Abstract
The mechanisms eliciting cancer cachexia are not well understood. Wasting of skeletal muscle is problematic because it is responsible for the clinical deterioration in cancer patients and the ability to tolerate cancer treatment. Animal studies suggest that nuclear factor of kappa B (NF-κB) signaling is important in the progression of muscle wasting due to several types of tumors. However, there are no published studies in humans on a role for NF-κB in cancer cachexia. In this project we studied the rectus abdominis muscle from patients with gastric tumors (n=14) and age matched control subjects (n=10) for markers of NF-κB activation. Nuclear levels of p65, p50, and Bcl-3 were the same in both groups of subjects. However, phospho-p65 was elevated by 25% in muscles of cancer patients. In addition, expression of the inhibitor of kappa B alpha (IκBα), was decreased by 25% in cancer patients. Decreased expression of IκBα reflects its degradation by one of the IκBα kinases and is a marker of NF-κB activation. Interestingly, there was no correlation between the stage of cancer and the extent of IκBα decrease, nor was there a correlation between the degree of cachexia and decreased IκBα levels. This suggests that the activation of NF-κB is an early and sustained event in gastric cancer. The work implicates the NF-κB signaling in the initiation and progression of cancer cachexia in humans and demonstrates the need for additional study of this pathway; it also recommends NF-κB signaling as a therapeutic target for the amelioration of cachexia as has been suggested from rodent studies.
Keywords: Bcl-3, NF-κB, IκBα skeletal muscle, cancer cachexia, gastric cancer patients
Introduction
Cancer cachexia is present in about 50% of cancer patients, its prevalence being higher in patients with tumours of the gastrointestinal tract and the lung, than in those with other solid neoplasms, such as breast and thyroid cancer, and haematological malignancies. Cachexia is present in most terminally-ill cancer patients, accounting for about 20% of all cancer deaths.(1-3) Cancer cachexia is characterised by progressive weight loss, anorexia, metabolic alterations, asthenia, depletion of lipid stores, and severe loss of skeletal muscle protein.(1-3)
Loss of skeletal muscle results from increased protein degradation, reduced protein synthesis, or both.(3) Proteolytic processes that contribute to muscle hypercatabolism in cancer are calcium-dependent calpain activity(4), caspase activity(5, 6), and ATP-dependent proteosomal activity.(7-9). The signaling pathways that underlie the changes in synthesis and degradation rates are also an active area of study because understanding the cellular pathways and the genes that form the wasting phenotype will allow us to develop rational therapeutic strategies. Several signaling mechanisms have been linked to cachexia including smads, the signaling arm of upstream regulator myostatin which is elevated in muscle wasting conditions(10), AP-1(11), IFNγ(12), and Foxo1(13). However, the involvment of NF-κB in cancer cachexia is the most well documented signaling pathway in rodent models..
Cai et al.(14) showed strong support for the involvement of NF-κB in the mechanism of cancer cachexia. NF-κB DNA binding activity was increased 6-fold in muscles of wild type mice bearing the Lewis lung carcinoma (LCC) whereas this DNA binding activity was blocked in mouse muscle overexpressing the IκB super repressor (“MISR” mice) with a similar tumor burden. This effect appeared to involve the binding of p65 because NF-κB activation was assessed by p65 binding to a κB oligonucleotide. Importantly, the blockade of NF-κB in MISR mice was associated with a 50% attenuation of muscle loss compared to wild type tumor-bearing mice and an increased survival time. In another study, expression levels of p65 in muscles of mice bearing the adenocarcinoma colon-26 (C-26) tumor did not change but increases in phospho-p65 were increased(15) In addition, gel shift assays showed increased protein binding to a κB oligo compared to that from non-tumor bearing mice. Thus nuclear levels of p65 alone are not a good indicator of NF-κB activation in cancer cachexia.
In another study, double stranded oligodeoxynucleotides were used as decoy molecules targeting NF-κB binding sites on genes. The oligonucleotides were directly injected into tumors of C-26 in mice.(16) Tumor growth was not affected but there was an attenuation of the loss of body weight, epididymal fat mass, and gastrocnemius muscle mass, again suggesting a role for NF-κB in controlling the muscle wasting process associated with cancer.
Studies in muscle cell culture also support a role for NF-κB in muscle wasting associated with cancer cachexia. Wyke and Tisdale.(17) studied the role of NF-κB in regulating muscle protein degradation and expression of the ubiquitin-proteasome pathway in response to proteolysis inducing factor (PIF, isolated from mouse MAC16 tumors) by the use of stable, transdominant-negative IκBα muscle cell lines. PIF induced degradation of IκBα, an increase in NF-κB dependent reporter activity, and muscle protein loss in myotubes but these changes were blocked in myotubes expressing the IκBα super repressor. These data show that PIF mediates protein loss at least in part via NF-κB activation in muscle cells. Similar types of studies have been carried out in myotubes in response to TNFα, a major catabolic cytokine involved in certain cancers. These studies show the same results as those with PIF in terms the activation of NF-κB and its role in muscle catabolism(18-20)
Our work on skeletal muscle disuse atrophy showed critical roles in for the NF-κB and IκB proteins p50 and Bcl-3, respectively, but not c-Rel.(21-23) We found nuclear protein from atrophied muscle showed increased binding to a κB oligonucleotide in a gel shift assay, but we could not see a further band shift using a p65 antibody as we could with anti-p50 and anti-Bcl-3.(22) Thus the role of NF-κB in cancer and disuse wasting appear to involve different components of NF-κB signaling.
Surprisingly, there are no studies in human cancer cachexia to determine if NF-κB signaling is activated in muscle, and if so, which κB transcription factors are involved. In the present study our aim was to determine if skeletal muscle from humans with gastric cancer showed signs of NF-κB activation by measurement of nuclear levels of p50, p65, and Bcl-3, as well as expression of phospho-p65 (Ser536) and levels of IκBα, the latter being decreased when κB signaling is activated.(21, 24) We found that cancer cachexia is dissimilar to disuse atrophy as there was no difference in nuclear p50 or Bcl-3 protein expression. However, similar results were found to that seen in mouse models of cancer cachexia where phospho-p65 is increased and IκBα is decreased regardless of cancer stage. These are consistent with activation of the classical NF-κB pathway.
Patients and Methods
Subjects
The study was approved by the local ethic committees. Fourteen consecutive patients with gastric cancer admitted to the Istituto di Clinica Chirurgica of the Universita Cattolica del Sacro Cuore of Rome, Italy, between January 2007 and December 2007 were included in the study. Diagnosis of gastric cancer was made by endoscopic biopsy. Ten patients undergoing surgery for benign abdominal diseases (laparocele: 7 case; inguinal hernia: 3 cases) served as a control group. Exclusion criteria for both groups were as follows: acute or chronic renal failure (serum creatinine ≥ 1.2 mg/dL), liver failure, diabetes, metabolic acidosis, sepsis, AIDS, inflammatory bowel disease, autoimmune disorders, chronic heart failure, acute and chronic hepatitis, hyperthyroidism, and chronic obstructive pulmonary disease.
Protocol
Written informed consent for the study procedures was obtained from the patients. All subjects were studied at 8 AM, after overnight fasting. Blood samples for subsequent biochemical analyses were obtained from an antecubital vein immediately before entering the operating room.
Nutritional Assessment
The nutritional assessment included anthropometric (actual body weight, usual body weight, percent weight loss) and biochemical (serum albumin) indices.
Muscle Biopsy
A biopsy specimen was obtained from the rectus abdominis muscle during the initial phase of the operation. After skin incision and dissection through the subcutaneous fat, the anterior sheet of the rectus abdominis muscle was opened with scissors and a muscle biopsy specimen weighing ~0.5 g was obtained. The biopsy specimen was immediately frozen in liquid nitrogen and then stored at −70°C until analyzed. After the muscle biopsy had been obtained, small bleeding vessels were carefully controlled with ligatures and cautery, where after the operation continued in a routine fashion. No complications occurred from the biopsy procedure.
Nuclear and Cytosolic Isolation
A protocol similar to Siu and Alway (25) was used. An ~200mg piece of biopsy material from the rectus abdominis of control and cancer patients was homogenized on ice using a mechanical tissue homogenizer in ice-cold buffer (10 mM NaCl, 1.5 mM MgCl2, 10 mM Hepes, pH 7.4, 20% glycerol, 0.1% Triton X-100, 1 mM DTT). The homogenate was briefly centrifuged (800g) to pellet nuclei. Nuclear pellets were washed in lysis buffer and re-suspended in lysis buffer plus protease inhibitor cocktail and NaCl (brought to 450mM) to lyse nuclei. Samples were rocked for 1 hr at 4°C. Lysed nuclei were spun at 16,000 g for 15 min at 4°C. The supernatant was used as the nuclear protein fraction and protein concentration was measured using a Bradford assay (Bio-Rad). Protein concentration was measured on the cytosolic fraction similarly. Both fractions were stored at −80°C for subsequent immunoblotting. The presence and absence of histone H2B protein using immunobloting was used to confirm the purity of the nuclear and cytosolic fractions, respectively.
Whole muscle lysates
Approximately 200mg of muscle biopsy material was mechanically homogenized in a lysis buffer containing 20 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM ß-glycerol phosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and 1 mM PMSF. The homogenate was sonicated in an ice cold Bioruptor 5 × 30s, with 30s rest between cycles. Homogenates were rocked for 45 min at 4°C, then centrifuged at 15,000 g for 10 min to pellet disrupted nuclear membranes. The supernatant was used as total cellular lysate. Protein concentration was measured using a detergent compatible assay (Bio-Rad).
Immunoblotting
Protein was denatured and separated on SDS-polyacrylamide gels (Bio-Rad). Proteins were transferred to an immobilon-FL polyvinylidene fluoride membrane (Millipore), blocked, and immunoblotted with the appropriate antibody diluted according to the manufacturer’s instructions. Primary antibodies were anti-p65 (Santa Cruz, sc-109), phospho-p65 (Ser536) (Cell Sigaling, 3033S), p50 (Santa Cruz, sc-8414), Bcl-3 (Santa Cruz, sc-185), IκBα (Santa Cruz, sc-371), histone H2B (Upstate Biotechnologies, 07-371) and α-tubulin (Sigma, T6074). Alexa Fluor 680 (Invitrogen) or IRDye800 (Rockland Immunochemicals) fluorescent dye-conjugated secondary antibodies were used for visualization. Signals on blots were quantified using an Odyssey system (Li-Cor Biosciences), which uses direct infrared fluorescence detection and has a wide linear dynamic range for accurate measurement of band intensity.
Statistics
A non-parametric t-statistic for unequal n was used to determine statistical differences between control and cancer groups for measurement of nuclear protein expression (P<0.05). A student’s t-statistic was used for measurements on whole muscle lysates and measurement of IκBα expression (P<0.05). For bar graphs, the values plotted are mean ± SEM. The number of samples used per assay is given in figure legends.
Results
Patient characteristics
As shown in Table 1, gastric cancer patients and controls were similar in terms of age and sex distribution. Nevertheless, gastric cancer patients showed a significantly higher percentage weight loss and lower serum albumin levels.
Table 1.
Characteristics of patients. Data are expressed as mean ± SD. Stage of disease is according to the UICC classification of tumor stage.(37)
| Controls (n=10) | Gastric Cancer (n=14) | P value | |
|---|---|---|---|
| Age (yrs) | 63.9±2.8 | 64.2±3.8 | 0.37 |
| Sex (M:F) | 6:4 | 8:6 | 0.78 |
| Weight loss (%)* | 0 | 10.9±4.1 | <0.0001 |
| Serum albumin (g/dl)* | 4.3±0.1 | 3.4±0.1 | <0.0001 |
|
Stage
Ib II IIIa IIIb IV |
- - - - - |
2 2 3 1 6 |
Nuclear protein expression in muscle from control and cancer subjects
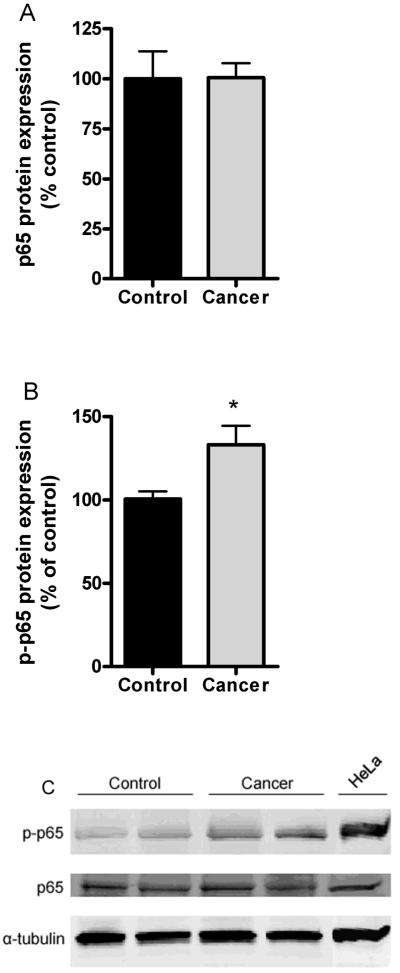
In disuse induced muscle atrophy nuclear levels of p50 and Bcl-3 are increased by 3-fold but p65 is unchanged (22). Thus we determined whether these changes are seen in muscle from patients with cancer cachexia. There were no differences in nuclear levels of any of these proteins demonstrating that the muscle wasting associated with cachexia is different from disuse (Fig. 1). Also muscle p65 levels are unchanged in skeletal muscle from mice bearing the C-26 tumor, but phospho-p65 levels are elevated.{Acharyya, 2005 #3633 Thus we went on to assess phospho-p65 in muscle in patients with cancer and found a significant increase of 25% (Fig. 2B).
Fig. 1.
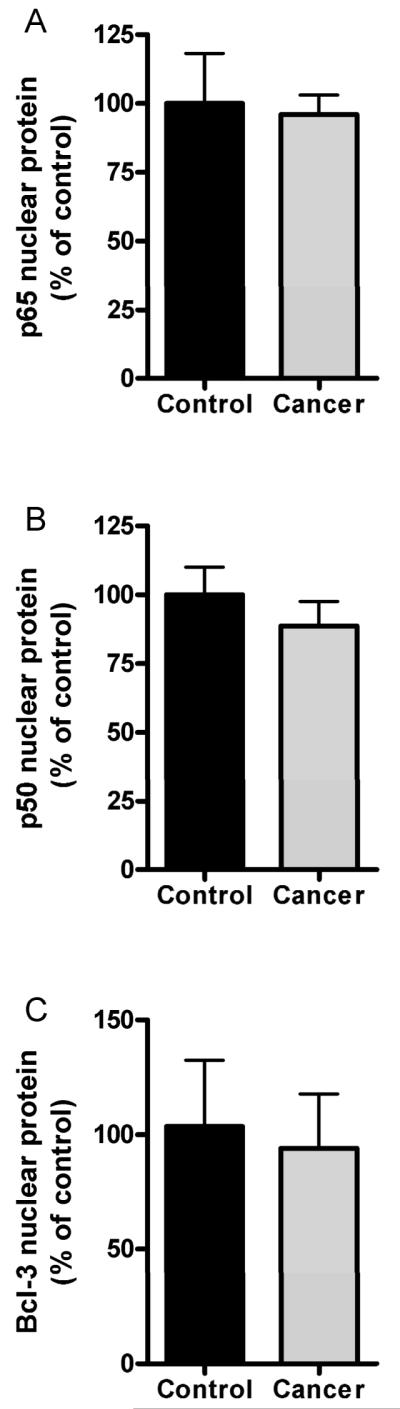
Protein expression using immunobloting of nuclear fractions isolated from control and cancer patients. Average signal intensity values are plotted for (A) p65 protein expression, (B) p50 protein expression, and (C) Bcl-3 protein expression. 40ug protein loaded per lane. All signals were normalized to histone H2B (not shown). Values are mean ± SEM. n=6 control and n=10 cancer patients. No statistically significant differences were found between control and cancer subjects.
Fig. 2.
Total muscle p65 and phospho-p65 expression isolated from control (n=5) and cancer patients (n=6). (A) whole muscle p65 expression and (B) phospho-p65 expression. Signals were normalized to α-tubulin. (C) Representative samples from immunoblots of phopho-p65, p65, and α-tubulin from samples in panels A and B. Hela lysates were used as a positive control. 70μg protein loaded per lane. * indicates significantly increased compared to control value (P<0.05).
IκBα expression in muscle from control and cancer subjects
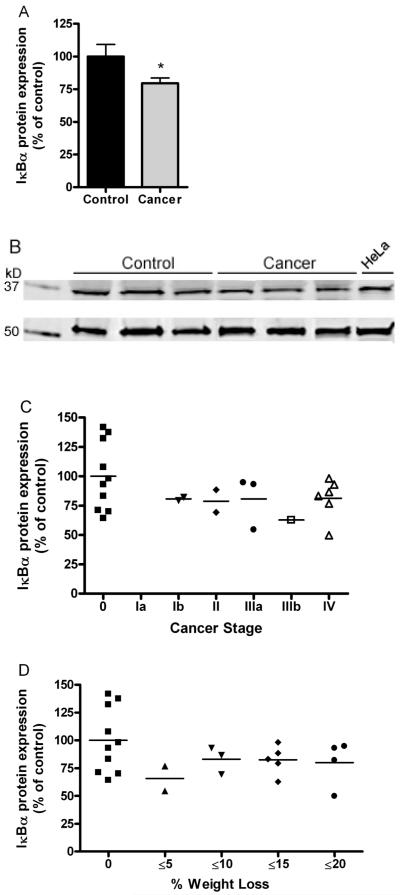
One of the most sensitive markers of NF-κB activation is the expression of IκBα protein. Proteasomal degradation of this protein is rapid in response to many stimuli that activate various NF-κB signaling pathways. We assessed expression in 10 control muscles and 14 patient muscles and found a significant 25% decrease in IκBα protein expression (Fig. 3A). This observation was independent of the stage of cancer (Fig. 3C) or the degree of cachexia (Fig. 3D) suggesting that degradation of IκBα and thus activation of NF-κB is an early and sustained event in humans with gastric cancer.
Fig. 3.
Cytosolic IκBα expression using immunoblotting in control and cancer patients. (A) Cancer patients showed a significant 25% decrease in IκBα expression compared to age-matched controls (P<0.01). 50μg protein loaded per lane. (B) Examples of signals for IκBα (top panel) and α-tubulin (lower panel), the latter used for signal normalization. HeLa lysates are positive control. (C) Individual values plotted for IκBα expression for control and cancer patients parsed by cancer stage. (D) Individual values for IκBα expression for control and cancer patients parsed by degree of cachexia. No differences in IκBα expression due to either cancer stage or amount of weight loss. n=10 controls, n=14 cancer patients.
Discussion
The present study shows that in skeletal muscle of gastric cancer patients nuclear levels of p50, Bcl-3, and p65 were similar to controls, but there was a 25% increase in phospho-p65 and a significant decrease in expression of IκBα (25%). To our knowledge, this is the first time that such results are reported suggesting activation of the classical NF-κB pathway in muscle from patients with cancer cachexia.
NF-κB represents a family of five transcription factors [p65 (Rel A), Rel B, c-Rel, p52 and p50] that mediates a variety of processes depending on cell type and upstream trigger.{Scheidereit, 2006 #3738} All these transcription factors are expressed in the skeletal muscle.(22) The activation of NF-κB is achieved by nuclear transport of heterodimers of NF-κB family members and often occurs by the ubiquitination and degradation of the inhibitory protein IκB. Normally, IκB binds NF-κB heterodimers and retains them in the cytoplasm. Activation of NF-κB transcription factors most often occurs by the phosphorylation, ubiquitination, and degradation of IκB by the IκB kinases, allowing Rel heterodimers to translocate to the nucleus to activate NF-κB-dependent genes.(26) Two Rel proteins, p50 and p52 do not have transactivation domains and when found to homodimerize, they induce transcriptional repression. However, Bcl-3, a mostly nuclear IκB protein that has a transactivation domain, can bind to these homodimers and induce transaction.(27-29)
Mouse studies have shown that the Rel protein p65 has a role in cancer induced muscle wasting due to an increase in its phosphorylation at Serine 536 in muscles from mice with C-26 tumor (15). Another study showed increased binding of nuclear protein to a κB oligonucleotide that also bound to a p65 antibody in an ELISA-based DNA binding assay in muscle from mice with LLC (14). Both these mouse studies also showed increased NF-κB binding activity using a gel shift assay.. In the present work, we found a 25% increase in phospho-p65, similar to that seen in mouse studies. On the other hand, nuclear levels of p65 were not different in the mouse study with C-26 tumors (15), nor were they different in the cancer patients in the present study, or even in atrophied muscles due to disuse.(22) Thus the nuclear level of p65 alone is not a good marker of κB activation.
On the other hand our previous work on muscle disuse showed more than a doubling of nuclear p50 and Bcl-3. Super shift assays of these nuclear extracts further implicated p50 and Bcl-3(22), and the requirement of these two proteins were confirmed in subsequent studies using knockout mice.(23) Any involvement of p50 or Bcl-3 in cancer cachexia has not been tested to our knowledge. Unlike disuse atrophy in rodents, muscle from cancer patients in the present study showed no difference in nuclear levels of p50 or Bcl-3.
The observation that IκBα protein expression was decreased by 25% in cancer patients’ skeletal muscle is in agreement with that seen in muscle from patients with COPD.(30). Decreased IκBα is a common feature of many different types of NF-κB signaling(31) and in conjunction with an increase in phospho-p65; our data suggest activation of the classical pathway. This is consistent with the fact that TNFα, a potent activator of NF-κB, induces increased phospho-p65 and decreased IκBα expression in muscle and is a likely a trigger of cancer cachexia.(32)
An interesting finding of the present study is that the decreased IκBα protein expression was independent of the stage of cancer or the degree of cachexia suggesting that this event is an early and sustained feature of NF-κB activation. These data also fit well with our recent observation that ubiquitin mRNA increased expression and enhanced proteasome activity are detectable in the skeletal muscle of gastric cancer patients even before clinical evidence of body wasting and cachexia, likely indicating that modulations of the muscle proteolytic machinery occur early in cancer patients.(9, 33)
A rise in cytokine production is common in patients with cancer(34) and is thought to be critical for initiating the loss of muscle mass. Cytokines and in particular TNFα activate NF-κB through the dissociation of IκBα with NF-κB proteins, in turn permitting NF-κB to translocate to the nucleus and influence gene transcription, including genes regulating the proteolytic pathways.(35, 36) The potential activation of the classical pathway of NF-κB in the muscle of cancer patients suggested by the present study provides the first evidence of involvement of NF-κB in the complicated pathogenesis of cancer-related muscle wasting.
In conclusion, the present data on muscle from patients with gastric cancer are similar to animal models of cancer cachexia, but not to animal models of disuse atrophy, although NF-κB activation is found in both conditions. Nevertheless further studies are needed to discover the specific NF-κB transcription factors involved in NF-κB activation in cancer cachexia. It will also be important to determine the NF-κB target genes as potential targets for therapeutic foci.
Acknowledgements
The authors wish to thank Angie Cornwell, Chia-ling Wu and Manuela Papacci for technical assistance. This work was supported by NIAMS R21 AR054446 to S.K.
Footnotes
Conflict of interest statement
None of the authors has any financial and personal relationships with other people or organisations that could inappropriately influence (bias) their work.
References
- 1.Bossola M, Pacelli F, Doglietto GB. Novel treatments for cancer cachexia. Expert Opin Investig Drugs. 2007;16(8):1241–53. doi: 10.1517/13543784.16.8.1241. [DOI] [PubMed] [Google Scholar]
- 2.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89(2):381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 3.Smith KL, Tisdale MJ. Increased protein degradation and decreased protein synthesis in skeletal muscle during cancer cachexia. Br J Cancer. 1993;67(4):680–5. doi: 10.1038/bjc.1993.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costelli P, Reffo P, Penna F, Autelli R, Bonelli G, Baccino FM. Ca(2+)-dependent proteolysis in muscle wasting. Int J Biochem Cell Biol. 2005;37(10):2134–46. doi: 10.1016/j.biocel.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Argiles JM, Lopez-Soriano FJ, Busquets S. Mechanisms to explain wasting of muscle and fat in cancer cachexia. Curr Opin Support Palliat Care. 2007;1(4):293–8. doi: 10.1097/SPC.0b013e3282f34738. [DOI] [PubMed] [Google Scholar]
- 6.Belizario JE, Lorite MJ, Tisdale MJ. Cleavage of caspases-1, -3, -6, -8 and -9 substrates by proteases in skeletal muscles from mice undergoing cancer cachexia. Br J Cancer. 2001;84(8):1135–40. doi: 10.1054/bjoc.2001.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bossola M, Pacelli F, Costelli P, Tortorelli A, Rosa F, Doglietto GB. Proteasome activities in the rectus abdominis muscle of young and older individuals. Biogerontology. 2008;9(4):261–8. doi: 10.1007/s10522-008-9135-9. [DOI] [PubMed] [Google Scholar]
- 8.Khal J, Hine AV, Fearon KC, Dejong CH, Tisdale MJ. Increased expression of proteasome subunits in skeletal muscle of cancer patients with weight loss. Int J Biochem Cell Biol. 2005;37(10):2196–206. doi: 10.1016/j.biocel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Bossola M, Muscaritoli M, Costelli P, et al. Increased muscle proteasome activity correlates with disease severity in gastric cancer patients. Ann Surg. 2003;237(3):384–9. doi: 10.1097/01.SLA.0000055225.96357.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costelli P, Muscaritoli M, Bonetto A, et al. Muscle myostatin signalling is enhanced in experimental cancer cachexia. Eur J Clin Invest. 2008;38(7):531–8. doi: 10.1111/j.1365-2362.2008.01970.x. [DOI] [PubMed] [Google Scholar]
- 11.Moore-Carrasco R, Garcia-Martinez C, Busquets S, et al. The AP-1/CJUN signaling cascade is involved in muscle differentiation: implications in muscle wasting during cancer cachexia. FEBS Lett. 2006;580(2):691–6. doi: 10.1016/j.febslet.2005.12.084. [DOI] [PubMed] [Google Scholar]
- 12.Matthys P, Heremans H, Opdenakker G, Billiau A. Anti-interferon-gamma antibody treatment, growth of Lewis lung tumours in mice and tumour-associated cachexia. Eur J Cancer. 1991;27(2):182–7. doi: 10.1016/0277-5379(91)90483-t. [DOI] [PubMed] [Google Scholar]
- 13.Liu CM, Yang Z, Liu CW, et al. Effect of RNA oligonucleotide targeting Foxo-1 on muscle growth in normal and cancer cachexia mice. Cancer Gene Ther. 2007;14(12):945–52. doi: 10.1038/sj.cgt.7701091. [DOI] [PubMed] [Google Scholar]
- 14.Cai D, Frantz JD, Tawa NE, Jr., et al. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119(2):285–98. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 15.Acharyya S, Butchbach ME, Sahenk Z, et al. Dystrophin glycoprotein complex dysfunction: a regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell. 2005;8(5):421–32. doi: 10.1016/j.ccr.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Kawamura I, Morishita R, Tomita N, et al. Intratumoral injection of oligonucleotides to the NF-kappaB binding site inhibits cachexia in a mouse tumor model. Gene Ther. 1999;6(1):91–7. doi: 10.1038/sj.gt.3300819. [DOI] [PubMed] [Google Scholar]
- 17.Wyke SM, Tisdale MJ. NF-kappaB mediates proteolysis-inducing factor induced protein degradation and expression of the ubiquitin-proteasome system in skeletal muscle. Br J Cancer. 2005;92(4):711–21. doi: 10.1038/sj.bjc.6602402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li YP, Schwartz RJ, Waddell ID, Holloway BR, Reid MB. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-kappaB activation in response to tumor necrosis factor alpha. Faseb J. 1998;12(10):871–80. doi: 10.1096/fasebj.12.10.971. [DOI] [PubMed] [Google Scholar]
- 19.Li YP, Reid MB. NF-kappaB mediates the protein loss induced by TNF-alpha in differentiated skeletal muscle myotubes [In Process Citation] Am J Physiol Regul Integr Comp Physiol. 2000;279(4):R1165–70. doi: 10.1152/ajpregu.2000.279.4.R1165. [DOI] [PubMed] [Google Scholar]
- 20.Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS., Jr. NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289(5488):2363–6. doi: 10.1126/science.289.5488.2363. see comments. [DOI] [PubMed] [Google Scholar]
- 21.Judge AR, Koncarevic A, Hunter RB, Liou HC, Jackman RW, Kandarian SC. Role for I{kappa}B{alpha}, but not c-Rel, in skeletal muscle atrophy. Am J Physiol Cell Physiol. 2007;292(1):C372–82. doi: 10.1152/ajpcell.00293.2006. [DOI] [PubMed] [Google Scholar]
- 22.Hunter RB, Stevenson E, Koncarevic A, Mitchell-Felton H, Essig DA, Kandarian SC. Activation of an alternative NF-kappaB pathway in skeletal muscle during disuse atrophy. Faseb J. 2002;16(6):529–38. doi: 10.1096/fj.01-0866com. [DOI] [PubMed] [Google Scholar]
- 23.Hunter RB, Kandarian SC. Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J Clin Invest. 2004;114(10):1504–11. doi: 10.1172/JCI21696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acharyya S, Ladner KJ, Nelsen LL, et al. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004;114(3):370–8. doi: 10.1172/JCI20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siu PM, Alway SE. Mitochondria-associated apoptotic signalling in denervated rat skeletal muscle. J Physiol. 2005;565:309–23. doi: 10.1113/jphysiol.2004.081083. 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25(51):6680–4. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 27.Fujita T, Nolan GP, Liou HC, Scott ML, Baltimore D. The candidate proto-oncogene bcl-3 encodes a transcriptional coactivator that activates through NF-kappa B p50 homodimers. Genes Dev. 1993;7(7B):1354–63. doi: 10.1101/gad.7.7b.1354. [DOI] [PubMed] [Google Scholar]
- 28.Bours V, Franzoso G, Azarenko V, et al. The oncoprotein Bcl-3 directly transactivates through kappaB motifs via association with DNA-binding p50B homodimers. Cell. 1993;72(5):729–39. doi: 10.1016/0092-8674(93)90401-b. [DOI] [PubMed] [Google Scholar]
- 29.Baek SH, Ohgi KA, Rose DW, Koo EH, Glass CK, Rosenfeld MG. Exchange of N-CoR corepressor and Tip60 coactivator complexes links gene expression by NF-kappaB and beta-amyloid precursor protein. Cell. 2002;110(1):55–67. doi: 10.1016/s0092-8674(02)00809-7. [DOI] [PubMed] [Google Scholar]
- 30.Agusti A, Morla M, Sauleda J, Saus C, Busquets X. NF-kappaB activation and iNOS upregulation in skeletal muscle of patients with COPD and low body weight. Thorax. 2004;59(6):483–7. doi: 10.1136/thx.2003.017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005;30(1):43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Ladner KJ, Caligiuri MA, Guttridge DC. Tumor necrosis factor-regulated biphasic activation of NF-kappa B is required for cytokine-induced loss of skeletal muscle gene products. J Biol Chem. 2003;278(4):2294–303. doi: 10.1074/jbc.M207129200. Epub 002 Nov 12. [DOI] [PubMed] [Google Scholar]
- 33.Bossola M, Muscaritoli M, Costelli P, et al. Increased muscle ubiquitin mRNA levels in gastric cancer patients. Am J Physiol Regul Integr Comp Physiol. 2001;280(5):R1518–23. doi: 10.1152/ajpregu.2001.280.5.R1518. [DOI] [PubMed] [Google Scholar]
- 34.Bossola M, Muscaritoli M, Bellantone R, et al. Serum tumour necrosis factor-alpha levels in cancer patients are discontinuous and correlate with weight loss. Eur J Clin Invest. 2000;30(12):1107–12. doi: 10.1046/j.1365-2362.2000.00751.x. [DOI] [PubMed] [Google Scholar]
- 35.Li YP, Lecker SH, Chen Y, Waddell ID, Goldberg AL, Reid MB. TNF-alpha increases ubiquitin-conjugating activity in skeletal muscle by up-regulating UbcH2/E220k. Faseb J. 2003;17(9):1048–57. doi: 10.1096/fj.02-0759com. [DOI] [PubMed] [Google Scholar]
- 36.Li YP, Chen Y, John J, et al. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. Faseb J. 2005;19(3):362–70. doi: 10.1096/fj.04-2364com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wittekind CSL. TNM classification of malignant tumours. 6th ed. Wiley-Liss; New York: 2002. [Google Scholar]