Abstract
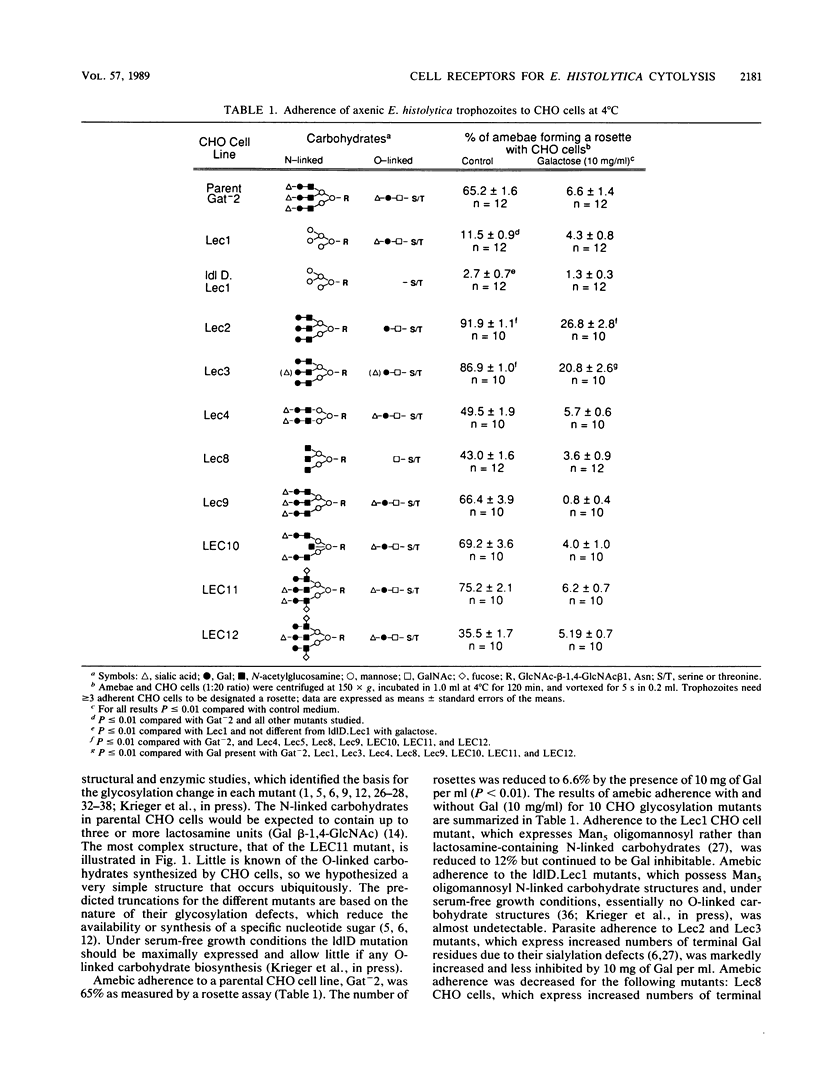
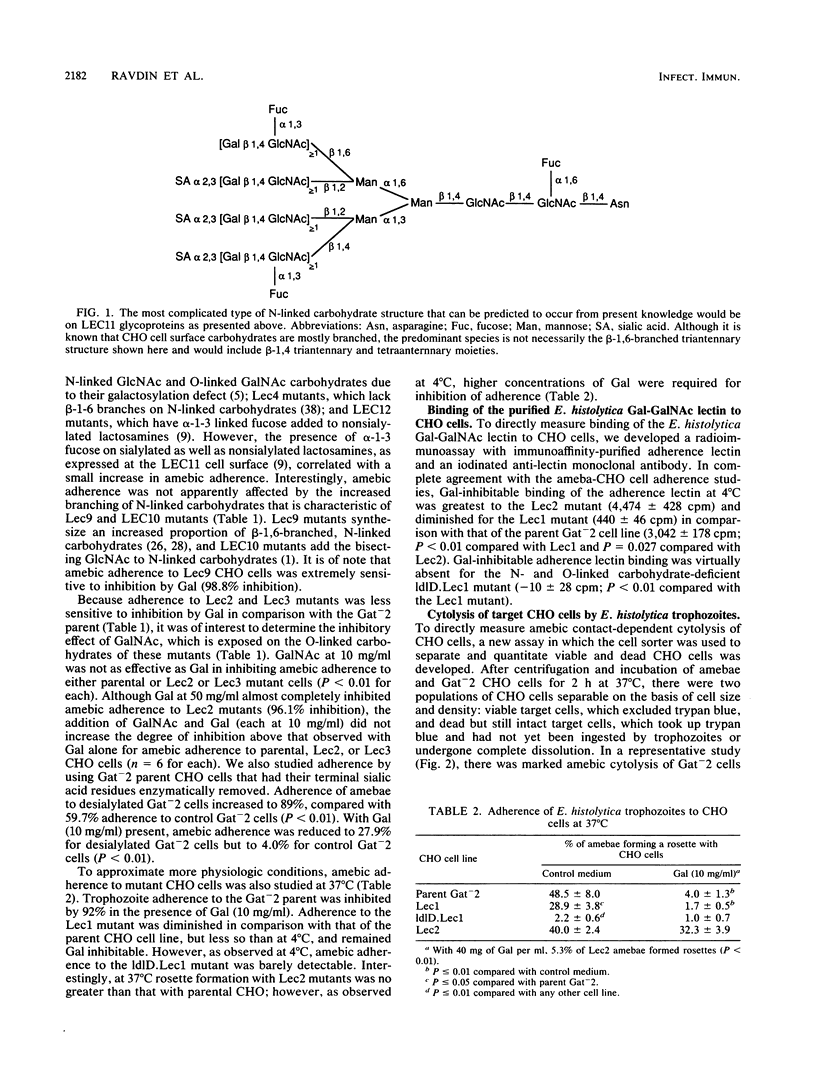
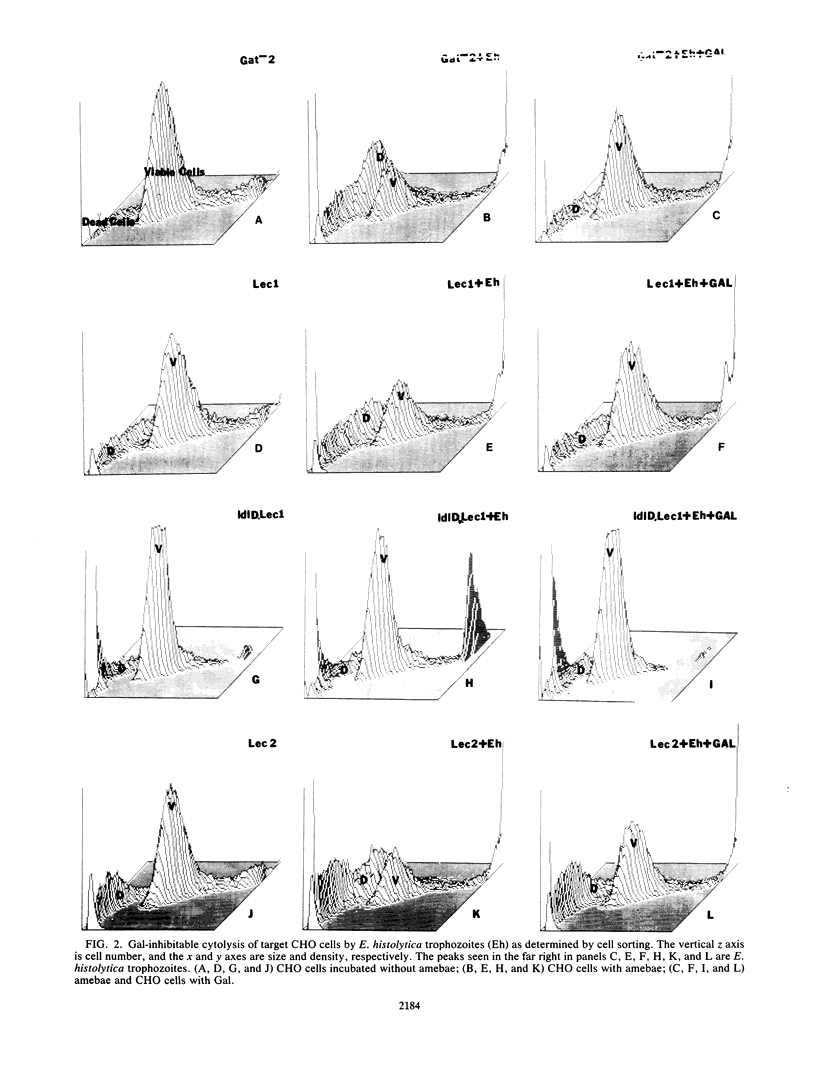
Binding and cytolysis of Chinese hamster ovary (CHO) cells by Entamoeba histolytica trophozoites is inhibitable by galactose (Gal) or N-acetyl-D-galactosamine (GalNAc). To better define the carbohydrate receptor for E. histolytica, we compared the binding and cytolytic target properties of 10 CHO glycosylation mutants. Each mutant expresses a uniquely altered array of N- and/or O-linked cell surface carbohydrates. Amebic adherence was reduced when lactosamine-containing N-linked carbohydrates were essentially absent (Lec1 mutant), almost undetectable when Gal and GalNAc residues were absent on both N- and O-linked carbohydrates (ldlD.Lec1 mutant), and enhanced for mutants with increased terminal Gal residues (Lec2 and Lec3). Parental CHO cells treated with neuraminidase to expose Gal residues behaved like Lec2 mutants. Binding of purified Gal or GalNAc lectin to parental, Lec1, ldlD.Lec1, and Lec2 mutant CHO cells corroborated the adherence results. The suitability of CHO cell mutants as targets for amebic cytolysis correlated with their glycosylation phenotype: the Lec1 mutants were less susceptible than parental CHO cells, the ldlD.Lec1 mutants were highly resistant, and the Lec2 mutants required higher concentrations of Gal for inhibition. The E. histolytica Gal or GalNAc adherence lectin bound preferentially to beta 1-6-branched, N-linked carbohydrates lacking terminal sialic acid or fucose residues. However, amebic lectin binding to either N- or O-linked cell surface carbohydrates was sufficient to initiate parasite cytolytic activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell C., Stanley P. A dominant mutation to ricin resistance in Chinese hamster ovary cells induces UDP-GlcNAc:glycopeptide beta-4-N-acetylglucosaminyltransferase III activity. J Biol Chem. 1984 Nov 10;259(21):13370–13378. [PubMed] [Google Scholar]
- Chadee K., Johnson M. L., Orozco E., Petri W. A., Jr, Ravdin J. I. Binding and internalization of rat colonic mucins by the galactose/N-acetyl-D-galactosamine adherence lectin of Entamoeba histolytica. J Infect Dis. 1988 Aug;158(2):398–406. doi: 10.1093/infdis/158.2.398. [DOI] [PubMed] [Google Scholar]
- Chadee K., Meerovitch E. The Mongolian gerbil (Meriones unguiculatus) as an experimental host for Entamoeba histolytica. Am J Trop Med Hyg. 1984 Jan;33(1):47–54. doi: 10.4269/ajtmh.1984.33.47. [DOI] [PubMed] [Google Scholar]
- Chadee K., Petri W. A., Jr, Innes D. J., Ravdin J. I. Rat and human colonic mucins bind to and inhibit adherence lectin of Entamoeba histolytica. J Clin Invest. 1987 Nov;80(5):1245–1254. doi: 10.1172/JCI113199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher S. L., Hirschberg C. B. Mechanism of galactosylation in the Golgi apparatus. A Chinese hamster ovary cell mutant deficient in translocation of UDP-galactose across Golgi vesicle membranes. J Biol Chem. 1986 Jan 5;261(1):96–100. [PubMed] [Google Scholar]
- Deutscher S. L., Nuwayhid N., Stanley P., Briles E. I., Hirschberg C. B. Translocation across Golgi vesicle membranes: a CHO glycosylation mutant deficient in CMP-sialic acid transport. Cell. 1984 Dec;39(2 Pt 1):295–299. doi: 10.1016/0092-8674(84)90007-2. [DOI] [PubMed] [Google Scholar]
- Diamond L. S., Harlow D. R., Cunnick C. C. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72(4):431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- Green E. D., Brodbeck R. M., Baenziger J. U. Lectin affinity high-performance liquid chromatography. Interactions of N-glycanase-released oligosaccharides with Ricinus communis agglutinin I and Ricinus communis agglutinin II. J Biol Chem. 1987 Sep 5;262(25):12030–12039. [PubMed] [Google Scholar]
- Howard D. R., Fukuda M., Fukuda M. N., Stanley P. The GDP-fucose:N-acetylglucosaminide 3-alpha-L-fucosyltransferases of LEC11 and LEC12 Chinese hamster ovary mutants exhibit novel specificities for glycolipid substrates. J Biol Chem. 1987 Dec 15;262(35):16830–16837. [PubMed] [Google Scholar]
- Joyce M. P., Ravdin J. I. Antigens of Entamoeba histolytica recognized by immune sera from liver abscess patients. Am J Trop Med Hyg. 1988 Jan;38(1):74–80. doi: 10.4269/ajtmh.1988.38.74. [DOI] [PubMed] [Google Scholar]
- Kingsley D. M., Kozarsky K. F., Hobbie L., Krieger M. Reversible defects in O-linked glycosylation and LDL receptor expression in a UDP-Gal/UDP-GalNAc 4-epimerase deficient mutant. Cell. 1986 Mar 14;44(5):749–759. doi: 10.1016/0092-8674(86)90841-x. [DOI] [PubMed] [Google Scholar]
- Li E., Becker A., Stanley S. L., Jr Chinese hamster ovary cells deficient in N-acetylglucosaminyltransferase I activity are resistant to Entamoeba histolytica-mediated cytotoxicity. Infect Immun. 1989 Jan;57(1):8–12. doi: 10.1128/iai.57.1.8-12.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E., Becker A., Stanley S. L., Jr Use of Chinese hamster ovary cells with altered glycosylation patterns to define the carbohydrate specificity of Entamoeba histolytica adhesion. J Exp Med. 1988 May 1;167(5):1725–1730. doi: 10.1084/jem.167.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E., Gibson R., Kornfeld S. Structure of an unusual complex-type oligosaccharide isolated from Chinese hamster ovary cells. Arch Biochem Biophys. 1980 Feb;199(2):393–399. doi: 10.1016/0003-9861(80)90295-7. [DOI] [PubMed] [Google Scholar]
- Libby P., Alroy J., Pereira M. E. A neuraminidase from Trypanosoma cruzi removes sialic acid from the surface of mammalian myocardial and endothelial cells. J Clin Invest. 1986 Jan;77(1):127–135. doi: 10.1172/JCI112266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty V. L., Downs F. J., Pigman W. Rat-colonic, mucus glycoprotein. Carbohydr Res. 1978 Mar;61:139–145. doi: 10.1016/s0008-6215(00)84474-2. [DOI] [PubMed] [Google Scholar]
- Petri W. A., Jr, Broman J., Healy G., Quinn T., Ravdin J. I. Antigenic stability and immunodominance of the Gal/GalNAc adherence lectin of Entamoeba histolytica. Am J Med Sci. 1989 Mar;297(3):163–165. doi: 10.1097/00000441-198903000-00006. [DOI] [PubMed] [Google Scholar]
- Petri W. A., Jr, Chapman M. D., Snodgrass T., Mann B. J., Broman J., Ravdin J. I. Subunit structure of the galactose and N-acetyl-D-galactosamine-inhibitable adherence lectin of Entamoeba histolytica. J Biol Chem. 1989 Feb 15;264(5):3007–3012. [PubMed] [Google Scholar]
- Petri W. A., Jr, Joyce M. P., Broman J., Smith R. D., Murphy C. F., Ravdin J. I. Recognition of the galactose- or N-acetylgalactosamine-binding lectin of Entamoeba histolytica by human immune sera. Infect Immun. 1987 Oct;55(10):2327–2331. doi: 10.1128/iai.55.10.2327-2331.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri W. A., Jr, Smith R. D., Schlesinger P. H., Murphy C. F., Ravdin J. I. Isolation of the galactose-binding lectin that mediates the in vitro adherence of Entamoeba histolytica. J Clin Invest. 1987 Nov;80(5):1238–1244. doi: 10.1172/JCI113198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin J. I., Croft B. Y., Guerrant R. L. Cytopathogenic mechanisms of Entamoeba histolytica. J Exp Med. 1980 Aug 1;152(2):377–390. doi: 10.1084/jem.152.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin J. I., Guerrant R. L. Role of adherence in cytopathogenic mechanisms of Entamoeba histolytica. Study with mammalian tissue culture cells and human erythrocytes. J Clin Invest. 1981 Nov;68(5):1305–1313. doi: 10.1172/JCI110377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin J. I., John J. E., Johnston L. I., Innes D. J., Guerrant R. L. Adherence of Entamoeba histolytica trophozoites to rat and human colonic mucosa. Infect Immun. 1985 May;48(2):292–297. doi: 10.1128/iai.48.2.292-297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin J. I., Moreau F., Sullivan J. A., Petri W. A., Jr, Mandell G. L. Relationship of free intracellular calcium to the cytolytic activity of Entamoeba histolytica. Infect Immun. 1988 Jun;56(6):1505–1512. doi: 10.1128/iai.56.6.1505-1512.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin J. I., Murphy C. F., Salata R. A., Guerrant R. L., Hewlett E. L. N-Acetyl-D-galactosamine-inhibitable adherence lectin of Entamoeba histolytica. I. Partial purification and relation to amoebic virulence in vitro. J Infect Dis. 1985 May;151(5):804–815. doi: 10.1093/infdis/151.5.804. [DOI] [PubMed] [Google Scholar]
- Ravdin J. I. Pathogenesis of disease caused by Entamoeba histolytica: studies of adherence, secreted toxins, and contact-dependent cytolysis. Rev Infect Dis. 1986 Mar-Apr;8(2):247–260. doi: 10.1093/clinids/8.2.247. [DOI] [PubMed] [Google Scholar]
- Ravdin J. I., Petri W. A., Murphy C. F., Smith R. D. Production of mouse monoclonal antibodies which inhibit in vitro adherence of Entamoeba histolytica trophozoites. Infect Immun. 1986 Jul;53(1):1–5. doi: 10.1128/iai.53.1.1-5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripka J., Shin S., Stanley P. Decreased tumorigenicity correlates with expression of altered cell surface carbohydrates in Lec9 CHO cells. Mol Cell Biol. 1986 Apr;6(4):1268–1275. doi: 10.1128/mcb.6.4.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M. A., Etchison J. R., Robertson J. S., Summers D. F., Stanley P. Specific changes in the oligosaccharide moieties of VSV grown in different lectin-resistnat CHO cells. Cell. 1978 Mar;13(3):515–526. doi: 10.1016/0092-8674(78)90325-2. [DOI] [PubMed] [Google Scholar]
- Rosenwald A. G., Stanley P., Krag S. S. Control of carbohydrate processing: increased beta-1,6 branching in N-linked carbohydrates of Lec9 CHO mutants appears to arise from a defect in oligosaccharide-dolichol biosynthesis. Mol Cell Biol. 1989 Mar;9(3):914–924. doi: 10.1128/mcb.9.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salata R. A., Pearson R. D., Ravdin J. I. Interaction of human leukocytes and Entamoeba histolytica. Killing of virulent amebae by the activated macrophage. J Clin Invest. 1985 Aug;76(2):491–499. doi: 10.1172/JCI111998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salata R. A., Ravdin J. I. The interaction of human neutrophils and Entamoeba histolytica increases cytopathogenicity for liver cell monolayers. J Infect Dis. 1986 Jul;154(1):19–26. doi: 10.1093/infdis/154.1.19. [DOI] [PubMed] [Google Scholar]
- Slomiany B. L., Murty V. L., Slomiany A. Isolation and characterization of oligosaccharides from rat colonic mucus glycoprotein. J Biol Chem. 1980 Oct 25;255(20):9719–9723. [PubMed] [Google Scholar]
- Stanley P. Chinese hamster ovary cell mutants with multiple glycosylation defects for production of glycoproteins with minimal carbohydrate heterogeneity. Mol Cell Biol. 1989 Feb;9(2):377–383. doi: 10.1128/mcb.9.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. Glycosylation mutants of animal cells. Annu Rev Genet. 1984;18:525–552. doi: 10.1146/annurev.ge.18.120184.002521. [DOI] [PubMed] [Google Scholar]
- Stanley P. Lectin-resistant CHO cells: selection of new mutant phenotypes. Somatic Cell Genet. 1983 Sep;9(5):593–608. doi: 10.1007/BF01574260. [DOI] [PubMed] [Google Scholar]
- Stanley P. Membrane mutants of animal cells: rapid identification of those with a primary defect in glycosylation. Mol Cell Biol. 1985 May;5(5):923–929. doi: 10.1128/mcb.5.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P., Sudo T., Carver J. P. Differential involvement of cell surface sialic acid residues in wheat germ agglutinin binding to parental and wheat germ agglutinin-resistant Chinese hamster ovary cells. J Cell Biol. 1980 Apr;85(1):60–69. doi: 10.1083/jcb.85.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P., Vivona G., Atkinson P. H. 1H NMR spectroscopy of carbohydrates from the G glycoprotein of vesicular stomatitis virus grown in parental and Lec4 Chinese hamster ovary cells. Arch Biochem Biophys. 1984 Apr;230(1):363–374. doi: 10.1016/0003-9861(84)90119-x. [DOI] [PubMed] [Google Scholar]
- Tsutsumi V., Mena-Lopez R., Anaya-Velazquez F., Martinez-Palomo A. Cellular bases of experimental amebic liver abscess formation. Am J Pathol. 1984 Oct;117(1):81–91. [PMC free article] [PubMed] [Google Scholar]
- Udezulu I. A., Leitch G. J. A membrane-associated neuraminidase in Entamoeba histolytica trophozoites. Infect Immun. 1987 Jan;55(1):181–186. doi: 10.1128/iai.55.1.181-186.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. A. Problems in recognition and diagnosis of amebiasis: estimation of the global magnitude of morbidity and mortality. Rev Infect Dis. 1986 Mar-Apr;8(2):228–238. doi: 10.1093/clinids/8.2.228. [DOI] [PubMed] [Google Scholar]


