Abstract
Carbon nanotubes (CNTs) cause perturbations in immune systems and limit the application of CNTs in biomedicine. Here we demonstrate that a surface chemistry modification on multiwalled CNTs (MWCNTs) reduces their immune perturbations in mice and in macrophages. The modified MWCNTs change their preferred binding pattern from mannose receptor to scavenger receptor. This switch significantly alleviates NFκB activation and reduces immunotoxicity of MWCNTs.
Keywords: multiwalled carbon nanotubes (MWCNTs), immunotoxicity, surface chemistry modification, mannose receptor, scavenger receptor, NFκB
Introduction
Nanomaterials such as functionalized carbon nanotubes (CNTs) have a wide range of promising applications in drug delivery,1–3 therapy4, 5 and biomedical imaging.6 However, a crucial step toward the application of CNTs in human body is to regulate their impacts on immune systems. Human immune systems safeguard the host from infection and malignancy. They may be perturbed at different levels, resulting in their suppression or overstimulation leading to pathological conditions.
Macrophages, also known as phagocytes, are abundant in organs like liver, lungs and spleen and they are the primary and most important cells responding to external stimuli and fighting against foreign substances. Macrophages respond to foreign substances by triggering inflammatory responses such as the secretion of cytokines to attract more cells to respond to the foreign invasion. It is well known that macrophages recognize the foreign substances by surface receptors that internalize foreign substances and activate the signaling pathways as key immune responses.
CNTs (both single and multiwalled CNTs) have been reported to cause perturbations of the immune system in vitro7–9 and in vivo.10–13 Such adverse effects severely limit the applications of CNTs in biomedicine. Although there have been reports on CNT’s perturbations on cellular signaling events such as NFκB14, 15 and AP-1,16, 17 the associated mechanism is not understood. Even more critical is the complete lack of strategy to remediate immunotoxicity of CNTs and other nanomaterials targeted for biomedical applications. In order to elucidate the upstream mechanisms of CNT-induced NFκB pathway activation and develop a strategy to modulate and reduce CNTs’ immunotoxicity, we carried out a series of investigations on surface-modified MWCNTs and the molecular and cellular events they perturbed in macrophages.
Here we demonstrate that THP-1 macrophage’s recognition of CNTs can be modulated by their surface chemistry modifications. The increased proportional binding of modified CNTs to scavenger receptor steered CNTs away from mannose receptor binding, alleviated NFκB activation, and reduced their immunotoxicity both in vitro and in vivo.
Results and Discussion
Surface chemistry modifications on MWCNTs reduces its immune perturbation
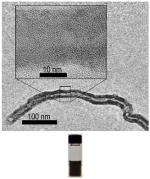
To test whether surface chemistry modification can modulate the immune perturbation of CNTs, we previously designed and synthesized an 80-member combinatorial MWCNTs library by placing most diverse molecules on the nanotubes surface (Figure 1).18 Screening of MWCNTs in this combinatorial library for the immune responsive nitric oxide (NO) generation showed that reduction of immune perturbation was achieved by surface chemistry modifications. The reduced cell immune response by surface chemistry modification poses some challenging questions: can this reduction be replicated in vivo? What is the mechanistic explanation of the modulation of immunotoxicity? To address these questions, MWCNT 1 and 2 were selected for a series of investigations. They were selected based on their markedly different immune responses in macrophages (NO production). Therefore they represent high- and low-toxicity materials for us to elucidate the associated mechanisms. Physicochemical characterization of MWCNTs is crucial for understanding their biological perturbations. We characterized MWCNT 1 and 2 by a wide range of analytical methods and results are summarized in Table 1.
Figure 1.
Heat map for NO generation of MWCNT 1 and a 80-member combinatorial MWCNT library and the molecular structures of selected MWCNTs for in this study. Colors from light blue to dark blue are for lower NO to higher NO generations (μmol/L, NO<7.5,
 ). MWCNT 1 and 2 were selected for investigation of their immune perturbations.
). MWCNT 1 and 2 were selected for investigation of their immune perturbations.
Table 1.
Characterization of Multiwalled Carbon Nanotubes (MWCNTs)
| MWCNT 1 | MWCNT 2 | |
|---|---|---|
| Chemical structure |

|

|
| TEM |
|

|
| Suspension in plasma (24 hrs) | ||
| Diameter (nm) | 30–50 nm (outer); 5–12 nm (inner) | 30–50 nm (outer); 5–12 nm (inner) |
| Zeta potential (mV in H2O and plasma) | −62.7, −6.1 | −60.2, −6.5 |
| Functional group loading (mmol/g) | 0.4 | 0.4 |
| C, Cl, Fe, Ni, S (%) | 97.37, 0.20, 0.55, 1.86, 0.02 | 97.37, 0.20, 0.55, 1.86, 0.02 |
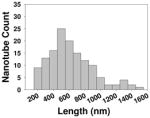
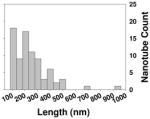
| Length distribution |
|

|
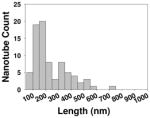
| Length distribution inside cells |
|
|
To clarify the possible association between the molecular properties such as hydrophobicity and steric hindrance of the ligands, we analyzed the data in terms of correlations between these physicochemical properties and the NO release. The MWCNTs’ molecule properties, MlogP and steric properties such as Verloop L, B1 and B3, were calculated and compared to NO release responses. No correlation was found between NO release and MlogP or steric properties. The results indicated that the different perturbations were not due to physicochemical or steric properties of ligands (Figure S1). Their sizes, shapes, densities of functional groups, uniform suspensions in plasma were all similar and they also have comparable length distribution (Table 1). It is likely that ligand’s interactions with cell membrane receptors may play a role in such a difference. To confirm our results in vitro, we went on to test whether this result can be replicated in vivo.
MWCNTs accumulated in lungs, liver and spleen in which TNF-α and IL-1β levels elicited by MWCNT 2 were reduced
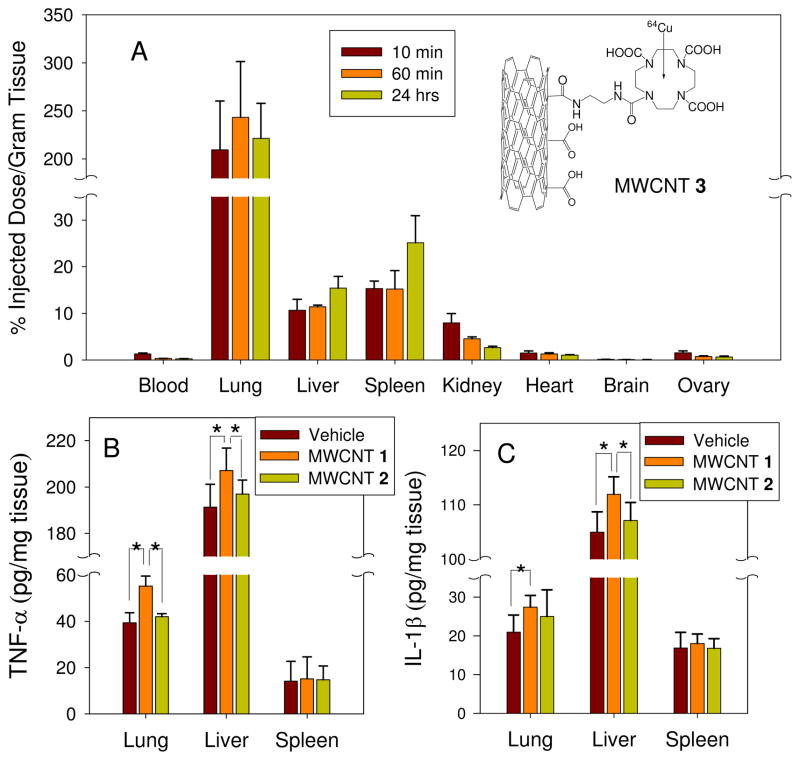
In order to assess the effect of MWCNTs on immune systems in mice, we first investigated their body distribution in mice. Due to the lack of quantitative analysis methods for MWCNTs, we labeled MWCNT 1 with a specific ligand that chelates 64Cu as a radioactive label, which was named MWCNT 3. The accumulations of MWCNTs in various organs were measured 10 min, 60 min and 24 hrs after tail vein injection. Results showed that MWCNTs were mostly accumulated in lungs, liver and spleen in 24 hrs (Figure 2A). This result was similar to the previous work.19–21 Carbon nanoparticles also have been reported to induce the inflammation in vivo. TNF-α and IL-1 in lungs bronchoalveolar lavage induced by C60 exposure were evaluated at day 1, 7, 14 and 28 after intratracheal instillation and the maximum response was at day 1.22 Pulmonary inflammation caused by MWCNTs was also examined at day 1, 28 and 56 and the maximum inflammation was also found at day 1.23 The main goal of our investigation is to compare the altered inflammatory reponses caused by MWCNT’s surface chemistry modification. Therefore, we assessed the inflammation reponses in organs generated by MWCNT 1 and MWCNT 2 at 24 hrs, when the effect was the highest.
Figure 2.
The distribution of MWCNTs in mice and the effect of MWCNT 1 and 2 on inflammatory cytokines in mice lung, liver and spleen in vivo. (A): MWCNT 1 were labeled with a specific ligand for 64Cu. Suspension of MWCNT 1 was injected in to CD-1 female mice (4 mice per group) via tail vein. For 10 min, 60 min and 24 hrs, the accumulation of MWCNT 1 in the tissues were measured. (B and C): Suspension of MWCNT 1 and 2 in PBS solution in the presence of 0.1% Tween 80 was injected in to female BALB/c mice (5 mice per group) via tail vein with a dose of 14 mg/kg. For 24 hrs, TNF-α and IL-1β in the tissue homogenate were determined by ELISA. (*p<0.05)
To investigate the immune perturbation elicited by MWCNT 1 and 2 in mice, we quantified inflammation cytokines, TNF-α and IL-1β in the tissue homogenates of mouse lungs, liver and spleen 24 hrs after injection (Figure 2B and 2C). MWCNT 1 elicited an elevated response of TNF-α and IL-1β in lung and liver compared to vehicle control. However MWCNT 2 did not induce evident immune response as measured by these cytokines.
It was reported that CNTs caused inflammation in mice11, 24 and rat10. CNTs predominantly accumulate in the lungs after the intratracheal instillation exposures.25 Here we found that lung accumulation was also dominant after i.v. injection. CNT stimulation of alveolar macrophages in the lung or the phagocytes in the reticuloendothelial system (RES) strongly induce inflammation responses.20, 26 CNTs promoted allergic responses with sharply increased numbers of inflammatory cells and cytokine levels.27 MWCNTs significantly increased the IL-1β and TNF-α level in bronchoalveolar lavage fluid and caused pulmonary damage in three days.28, 29 Inhalation of MWCNTs caused systemic immune function alterations which was characterized by reduced T-cell-dependent antibody response and T-cell proliferation.24 However, here we discovered that a surface chemistry modification reduced MWCNT’s immune perturbations both in vitro and in vivo. It is well known that macrophages are abundant in liver and lungs and play an important role in the first line defense against foreign particles. In order to elucidate the mechanism of action of the modified MWCNTs, we carried out further investigations using macrophages.
Define the effective MWCNTs concentration in cell culture
Since CNTs intrinsically tend to aggregate, the actual effective concentration of CNTs may be different from the amount of CNTs used to prepare the suspension. In order to get an accurate account of the effective MWCNT concentration, we sonicated the suspensions for 5 minutes to disperse the nano-materials, removed the heavily aggregated particles by centrifugation (1500 rpm, 5 min) and then determined the concentration of well suspended MWCNT 1 and 2 by UV spectroscopy using a calibration curve prepared separately. Concentrations of MWCNT 1 were determined to be 9.88, 48.8, 97.6, 192 and 379 μg/mL and MWCNT 2 were 9.89, 48.1, 95.9 189 and 374 μg/mL when 10, 50, 100, 200, 400 μg/mL of MWCNTs were dissolved in cell culture medium (see the Supporting Information, Figure S2 and Table S1).
MWCNT 2 caused less immune perturbations in macrophages compared to MWCNT 1
Previous studies have shown that CNTs induced nitric oxide (NO) and pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-8 and IL-1β in macrophages and monocytes.8, 24, 30 The induction of nitric oxide (NO) and cytokine (TNF-α) production has been suggested to be molecular markers for immunotoxicity of engineered nanoparticle.31, 32 In order to elucidate the mechanisms of MWCNT’s perturbation on macrophages, we first investigated the effect of MWCNTs on generation of NO, TNF-α and reactive oxygen species (ROS).
TNF-α and IL-1 in lungs bronchoalveolar lavage induced by C60 exposure were evaluated at day 1, 7, 14 and 28 after intratracheal instillation and the maximum response was at day 1.22 Pulmonary inflammation caused by MWCNTs was also examined at day 1, 28 and 56 and the maximum inflammation was also found at day 1.23 The main goal of our investigation is to compare the altered inflammatory responses caused by MWCNT surface chemistry modifications. Therefore, we assessed the inflammation responses in organs generated by MWCNT 1 and MWCNT 2 at 24 hrs.
The bacterial endotoxin LPS (as a control) is known to induce the release of inflammatory factors in macrophages as an innate immune response. MWCNT 1-induced NO release was less than that of LPS at lower doses, but this value exceed LPS at higher doses. Compared to MWCNT 1, MWCNT 2 elicited lower NO release (Figure 3A). Similarly, treatment with MWCNT 1 and 2 elicited different amounts of TNF-α release after 24 hrs. MWCNT 1 induced a significant TNF-α release especially at the high dose which was comparable to the LPS-induced response. However, TNF-α release triggered by MWCNT 2 was less than 27% of the LPS response at the highest dose tested (374 μg/mL) (Figure 3B).
Figure 3.
Immune perturbations of MWCNTs in macrophages. (A) and (B): macrophages were treated with different concentrations of MWCNTs for 24 h and the nitrite concentration (A) and TNF-α (B) in the culture supernatant was analyzed by Griess reaction and ELISA separately. LPS (20 ng/mL) was used as control. (C): reactive oxygen species in macrophages was determined through increases in fluorescence intensity of dichlorofluorescin (DCF) after incubation with MWCNTs for 24 hrs. (D): The lowered mitochondrial membrane potentials elicited by MWCNTs (97.6 μg/mL for MWCNT 1 or 95.9 μg/mL for MWCNT 2, 24 hrs) were measured by JC-1 fluorescence. Mitochondria depolarization is specifically indicated by a decrease in the red (610±10 nm) to green (525±10 nm) fluorescence intensity ratio. The fluorescence was measured by confocal laser scanning microscopy.
ROS generation is a part of the primary immune defense in macrophages against foreign materials. The induction of oxidative stress has also been suggested to be a major toxicological paradigm for engineered nanoparticles.33–35 Nanoparticles were reported to cause pulmonary diseases by generating oxidative injury.35 In vitro studies have shown that CNTs induced oxidative stress in rat alveolar macrophages and monocytic cells,30, 36 and SWCNTs also showed an adverse effect on keratinocytes through an oxidative mechanism.15
The MWCNT-induced intracellular ROS was assayed by monitoring the increase of fluorescence intensity of dichlorofluorescein (DCF) in cells (Figure 3C). 2′,7′-dichlorofluorescein diacetate (H2DCF) is a nonfluorescent compound that accumulates in cells. It reacts with ROS to form the fluorescent compound dichlorofluorescein (DCF). In our experiments, cells were treated with different concentrations of MWCNTs for 24 hrs and the increase in fluorescence was determined in the presence of DCF by a fluorometer. The results showed a dose-dependent generation of ROS by both MWCNTs. The intracellular ROS generated by MWCNT 1 and 2 was negligible at the lowest concentration, which was similar to previous study that SWCMT-COOH did not induce the ROS generation below 20 μg/mL.37 However the increased intracellular ROS generated by MWCNT 1 was sharper than that by MWCNT 2. The behavior of MWCNT 1 is consistent with previous findings.15 The MWCNT 2-induced increase in intracellular ROS at the highest concentration (374 μg/mL) was only 36% of that induced by MWCNT 1 at 379 μg/mL in comparison to untreated cells.
Loss of mitochondrial membrane potential also leads to oxidative stress. The mitochondrial membrane potential was assessed using the fluorescent dye JC-1 (Figure 3D). This cationic dye exhibits potential dependent accumulation in mitochondria indicated by a fluorescence emission shift from green (525±10 nm) to red (610±10 nm). Following treatment with MWCNTs, mitochondrial membrane potential was monitored by measuring the red/green ratio using confocal laser scanning microscopy. At the concentration of 97.6 μg/mL, MWCNT 1 reduced the mitochondrial membrane potential by 34% while MWCNT 2 at the concentration of 95.9 μg/mL only by 14%.
Above results demonstrated that MWCNT 2 exhibited reduced immune perturbations in macrophages through multiple assays. To determine whether these effects were results of CNT surface modification or a artifact such as difference in internalization in macrophages between MWCNT 1 and 2, we quantitatively investigated MWCNTs internalization and investigated their cellular locations.
MWCNT 1 and 2 are taken up by macrophages in a similar amount and distributed in identical cellular locations
Since there is no available method to quantify the amount of MWCNTs inside cells, we developed a quantitative analysis protocol to determine the absolute amount of MWCNTs in macrophages. CNTs bind proteins with high affinity38 that CNTs are all in protein-bound state (mostly BSA) in cell culture. FITC-labeled-BSA spontaneously binds to MWCNTs and the bound proteins remain associated with MWCNTs even after three wash-and-centrifuge cycles. Only 6% of labeled BSA was dissociated after 24 hrs. In this way, we were able to label both MWCNT 1 and 2 with fluorescence tag and quantify their amounts inside macrophages by measuring fluorescence of fragmented cells after sonication. A quantification calibration curve was generated using known amounts of fluorescent labeled MWCNT 1 or 2 in the presence of the same number of cells. Macrophages incubated with MWCNT 1 and 2 for 24 hrs were sonicated and fluorescence of the contents was measured. Experimental results showed that the total cell uptakes of MWCNT 1 and 2 were highly comparable (63.7±8.04 and 62.1±7.39 pg/cell, Table 2).
Table 2.
Amount of MWCNTs internalized into macrophages *
| Sample | Cell (×106) | Uptake (μg) | Uptake/Cell (pg) |
|---|---|---|---|
| MWCNT 1 | 1.5 | 95.6±12.1 | 63.7±8.04 |
| MWCNT 2 | 1.5 | 93.1±11.1 | 62.1±7.39 |
Quantitative method for determination of MWCNTs internalization is described in supporting information.
To objectively evaluate the mechanisms of MWCNT’s effects on macrophages, we also determined the cellular locations of MWCNT 1 and 2 inside macrophages. CNTs were known to be taken up by mammalian cells and distributed in cytoplasm, endosomes and lysosome.7, 39, 40 TEM images showed the ultrastructural features of macrophages exposed to MWCNT 1 and 2 for 24 hrs (Figure 4). Both MWCNT 1 and 2 were found in the same subcellular organelles: phagosomes, cytoplasm and lysosomes. Phagosomes and lysosomes were distinguished by the shade inside due to the relative emptiness of early phagosomes while lysosomes contain a lot more biological materials that produced darker images.
Figure 4.
TEM characterization of MWCNTs internalization and their distribution in subcellular organelles. Macrophages were incubated with MWCNT 1 (A, B, C, 97.6 μg/mL) or 2 (D, E, F, 95.9 μg/mL) for 24 hrs. A and D: cytoplasm; B and E: endosome; C and F: lysosome. Arrows point to MWCNTs. Scale bars represent 100 nm.
MWCNT 1 and 2 are recognized by different receptors
The first strategy of macrophages to fight against foreign particles is to recognize them by cell surface receptors and engulf them via phagocytosis. This process involves receptors like the mannose receptor (MR), toll-like receptor (TLR) and scavenger receptor (SR).41, 42 Studies have shown that the internalization of mannosylated gelatin nanoparticles were two-fold higher than unconjugated nanoparticles.43 The uptake of albumin-coated SWCNTs were reduced by a scavenger receptor inhibitor.44
To delineate the role of specific phagocytotic pathways and receptors involved in MWCNTs internalization and immune modulation, macrophages were treated with known biochemical inhibitors of phagocytosis and macrophage receptors (Fig. 5). Cytochalasin D (5 μg/mL), fucoidan (25 μg/mL), mannan (2 mg/mL) and OxPAPC (30 μg/mL) are inhibitors for macropinocytosis/phagocytosis, scavenger receptor, mannose receptor and Toll-like receptor 4 (TLR4) respectively. Macrophages were preincubated with inhibitors for 30 min and then treated with MWCNTs-BSA-FITC. Cytochalasin D resulted in a marked decrease in the cellular internalization of both MWCNT 1 and 2 (about 69% and 67% compared with un-pretreated cells) indicating that the main internalization mechanism for both MWCNTs was macropinocytosis/phagocytosis. The pretreatment of TLR4 inhibitor affected the internalization of MWCNT 1 and 2 similarly (15% and 14% respectively). However, treatments with MR and SR inhibitors showed striking differences. Internalization of MWCNT 1 was inhibited similarly by both MR and SR inhibitors (19 and 25%), while MWCNT 2 was inhibited mainly by SR inhibitor (45%). MR inhibitor only caused 9% inhibition of MWCNT 2.
Figure 5.
Probing the mechanisms of macrophages’ internalization of MWCNTs. Cytochalasin D, fucoidan, mannan and OxPAPC are generally classified as inhibitor of macropinicytosis/phagocytosis, scavenger receptor, mannose receptor and Toll-like receptor 4. Macrophages were first treated with various inhibitors for 30 min. treated and untreated macrophages then were incubated with MWCNT 1-FITC (48.8 μg/mL) or MWCNT 2-FITC (48.1 μg/mL) for 45 min before the amounts of internalization were quantitatively determined. The percent internalization was normalized to particle internalization in the absence of inhibitors. (Comparison between pretreated and untreated macrophages, *p<0.05. Comparison between scavenger and mannose receptor inhibitor pretreated macrophages, **p<0.05)
Phagocytosis of NPs via MR-mediated pathways results in a variety of downstream events, including the release of lysosomal enzymes, reactive oxygen intermediates, arachidonic acid metabolites, cytokines such as IL-1, IL-6, TNF-a, and NFκB activation.45–48 Nevertheless, SR-mediated uptake is not accompanied by pro-inflammatory cytokine secretion and NFκB activation.42 Therefore, to further elucidate whether MWCNT 1 and 2 trigger different receptor-mediated pathways, we investigated the activation of their downstream signaling proteins.
MWCNT 2 alleviates activation of the NFκB pathway due to preference to SR binding
Generally, inflammatory effects induced by foreign materials are mostly mediated by the NF-κB pathway.49 CNTs was reported to generate ROS, activate NF-κB, and to release proinflammatory cytokines in human bronchial epithelial cells and mesothelial cells,14, 15, 17 as well as in mice16.
The active NFκB complex consists of P50, P65, and IκB kinase. The activation of NFκB leads to IκB phosphorylation and degradation, as well as nuclear translocation of the p50/p65 dimer.50 After MWCNT treatment, the cytoplasmic and nuclear extracts of macrophages were analyzed by Western blot. Results showed both IκBα degradation and the translocation of p65 proteins into the nucleus (Figure 6A). Such degradation and nucleus translocation elicited by MWCNT 2 was much lower than MWCNT 1. The results indicated that the chemistry modification on MWCNT 2 surface evidently alleviated the activation of the NFκB pathway.
Figure 6.
Effect of MWNCTs on NFκB activation (A), iNOS (B) and Src-family of PTK (C) expression. Degradation of IκBα and translocation of p65 protein into the nucleus are indicators for activation of NFκB signaling pathway. Macrophages were treated with 97.6 μg/mL of MWNCT 1 or 95.9 μg/mL of MWNCT 2 for 24 hrs. The cytoplasm or nuclear extracts were analyzed by western blot for IκBα, p65, iNOS and Src-family of PTK in cytoplasm and p65 in nuclear. For equal protein loading of cytoplasmic extracts, the cytoplasm extracts were analyzed with anti-β-actin antibody and for nuclear extracts with anti-PARP antibody.
NO is synthesized from L-arginine and molecular oxygen by NO synthase (NOS). Inducible nitric oxide synthase (iNOS) is a major inducible isoform that is regulated by the NFκB pathway. Depending on the different upstream signaling pathway activation, iNOS expression may be promoted or inhibited.32, 51 Its expression is upregulated by NFκB activation. To further confirm that MWCNT 1 activated NFκB while MWCNT 2 reduced such activation, we measured the level of iNOS expression by Western blot (Figure 6B). Our results showed that iNOS expression was reduced by MWCNT 2 compared to that induced by MWCNT 1.
On the other hand, a previous study has reported that tyrosine phosphorylation of pp60Src are key components of SR-mediated signal transduction cascades.52, 53 In order to confirm the SR recognition predominantly by MWCNT 2, the expression of Src-family of protein tyrosine kinase (PTK) in macrophages treated with MWCNTs was determined by Western blot (Figure 6C). The level of Src-family PTK activation by MWCNT 2 surpassed that by MWCNT 1. This result confirmed that MWCNT 2 was internalized mainly through scavenger receptor recognition that activates the downstream Src-family of PTK. The scavenger receptor-mediated pathway did not activate the NFκB pathway and, therefore, the inflammatory responses were reduced. This finding demonstrated that surface property changes on CNTs could significantly alter the receptor recognition events and reverse their immune perturbations.
Conclusion
In summary, we demonstrated that the surface chemistry modification on MWCNTs can regulates the immune perturbation in mice and in macrophages. MWCNT 2 caused less immune perturbations compared to MWCNT 1 both in mice and in macrophages. The mechanistic explanation is that the chemistry modification on MWCNT 2 surface increased its binding to scavenger receptor. The recognition by scavenger receptor alleviated NFκB activation and reduced immunotoxicity of MWCNT 2. The elucidation of immunotixicity of nanomaterials are exemplifies here and, more importantly, we establish an approach to modify nanomaterials to reduce their immune perturbations will facilitate the wide applications of nanomaterials in medicane and life sciences.
Materials and Methods
Functionalized multi-walled carbon nanotubes
MWCNTs were synthesized as we previously reported.18 For experiments in vitro, MWCNTs were sterilized at 121 °C for 3 hrs and were suspended in RPMI 1640 (Invitrogen, CA, USA) plus 2 mM L-glutamine (Invitrogen, CA, USA), 25 mM HEPES (Invitrogen, CA, USA), 100 U/mL penicillin, 100 μg/mL streptomycin and 10% fetal bovine serum. Stock solutions were sonicated for 5 min in order to well suspending befor they were diluted by complete RPMI 1640 culture medium to 10, 50, 100, 200 and 400 μg/mL. Aggregated particles were removed by centrifugation at 1500 rpm for 5 min and the effective concentrations of MWCNTs were determined by UV-Vis spectroscopy using a calibration curve obtained separately. The suspensions were sonicated for 5 min again before the treatment on cells and they were well homogenous in culture medium (Figure S3).
For experiments in vivo, sterilized MWCNTs were dispersed in PBS (pH 7.4) with 0.1% Tween 80 to a final concentration of 1.0 mg/mL. The suspensions were sonicated for 5 min before i.v. injection.
Macrophages
The cells used in this study were THP-1 (human monocyte) cell line. THP-1 cells were cultivated in complete RPMI 1640 culture medium and grown in a humidified incubator at 37 °C (95% room air, 5% CO2). Differentiation into macrophages was triggered by the addition of Phorbol 12-myristate 13-acetate (Promega, WI, USA) at a concentration of 50 ng/mL for 48 hrs. Differentiated cells were characterized by adhering to the plastic surface. The non-adherent monocytes were carefully removed and the adherent macrophages left in the original plate were washed twice by RPMI 1640.
In vivo biodistribution studies
To maintain the integrity of the MWCNT 1, less than 5% of the nanotubes were reacted with diaminopropane and then coupled with 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA), which is then used to chelate 64Cu to form the radioactive MWCNT 1. Radiochemical purity was determined by ITLC-SG using silica gel impregnated paper (Gelman Science Inc., Ann Arbor, MI). Radiolabeled 64Cu-DOTA-MWCNT 1, which was named as MWCNT 3, was injected in to CD-1 female mice at a dose of 67–123 μCi of radiotracer via the tail vein. Animals (n= 4 at each group) were sacrificed, the organs were weighed and radioactivity of 64Cu was counted by using an automatic γ-counter. All animal experiments were carried out in accordance with the NIH guidelines (Guide for the care and use of laboratory animals) and experimental protocols approved by an institutional animal care and use committee.
MWCNT’s effect in mice
After acclimation for one week, 15 famale BALB/c mice (19–23 g) were randomly divided into 3 groups (vehicle control, MWCNT 1 and MWCNT 2) with 5 mice per group. Mice were given injections of vehicle and MWCNTs via the tail vein at a dose of 15 mg/kg and sacrificed after 24 hrs. The methods for homogenate preparation and analyses were carried out according to established procedures.54 The non-tendon segments of lungs, liver and spleen were minced and homogenized in ice cold PBS. Homogenates were diluted to 1.7% (w/v) with PBS and centrifuged at 3,000 rpm for 10 min at 4 °C to collect the supernatants for TNF-α and IL-1β measurement by ELISA assays.
NO and TNF-α assay in macrophages
THP-1 macrophages (1×105/well in a 96-well plate) were treated with MWCNTs at various concentrations for 24 hrs at 37 °C. LPS (20 ng/mL) was used as control. After incubation, the supernatants were collected for NO and TNF-α assays by Griess reaction and ELISA separately according to the manufacturer’s protocol.
Oxidative Stress in macrophages
THP-1 macrophages (1×105/well in a 96-well plate) were exposed to different concentrations of MWCNTs for 24 hrs at 37 °C. After treatment and subsequent washing steps to remove the excess MWCNTs, the intracellular ROS were assayed by the increases in fluorescence intensity of dichlorofluorescein in cells following to the manufacturer’s protocol.
Assessment of mitochondrial membrane potential changes
The macrophages mitochondrial membrane potential was determined after MWCNTs treatment for 24 hrs. After washing twice to remove MWCNTs outside the cells, JC-1 was incubated with cells for 20 min at 37°C. JC-1 is a lipophilic, cationic dye that can selectively enter mitochondria and reversibly change color from green (525±10 nm) to red (610±10 nm) as the membrane potential increases. The JC-1 dye accumulates in the mitochondria of cells with normal membrane and display red fluorescence. In altered cells with lower membrane potential, JC-1 dye can no longer accumulate in the mitochondria and remains in the cytoplasm with green fluorescence. Followed by washing twice carefully, the fluorescence was imaged and measured by confocal laser scanning microscopy (CLSM, Leica, Wetzlar, Germany). Mitochondria depolarization is indicated by a decrease in the red to green fluorescence intensity ratio.
Transmission electron microscopy (TEM)
Macrophages were incubated with MWCNT 1 (97.6 μg/mL) or MWCNT 2 (95.9 μg/mL) for 24 hrs in culture dishes (1×107 cells/dish). Then cells were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) and rinsed. Cells were fixed for one hour in 2% osmium tetroxide with 3% potassium ferriocyanide and rinsed. They were treated with enbloc staining with a 2% aqueous uranyl acetate solution and dehydrated through a graded series of alcohol. They were then put into propylene oxide, a series of propylene/epon dilutions and embedded. The thin (70 microns) sections were cut on a Leica UC6 ultramicrotome and images were taken on a JEOL 1200 EX (JEOL, Ltd. Tokyo, Japan) using an AMT 2k digital Camera.
Quantitative analysis of cell uptake
Cell uptake of MWCNTs was quantified using FITC-BSA (Sigma Aldrich, MO, USA) labeled MWCNTs. MWCNTs (1 mg/mL) were incubated with FITC-albumin (2 mg/mL) for 36 hrs at 4 °C, and unabsorbed FITC-albumin was removed by washing (x3) and centrifuging at 20,000 rpm for 10 min. The stability of MWCNT-BSA-FITC was determined and the dissociation of BSA-FITC was only 6% compared with the total MWCNT-BSA-FITC even at 24 hrs (Figure S4). Then FITC-BSA-labeled MWCNT 1 (97.6 μg/mL) or MWCNT 2 (95.9 μg/mL) were added to macrophages respectively. After 24 hrs incubation, cells were washed and then sonicated to break cells into smaller fragments. The fluorescence of cell debris was measured on a fluorospectrophotometer (Thermo Scientific, MA, USA) with excitation at 488 nm. At the same time, a series of suspensions of MWCNT 1-BSA-FITC and MWCNT 2-BSA-FITC were prepared to determine calibration curves (Figure S5).
Macrophages receptors recognization
THP-1 macrophages (1×105/well in a 96-well plate) were treated with Cytochalasin D (5 μg/mL, Sigma Aldrich, MO, USA), fucoidan (25 μg/mL, Sigma Aldrich, MO, USA), mannan (2 mg/mL, Sigma Aldrich, MO, USA) and OxPAPC (30 μg/mL, InvivoGen, CA, USA) for 30 min at 37 °C. After removing the supernatant and washing the cells twice by RPMI 1640, macrophages were treated with MWCNT 1-BSA-FITC (48.8 μg/mL) or MWCNT 2-BSA-FITC (48.1 μg/mL) for 45 min and measured as the previous section.
Western blot analysis
THP-1 macrophages were treated with MWCNT 1 (97.6 μg/mL) or MWCNT 2 (95.9 μg/mL) for 24 hrs. The cells were washed with ice cold PBS for three times and collected. The cytoplasm and nuclear extracts were prepared and the concentration of solubilized protein was determined following the manufacturer’s protocol (Bio-Rad, CA, USA). The cytoplasm or nuclear extracts were separated by 10% SDS-PAGE and electrophoretically transferred onto a polyvinylidene difluoride membrane (Bio-Rad, CA, USA). The membrane was blocked with 5% nonfat milk in TBS containing 0.1% Tween 20 at room temperatures for 2 hrs followed by incubation overnight at 4°C with antibodies against IκBα (Santa Cruz, CA, USA), p65 (Santa Cruz, CA, USA), iNOS (Santa Cruz, CA, USA), Src-family of PTK (Cell Signaling, MA, USA), β-actin (Santa Cruz, CA, USA) and PARP (Cell Signaling, MA, USA). The membranes were washed with TBS containing 0.1% Tween 20 and probed with horseradish peroxidase-coupled secondary goat anti-rabbit or anti-mouse immunoglobulin G antibodies (Santa Cruz, CA, USA). The proteins were detected with the Immun-Star Wsetern Chemiluminescence Kit (Bio-Rad, CA, USA) according to the manufacturer’s instructions.
Statistical analysis
Mean and standard deviation (s.d.) were calculated for each parameter. Results were expressed as mean±s.d of multiple determinations. Comparisons of each group were evaluated by two-side student’s t-test. A statistically significant difference was assumed to exist when p values were less than 0.05.
Supplementary Material
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program 2010CB933504), National Natural Science Foundation of China (21077068), National Cancer Institute (P30CA027165) and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Supporting Information Available: Additional figures depicting correlation of NO release and steric properties of MWCNT-attached ligand, determine the concentration of suspensions, stability of suspensions, stability of MWCNT-BSA-FITC and quantification of cell uptakewere described in the text. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Morinobu Endo MSS, Ajayan Pulickel M. Potential Applications of Carbon Nanotubes. Top Appl Phys. 2008;111:13–62. [Google Scholar]
- 2.Bianco A, Kostarelos K, Partidos CD, Prato M. Biomedical Applications of Functionalised Carbon Nanotubes. Chem Commun. 2005:571–577. doi: 10.1039/b410943k. [DOI] [PubMed] [Google Scholar]
- 3.Sun Z, Wang W, Meng J, Chen SC, Xu HY, Yang XD. Multi-Walled Carbon Nanotubes Conjugated to Tumor Protein Enhance the Uptake of Tumor Antigens by Human Dendritic Cells in vitro. Cell Res. 2010;20:1170–1173. doi: 10.1038/cr.2010.133. [DOI] [PubMed] [Google Scholar]
- 4.Kam NW, O’Connell M, Wisdom JA, Dai H. Carbon Nanotubes as Multifunctional Biological Transporters and Near-Infrared Agents for Selective Cancer Cell Destruction. Proc Natl Acad Sci USA. 2005;102:11600–11605. doi: 10.1073/pnas.0502680102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng J, Yang M, Jia FM, Kong H, Zhang WQ, Wang CY, Xing JM, Xie SS, Xu HY. Subcutaneous Iinjection of Water-Soluble Multi-Walled Carbon Nanotubes in Tumor-Bearing Mice Boosts the Host Immune Activity. Nanotechnology. 2010;21:145104–145112. doi: 10.1088/0957-4484/21/14/145104. [DOI] [PubMed] [Google Scholar]
- 6.Smith AM, Mancini MC, Nie SM. BIOIMAGING Second Window for in vivo Imaging. Nat Nanotechnology. 2009;4:710–711. doi: 10.1038/nnano.2009.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng C, Muller KH, Koziol KK, Skepper JN, Midgley PA, Welland ME, Porter AE. Toxicity and Imaging of Multi-Walled Carbon Nanotubes in Human Macrophage Cells. Biomaterials. 2009;30:4152–4160. doi: 10.1016/j.biomaterials.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 8.Hirano S, Kanno S, Furuyama A. Multi-Walled Carbon Nanotubes Injure the Plasma Membrane of Macrophages. Toxicol Appl Pharmacol. 2008;232:244–251. doi: 10.1016/j.taap.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Dumortier H, Lacotte S, Pastorin G, Marega R, Wu W, Bonifazi D, Briand JP, Prato M, Muller S, Bianco A. Functionalized Carbon Nanotubes are Non-Cytotoxic and Preserve the Functionality of Primary Immune Cells. Nano Lett. 2006;6:1522–1528. doi: 10.1021/nl061160x. [DOI] [PubMed] [Google Scholar]
- 10.Minnikanti S, Pereira M, Jaraiedi S, Jackson K, Costa-Neto CM, Li QL, Peixoto N. In vivo Electrochemical Characterization and Inflammatory Response of Multiwalled Carbon Nanotube-Based Electrodes in Rat Hippocampus. J Neural Eng. 2010;7:11. doi: 10.1088/1741-2560/7/1/016002. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell LA, Lauer FT, Burchiel SW, McDonald JD. Mechanisms for How Inhaled Multiwalled Carbon Nanotubes Suppress Systemic Immune Function in Mice. Nat Nanotechnology. 2009;4:451–456. doi: 10.1038/nnano.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schipper ML, Nakayama-Ratchford N, Davis CR, Kam NW, Chu P, Liu Z, Sun X, Dai H, Gambhir SS. A Pilot Toxicology Study of Single-Walled Carbon Nanotubes in a Small Sample of Mice. Nat Nanotechnol. 2008;3:216–221. doi: 10.1038/nnano.2008.68. [DOI] [PubMed] [Google Scholar]
- 13.Meng J, Yang M, Jia F, Xu Z, Kong H, Xu H. Immune Responses of BALB/c Mice to Subcutaneously Injected Multi-Walled Carbon Nanotubes. Nanotoxicology. 2010:1–10. doi: 10.3109/17435390.2010.523483. [DOI] [PubMed] [Google Scholar]
- 14.Hirano S, Fujitani Y, Furuyama A, Kanno S. Uptake and Cytotoxic Effects of Multi-Walled Carbon Nanotubes in Human Bronchial Epithelial Cells. Toxicol Appl Pharmacol. 2010;249:8–15. doi: 10.1016/j.taap.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Manna SK, Sarkar S, Barr J, Wise K, Barrera EV, Jejelowo O, Rice-Ficht AC, Ramesh GT. Single-Walled Carbon Nanotube Induces Oxidative Stress and Activates Nuclear Transcription Factor-kappaB in Human Keratinocytes. Nano Lett. 2005;5:1676–1684. doi: 10.1021/nl0507966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou CC, Hsiao HY, Hong QS, Chen CH, Peng YW, Chen HW, Yang PC. Single-Walled Carbon Nanotubes Can Induce Pulmonary Injury in Mouse Model. Nano Lett. 2008;8:437–445. doi: 10.1021/nl0723634. [DOI] [PubMed] [Google Scholar]
- 17.Pacurari M, Yin XJ, Zhao JS, Ding M, Leonard SS, Schwegier-Berry D, Ducatman BS, Sbarra D, Hoover MD, Castranova V, et al. Raw Single-Wall Carbon Nanotubes Induce Oxidative Stress and Activate MAPKs, AP-1, NF-kappa B, and Akt in Normal and Malignant Human Mesothelial Cells. Environ Health Persp. 2008;116:1211–1217. doi: 10.1289/ehp.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou H, Mu Q, Gao N, Liu A, Xing Y, Gao S, Zhang Q, Qu G, Chen Y, Liu G, et al. A Nano-Combinatorial Library Strategy for the Discovery of Nanotubes with Reduced Protein-Binding, Cytotoxicity, and Immune Response. Nano Lett. 2008;8:859–865. doi: 10.1021/nl0730155. [DOI] [PubMed] [Google Scholar]
- 19.Miyawaki J, Matsumura S, Yuge R, Murakami T, Sato S, Tonnida A, Tsuruo T, Ichihashi T, Fujinami T, Irie H, et al. Biodistribution and Ultrastructural Localization of Single-Walled Carbon Nanohorns Determined in vivo with Embedded Gd2O3 Labels. ACS Nano. 2009;3:1399–1406. doi: 10.1021/nn9004846. [DOI] [PubMed] [Google Scholar]
- 20.Yang ST, Guo W, Lin Y, Deng XY, Wang HF, Sun HF, Liu YF, Wang X, Wang W, Chen M, et al. Biodistribution of Pristine Single-Walled Carbon Nanotubes in vivo. J Phys Chem C. 2007;111:17761–17764. [Google Scholar]
- 21.Singh R, Pantarotto D, Lacerda L, Pastorin G, Klumpp C, Prato M, Bianco A, Kostarelos K. Tissue Biodistribution and Blood Clearance Rates of Intravenously Administered Carbon Nanotube Radiotracers. Proc Natl Acad Sci USA. 2006;103:3357–3362. doi: 10.1073/pnas.0509009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park EJ, Kim H, Kim Y, Yi J, Choi K, Park K. Carbon Fullerenes (C60s) Can Induce Inflammatory Responses in the Lung of Mice. Toxicol Appl Pharmacol. 2010;244:226–233. doi: 10.1016/j.taap.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 23.Porter DW, Hubbs AF, Mercer RR, Wu NQ, Wolfarth MG, Sriram K, Leonard S, Battelli L, Schwegler-Berry D, Friend S, et al. Mouse Pulmonary Dose- and Time Course-Responses Induced by Exposure to Multi-Walled Carbon Nanotubes. Toxicology. 2010;269:136–147. doi: 10.1016/j.tox.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell LA, Gao J, Wal RV, Gigliotti A, Burchiel SW, McDonald JD. Pulmonary and Systemic Immune Response to Inhaled Multiwalled Carbon Nanotubes. Toxicol Sci. 2007;100:203–214. doi: 10.1093/toxsci/kfm196. [DOI] [PubMed] [Google Scholar]
- 25.Deng X, Jia G, Wang H, Sun H, Wang X, Yang S, Wang T, Liu Y. Translocation and Fate of Multi-Walled Carbon Nanotubes in vivo. Carbon. 2007;45:1419–1424. [Google Scholar]
- 26.Qu GB, Bai YH, Zhang Y, Jia Q, Zhang WD, Yan B. The Effect of Multiwalled Carbon Nanotube Agglomeration on Their Accumulation in and Damage to Organs in Mice. Carbon. 2009;47:2060–2069. [Google Scholar]
- 27.Nygaard UC, Hansen JS, Samuelsen M, Alberg T, Marioara CD, Lovik M. Single-Walled and Multi-Walled Carbon Nanotubes Promote Allergic Immune Responses in Mice. Toxicol Sci. 2009;109:113–123. doi: 10.1093/toxsci/kfp057. [DOI] [PubMed] [Google Scholar]
- 28.Fenoglio I, Greco G, Tornatis M, Muller J, Rayrnundo-Pinero E, Beguin F, Fonseca A, Nagy JB, Lison D, Fubini B. Structural Defects Play a Major Role in the Acute Lung Toxicity of Multiwall Carbon Nanotubes: Physicochemical Aspects. Chem Res Toxicol. 2008;21:1690–1697. doi: 10.1021/tx800100s. [DOI] [PubMed] [Google Scholar]
- 29.Han SG, Andrews R, Gairola CG. Acute Pulmonary Response of Mice to Multi-wall Carbon Nanotubes. Inhal Toxicol. 2010;22:340–347. doi: 10.3109/08958370903359984. [DOI] [PubMed] [Google Scholar]
- 30.Brown DM, Kinloch IA, Bangert U, Windle AH, Walter DM, Walker GS, Scotchford CA, Donaldson K, Stone V. An in vitro Study of the Potential of Carbon Nanotubes and Nanofibres to Induce Inflammatory Mediators and Frustrated Phagocytosis. Carbon. 2007;45:1743–1756. [Google Scholar]
- 31.Old LJ. Tumor Necrosis Factor (TNF) Science. 1985;230:630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- 32.Bogdan C, Rollinghoff M, Diefenbach A. Reactive Oxygen and Reactive Nitrogen Intermediates in Innate and Specific Immunity. Curr Opin Immunol. 2000;12:64–76. doi: 10.1016/s0952-7915(99)00052-7. [DOI] [PubMed] [Google Scholar]
- 33.Nel A, Xia T, Madler L, Li N. Toxic Potential of Materials at the Nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 34.Nel A. Air Pollution-Related Illness: Effects of Particles. Science. 2005;308:804–806. doi: 10.1126/science.1108752. [DOI] [PubMed] [Google Scholar]
- 35.Li N, Xia T, Nel AE. The Role of Oxidative Stress in Ambient Particulate Matter-Induced Lung Diseases and Its Implications in the Toxicity of Engineered nNanoparticles. Free Radical Biol Med. 2008;44:1689–1699. doi: 10.1016/j.freeradbiomed.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schrand AM, Dai L, Schlager JJ, Hussain SM, Osawa E. Differential Biocompatibility of Carbon Nanotubes and Nanodiamonds. Diam Relat Mater. 2007;16:2118–2123. [Google Scholar]
- 37.Zeinali M, Jammalan M, Ardestani SK, Mosaveri N. Immunological and Cytotoxicological Characterization of Tuberculin Purified Protein Derivative (PPD) Conjugated to Single-Walled Carbon Nanotubes. Immunol Lett. 2009;126:48–53. doi: 10.1016/j.imlet.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 38.Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE. Preclinical Studies to Understand Nanoparticle Interaction with the Immune System and Its Potential Effects on Nanoparticle Biodistribution. Mol Pharm. 2008;5:487–495. doi: 10.1021/mp800032f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mu QX, Broughton DL, Yan B. Endosomal Leakage and Nuclear Translocation of Multiwalled Carbon Nanotubes: Developing a Model for Cell Uptake. Nano Lett. 2009;9:4370–4375. doi: 10.1021/nl902647x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.VanHandel M, Alizadeh D, Zhang L, Kateb B, Bronikowski M, Manohara H, Badie B. Selective Uptake of Multi-Walled Carbon Nanotubes by Tumor Macrophages in a Murine Glioma Model. J Neuroimmunol. 2009;208:3–9. doi: 10.1016/j.jneuroim.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Dobrovolskaia MA, McNeil SE. Immunological Properties of Engineered Nanomaterials. Nat Nanotechnology. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 42.Aderem A, Underhill DM. Mechanisms of Phagocytosis in Macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 43.Mahajan S, Prashant CK, Koul V, Choudhary V, Dinda AK. Receptor Specific Macrophage Targeting by Mannose-Conjugated Gelatin Nanoparticles-An in vitro and in vivo Study. Curr Nanosci. 2010;6:413–421. [Google Scholar]
- 44.Dutta D, Sundaram SK, Teeguarden JG, Riley BJ, Fifield LS, Jacobs JM, Addleman SR, Kaysen GA, Moudgil BM, Weber TJ. Adsorbed Proteins Influence the Biological Activity and Molecular Targeting of Nanomaterials. Toxicol Sci. 2007;100:303–315. doi: 10.1093/toxsci/kfm217. [DOI] [PubMed] [Google Scholar]
- 45.Fraser IP, Koziel H, Ezekowitz RAB. The Serum Mannose-Binding Protein and the Macrophage Mannose Receptor are Pattern Recognition Molecules that Link Innate and Adaptive Immunity. Semin Immunol. 1998;10:363–372. doi: 10.1006/smim.1998.0141. [DOI] [PubMed] [Google Scholar]
- 46.Zhang JM, Zhu JP, Imrich A, Cushion M, Kinane TB, Koziel H. Pneumocystis Activates Human Alveolar Macrophage NF-KB Signaling through Mannose Receptors. Infect Immun. 2004;72:3147–3160. doi: 10.1128/IAI.72.6.3147-3160.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.East L, Isacke CM. The Mannose Receptor Family. Biochim Biophys Acta-Gen Subj. 2002;1572:364–386. doi: 10.1016/s0304-4165(02)00319-7. [DOI] [PubMed] [Google Scholar]
- 48.Stahl PD, Ezekowitz RAB. The Mannose Receptor is a Pattern Recognition Receptor Involved in Host Defense. Curr Opin Immunol. 1998;10:50–55. doi: 10.1016/s0952-7915(98)80031-9. [DOI] [PubMed] [Google Scholar]
- 49.Hayden MS, West AP, Ghosh S. NF-kappa B and the Immune Response. Oncogene. 2006;25:6758–6780. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 50.Baichwal VR, Baeuerle PA. Activate NF-kappa B or die? Curr Biol. 1997;7:R94–96. doi: 10.1016/s0960-9822(06)00046-7. [DOI] [PubMed] [Google Scholar]
- 51.MacMicking J, Xie QW, Nathan C. Nitric Oxide and Macrophage Function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 52.Coller SP, Paulnock DM. Signaling Pathways Initiated in Macrophages after Engagement of Type A Scavenger Receptors. J Leukocyte Biol. 2001;70:142–148. [PubMed] [Google Scholar]
- 53.Hsu HY, Chiu SL, Wen MH, Chen KY, Hua KF. Ligands of Macrophage Scavenger Receptor Induce Cytokine Expression via Differential Modulation of Protein Kinase Signaling Pathways. J Biol Chem. 2001;276:28719–28730. doi: 10.1074/jbc.M011117200. [DOI] [PubMed] [Google Scholar]
- 54.Deng XY, Wu F, Liu Z, Luo M, Li L, Ni QS, Jiao Z, Wu MH, Liu YF. The Splenic Toxicity of Water Soluble Multi-Walled Carbon Nanotubes in Mice. Carbon. 2009;47:1421–1428. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.