.2006.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giehl KA, Nagele U, Volkenandt M, et al. Protein expression of melanocyte growth factors (bFGF, SCF) and their receptors (FGFR-1, c-kit) in nevi and melanoma. J Cutan Pathol. 2007;34:7–14. doi: 10.1111/j.1600-0560.2006.00569.x. [DOI] [PubMed] [Google Scholar]
- Kunisada T, Lu SZ, Yoshida H, et al. Murine cutaneous mastocytosis and epidermal melanocytosis induced by keratinocyte expression of transgenic stem cell factor. J Exp Med. 1998;187:1565–73. doi: 10.1084/jem.187.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longley BJ, Carter EL. SCF-KIT pathway in human epidermal melanocyte homeostasis. J Invest Dermatol. 1999;113:139–40. doi: 10.1046/j.1523-1747.1999.00643.x. [DOI] [PubMed] [Google Scholar]
- Noonan FP, Recio JA, Takayama H, et al. Neonatal sunburn and melanoma in mice. Nature. 2001;413:271–2. doi: 10.1038/35095108. [DOI] [PubMed] [Google Scholar]
- Yamazaki F, Okamoto H, Miyauchi-Hashimoto H, et al. XPA gene-deficient, SCF-transgenic mice with epidermal melanin are resistant to UV-induced carcinogenesis. J Invest Dermatol. 2004;123:220–8. doi: 10.1111/j.0022-202X.2004.22710.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki F, Okamoto H, Matsumura Y, et al. Development of a new mouse model (xeroderma pigmentosum a-deficient, stem cell factor-transgenic) of ultraviolet B-induced melanoma. J Invest Dermatol. 2005;125:521–5. doi: 10.1111/j.0022-202X.2005.23753.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.