Abstract
3-Hydroxy-3-methylglutaryl–coenzyme A (HMG-CoA) reductase inhibitors (statins) are among the most prescribed medications in the United States. Statins act on the rate-limiting step in cholesterol biosynthesis (the conversion of HMG-CoA to mevalonate) and are effective in treating dyslipidemia. However, statins decrease other downstream products of the mevalonate pathway, and it is via these pathways that statins may affect inflammation, nitric oxide synthesis, the coagulation cascade, and other processes. Through these pleiotropic effects, statins may have an effect on neurologic diseases, including ischemic and hemorrhagic stroke, Alzheimer disease, Parkinson disease, and multiple sclerosis. This article reviews the basic biochemistry of statins as it relates to these pleiotropic effects, the potential role of statins in several neurologic disorders, and the results of clinical trials performed for several of these conditions.
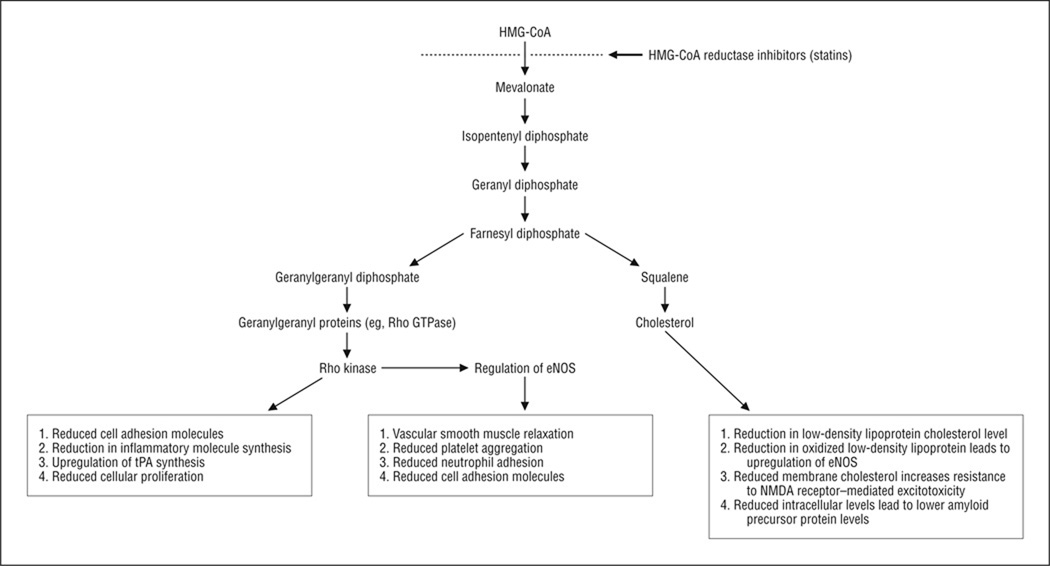
3-Hydroxy-3-methylglutaryl–coenzyme A (HMG-CoA) reductase inhibitors, also known as statins, act on the rate-limiting step in the pathway by which HMG-CoA is converted to mevalonate.1 Through their effect on this pathway (Figure), as well as an increase in low-density lipoprotein cholesterol (LDL-C) receptors and uptake, statins reduce the production of cholesterol, thereby modifying dyslipidemia, which is the most common use for this class of medications. Statins reduce other by-products of the mevalonate pathway, including ubiquinone, dolichol, and the isoprenoids farnesyl pyrophosphate and geranylgeranylpyrophosphate. In turn, farnesyl pyrophosphate and geranylgeranylpyrophosphate are necessary for the posttranslational lipid modification (prenylation) of several proteins that are tethered to the cell wall. Among these key membrane proteins are small guanosine triphosphate–binding proteins such as the Rho family of guanosine triphosphatases, which acts on Rho kinase. Rho kinase downregulates the expression of endothelial nitric oxide synthase (eNOS). This and other proteins have important roles in apoptosis, intracellular vesicular transport, cellular proliferation and differentiation, and the expression of additional membrane proteins (including cell adhesion molecules). Treatment with statins reduces prenylation and modifies several of these cellular functions, with the potential for therapeutic benefit in many neurologic diseases.2
Figure.
Summary of important biochemical pathways for statins and their reported mechanisms of action. Text boxes indicate potential mechanisms of action for the benefit of statins. eNOS indicates endothelial nitric oxide synthase; HMG-CoA, 3-hydroxy-3-methylglutaryl–coenzyme A; NMDA, N-methyl-d-aspartate; Rho GTPase, Rho family of guanosine triphosphatases; and tPA, tissue plasminogen activator.
CEREBROVASCULAR DISEASE
Stroke Prevention
Statins have a clear role in primary and secondary prevention of ischemic stroke. The reduction in LDL-C level and other atherogenic moieties decreases the risk of atherothrombotic stroke. The effects of statins on primary and secondary stroke prevention have been well established in several large clinical trials,3 although they remain underused in clinical practice.4
The protective effects of statins on ischemic stroke prevention seem in part to be unrelated to their effect on the plasma lipid panel, as clinical trial data have supported the protective effect of statins independent of dyslipidemia correction.3 The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) study5 enrolled subjects with a minimum LDL-C level of 100 mg/dL and a mean level of 140 mg/dL (to convert cholesterol level to millimoles per liter, multiply by 0.0259); there was a 16% reduction in the risk of stroke with high-dose atorvastatin calcium, an effect that was independent of baseline LDL-C levels. Statins have also been shown to reduce levels of Lp(a) 1, which has been implicated as an inhibitor of tissue plasminogen.6 Therefore, Lp(a) 1 may promote not only atherosclerosis but also thrombosis.7
Acute Ischemic Stroke
Statins could act as neuroprotectants through several mechanisms.8 The inflammatory cascade has an important role in ischemic stroke via the immune response to brain infarction. Statins suppress the upregulation of major histocompatibility complex class II expression,9 inhibit inflammatory cell migration into the central nervous system,10 and reduce inflammatory biomarkers such as C-reactive protein in a cholesterol-independent manner. 11 Statins also modulate endothelial function, with several studies demonstrating that statins enhance NO production. Endothelial NOS is inhibited by the presence of oxidized LDL-C12; the latter can be reduced by treatment with statins. In addition, statins reduce Rho kinase activity in a cholesterol-independent manner, which further improves eNOS expression.13 The production of NO may affect cerebrovascular disease by enhancing vascular smooth muscle relaxation and by increasing cerebral blood flow.14 In addition, NO may be protective through inhibition of platelet aggregation15 and through impairment of leukocyte adhesion receptor expression, reducing recruitment of inflammatory cells.16 In animal models of stroke, treatment with statins before and up to 3 hours after stroke has resulted in reduced infarct size, a result that was mediated in part by eNOS expression. 17,18 Statins have also been shown to reduce inflammation and to increase angiogenesis, synaptogenesis, and neurogenesis when started up to 24 hours after stroke.19 In addition, the reduction of membrane cholesterol synthesis in neurons may render them more resistant to glutamate N-methyl-d-aspartate receptor–mediated excitotoxic effects, one of the principal mechanisms of neuronal death in ischemic brain.20
The benefits of statins in ischemic stroke seem also to be related to modulation of platelet function,21 the coagulation cascade,22 and increased fibrinolysis.23 The anti-inflammatory properties of statins modulate the initiation of the coagulation cascade, although there are other important mechanisms by which thrombus formation can be blunted such as upregulation of tissue plasminogen activator,23 inhibition of plasminogen activator inhibitor, 6 and reduction in Lp(a) 1 levels.7
In observational investigations, patients already taking statins at the time of their stroke have lower likelihoods of mortality, poor functional outcome, and worsening after their initial event.24 Discontinuation of statins after ischemic stroke has been associated with worse outcomes in a clinical trial.25 These results have prompted the investigation of high-dose statins as possible neuroprotective agents. A novel dose-escalating phase I study26 has indicated acceptable safety at dosages of lovastatin as high as 8 mg/kg of body weight, and a phase II study is under way.
However, prior statin use could have a deleterious effect on the risk of hemorrhagic transformation after ischemic infarction. In patients having acute ischemic stroke treated with intra-arterial thrombolysis, prior statin use was associated with a higher risk of hemorrhage while plasma cholesterol level was not,27 although there was no effect on clinical outcomes. Studies on cerebrovascular disease, especially in the acute setting, will need to consider possible effects on the development of intracerebral hemorrhage (ICH) and hemorrhagic transformation.
Intracerebral Hemorrhage
The association between plasma lipid components and the risk for ICH seems to be opposite that for ischemic stroke.28 In the SPARCL study,5 which enrolled patients with ischemic stroke and patients with ICH, statin use was associated with increased risk of ICH, particularly in patients with ICH at enrollment, although greater reduction in LDL-C level was unassociated with increased risk of ICH. However, there may be some benefits to treatment with statins in the setting of ICH. In a retrospective review of 312 patients with ICH, investigators found that 89 patients who were taking statins before the event were more likely to have a milder stroke, be discharged home, and have less functional disability.29 Another group, however, found that although hematoma volumes were lower in patients taking statins, there was no difference in functional outcomes at 3 months.30
Subarachnoid Hemorrhage
Observational studies among patients with subarachnoid hemorrhage (SAH) provide evidence that those who were taking statins at the time of the hemorrhage have better outcomes. The mechanisms of action in statins for preventing SAH-related complications could include their anti-inflammatory properties, inhibition of leukocyte migration, and induction of eNOS. In an observational study,31 patients who were taking statins on admission had a lower risk of vasospasm and delayed cerebral infarction, with no clear effect on long-term functional outcomes. This finding was not replicated by another group in a retrospective review of outcomes before and after routinely administering simvastatin (80 mg) for 14 days32 and in 1 clinical trial using the same statin in 32 participants. 33 In a pilot clinical trial, 39 patients with aneurysmal SAH were randomized to receive simvastatin (80 mg) vs placebo, with a primary clinical outcome of symptomatic vasospasm. Symptomatic vasospasm and reduced middle cerebral artery velocities were observed in the statin treatment arm, with no observed liver or muscle safety end points.34 Another small trial of patients with Fisher grade III SAH who were not previously taking statins found that fewer subjects met the primary outcome of death or drug morbidity in the statin treatment arm (1 of 20 vs 4 of 21 patients), with a trend toward improvement in angiographic and clinically apparent vasospasm.35 Whether statins should be started in all patients with SAH to prevent vasospasm and delayed cerebral ischemia remains to be established by larger clinical trials, although a recent meta-analysis failed to show a benefit of statins for prevention of vasospasm, delayed cerebral ischemia, or mortality.36
NEURODEGENERATIVE CONDITIONS
Alzheimer Disease
Statins may decrease the risk of Alzheimer disease (AD) via their effects on dyslipidemia and on the development of cerebrovascular disease, which contributes to AD risk. Statins may also decrease AD risk through their direct effects on neurodegeneration.
The possible protective effects of statins have been noted in several observational studies. Statins have been associated with reduced neurofibrillary tangle burden at autopsy37 and with lower risk of AD in case-control investigations, an effect that seemed to be independent of the plasma lipid profile.38 However, these studies were retrospective, and the selection of cases and controls could have been biased such that patients who are prescribed statins are more likely to have other known protective conditions against AD (such as higher socioeconomic status or educational achievement). In the prospective Cardiovascular Health Study,39 statin use was associated with a slower rate of decline in Mini-Mental State Examination (MMSE) scores independent of serum cholesterol panels; however, statin use was unassociated with the risk incident dementia.40
Large clinical trials have been limited to studies designed to test reduced vascular outcomes in which cognitive measures were also obtained. In the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER),41 subjects (n = 5804) with preexisting vascular disease were randomized to receive pravastatin sodium vs placebo,with an initial outcome hypothesis of major vascular events. Subjects had multiple measures of cognitive function over a mean follow-up period of 42 months. The investigators found no effect of pravastatin use on any of the cognitive outcome measures, although no measures were specific to AD and the sample had no predefined mild cognitive impairment or AD (the mean MMSE score was 28, and subjects with an MMSE score of <24 were excluded from further study). In the Medical Research Council/British Heart Foundation Heart Protection Study,42 use of simvastatin (40 mg) vs placebo resulted in no change in the incidence of dementia or alteration in the Telephone Interview for Cognitive Status score.
Fewer trials have been performed among patients who are at high risk or who already have AD. The Alzheimer’s Disease Cholesterol-Lowering Treatment trial randomized 67 patients with mild or moderate AD based on an MMSE score of 12 to 28 to receive atorvastatin (80 mg) or placebo.43 The primary outcome of the study was a change in the Alzheimer Disease Assessment Scale–Cognitive subscale score after 6 months, and secondary outcomes included a change in 6-month MMSE scores and circulating serum cholesterol levels. Overall, treatment with atorvastatin was associated with a 3.5-point improvement at 6 months compared with placebo, although the benefit seemed greatest for individuals with a total cholesterol level exceeding 200 mg/dL and for those who harbored an apolipoprotein E 4 allele. The magnitude of change is similar to that in trials for acetylcholinesterase inhibitors, whereby a 4-point change is considered positive and clinically meaningful in the sense that patients who improve their score seem unlikely to have score decreases on other dementia scales.44 This was a small randomized trial that excluded individuals being treated for depression or those previously taking statins, did not measure decreases in other scale scores or in the incidence of dementia, and needs to be replicated. The Lipitor’s Effect in Alzheimer Dementia study enrolled 640 subjects already taking donepezil hydrochloride (10 mg) and randomized subjects in a double-blind fashion to receive atorvastatin (80 mg) vs placebo with a baseline LDL-C of 95 to 195 mg/dL.45 The investigators failed to find a benefit in cognition (as measured by the Alzheimer Disease Assessment Scale–Cognitive subscale) or global function (as measured by the Alzheimer’s Disease Cooperative Study Clinical Global Impression of Change).
The mechanisms of a potential statin benefit on AD remain uncertain. Intracellular cholesterol levels may influence the production of amyloid precursor protein such that the production of amyloid β (Aβ) protein is increased. Whether statins can affect the intracellular cholesterol levels or modulate the pathologic processes in AD remains controversial. One study46 group examined whether simvastatin (80 mg) was effective in reducing cerebrospinal fluid levels of Aβ-40 or Aβ-42 compared with placebo over 26 weeks among subjects with AD and normal cholesterol levels. There was no significant difference in the cerebrospinal fluid levels of either Aβ moiety, although there was a trend toward reduced Aβ-40 levels in secondary analyses, especially among subjects with an MMSE score of 21 to 26. It is possible that many observed protective effects of statins are in fact due to modulation of vascular disease on dementia. It remains to be proven that statins have usefulness in the treatment or prevention of AD, whether certain genetic mutations could modify these effects, and if any such effects depend on the lipid solubility of particular statins.
Parkinson Disease
Preclinical investigations have indicated a potential role for inflammation, mitochondrial dysfunction, and free radical formation in the pathogenesis of Parkinson disease.47 Therefore, the anti-inflammatory properties of statins could be useful to prevent worsening of the disease, perhaps by decreased activation of microglia and by the loss of dopaminergic neurons due to free radical formation.48
Epidemiologic investigations have revealed an association between low LDL-C levels and the risk of Parkinson disease.49 However, clinical studies to date have been unable to draw definitive conclusions. An extensive review of patients in the Veterans Affairs health care system found that simvastatin (but not atorvastatin or lovastatin) use was associated with lower risk of Parkinson disease,50 while another study51 found a strong protective effect from any statin use, especially when used for more than 5 years. The authors of the latter study cautioned against drawing any conclusions of causality, as the observed effect of statins could instead have been related to the possibility that patients with high LDL-C levels were prescribed these agents. There are no clear indications for the routine use of statins in patients with Parkinson disease.
Multiple Sclerosis
The anti-inflammatory properties of statins generated interest in their being potentially effective in multiple sclerosis (MS). Modulation of major histocompatibility complex class II expression and T-cell adhesion molecules, reduction in B-cell and T-cell chemokine receptors, decrease in natural killer cell activity, and inhibition of proinflammatory cytokine release by microglia and astrocytes have been proposed as effects of statins that could be biologically useful in MS.52 On the other hand, in vivo and in vitro models have indicated that statins may impair remyelination via inhibition of oligodendrocyte maturation from progenitor cells53 or via myelin formation in mature oligodendrocytes.54 In the experimental allergic encephalomyelitis rat model for human MS, statins are associated with clinical improvement and with reduced pathologic changes.55
A phase II open-label study56 of 28 subjects with treatment-naive relapsing-remitting MS showed a statistically significant reduction in the number and volume of new enhancing lesions on magnetic resonance imaging with simvastatin (80 mg)when comparing the 3-month lead-in phase with the 3-month postmedication phase, with no difference in safety outcomes. This finding was replicated by another group administering atorvastatin (80 mg) in 41 subjects, of whom 16 were taking interferon beta.57 The number of enhancing lesions was reduced in the 6- to 9-month treatment phase compared with the 3-month lead-in phase, and there was possible synergy with interferon beta treatment (interferon beta-1a [22 µg 3 times weekly] or interferon beta-1b [dosage not specified]) such that the benefit was primarily found in patients receiving both statins and interferon therapy. A retrospective review of statin use for dyslipidemia among patients with MS in the Safety and Efficacy of Natalizumab in Combination With Interferon Beta-1a in Patients With Relapsing-Remitting Multiple Sclerosis (SENTINEL) study58 found no benefit of treatment with statins (most were taking atorvastatin or simvastatin) on disability progression, the number of enhancing lesions, or adjusted annualized relapse rate. In addition, a blinded study59 comparing atorvastatin (40 or 80 mg) vs placebo for 6 months in subjects already receiving interferon beta-1a (44 µg 3 times per week) found evidence of an increase in clinical activity and the number of new enhancing lesions in the statin arm. The reasons for the different conclusions relative to those of animal studies, as well as among these preliminary human studies, are unclear but may include differences in statin types and dosages, variations in interferon beta treatment regimens, statistical chance, and the incomplete correlation between murine experimental allergic encephalomyelitis and human MS.52 Studies are under way to examine statins in various combinations as primary or adjunct treatments, but data are incomplete to recommend statin treatment for disease modulation in MS, and caution is warranted.
CONCLUSIONS
Statins have been proposed in the treatment of multiple central nervous system diseases. Beyond primary and secondary stroke prevention, effectiveness and safety questions remain unanswered. The usefulness of statins in inflammatory and degenerative disease of the central nervous system needs to be tested further in animal models of disease and in well-designed prospective clinical trials using clinically meaningful outcomes. At this time, there is no evidence to support routine clinical use of statins except for cerebrovascular disease indications.
Footnotes
Author Contributions: Study concept and design: Willey and Elkind. Acquisition of data: Willey and Elkind. Analysis and interpretation of data: Elkind. Drafting of the manuscript: Willey. Critical revision of the manuscript for important intellectual content: Elkind. Administrative, technical, and material support: Willey. Study supervision: Elkind.
Financial Disclosure: Dr Elkind has received funding by grant P50 NS049060 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, to conduct the Neuroprotection With Statin Therapy for Acute Recovery Trial (NeuSTART), a drug development program for the use of lovastatin therapy in acute ischemic stroke.
REFERENCES
- 1.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 2.Stüve O, Youssef S, Steinman L, Zamvil SS. Statins as potential therapeutic agents in neuroinflammatory disorders. Curr Opin Neurol. 2003;16(3):393–401. doi: 10.1097/01.wco.0000073942.19076.d1. [DOI] [PubMed] [Google Scholar]
- 3.Collins R, Armitage J, Parish S, Sleight P, Peto R Heart Protection Study Collaborative Group. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004;363(9411):757–767. doi: 10.1016/S0140-6736(04)15690-0. [DOI] [PubMed] [Google Scholar]
- 4.Ovbiagele B, Saver JL, Bang H, et al. VISP Study Investigators. Statin treatment and adherence to national cholesterol guidelines after ischemic stroke. Neurology. 2006;66(8):1164–1170. doi: 10.1212/01.wnl.0000208403.18885.0e. [DOI] [PubMed] [Google Scholar]
- 5.Amarenco P, Bogousslavsky J, Callahan A, III, et al. Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 6.Bourcier T, Libby P. HMG CoA reductase inhibitors reduce plasminogen activator inhibitor-1 expression by human vascular smooth muscle and endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20(2):556–562. doi: 10.1161/01.atv.20.2.556. [DOI] [PubMed] [Google Scholar]
- 7.van Wissen S, Smilde TJ, Trip MD, de Boo T, Kastelein JJ, Stalenhoef AF. Long term statin treatment reduces lipoprotein(a) concentrations in heterozygous familial hypercholesterolaemia. Heart. 2003;89(8):893–896. doi: 10.1136/heart.89.8.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elkind MS. Statins as acute-stroke treatment. Int J Stroke. 2006;1(4):224–225. doi: 10.1111/j.1747-4949.2006.00061.x. [DOI] [PubMed] [Google Scholar]
- 9.Kuipers HF, van den Elsen PJ. Immunomodulation by statins: inhibition of cholesterol vs. isoprenoid biosynthesis. Biomed Pharmacother. 2007;61(7):400–407. doi: 10.1016/j.biopha.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Kaneider NC, Reinisch CM, Dunzendorfer S, Meierhofer C, Djanani A, Wiedermann CJ. Induction of apoptosis and inhibition of migration of inflammatory and vascular wall cells by cerivastatin. Atherosclerosis. 2001;158(1):23–33. doi: 10.1016/s0021-9150(00)00764-4. [DOI] [PubMed] [Google Scholar]
- 11.Albert MA, Danielson E, Rifai N, Ridker PM. PRINCE Investigators. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort studyPRINCE Investigators. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286(1):64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 12.Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97(12):1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 13.Liu PY, Liu YW, Lin LJ, Chen JH, Liao JK. Evidence for statin pleiotropy in humans: differential effects of statins and ezetimibe on rho-associated coiled-coil containing protein kinase activity, endothelial function, and inflammation. Circulation. 2009;119(1):131–138. doi: 10.1161/CIRCULATIONAHA.108.813311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterzer P, Meintzschel F, Rösler A, Lanfermann H, Steinmetz H, Sitzer M. Pravastatin improves cerebral vasomotor reactivity in patients with subcortical small-vessel disease. Stroke. 2001;32(12):2817–2820. doi: 10.1161/hs1201.099663. [DOI] [PubMed] [Google Scholar]
- 15.Roberts W, Riba R, Homer-Vanniasinkam S, Farndale RW, Naseem KM. Nitric oxide specifically inhibits integrin-mediated platelet adhesion and spreading on collagen. J Thromb Haemost. 2008;6(12):2175–2185. doi: 10.1111/j.1538-7836.2008.03190.x. [DOI] [PubMed] [Google Scholar]
- 16.De Caterina R, Libby P, Peng HB, et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96(1):60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engelhorn T, Doerfler A, Heusch G, Schulz R. Reduction of cerebral infarct size by the AT1-receptor blocker candesartan, the HMG-CoA reductase inhibitor rosuvastatin and their combination: an experimental study in rats. Neurosci Lett. 2006;406(1–2):92–96. doi: 10.1016/j.neulet.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 18.Sironi L, Cimino M, Guerrini U, et al. Treatment with statins after induction of focal ischemia in rats reduces the extent of brain damage. Arterioscler Thromb Vasc Biol. 2003;23(2):322–327. doi: 10.1161/01.atv.0000044458.23905.3b. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Zhang ZG, Li Y, et al. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53(6):743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 20.Zacco A, Togo J, Spence K, et al. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors protect cortical neurons from excitotoxicity. J Neurosci. 2003;23(35):11104–11111. doi: 10.1523/JNEUROSCI.23-35-11104.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obi C, Wysokinski W, Karnicki K, Owen WG, McBane RD., II Inhibition of platelet-rich arterial thrombus in vivo: acute antithrombotic effect of intravenous HMG-CoA reductase therapy. Arterioscler Thromb Vasc Biol. 2009;29(9):1271–1276. doi: 10.1161/ATVBAHA.109.190884. [DOI] [PubMed] [Google Scholar]
- 22.Laufs U, Gertz K, Huang P, et al. Atorvastatin upregulates type III nitric oxide synthase in thrombocytes, decreases platelet activation, and protects from cerebral ischemia in normocholesterolemic mice. Stroke. 2000;31(10):2442–2449. doi: 10.1161/01.str.31.10.2442. [DOI] [PubMed] [Google Scholar]
- 23.Asahi M, Huang Z, Thomas S, et al. Protective effects of statins involving both eNOS and tPA in focal cerebral ischemia. J Cereb Blood Flow Metab. 2005;25(6):722–729. doi: 10.1038/sj.jcbfm.9600070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elkind MS, Flint AC, Sciacca RR, Sacco RL. Lipid-lowering agent use at ischemic stroke onset is associated with decreased mortality. Neurology. 2005;65(2):253–258. doi: 10.1212/01.wnl.0000171746.63844.6a. [DOI] [PubMed] [Google Scholar]
- 25.Blanco M, Nombela F, Castellanos M, et al. Statin treatment withdrawal in ischemic stroke: a controlled randomized study. Neurology. 2007;69(9):904–910. doi: 10.1212/01.wnl.0000269789.09277.47. [DOI] [PubMed] [Google Scholar]
- 26.Elkind MS, Sacco RL, MacArthur RB, et al. The Neuroprotection with Statin Therapy for Acute Recovery Trial (NeuSTART): an adaptive design phase I dose-escalation study of high-dose lovastatin in acute ischemic stroke. Int J Stroke. 2008;3(3):210–218. doi: 10.1111/j.1747-4949.2008.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meier N, Nedeltchev K, Brekenfeld C, et al. Prior statin use, intracranial hemorrhage, and outcome after intra-arterial thrombolysis for acute ischemic stroke. Stroke. 2009;40(5):1729–1737. doi: 10.1161/STROKEAHA.108.532473. [DOI] [PubMed] [Google Scholar]
- 28.Ariesen MJ, Claus SP, Rinkel GJ, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003;34(8):2060–2065. doi: 10.1161/01.STR.0000080678.09344.8D. [DOI] [PubMed] [Google Scholar]
- 29.Leker RR, Khoury ST, Rafaeli G, Shwartz R, Eichel R, Tanne D NASIS Investigators. Prior use of statins improves outcome in patients with intracerebral hemorrhage: prospective data from the National Acute Stroke Israeli Surveys (NASIS) Stroke. 2009;40(7):2581–2584. doi: 10.1161/STROKEAHA.108.546259. [DOI] [PubMed] [Google Scholar]
- 30.Eichel R, Khoury ST, Ben-Hur T, Keidar M, Paniri R, Leker RR. Prior use of statins and outcome in patients with intracerebral haemorrhage. Eur J Neurol. 2010;17(1):78–83. doi: 10.1111/j.1468-1331.2009.02747.x. [DOI] [PubMed] [Google Scholar]
- 31.Parra A, Kreiter KT, Williams S, et al. Effect of prior statin use on functional outcome and delayed vasospasm after acute aneurysmal subarachnoid hemorrhage: a matched controlled cohort study. Neurosurgery. 2005;56(3):476–484. doi: 10.1227/01.neu.0000153925.96889.8a. [DOI] [PubMed] [Google Scholar]
- 32.Kramer AH, Gurka MJ, Nathan B, Dumont AS, Kassell NF, Bleck TP. Statin use was not associated with less vasospasm or improved outcome after subarachnoid hemorrhage. Neurosurgery. 2008;62(2):422–430. doi: 10.1227/01.neu.0000316009.19012.e3. [DOI] [PubMed] [Google Scholar]
- 33.Vergouwen MD, Meijers JC, Geskus RB, et al. Biologic effects of simvastatin in patients with aneurysmal subarachnoid hemorrhage: a double-blind, placebocontrolled randomized trial. J Cereb Blood Flow Metab. 2009;29(8):1444–1453. doi: 10.1038/jcbfm.2009.59. [DOI] [PubMed] [Google Scholar]
- 34.Lynch JR, Wang H, McGirt MJ, et al. Simvastatin reduces vasospasm after aneurysmal subarachnoid hemorrhage: results of a pilot randomized clinical trial. Stroke. 2005;36(9):2024–2026. doi: 10.1161/01.STR.0000177879.11607.10. [DOI] [PubMed] [Google Scholar]
- 35.Chou SH, Smith EE, Badjatia N, et al. A randomized, double-blind, placebo-controlled pilot study of simvastatin in aneurysmal subarachnoid hemorrhage. Stroke. 2008;39(10):2891–2893. doi: 10.1161/STROKEAHA.107.505875. [DOI] [PubMed] [Google Scholar]
- 36.Vergouwen MD, de Haan RJ, Vermeulen M, Roos YB. Effect of statin treatment on vasospasm, delayed cerebral ischemia, and functional outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis update. Stroke. 2010;41(1):e47–e52. doi: 10.1161/STROKEAHA.109.556332. [DOI] [PubMed] [Google Scholar]
- 37.Li G, Larson EB, Sonnen JA, et al. Statin therapy is associated with reduced neuropathologic changes of Alzheimer disease. Neurology. 2007;69(9):878–885. doi: 10.1212/01.wnl.0000277657.95487.1c. [DOI] [PubMed] [Google Scholar]
- 38.Dufouil C, Richard F, Fiévet N, et al. APOE genotype, cholesterol level, lipid-lowering treatment, and dementia: the Three-City Study. Neurology. 2005;64(9):1531–1538. doi: 10.1212/01.WNL.0000160114.42643.31. [DOI] [PubMed] [Google Scholar]
- 39.Bernick C, Katz R, Smith NL, et al. Cardiovascular Health Study Collaborative Research Group. Statins and cognitive function in the elderly: the Cardiovascular Health Study. Neurology. 2005;65(9):1388–1394. doi: 10.1212/01.wnl.0000182897.18229.ec. [DOI] [PubMed] [Google Scholar]
- 40.Rea TD, Breitner JC, Psaty BM, et al. Statin use and the risk of incident dementia: the Cardiovascular Health Study. Arch Neurol. 2005;62(7):1047–1051. doi: 10.1001/archneur.62.7.1047. [DOI] [PubMed] [Google Scholar]
- 41.Trompet S, van Vliet P, de Craen AJ, et al. Pravastatin and cognitive function in the elderly: results of the PROSPER study. J Neurol. 2010;257(1):85–90. doi: 10.1007/s00415-009-5271-7. [DOI] [PubMed] [Google Scholar]
- 42.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 43.Sparks DL, Connor DJ, Sabbagh MN, Petersen RB, Lopez J, Browne P. Circulating cholesterol levels, apolipoprotein E genotype and dementia severity influence the benefit of atorvastatin treatment in Alzheimer’s disease: results of the Alzheimer’s Disease Cholesterol-Lowering Treatment (ADCLT) trial. Acta Neurol Scand Suppl. 2006;185:3–7. doi: 10.1111/j.1600-0404.2006.00690.x. [DOI] [PubMed] [Google Scholar]
- 44.Rockwood K, Fay S, Gorman M, Carver D, Graham JE. The clinical meaningfulness of ADAS-Cog changes in Alzheimer’s disease patients treated with donepezil in an open-label trial. BMC Neurol. 2007;7:26. doi: 10.1186/1471-2377-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feldman HH, Doody RS, Kivipelto M, et al. LEADe Investigators. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology. 2010;74(12):956–964. doi: 10.1212/WNL.0b013e3181d6476a. [DOI] [PubMed] [Google Scholar]
- 46.Simons M, Schwärzler F, Lütjohann D, et al. Treatment with simvastatin in normocholesterolemic patients with Alzheimer’s disease: a 26-week randomized, placebo-controlled, double-blind trial. Ann Neurol. 2002;52(3):346–350. doi: 10.1002/ana.10292. [DOI] [PubMed] [Google Scholar]
- 47.Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol. 2008;4(11):600–609. doi: 10.1038/ncpneuro0924. [DOI] [PubMed] [Google Scholar]
- 48.Selley ML. Simvastatin prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced striatal dopamine depletion and protein tyrosine nitration in mice. Brain Res. 2005;1037(1–2):1–6. doi: 10.1016/j.brainres.2004.02.083. [DOI] [PubMed] [Google Scholar]
- 49.de Lau LM, Stricker BH, Breteler MM. Serum cholesterol, use of lipid-lowering drugs, and risk of Parkinson disease. Mov Disord. 2007;22(13):1985. doi: 10.1002/mds.21582. [DOI] [PubMed] [Google Scholar]
- 50.Wolozin B, Wang SW, Li NC, Lee A, Lee TA, Kazis LE. Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease. BMC Med. 2007;5(20) doi: 10.1186/1741-7015-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wahner AD, Bronstein JM, Bordelon YM, Ritz B. Statin use and the risk of Parkinson disease. Neurology. 2008;70(16, pt 2):1418–1422. doi: 10.1212/01.wnl.0000286942.14552.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldman MD, Cohen JA. Statins to treat multiple sclerosis: friend or foe? Neurology. 2008;71(18):1386–1387. doi: 10.1212/01.wnl.0000327876.72639.e7. [DOI] [PubMed] [Google Scholar]
- 53.Miron VE, Zehntner SP, Kuhlmann T, et al. Statin therapy inhibits remyelination in the central nervous system. Am J Pathol. 2009;174(5):1880–1890. doi: 10.2353/ajpath.2009.080947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klopfleisch S, Merkler D, Schmitz M, et al. Negative impact of statins on oligodendrocytes and myelin formation in vitro and in vivo. J Neurosci. 2008;28(50):13609–13614. doi: 10.1523/JNEUROSCI.2765-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Youssef S, Stüve O, Patarroyo JC, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420(6911):78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 56.Vollmer T, Key L, Durkalski V, et al. Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet. 2004;363(9421):1607–1608. doi: 10.1016/S0140-6736(04)16205-3. [DOI] [PubMed] [Google Scholar]
- 57.Paul F, Waiczies S, Wuerfel J, et al. Oral high-dose atorvastatin treatment in relapsing-remitting multiple sclerosis. PLoS ONE. 2008;3(4):e1928. doi: 10.1371/journal.pone.0001928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rudick RA, Pace A, Rani MR, et al. Effect of statins on clinical and molecular responses to intramuscular interferon beta-1a. Neurology. 2009;72(23):1989–1993. doi: 10.1212/WNL.0b013e3181a92b96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Birnbaum G, Cree B, Altafullah I, Zinser M, Reder AT. Combining beta interferon and atorvastatin may increase disease activity in multiple sclerosis. Neurology. 2008;71(18):1390–1395. doi: 10.1212/01.wnl.0000319698.40024.1c. [DOI] [PubMed] [Google Scholar]