Abstract
Procedures for the synthesis of thirty-six 5-methyl-3-(substituted)-[1,2,4]triazines have been described. These compounds were evaluated for antagonism at metabotropic glutamate receptor subtype 5. Two compounds, 5b and 3c, were determined to be low micromolar inhibitors of mGluR5.
Keywords: Triazine, mGluR5 Antagonist, Addiction, Heterocyclic synthesis
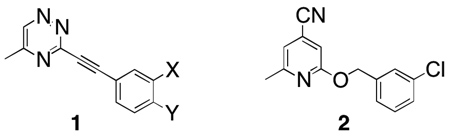
There are several studies that show that metabotropic glutamate receptor subtype 5 (mGluR5) plays a role in central nervous system disorders including addiction, pain, fragile X syndrome, and anxiety.1–5 In a previous study, we discovered that 3-(substituted phenylethynyl)-5-methy[1,2,4]triazines (1) were potent antagonists of glutamate-mediated mobilization of internal calcium in an mGluR5 in vitro efficacy assay.6
In 2006, Buttelmann et al.7 reported that 2 had Ki of 1 nM in an mGluR5 binding assay and was active in an anxiolytic test after oral administration. We, therefore, considered it would be of interest to synthesize and evaluate triazine analogues of compound 2 (3a–f and 4a–f) for antagonism at mGluR5. In addition, we prepared analogues 5a–f, 6a–f, 7a–f, and 8a–f where the oxygen atom was replaced by a sulfur or amino group.
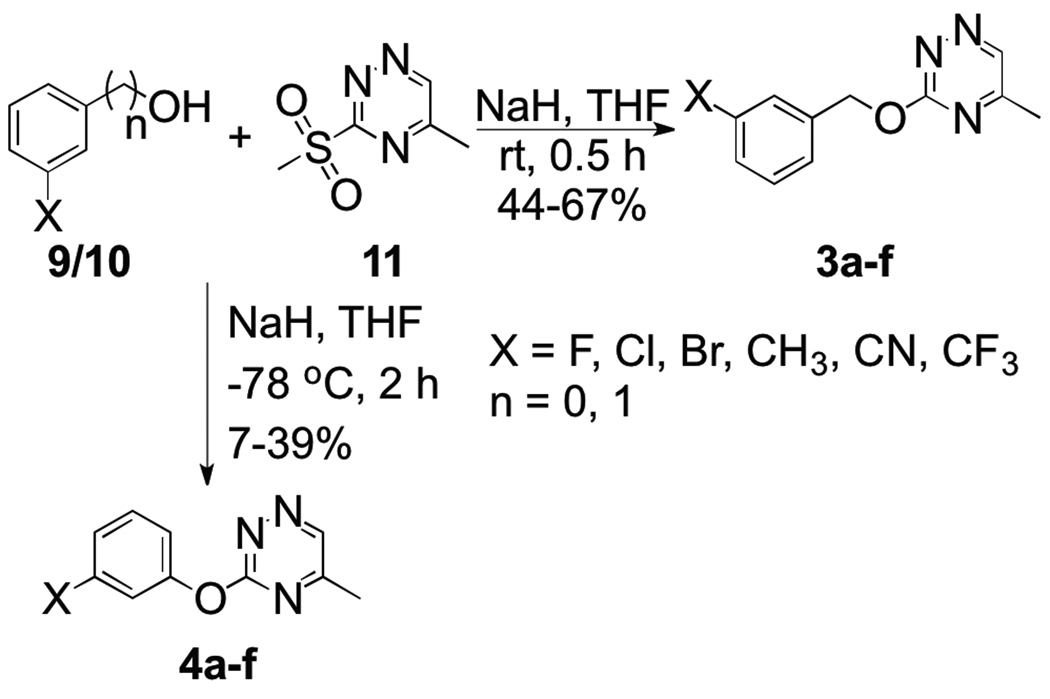
The phenoxy and benzyloxy substituted methyl-[1,2,4]triazines were synthesized from the corresponding phenols (9a–f) and benzyl alcohols (10a–f), which were first deprotonated with sodium hydride in THF and added to 5-methyl-3-sulfonyl[1,2,4]triazine (11) (Scheme 1). 5-Methyl-3-sulfonyl[1,2,4]triazine (11) was prepared as described previously.6 The products 3a–f and 4a–f were isolated in moderate yields after chromatographic separation.
Scheme 1.
Synthesis of substituted phenoxy and benzyloxy methyl[1,2,4]triazines
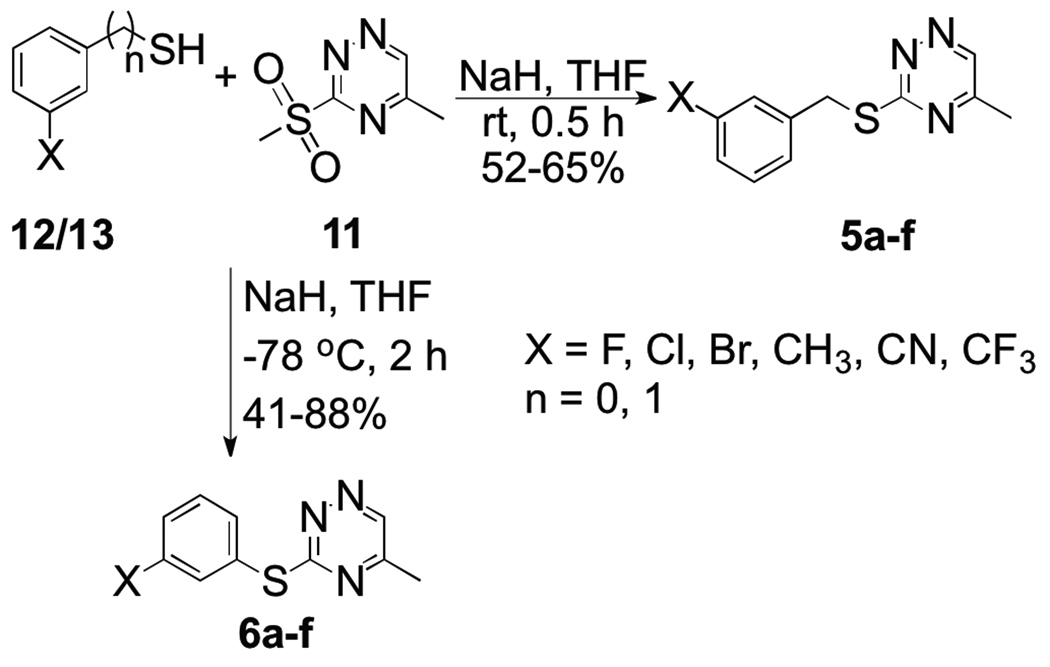
The thio-linked compounds 5a–f and 6a–f were synthesized after deprotonation of the corresponding thiols (12a–f) and benzyl thiols (13a–f) with sodium hydride in THF followed by displacement of the methylsulfonyl group in 11 (Scheme 2).
Scheme 2.
Synthesis of thiophenol and benzylthiol substituted methyl[1,2,4]triazines
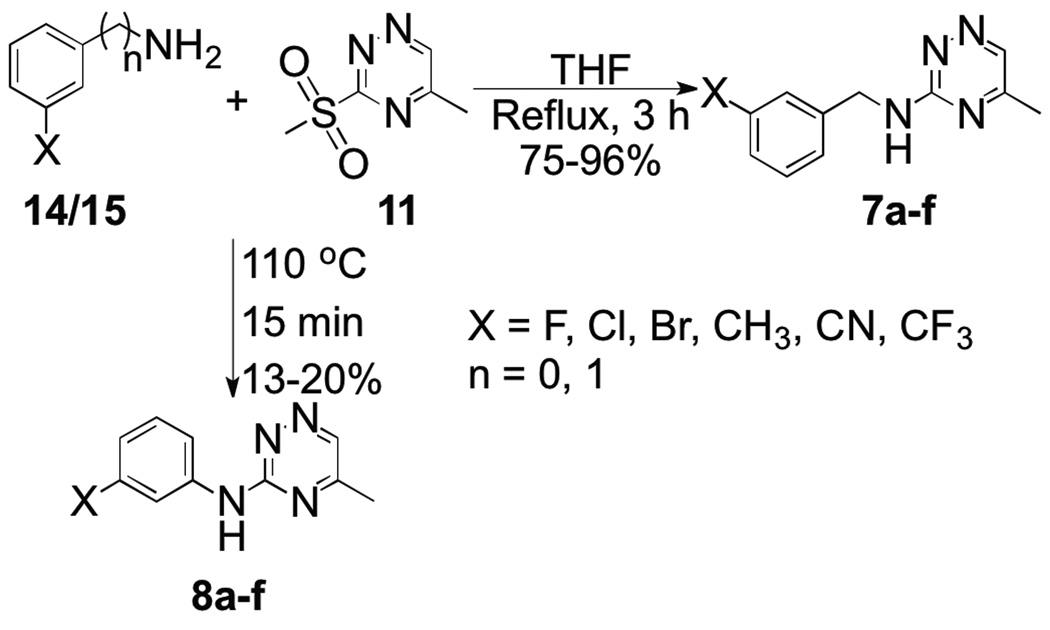
Preparation of either aniline derivatives or benzyl amines did not require basic conditions to facilitate the displacement of the methylsulfonyl group in 11 (Scheme 3). Benzyl amines (14a–f) reacted rapidly in refluxing THF to provide the desired analogues (7a–f) in good yields. Reaction of various substituted anilines (15a–f) with 11 did not occur in refluxing THF but the desired products (8a–f) could be isolated by heating the reactants without solvent at 110 °C under N2 blanket after chromatographic separation. The low yields (13 – 20%) were as a result of decomposition of 11 under heat.
Scheme 3.
Synthesis of aniline and benzylamine substituted methyl[1,2,4]triazines
Fluorimetric Ca2+/flux assay was used to evaluate the compounds for antagonism at mGluR5. Compounds were screened for in vitro efficacy using CHO-K1 cells stably transfected with the human mGluR5 cells. Through the screening process it was found that 5b, 5f, and 3c were weakly to moderately active, producing >35% inhibition of calcium mobilization in CHOK1-mGluR5 cells. Of these three compounds, the two compounds that showed higher levels of inhibition were reevaluated to determine the IC50 values in a calcium efflux assay (Table 1). Compounds 5b and 3c were determined to be low micromolar inhibitors of mGluR5.
Table 1.
Selected Inhibition of Human mGluR5-Mediated Intracellular Calcium Mobilization for Heterocyclic-Linked Analogues
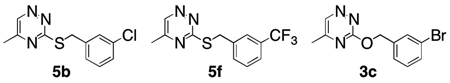 | |||
|---|---|---|---|
| Compound | 5b | 5f | 3c |
| % inhibition at 10 µM | 44 | 35 | 51 |
| IC50 (µM) | 13.4 ± 2.3 | n.d. | 15.2 ± 4.0 |
Supplementary Material
Acknowledgments
This research was supported by the National Institute on Drug Abuse grant DA05477.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Experimental details including 1H NMR, 13C NMR, MS, and elemental analysis data and calcium mobilization assay procedure are provided.
References and notes
- 1.Li X, Need AB, Baez M, Witkin JM. Metabotropic Glutamate 5 Receptor Antagonism Is Associated with Antidepressant-Like Effects in Mice. Journal of Pharmacology and Experimental Therapeutics. 2006;319(1):254–259. doi: 10.1124/jpet.106.103143. [DOI] [PubMed] [Google Scholar]
- 2.Tatarczyńska E, Kłodzińska A, Chojnacka-Wójcik E, Pałucha A, Gasparini F, Kuhn R, Pilc A. Potential anxiolytic- and antidepressant-like effects of MPEP, a potent, selective and systemically active mGlu5 receptor antagonist. British Journal of Pharmacology. 2001;132(7):1423–1430. doi: 10.1038/sj.bjp.0703923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4(9):873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- 4.Bear MF. Therapeutic implications of the mGluR theory of fragile X mental retardation. Genes, Brain and Behavior. 2005;4(6):393–398. doi: 10.1111/j.1601-183X.2005.00135.x. [DOI] [PubMed] [Google Scholar]
- 5.Dolan S, Kelly JG, Monteiro AM, Nolan AM. Up-regulation of metabotropic glutamate receptor subtypes 3 and 5 in spinal cord in a clinical model of persistent inflammation and hyperalgesia. Pain. 2003;106(3):501–512. doi: 10.1016/j.pain.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Carroll FI, Kotturi SV, Navarro HA, Mascarella SW, Gilmour BP, Smith FL, Gabra BH, Dewey WL. Synthesis and pharmacological evaluation of phenylethynyl[1,2,4]methyltriazines as analogues of 3-methyl-6-(phenylethynyl)pyridine. J. Med. Chem. 2007;50:3388–3391. doi: 10.1021/jm070078r. [DOI] [PubMed] [Google Scholar]
- 7.Buttelmann B, Peters JU, Ceccarelli S, Kolczewski S, Vieira E, Prinssen EP, Spooren W, Schuler F, Huwyler J, Porter RH, Jaeschke G. Arylmethoxypyridines as novel, potent and orally active mGlu5 receptor antagonists. Bioorg. Med. Chem. Lett. 2006;16:1892–1897. doi: 10.1016/j.bmcl.2005.12.088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.