Abstract
Head and neck cancer arises from a series of molecular alterations progressive from dysplasia to carcinoma in situ, and finally invasive carcinoma. Risk factors associated with head and neck cancer include tobacco, alcohol and viral infection. There are genetic alterations in pre-cancerous cells that contribute to transformation. The accumulation of these alterations facilitates tumor development. Additionally, the tumor microenvironment enables tumor progression. The cooperative effect of molecular alterations in the tumor cells and compensatory microenvironment changes enable tumors to invade and metastasize. This review focuses on the genes and molecules altered during the progression of head and neck cancer with an emphasis on the genetic, molecular and phenotypic changes during the pathogenesis of head and neck cancer. Therapeutic strategies that target key changes in the tumor cells and/or stromal cells in the tumor microenvironment are discussed.
Keywords: head and neck squamous cell carcinoma, epidermal growth factor receptor, vascular endothelial growth factor receptor, tumor microenvironment, pathogenesis, molecular targeted therapy
Introduction
Head and neck cancer is the 6th most common cancer worldwide and poses a significant health problem with more than 45,000 new cases in the United States each year.1,2 Head and neck cancer refers to malignancies arising in the mucosal surfaces of the oral cavity, pharynx and larynx, of which 90% are squamous cell carcinomas (HNSCC).1 Risk factors associated with HNSCC carcinogenesis include tobacco and alcohol use, poor diet and human papilloma virus (HPV) infection.3–6 While the presence of HPV in the tumor has been implicated as a positive prognostic factor, the use of alcohol and tobacco are correlated with the acceleration of invasive carcinoma and a poorer prognosis. The most common treatment modalities for HNSCC include chemotherapy, radiation and surgery, often in combination. Such multimodality treatment often leads to permanent cosmetic and functional defects. Radiation is used in patients with early stage malignancies as either a single-modality or as an adjuvant treatment. For patients with advanced stage disease, chemotherapy is generally administered in combination with radiation, either as a primary treatment strategy or following surgical resection. For patients with recurrent disease or distant metastasis, treatment options are more limited and the overall survival is poor.7 Despite new treatment options for HNSCC patients, the prognosis has remained unchanged over the past 40 years with approximately 50% of patients succumbing to their disease.8
Molecular targeting approaches are aimed at isolating the treatment effects to critical targets in the tumor and surrounding microenvironment, thereby limiting treatment associated toxicity, and improving the overall survival. These newer treatments target genetic and epigenetic alterations that arise during carcinogenesis.9 In addition to approaches that are specific to the tumor, it is also possible to target the surrounding stromal cells and neoangiogenesis. There is preclinical and clinical evidence that targeting critical pathways in the tumor and/or stroma may improve the outcome for patients with HNSCC. This review will describe the pathogenesis of head and neck cancer with an emphasis on the genetic and molecular alterations that characterize HNSCC cells and surrounding stroma in the tumor microenvironment. Targeting molecular interactions between stromal cells and tumor cells may abrogate tumor progression.
Molecular Genetics of Head and Neck Cancer
Genetic and epigenetic alterations may lead to protein changes including decreased or increased expression. The accumulation of these alterations in oncogenes, proto-oncogenes and tumor suppressors can lead to the formation of a malignancy. Critically altered pathways in HNSCC include p53, epidermal growth factor receptor (EGFR), signal transducer and activator of transcription 3 (STAT3) and vascular endothelial growth factor receptor (VEGFR), among other important molecules that may serve as therapeutic targets.
p53
The p53 gene is one of the most commonly mutated genes in head and neck cancer, with mutations detected in over 50% of HNSCC malignances.10,11 Inactivation of the tumor suppressor p53 on chromosome 17q13 leads to a lack of growth control and renders cells unable to respond to stress or DNA damage.12 In addition to mutated p53, other genes in the p53 pathway are often mutated or dysregulated causing dysfunction of the p53 pathway. Ataxia telangiectasia mutated (ATM) is a kinase, activated primarily by double-strand DNA breaks, which activates p53. Silencing of ATM is a mechanism of loss-of-function of p53.13 p14arf lies upstream of p53 and binds the p53 inhibitor HDM2 in order to activate p53. Mutation in the ARF locus controlling p14arf expression is a common molecular event in the pathogenesis of HNSCC.14 In addition to upstream effectors of p53, there can also be mutations in downstream molecules such as the apoptotic proteins Bcl-2 and Bax.15 Endogenous genetic alterations are not the only disrupters of p53 function. HPV, specifically HPV16, is a risk factor for oropharyngeal HNSCC.16 E6 is a viral oncoprotein of HPV16, and E6 inactivates p53 via ubiquitination.17
Epidermal growth factor receptor
Molecular pathogenesis of HNSCC is additionally driven by alterations in growth factor signaling pathways. EGFR (ErbB-1 or HER1) is a tyrosine kinase receptor that is highly expressed on epithelial cells. EGFR is ubiquitously overexpressed in HNSCC on the basis of gene amplification and transcriptional activation.18 EGFR is activated by several autocrine ligands including transforming growth factor alpha (TGFα) and amphiregulin, in addition to EGF. Upon ligand binding, EGFR dimerizes, autophosphorylation occurs and downstream signaling is activated. EGFR is overexpressed in the majority of HNSCC malignancies.19 In addition to overexpression, a mutant form of EGFR known as EGFRvIII is characterized by a deletion mutation in exons 2–7 leading to a truncated ligand binding domain, rendering it constitutively active.20 EGFR is upstream of phosphoinositol-3-kinase (PI3K) and Akt. EGFR is also upstream of mitogen activated protein kinase (MAPK), which is stimulated through the Ras/Raf pathway.
Signal transducers and activators of transcription
STAT3 is a transcription factor that is activated downstream of both EGFR and EGFRvIII and mediates, at least in part, the tumorigenic effects of EGFR signaling. STAT3 can also be activated by Src family kinases as well as the interleukin-6 (IL-6)/gp130 receptor pathway through janus kinase (JAK) mediated phosphorylation.21,22 STAT3 can be constitutively active in HNSCC where increased levels of tyrosine phosphorylated STAT3 are found in HNSCC tumors compared to levels in normal epithelium.21 STAT3 leads to transcriptional activation of genes that control cell cycle progression, apoptosis and angiogenesis including Cyclin D1, Bcl-XL and VEGF.
Hepatocyte growth factor and cMET
The hepatocyte growth factor (HGF), also known as scatter factor (SF) is the ligand for c-Met and has been implicated as an important cytokine in tumor progression. C-Met has been shown to have activating mutations in HNSCC.23 Increased production of HGF contributes to enhanced tumor cell proliferation, invasion, motility and metastasis.23 HGF has also been shown to be a regulator of both the MEK and PI3K pathways subsequently contributing to the expression of the proangiogenic factors VEGF and interleukin-8 (IL-8).24 HGF is produced in the HNSCC tumor microenvironment primarily by stromal fibroblasts rather than HNSCC cells and contributes to the activation of Akt and MAPK in tumor cells leading to expression of IL-8 and the promotion of tumor progression and angiogenesis.25,26
Insulin-like growth factor-1 receptor
The insulin-like growth factor-1 receptor (IGF-1R), its ligands IGF-I and IGF-II have been implicated in the progression and survival of multiple tumor types including HNSCC. Similar to c-Met, IGF-1R signals via Akt and MAPK, contributing to rapid tumor progression through apoptosis evasion, increased motility, changes in cell adhesion and enhancement of proliferation.27 IGF-1R controls focal-adhesion kinase (FAK) expression contributing to increased motility and metastatic potential with IGF-1R overexpression.27 In HNSCC, IGF-1R has been shown to be overexpressed and its inhibition leads to decreased MAPK and PI3K signaling and is associated with a decrease in growth and motility.27 Additionally, it has been shown in HNSCC that upon ligand binding (either IGF or EGF), IGF-1R can heterodimerize with EGFR, and the therapeutic benefit of this is currently being evaluated.27,28
Mammalian target of rapamycin
Mammalian target of rapamycin (mTOR) is a signaling molecule downstream of the PI3K/Akt pathway. Alterations in the mTOR pathway are prevalent in HNSCC independent of EGFR or p53 status.29 Expression of phosphorylated Akt is increased in HNSCC contributing to mTOR activation and cell proliferation.30 Activation of the mTOR pathway contributes to phosphorylation of p70S6K, which directs the translation of cell cycle regulatory proteins such as Cyclin D1 and myc.31 The eukaryotic translation initiation factor 4E (eIF4E) is controlled by the mTOR pathway and eIF4E gene amplification in HNSCC has been shown to be associated with malignant progression.32 IGF-1R is upstream of mTOR and can also regulate mTOR activity.33
Vascular endothelial growth factor and receptors
It has been well established that in several tumor types including HNSCC, STAT3 induces the expression of VEGF which binds VEGFR2 on endothelial cells initiating angiogenesis.34,35 In addition to genetic changes in the tumor cells, there are also pathological alterations in the surrounding stromal cells. Lymphatic endothelial cells demonstrate increased expression of endothelial specific adhesion molecule (esam1), VEGFR-3, platelet-derived growth factor beta (PDGF-β) and transforming growth factor beta 2 (TGF β2).36 In tumor-derived vascular endothelial cells, there is elevated expression of Bcl-2 and VEGFR-2.37,38
Development of Tumorigenic Phenotypes
Alterations in both tumor suppressors and oncogenes in tumor cells and stromal cells lead to the transformation of normal epithelium and the promotion of transformed epithelial cells to carcinoma in situ and invasive carcinoma. The pathogenesis of head and neck cancer is considered a multistep process with an accumulation of genetic mutations, altered protein expression, leading to the development of a unique microenvironment designed to support tumor growth. The function of p53 in normal epithelium is to act as a tumor suppressor. p53 is activated by stress signals such as double-stranded DNA breaks or aberrant growth signals and activates the transcription of genes necessary for inhibition of cell cycle progression and induction of apoptosis. The expression of p21 is driven by p53, and p21 inhibits cyclin-dependent kinases (CDKs) leading to inhibition of cell cycle progression from the G1 phase to the S phase.12 In the setting of DNA damage and/or a multitude of stress signals, p53 can induce expression of Bax leading to apoptosis. Therefore, if the control of p53 is lost, via mutation or virus-mediated inhibition through the E6 protein of HPV16, there is a subsequent lack of cell cycle inhibition and a lack of apoptosis, both of which contribute to the malignant phenotype. In addition to p53, p16 is often inactivated and cyclin D1 is often overexpressed in HNSCC contributing to increased proliferation.29,39
EGFR functions as an upstream branch point mediating multiple signaling pathways controlling proliferation, invasion, migration, survival and angiogenesis. An alteration in EGFR, via receptor overexpression or an activating mutation in the ligand binding domain, can initiate a loss of control of its downstream signaling. Loss of control of EGFR or its mediators enables cells to take on a malignant phenotype. EGFR signals through PI3K and its downstream components including Akt, which is activated by phoshoinositide-dependent kinase 1 (PDK1) mediated phosphorylation. Akt mediates further downstream cellular pathways that primarily control cell growth and survival including the mTOR pathway.40,41
EGFR induced STAT3 signaling initiates transcription of genes necessary for cell growth (Cyclin D1), survival (Bcl-XL) and angiogenesis (VEGF). These genes are often found overexpressed in HNSCC promoting these malignant phenotypes.34,42 STAT3 additionally regulates the expression of MMP-2 and MMP-9 necessary for migration and invasion via digestion of the basement membrane.43,44 Constitutive expression of STAT3 enables continuous promotion of angiogenesis, migration and invasion enabling a supportive tumor microenvironment.
Phenotypic changes occuring in stromal cells result from alterations in tumor-secreted molecules as well as stromal cell gene expression. VEGF is secreted by tumor cells in response to hypoxic environments, in addition to the consequences of EGFR overexpression and constitutive STAT3 activation. VEGF, primarily the VEGFA165 isoform, binds the VEGFR2 on vascular endothelial cells, promoting the proliferation of endothelial cells and migration toward the tumor.35,38 Additionally, vascular endothelial cells secrete chemokines that induce tumor cell invasion and field cancerization.37 Interactions between tumor cells and the extra-cellular matrix (ECM) facilitate remodeling of the ECM to support tumor growth. Expression of integrins and cadherins in HNSCC tumor cells mediates adhesion and migration within the ECM.45
Molecular Targeted Therapy
Molecular targeted therapy is directed against specific proteins rather than dividing cells, in general. Cetuximab (™Erbitux) is a chimeric monoclonal antibody directed against the EGFR and is the only FDA-approved molecular targeted agent for the treatment of HNSCC. Emerging molecular targeting strategies are being tested in HNSCC patients (Tables 1 and 2). These targeted therapies are often used in combination with chemotherapy and/or radiation. Most studies to date have focused on varying mechanisms of EGFR inhibitors and anti-angiogenic agents.46
Table 1.
Tumor cell molecular targets and targeting agents evaluated in HNSCC
| Molecular target | Inhibitor | Mechanism of action | Stage of clinical development |
Citation |
|---|---|---|---|---|
| Epidermal Growth Factor Receptor (EGFR) | Cetuximab (Erbitux, C225) | Chimeric Monoclonal Antibody | FDA approved combined with radiation therapy for locally advanced | Bonner et al. N Eng J Med 200660 |
| Panitumumab (Vectibix, ABX-EGF) | Human Monoclonal Antibody | Ongoing Phase I combined with chemoradiation for stage III/IV | Capdevila et al. Cancer Treat Rev 200961 | |
| Gefitinb (Iressa, ZD1839) | Small Molecule Tyrosine Kinase Inhibitor | Phase II for recurrent or metastatic | Cohen et al. J Clin Oncol 200362 | |
| Erlotinib (Terceva, OSI-774) | Small Molecule Tyrosine Kinase Inhibitor | Phase I/II combined with cislpatin for metastatic or recurrent | Siu et al. J Clin Oncol 200763 | |
| Lapatinib (Tykerb/Tyverb, GW572016) | Small Molecule Tyrosine Kinase Inhibitor | Phase II for metastatic or recurrent ACC or salivary gland | Agulnik et al. J Clin Oncol 200764 | |
| Signal Transducer and Activator of Transcription 3 (STAT3) | STAT3 Decoy | Oligonucleotide Transcription Factor Decoy | Ongoing Phase 0 safety and biological activity study for surgically resectable | Sen et al. Cancer Chemother Pharmacol 200949 |
| Hepatocyte Growth Factor Receptor (HGFR or cMET) | Foretinib (GSK1363089) | Small Molecule Tyrosine Kinase Inhibitor | Phase II for recurrent or metastatic | Eder et al. J Clin Oncol 200765 |
| Mammalian Target of Rapamycin (mTOR) | Everolimus (RAD001) | Oral inhibitor of mTORC2 | Phase II for monotherapy after previous chemotherapy | Papadimitrakopoulou et al. J Clin Oncol 200766 |
| p53 | Ad5CMV-p53 gene (RPR/INGN 201) | Insertion of p53 gene | Phase II post surgery followed by chemoradiation for resectable | Yoo et al. Arch Otolaryngol Head Neck Surg 200950 |
| Src | Dasatinib (BMS-354825) | Small Molecule Tyrosine Kinase Inhibitor | Phase II for recurrent or metastatic | Brooks et al. J Clin Oncol 200967 |
| Saracatinib (AZD0530) | Dual Src and ABL kinase inhibitor | Phase II for recurrent or metastatic | Fury et al. www.clinicaltrials.gov 200968 | |
| Insulin-like Growth Factor-1 Receptor (IGF-1R) | IMC-A12 | Human Monoclonal Antibody | Phase II for preoperative treatment with or without cetuximab | Cruz et al. Ann Oncol 200769 |
Table 2.
Endothelial cell molecular targets and targeting evaluated in HNSCC
| Molecular target | Inhibitor | Mechanism of action | Stage of clinical development |
Citation |
|---|---|---|---|---|
| Vascular endothelial growth factor receptor (VEGFR) | Sunitinib (Sutent, SU11248) | Small Molecule Tyrosine Kinase Inhibitor | Phase II for recurrent or metastatic | Choong et al. Invest New Drugs 200970 |
| Sorafenib (Nexavar NSC-724772) | Small Molecule Tyrosine Kinase Inhibitor | Phase II for recurrent or metastatic | Williamson et al. J Clin Oncol 200771 | |
| Cediranib (Recentin, AZD2171) | Small Molecule Tyrosine Kinase Inhibitor | Ongoing Phase II as monotherapy for recurrent or metastatic | Saura et al. J Clin Oncol 200972 | |
| Vascular endothelial growth factor (VEGF) | Bevacizumab (Avastin) | Humanized Monoclonal Antibody | Phase I with chemoradiation and 5-FU for stage IV and intermediate | Seiwert et al. J Clin Oncol 200873 |
| VEGF/R and EGFR | Bevacizumab (Avastin) Erlotinib (Terceva, OSI-774) | Monoclonal Antibody Tyrosine Kinase Inhibitor | Phase I/II combined with chemotherapy for recurrent or metastatic | Cohen et al. Lancet Oncol 200974 |
| Zactima (Vandetanib,ZD6474) | Small Molecule Tyrosine Kinase Inhibitor | Phase I with chemoradiation for unresected stage III/IV | Papadimitrakopoulou et al. J Clin Oncol 200975 |
In addition to cetuximab, panitumumab (™Vectibix) is another monoclonal antibody against EGFR in clinical trial in combination with chemoradiation. EGFR tyrosine kinase inhibitors (TKIs) are also in under investigation including gefitinib (™Iressa), erlotinib (™Tarceva) and lapatinib (™Tykerb). The anti-angiogenic agents currently in clinical trial target VEGF and VEGFR2. Sunitinib (™Sutent), sorafenib (™Nexavar) and cediranib (™Recentin) are tyrosine kinase inhibitors specific for VEGFR2. Bevacizumab (™Avastin), a monoclonal antibody to VEGF, is FDA approved in metastatic colon, breast and non-small cell lung cancer (NSCLC) and is in clinical trials for recurrent or metastatic HNSCC.47,48 There are also ongoing HNSCC studies assessing dual targeting of EGFR and VEGF/R, including vandetanib (™Zactima), a TKI that has activity against both EGFR and VEGFR2.
In addition to EGFR and angiogenesis targeted therapy, several of the dysregulated proteins discussed above are currently being assessed as drug targets (Table 1). STAT3 is being targeted via an oligonucleotide decoy.49 Src is a tyrosine kinase that is upstream of the PI3K/Akt/mTOR pathway and the MAPK pathway. Dasatinib (™Sprycel) and AZD0530 (™Saracatinib) are small molecule TKIs targeting Src family kinases that are under investigation for recurrent or metastatic HNSCC. Agents inhibiting other molecular targets are being tested clinically in HNSCC and include drugs that block mTOR or IGF-1R. There is also an adenoviral gene therapy trial which is being used to increase wild-type p53 in tumors expressing wild-type or mutated p53.50,51
Stromal Cells in Head and Neck Cancer
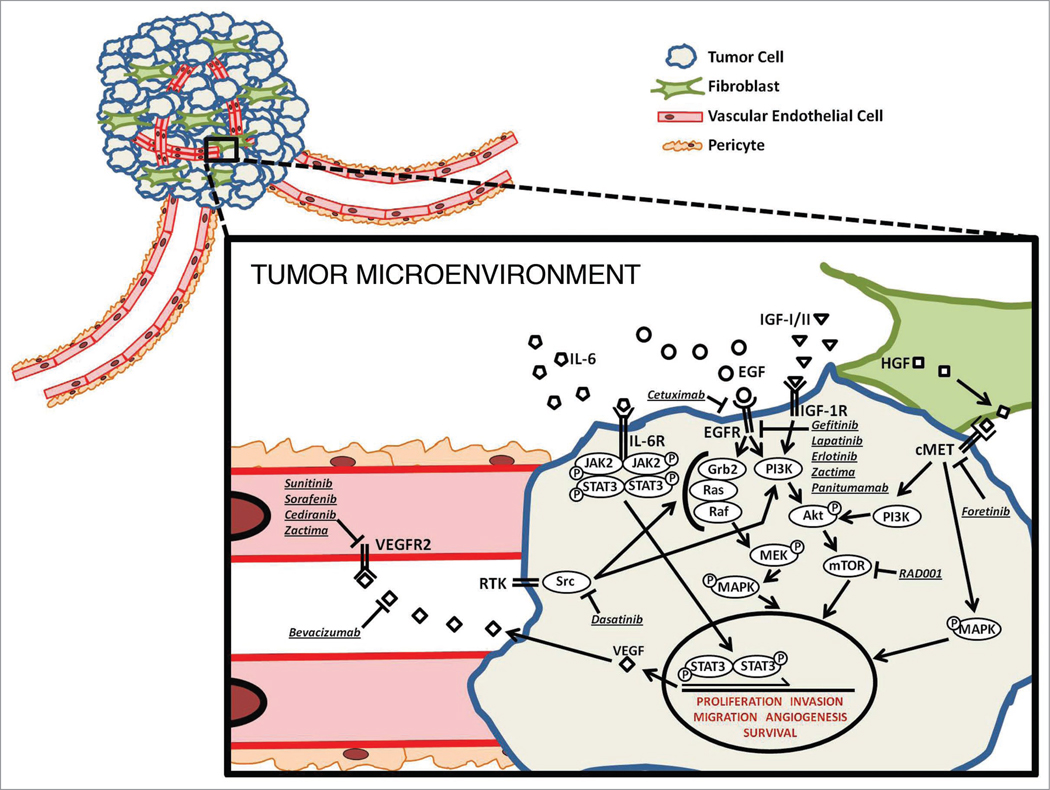
While there is limited data regarding the importance of the tumor microenvironment in head and neck cancer, it is currently a topic of much interest. Cytokines control the cross-talk between tumor cells and stromal cells (Fig. 1). Tumor cells released cytokines that induce ECM remodeling, basement membrane degradation, tumor cell proliferation and angiogenesis. Fibroblasts and endothelial cells in the microenvironment play a crucial role in the response to tumor derived cytokines. Not only do stromal cells respond to signals from tumor cells, but the stromal cells themselves can induce tumor growth.
Figure 1.
HNSCC tumor microenvironment. This model depicts the potential signaling pathways for targeting in HNSCC, including ligands, receptors and signaling intermediates of the tumor cells and the contributing vascular endothelial cells and fibroblasts. Also shown are the molecular inhibitors currently in clinical investigation for the treatment of HNSCC. This model shows pathway convergence, pathway divergence and microenvironment signaling in order to demonstrate the potential of both single-targeting and multi-targeting drug approaches.
One of the most well characterized interactions is that between HNSCC tumor cells and vascular endothelial cells. In the HNSCC tumor cell, STAT3 is activated via EGFR, IL-6 and/or Src. Phosphorylated STAT3 enters the nucleus and induces transcription of VEGF. VEGF is then secreted from the tumor cells, binds VEGFR on the vascular endothelial cells and promotes proliferation and recruitment of vascular endothelial cells initiating neoangiogenesis necessary for tumor survival.34 When IL-6 receptor signaling is abrogated via monoclonal antibody binding to the receptor, there is a decrease in activated STAT3 with a concomitant abrogation of neoangiogenesis in oral squamous cell carcinoma.52 Head and neck tumors are highly inflammatory neoplasms.53 IL-6 is a protumorigenic cytokine that also regulates inflammation via secretion from T-lymphocytes and macrophages. Increased IL-6 in the tumor microenvironment can lead to inflammation and promotion of tumor growth and survival.54
Fibroblasts are another stromal cell necessary for tumor progression. Fibroblasts within a tumor secrete MMPs and other ECM remodeling proteins. The expression of enzymes by tumor fibroblasts is induced by cytokines that are secreted by the tumor cells. Tumor cells also respond to fibroblast-induced signals. For example, HGF and its receptor c-Met are overexpressed in HNSCC tissues and contribute to malignant transformation.55 Tumor-derived fibroblasts demonstrate increased expression of matrix metaloproteases (MMPs) and production of HGF, which stimulates tumor growth and spread.26,56 The source of HGF in the HNSCC microenvironment appears to be the fibroblasts where HGF induces tumor cell proliferation and angiogenesis.26
Summary and Future Outlook
Molecular pathogenesis of head and neck cancer is a multistep process consisting of genetic mutations and altered protein expression. Some of the most prevalent molecular events contributing to transformation include alterations in p53 and EGFR, manifested as a lack of growth control and increased proliferation, survival, migration and angiogenesis. Efforts are underway investigating the efficacy of targeting molecules that are dysregulated in HNSCC, including changes in tumor cells and stroma.
There is evidence that normal stromal cells are distinct from tumor-derived stromal cells, although data are limited regarding alterations in stromal cells in head and neck cancer.57–59 The mechanism by which changes in stromal cells facilitate growth is incompletely understood. The growth and survival of tumors likely results from complex interactions between tumor cells and surrounding stroma. Increased understanding of the mechanisms regulating tumor-stromal interactions will enable the development of more effective molecular targeted approaches.
Acknowledgements
Supported by P50CA097190 and an American Cancer Society Clinical Research Professorship (to J.R.G.).
Abbreviations
- ATM
ataxia telangiectasia mutated
- EGF(R)
epidermal growth factor (receptor)
- eIF4E
eukaryotic translation initation factor 4E
- IGF-1R
insulin-like growth factor 1 receptor
- HGF
hepatocyte growth factor
- HNSCC
head and neck squamous cell carcinoma
- HPV
human papilloma virus
- IL-6/8
interleukin 6/8
- JAK
janus kinase
- MAPK
mitogen activating protein kinase
- MMP
matrix metaloprotease
- mTOR
mammalian target of rapamycin
- PDGF-β
platelet-derived growth factor beta
- PDK1
phoshoinositide-dependent kinase 1
- PI3K
phosphoinositol-3-kinase
- SF
scatter factor
- STAT3
signal transducer and activator of transcription 3
- TGFβ2
transforming growth factor beta2
- TKI
tyrosine kinase inhibitor
- VEGF(R)
vascular endothelial growth factor (receptor)
References
- 1.Curado M, Hashibe M. Recent changes in the epidemiology. Curr Opin Oncol. 2009;21:194–200. doi: 10.1097/CCO.0b013e32832a68ca. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer Statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Gillison ML. Current topics in the epidemiology of oral cavity and oropharyngeal cancers. Head Neck. 2007;29:779–92. doi: 10.1002/hed.20573. [DOI] [PubMed] [Google Scholar]
- 4.Hashibe M, Brennan P, Benhamou S, Castellsague X, Chen C, Curado MP, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the international head and neck cancer epidemiology consortium. J Natl Cancer Inst. 2007;99:777–89. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Sturgis EM, Etzel CJ, Wei Q, Li G. p73 G4C14-to-A4T14 polymorphism and risk of human papillomavirus-associated squamous cell carcinoma of the oropharynx in never smokers and never drinkers. Cancer. 2008;113:3307–3314. doi: 10.1002/cncr.23976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ragin CCR, Modugno F, Gollin SM. The epidemiology and risk factors of head and neck cancer: a focus on human papillomavirus. J Dent Res. 2007;86:104–114. doi: 10.1177/154405910708600202. [DOI] [PubMed] [Google Scholar]
- 7.Cognetti DM, Weber RS, Lai SY. Head and neck cancer: An evolving treatment paradigm. Cancer. 2008;113:1911–1932. doi: 10.1002/cncr.23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhuvanesh S. Molecular pathogenesis of head and neck cancers. J Surg Oncol. 2008;97:634–639. doi: 10.1002/jso.21024. [DOI] [PubMed] [Google Scholar]
- 9.Marsit CJ, Christensen BC, Houseman EA, Karagas MR, Wrensch MR, Yeh RF, et al. Epigenetic profiling reveals etiologically distinct patterns of DNA methylation in head and neck squamous cell carcinoma. Carcinogenesis. 2009;30:416–422. doi: 10.1093/carcin/bgp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brachman DG, Graves D, Vokes E, Beckett M, Haraf D, Montag A, et al. Occurrence of p53 gene deletions and human papilloma virus infection in human head and neck cancer. Cancer Res. 1992;52:4832–4836. [PubMed] [Google Scholar]
- 11.Hoffmann TK, Sonkoly E, Hauser U, van Lierop A, Whiteside TL, Klussmann JP, et al. Alterations in the p53 pathway and their association with radio- and chemosensitivity in head and neck squamous cell carcinoma. Oral Oncol. 2008;44:1100–1109. doi: 10.1016/j.oraloncology.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 13.Bolt J, Vo QN, Kim W-J, McWhorter AJ, Thomson J, Hagensee ME, et al. The ATM/p53 pathway is commonly targeted for inactivation in squamous cell carcinoma of the head and neck (SCCHN) by multiple molecular mechanisms. Oral Oncol. 2005;41:1013–1020. doi: 10.1016/j.oraloncology.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Kresty LA, Mallery SR, Knobloch TJ, Song H, Lloyd M, Casto BC, et al. Alterations of p16INK4a and p14ARF in patients with severe oral epithelial dysplasia. Cancer Res. 2002;62:5295–5300. [PubMed] [Google Scholar]
- 15.Chen K, Hu Z, Wang LE, Sturgis EM, El-Naggar AK, Zhang W, et al. Single-nucleotide polymorphisms at the TP53-binding or responsive promoter regions of BAX and BCL2 genes and risk of squamous cell carcinoma of the head and neck. Carcinogenesis. 2007;28:2008–2012. doi: 10.1093/carcin/bgm172. [DOI] [PubMed] [Google Scholar]
- 16.Hennessey PT, Westra WH, Califano JA. Human papillomavirus and head and neck squamous cell carcinoma: recent evidence and clinical implications. J Dent Res. 2009;88:300–306. doi: 10.1177/0022034509333371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung CH, Gillison ML. Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clin Cancer Res. 2009;15:6758–6762. doi: 10.1158/1078-0432.CCR-09-0784. [DOI] [PubMed] [Google Scholar]
- 18.Grandis JR, Zeng Q, Tweardy DJ. Retinoic acid normalizes the increased gene transcription rate of TGFalpha and EGFR in head and neck cancer cell lines. Nat Med. 1996;2:237–240. doi: 10.1038/nm0296-237. [DOI] [PubMed] [Google Scholar]
- 19.Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24:2666–2672. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- 20.Sok JC, Coppelli FM, Thomas SM, Lango MN, Xi S, Hunt JL, et al. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin Cancer Res. 2006;12:5064–5073. doi: 10.1158/1078-0432.CCR-06-0913. [DOI] [PubMed] [Google Scholar]
- 21.Leeman RJ, Lui VWY, Grandis JR. STAT3 as a therapeutic target in head and neck cancer. Expert Opin Biol Th. 2006;6:231–241. doi: 10.1517/14712598.6.3.231. [DOI] [PubMed] [Google Scholar]
- 22.Sriuranpong V, Park JI, Amornphimoltham P, Patel V, Nelkin BD, Gutkind JS. Epidermal growth factor receptor-independent constitutive activation of STAT3 in head and neck squamous cell carcinoma is mediated by the autocrine/paracrine stimulation of the interleukin 6/gp130 cytokine system. Cancer Res. 2003;63:2948–2956. [PubMed] [Google Scholar]
- 23.Ma PC, Maulik G, Christensen J, Salgia R. c-Met: Structure, functions and potential for therapeutic inhibition. Cancer Metast Rev. 2003;22:309–325. doi: 10.1023/a:1023768811842. [DOI] [PubMed] [Google Scholar]
- 24.Dong G, Chen Z, Li Z-Y, Yeh NT, Bancroft CC, Van Waes C. Hepatocyte growth factor/scatter factor-induced activation of MEK and PI3K signal pathways contributes to expression of proangiogenic cytokines interleukin-8 and vascular endothelial growth factor in head and neck squamous cell carcinoma. Cancer Res. 2001;61:5911–5918. [PubMed] [Google Scholar]
- 25.Dong G, Lee TL, Yeh NT, Geoghegan J, Waes CV, Chen Z. Metastatic squamous cell carcinoma cells that overexpress c-Met exhibit enhanced angiogenesis factor expression, scattering and metastasis in response to hepatocyte growth factor. Oncogene. 2004;23:6199–6208. doi: 10.1038/sj.onc.1207851. [DOI] [PubMed] [Google Scholar]
- 26.Knowles LM, Stabile LP, Egloff AM, Rothstein ME, Thomas SM, Gubish CT, et al. HGF and c-Met participate in paracrine tumorigenic pathways in head and neck squamous cell cancer. Clin Cancer Res. 2009;15:3740–3750. doi: 10.1158/1078-0432.CCR-08-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnes CJ, Ohshiro K, Rayala SK, El-Naggar AK, Kumar R. Insulin-like Growth Factor Receptor as a Therapeutic Target in Head and Neck Cancer. Clin Cancer Res. 2007;13:4291–4299. doi: 10.1158/1078-0432.CCR-06-2040. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X, Yuan Y, Song J, Rothstein ME, Thomas SM, Gubish CT, et al. Dual silencing of epidermal growth factor and insulin-like growth factor 1 receptors significantly limits growth of nasopharyngeal carcinoma in nude mice. J Laryngol Otolo. 2009;123:208–222. doi: 10.1017/S0022215108002211. [DOI] [PubMed] [Google Scholar]
- 29.Molinolo AA, Hewitt SM, Amornphimoltham P, Keelawat S, Rangdaeng S, Meneses García A, et al. Dissecting the Akt/Mammalian target of rapamycin signaling network: emerging results from the head and neck cancer tissue array initiative. Clin Cancer Res. 2007;13:4964–4973. doi: 10.1158/1078-0432.CCR-07-1041. [DOI] [PubMed] [Google Scholar]
- 30.Amornphimoltham P, Patel V, Sodhi A, Nikitakis NG, Sauk JJ, Sausville EA, et al. Mammalian target of rapamycin, a molecular target in squamous cell carcinomas of the head and neck. Cancer Res. 2005;65:9953–9961. doi: 10.1158/0008-5472.CAN-05-0921. [DOI] [PubMed] [Google Scholar]
- 31.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 32.Sorrells DL, Meschonat C, Black D, Li BD. Pattern of amplification and overexpression of the eukaryotic initiation factor 4E gene in solid tumor. J Surg Res. 1999;85:37–42. doi: 10.1006/jsre.1999.5653. [DOI] [PubMed] [Google Scholar]
- 33.Oh SH, Jin Q, Kim ES, Khuri FR, Lee HY. Insulin-like growth factor-I receptor signaling pathway induces resistance to the apoptotic activities of SCH66336 (Lonafarnib) through Akt/Mammalian target of rapamycinâ, mediated increases in survivin expression. Clin Cancer Res. 2008;14:1581–1589. doi: 10.1158/1078-0432.CCR-07-0952. [DOI] [PubMed] [Google Scholar]
- 34.Masuda M, Ruan HY, Ito A, Nakashima T, Toh S, Wakasaki T, et al. Signal transducers and activators of transcription 3 upregulates vascular endothelial growth factor production and tumor angiogenesis in head and neck squamous cell carcinoma. Oral Oncol. 2007;43:785–790. doi: 10.1016/j.oraloncology.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Gabhann FM, Popel AS. Systems biology of vascular endothelial growth factors. Microcirculation. 2008;15:715–738. doi: 10.1080/10739680802095964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clasper S, Royston D, Baban D, Cao Y, Ewers S, Butz S, et al. A novel gene expression profile in lymphatics associated with tumor growth and nodal metastasis. Cancer Res. 2008;68:7293–7303. doi: 10.1158/0008-5472.CAN-07-6506. [DOI] [PubMed] [Google Scholar]
- 37.Warner KA, Miyazawa M, Cordeiro MM, Love WJ, Pinsky MS, Neiva KG, et al. Endothelial cells enhance tumor cell invasion through a crosstalk mediated by CXC chemokine signaling. Neoplasia. 2008;10:131–139. doi: 10.1593/neo.07815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yigitbasi OG, Younes MN, Doan D, Jasser SA, Schiff BA, Bucana CD, et al. Tumor cell and endothelial cell therapy of oral cancer by dual tyrosine kinase receptor blockade. Cancer Res. 2004;64:7977–7984. doi: 10.1158/0008-5472.CAN-04-1477. [DOI] [PubMed] [Google Scholar]
- 39.Papadimitrakopoulou VA, Izzo J, Mao L, Keck J, Hamilton D, Shin DM, et al. Cyclin D1 and p16 alterations in advanced premalignant lesions of the upper aerodigestive tract. Clin Cancer Res. 2001;7:3127–3134. [PubMed] [Google Scholar]
- 40.Molinolo AA, Amornphimoltham P, Squarize CH, Castilho RM, Patel V, Gutkind JS. Dysregulated molecular networks in head and neck carcinogenesis. Oral Oncol. 45:324–334. doi: 10.1016/j.oraloncology.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ratushny V, Astsaturov I, Burtness BA, Golemis EA, Silverman JS. Targeting EGFR resistance networks in head and neck cancer. Cell Signal. 2009;21:1255–1268. doi: 10.1016/j.cellsig.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masuda M, Suzui M, Yasumatu R, Nakashima T, Kuratomi Y, Azuma K, et al. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002;62:3351–3355. [PubMed] [Google Scholar]
- 43.Dechow TN, Pedranzini L, Leitch A, Leslie K, Gerald WL, Linkov I, et al. Requirement of matrix metalloproteinase-9 for the transformation of human mammary epithelial cells by Stat3-C. Proc Nat Acad Sci USA. 2004;101:10602–10607. doi: 10.1073/pnas.0404100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F, Sawaya R, et al. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene. 23:3550–3560. doi: 10.1038/sj.onc.1207383. [DOI] [PubMed] [Google Scholar]
- 45.Kramer RH, Shen X, Zhou H. Tumor cell invasion and survival in head and neck cancer. Cancer Metast Rev. 2005;24:35–45. doi: 10.1007/s10555-005-5046-2. [DOI] [PubMed] [Google Scholar]
- 46.Bernier J, Bentzen SM, Vermorken JB. Molecular therapy in head and neck oncology. Nat Rev Clin Oncol. 2009;6:266–277. doi: 10.1038/nrclinonc.2009.40. [DOI] [PubMed] [Google Scholar]
- 47.Los M, Roodhart JM, Voest EE. Target Practice: Lessons from phase III trials with bevacizumab and vatalanib in the treatment of advanced colorectal cancer. Oncologist. 2007;12:443–450. doi: 10.1634/theoncologist.12-4-443. [DOI] [PubMed] [Google Scholar]
- 48.Sachdev J, Jahanzeb M. Evolution of Bevacizumab-Based therapy in the management of breast cancer. Clin Breast Cancer. 2008;8:402–410. doi: 10.3816/CBC.2008.n.048. [DOI] [PubMed] [Google Scholar]
- 49.Sen M, Tosca P, Zwayer C, Ryan MJ, Johnson JD, Knostman KA, et al. Lack of toxicity of a STAT3 decoy oligonucleotide. Cancer Chemoth Pharm. 2009;63:983–995. doi: 10.1007/s00280-008-0823-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoo GH, Moon J, LeBlanc M, Lonardo F, Urba S, Kim H, et al. A phase 2 trial of surgery with perioperative INGN 201 (Ad5CMV-p53) gene therapy followed by chemoradiotherapy for advanced, resectable squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx and larynx: report of the southwest oncology group. Arch Otolaryngol Head Neck Surg. 2009;135:869–874. doi: 10.1001/archoto.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clayman GL, Frank DK, Bruso PA, Goepfert H. Adenovirus-mediated wild-type p53 gene transfer as a surgical adjuvant in advanced head and neck cancers. Clin Cancer Res. 1999;5:1715–1722. [PubMed] [Google Scholar]
- 52.Shinriki S, Jono H, Ota K, Ueda M, Kudo M, Ota T, et al. Humanized anti-interleukin-6 receptor antibody suppresses tumor angiogenesis and in vivo growth of human oral squamous cell carcinoma. Clin Cancer Res. 2009;15:5426–5434. doi: 10.1158/1078-0432.CCR-09-0287. [DOI] [PubMed] [Google Scholar]
- 53.Wang F, Arun P, Friedman J, Chen Z, Van Waes C. Current and potential inflammation targeted therapies in head and neck cancer. Curr Opin Pharmacol. 2009;9:389–395. doi: 10.1016/j.coph.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pries R, Wollenberg B. Cytokines in head and neck cancer. Cytokine Growth Factor Rev. 2006;17:141–146. doi: 10.1016/j.cytogfr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 56.Zhang W, Matrisian L, Holmbeck K, Vick C, Rosenthal E. Fibroblast-derived MT1-MMP promotes tumor progression in vitro and in vivo. BMC Cancer. 2006;6:52. doi: 10.1186/1471-2407-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. P National Acad Sci. 2009;106:3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song N, Huang Y, Shi H, Yuan S, Ding Y, Song X, et al. Overexpression of platelet-derived growth factor-BB increases tumor pericyte content via stromal-derived factor-1alpha/CXCR4 Axis. Cancer Res. 2009;69:6057–6064. doi: 10.1158/0008-5472.CAN-08-2007. [DOI] [PubMed] [Google Scholar]
- 59.Zhuang Z, Jian P, Longjiang L, Bo H, Hongwei Z. Identification of Oral cancer cell-induced changes in gene expression profile of lymphatic endothelial cell. Cancer Invest. 2008;26:1002–1007. doi: 10.1080/07357900802087234. [DOI] [PubMed] [Google Scholar]
- 60.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 61.Capdevila J, Elez E, Macarulla T, Ramos FJ, Ruiz-Echarri M, Tabernero J. Anti-epidermal growth factor receptor monoclonal antibodies in cancer treatment. Cancer Treat Rev. 2009;35:354–363. doi: 10.1016/j.ctrv.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 62.Cohen EE, Rosen F, Stadler WM, Recant W, Stenson K, Huo D, et al. Phase II Trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2003;21:1980–1987. doi: 10.1200/JCO.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 63.Siu LL, Soulieres D, Chen EX, Pond GR, Chin SF, Francis P, et al. Phase I/II trial of erlotinib and cisplatin in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: a princess margaret hospital phase II consortium and national cancer institute of canada clinical trials group study. J Clin Oncol. 2007;25:2178–2183. doi: 10.1200/JCO.2006.07.6547. [DOI] [PubMed] [Google Scholar]
- 64.Agulnik M, Cohen EW, Cohen RB, Chen EX, Vokes EE, Hotte SJ, et al. Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbB2 expressing adenoid cystic carcinoma and non adenoid cystic carcinoma malignant tumors of the salivary glands. J Clin Oncol. 2007;25:3978–3984. doi: 10.1200/JCO.2007.11.8612. [DOI] [PubMed] [Google Scholar]
- 65.Eder JP, Heath E, Appleman L, Shapiro G, Wand D, Malburg L, et al. Phase I experience with c-MET inhibitor XL880 administered orally to patients (pts) with solid tumors. J Clin Oncol. 2007;25 [Google Scholar]
- 66.Papadimitrakopoulou V, Soria JC, Douillard JY, Giaccone G, Wolf J, Crino L, et al. A phase II study of RAD001 (R) (everolimus) monotherapy in patients (pts) with advanced non-small cell lung cancer (NSCLC) failing prior platinum-based chemotherapy (C) or prior C and EGFR inhibitors (EGFR-I) J Clin Oncol. 2007;25 [Google Scholar]
- 67.Brooks HD, Glisson B, Lu C, Sabichi A, Johnson F, Ginsberg L, et al. Phase II study of dasatinib in the treatment of head and neck squamous cell carcinoma (HNSCC) J Clin Oncol. 2009;27 [Google Scholar]
- 68.Fury MG, Pfister DG. AZD0530 in treating patients with recurrent or metastatic head and neck cancer. 2009 www.clinicaltrials.gov.
- 69.Cruz JJ, Ocana A, Del Barco E, Pandiella A. Targeting receptor tyrosine kinases and their signal transduction routes in head and neck cancer. Ann Oncol. 2007;18:421–430. doi: 10.1093/annonc/mdl175. [DOI] [PubMed] [Google Scholar]
- 70.Choong N, Kozloff M, Taber D, Hu HS, Wade J, 3rd, Ivy P, et al. Phase II study of sunitinib malate in head and neck squamous cell carcinoma. Inves New Drugs. 2009 doi: 10.1007/s10637-009-9296-7. In press. [DOI] [PubMed] [Google Scholar]
- 71.Williamson SK, Moon J, Huang CH, Guaglianone P, Wolf GT, Urba SG. A phase II trial of sorafenib in patients with recurrent and/or metastatic head and neck squamous cell carcinoma (HNSCC): A Southwest Oncology Group (SWOG) trial. J Clin Oncol. 2007;25 doi: 10.1200/JCO.2009.25.6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saura C, Baselga J, Herbst R, Campo JD, Marotti M, Tessier J, et al. Antitumor activity of cediranib in patients with metastatic or recurrent head and neck cancer (HNC) or recurrent non-small cell lung cancer (NSCLC): An open-label exploratory study. J Clin Oncol. 2009;27 [Google Scholar]
- 73.Seiwert TY, Haraf DJ, Cohen EE, Stenson K, Witt ME, Dekker A, et al. Phase I study of bevacizumab added to fluorouracil- and hydroxyurea-based concomitant chemoradiotherapy for poor-prognosis head and neck cancer. J Clin Oncol. 2008;26:1732–1741. doi: 10.1200/JCO.2007.13.1706. [DOI] [PubMed] [Google Scholar]
- 74.Cohen EE, Davis DW, Karrison TG, Seiwert TY, Wong SJ, Nattam S, et al. Erlotinib and bevacizumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck: a phase I/II study. Lancet Oncol. 2009;10:247–257. doi: 10.1016/S1470-2045(09)70002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Papadimitrakopoulou V, Frank SJ, Blumenschein GR, Chen C, Kane M, Cohen EE, et al. Phase I evaluation of vandetanib with radiation therapy (RT) ± cisplatin in previously untreated advanced head and neck squamous cell carcinoma (HNSCC) J Clin Oncol. 2009;27 [Google Scholar]