Abstract
Background
Many newly diagnosed patients present to outpatient care with advanced HIV infection. More timely HIV diagnosis and initiation of care has the potential to improve individual health outcomes and has public health implications.
Objective
To assess temporal trends in late presentation for outpatient HIV medial care as measured by CD4 count <200 cells/mm3 and the implications on short-term (1-year) mortality.
Design
We conducted a cohort study nested in a prospective HIV clinical cohort including patients establishing initial outpatient HIV treatment between 2000–2010. Time series regression analysis evaluated temporal trends in late presentation for care measured by the proportion of patients with a CD4 count <200 cells/mm3 or an opportunistic infection at enrollment, and also evaluated trends in short-term mortality.
Participants
Patients establishing initial outpatient HIV treatment between 2000–2010 at an academic HIV clinic.
Main Measures
The proportion of patients with a CD4 count <200 cells/mm3 or an opportunistic infection at initial presentation and short-term (1-year) mortality following clinic enrollment.
Key Results
Among 1121 patients, 41% had an initial CD4 count <200 cells/mm3, 25% had an opportunistic infection and 2.4% died within 1-year of their initial visit. Time series regression analysis demonstrated significant reductions in late presentation for HIV care and decreases in short-term mortality with temporal improvement preceding updated CDC HIV testing recommendations.
Conclusion
We observed a significant decline in the number of patients presenting for outpatient HIV care with advanced disease, particularly in 2006–2010. A significant trend in improved short-term survival among patients establishing HIV care was also observed, likely related to more timely presentation for outpatient care in more recent years.
KEY WORDS: HIV, HIV testing, mortality, disparity, health policy
INTRODUCTION
According to the CDC, an estimated 21% of the 1,106,400 adults and adolescents living with HIV in the United States at the end of 2006 were unaware of their infection.1 Furthermore, an estimated 56,000 new HIV infections occur annually.2 Sequelae of undiagnosed infection are numerous, including late entry into HIV care and the consequent rapid progression to AIDS. Upwards of 39% of newly diagnosed HIV patients develop AIDS within one year of their HIV diagnosis and more than 50% of newly diagnosed patients enter care with initial CD4 counts ≤ 200 cells/mm3.3–8 Such advanced disease in the setting of high rates of undiagnosed HIV infection has detrimental effects at both the individual and public health levels. These include HIV-associated morbidity and mortality, increased healthcare costs with advanced infection and increased transmission risk of undiagnosed infection.9,10
Prior to 2006, the United States Centers for Disease Control and Prevention (CDC) recommended risk-based opt-in human immunodeficiency virus (HIV) testing for the diagnosis of HIV infection. The CDC published updated HIV testing recommendations calling for routine opt-out testing in all health care settings in September 2006, in part in response to the high rates of undiagnosed HIV infection and sizeable problem of late diagnosis with advanced disease.11 The new recommendations advocate HIV screening for all patients 13–64 years of age after informing the patients that HIV testing will be done unless refused by the patient (opt-out testing) with the goal of improving timely access to HIV prevention and treatment services as well as decreasing HIV transmission. If widely implemented, the updated CDC guidelines have the potential to foster earlier HIV diagnosis, thereby improving individual and public health outcomes.
We evaluated patients initiating outpatient HIV medical care at a dedicated HIV Clinic in the southeastern U.S. from 2000–2010 to assess trends in late diagnosis of HIV and presentation to care. We evaluated temporal trends in: 1) late presentation for outpatient HIV medical care as measured by the proportion of patients with an initial CD4 count <200 cells/mm3 and the proportion with an opportunistic infection (OI) upon enrollment, and 2) short-term mortality (1-year) following initiation of outpatient HIV care. We hypothesized that reductions in late presentation for HIV care would be temporally related to the updated CDC testing recommendations and translate to a decrease in short-term mortality.
METHODS
Our HIV/AIDS Clinic provides comprehensive HIV services to over 1800 active patients. Our 1917 Clinic Cohort Observational Database Project is an IRB approved prospective clinical cohort study that has previously been described in detail.12–14 Briefly, sociodemographic, psychosocial, clinical and health services utilization data are captured at the point-of-care through a locally programmed electronic medical record linked to administrative and clinical databases at our institution. The database is 100% quality controlled in real time and was recognized for excellence in information integrity.15
Study Design
A cohort study nested in the HIV Clinic Cohort included patients establishing initial outpatient HIV treatment at a Southeastern HIV Clinic between 1 January 2000 and 31 December 2010. As described in detail previously, the UAB 1917 Clinic Cohort has a systematic process to identify patients newly establishing outpatient HIV clinical care, such that patients transferring care from another treatment facility (including prison) or re-enrolling following a gap in care at the 1917 Clinic were excluded.8 Roughly 28% of new clinic patients are referred following HIV testing in emergency department or hospital settings, with the remaining 72% being largely self-referred following HIV diagnosis in a range of ambulatory settings.
An electronic query of the Clinic Cohort Database was performed to create an analyzable study dataset. Independent variables were selected a priori and included age, race, sex, location of residence, and HIV risk factor [injection drug use (IDU), men who have sex with men (MSM), and heterosexual sex]. Dependent variables included: (1) Initial CD4 count at clinic enrollment, categorized dichotomously as <200 cells/mm3 vs. ≥200 cells/mm3; (2) Diagnosis of an opportunistic infection at the time of presentation for clinical care; and (3) Short-term mortality, defined as death within 1-year of the initial HIV clinic visit. Mortality was ascertained by electronic query of the Social Security Death Index as of 31 December 2008.
Statistical Analyses
Frequency distributions, means and standard deviations were calculated to describe the study sample. The attributes of patients who enrolled in care at the HIV Clinic in 2000–2002, 2003–2005, 2006–2008, and 2009–2010 were compared using the Fisher’s exact test for categorical variables and one-way analysis of variance for continuous variables.
We used time series logistic regression analysis without a control group to assess temporal trends in late presentation for HIV care and short-term mortality during the observation period. We chose “calendar quarter” as a time unit for this analysis as a compromise between “month” (wide random fluctuations in outcome measures) and “year” (too few observations). To limit a priori assumptions regarding the form of the time trend and when a change in curve slope may have taken place, the calendar quarter variable was modeled using restricted cubic spline with four knots placed at percentiles 5, 35, 65 and 95, of the quarter distribution (2nd quarter 2000, 3 rd quarter 2003, 2nd quarter 2006, 3rd quarter 2008).16 Smoothing splines are special mathematical functions that are increasingly employed as flexible tools to describe the shape of continuous exposure-response relationships with minimal pre-specification.17 To account for possible serial correlation among the observations, standard errors for the parameters of interest were estimated using bootstrap method (1000 replications); bias-corrected and accelerated 95% confidence intervals were also calculated.18
We evaluated time series logistic regression models for both measures of late presentation for HIV medical care; the proportion of patients with an initial CD4 count <200 cells/mm3 and the proportion with an OI at clinic enrollment. Unadjusted associations between death within one year of enrollment in outpatient HIV care and patient characteristics were assessed using simple logistic regression with particular interest in the role of initial CD4 count on short-term mortality. Finally, we evaluated time series logistic regression models of short-term mortality that adjusted for patient’s baseline characteristics. The fit of adjusted models were assessed with the Hosmer-Lemeshow goodness-of-fit test, the Akaike’s information criterion, and the Bayesian information criterion. All statistical models were performed using STATA.
RESULTS
Between 2000–2010, 1121 newly diagnosed patients initiated outpatient HIV care at our HIV Clinic. Of them 34.2% were white males, 42.2% were non-white males and the mean age was 36.9 years (Table 1) Consistent with the southeast and national HIV epidemics, racial/ethnic minority women (18.4% of the overall sample) represented a disproportionately high fraction (78%) of all women. Seventy-two percent of patients lived within the county where the clinic is located, or in one of seven adjacent counties. Sexual activity was the predominant risk factor for reported HIV transmission (MSM, 43.4%; heterosexual sex 33.3%). At enrollment, 40.9% of patients had a baseline CD4 count <200 cells/mm3, 34.3% had a baseline viral load >100,000 c/mL and 25% had been diagnosed with an opportunistic infection. Slightly more than 2% of the patients died within one year of their initial visit (Table 1). The mean annual number of newly diagnosed patients enrolling into care was 87.0 in 2000–2002, 90.7 in 2003–2005, 110 in 2006–2008, and 129 in 2009–10. Over the course of the study period, significant shifts in HIV risk factor among new patients was observed, and the mean age of new clinic patients decreased over time (Table 1).
Table 1.
Overall and Calendar-Period-Specific Characteristics of 1121 Patients Establishing Initial Outpatient HIV Care at the University of Alabama at Birmingham 1917 HIV Clinic, 2000-2010
| Characteristic | 2000–2010 | 2000–2002 | 2003–2005 | 2006–2008 | 2009–2010 | P-value |
|---|---|---|---|---|---|---|
| n = 1121 | n = 261 | n = 272 | n = 330 | n = 258 | ||
| n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Baseline CD4 count <200 cells/mm3 | 458 (40.9) | 127 (48.7) | 131 (48.2) | 121 (36.7) | 79 (30.6) | <0.001 |
| Baseline VL > 100,000 c/mL | 384 (34.3) | 84 (32.2) | 98 (36.0) | 108 (32.7) | 94 (34.3) | 0.62 |
| Opportunistic infection | 280 (25.0) | 86 (33.0) | 88 (32.4) | 67 (20.3) | 39 (15.1) | <0.001 |
| Death within one year of initial visit *,† | 24 (2.1) | 11 (4.2) | 9 (3.3) | 4 (1.2) | N/A | 0.01 |
| Local residence | 801 (71.5) | 175 (67.1) | 191 (70.2) | 251 (76.1) | 184 (71.3) | 0.10 |
| HIV risk factor | ||||||
| MSM | 486 (43.4) | 108 (41.4) | 108 (39.7) | 123 (37.3) | 147 (57.0) | <0.001 |
| Heterosexual | 373 (33.3) | 107 (41.0) | 86 (31.6) | 100 (30.3) | 80 (31.0) | |
| IDU | 21 (1.9) | 5 (1.9) | 0 (0.0) | 1 (0.3) | 15 (5.8) | |
| Unknown | 241 (21.5) | 41 (15.7) | 78 (28.7) | 106 (32.1) | 16 (6.2) | |
| Race and sex | ||||||
| White Male | 381 (34.2) | 101 (38.7) | 100 (36.8) | 105 (32.2) | 75 (29.4) | 0.07 |
| Non-White Male | 470 (42.2) | 94 (36.0) | 102 (37.5) | 146 (44.6) | 128 (50.2) | |
| White Female | 58 (5.2) | 12 (4.6) | 15 (5.5) | 20 (6.2) | 11 (4.3) | |
| Non-White Female | 205 (18.4) | 54 (20.7) | 55 (20.2) | 55 (16.9) | 41 (16.1) | |
| Age (mean ± SD)‡ | 36.9 (10.4) | 37.5 (9.1) | 37.9 (9.7) | 37.0 (10.9) | 34.4 (11.2) | <0.001 |
*Fisher’s exact test
†Estimates obtained for the period 2000–2008
‡One-way ANOVA
Initial CD4 Count at Presentation
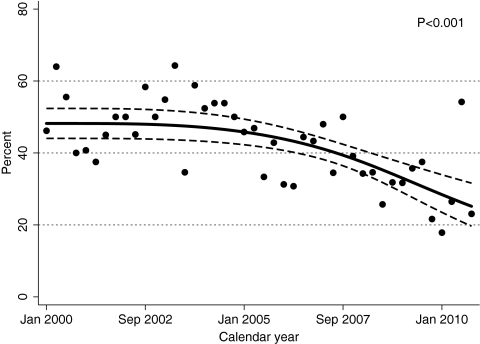
As indicated by an initial CD4 count <200 cells/mm3, the proportion of patients presenting with advanced immunosuppression was similar in 2000–2002 (48.7%) and 2003–2005 (48.2%), but significantly lower in 2006–2008 (36.7%) and 2009–10 (30.6%, P < 0.001; Table 1). This preliminary finding was confirmed by time series logistic regression analysis. As shown in Figure 1, results suggested that a statistically significant downward trend (P < 0.001) began in advance of the release of the revised CDC HIV testing recommendations in September 2006.
Figure 1.
Temporal trends in the percentage of patients with a CD 4 count < 200 cells/mm3 upon initial presentation to outpatient HIV medical care, UAB 1917 HIV Clinic, 2000–2010. Temporal trends in late presentation for outpatient HIV medical care using time series logistic regression analysis without a control group to evaluate the proportion of patients with an initial CD4 count <200 cells/mm3 at clinic enrollment. Each point on the figure represents “calendar quarter” as a time unit, representing the proportion of patients with late presentation during each 3-month interval from 2000–2010. To limit a priori assumptions regarding the form of the time trend and when a change in curve slope may have taken place, the calendar quarter variable was modeled using restricted cubic spline with four knots placed at percentiles 5, 35, 65 and 95, of the quarter distribution (2nd quarter 2000, 3rd quarter 2003, 2nd quarter 2006, 3rd quarter 2008).16 To account for possible serial correlation among the observations, standard errors for the parameters of interest were estimated using bootstrap method (1000 replications); bias-corrected and accelerated 95% confidence intervals were also calculated (dashed line).18
Opportunistic Infection at Presentation
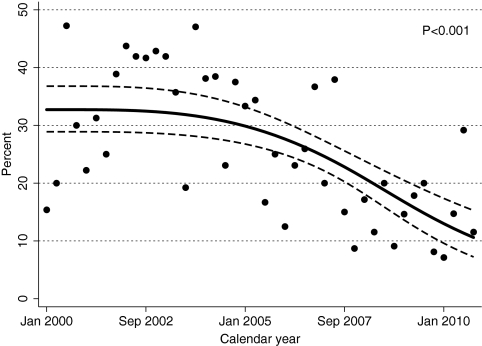
As observed with the proportion of patients with an initial CD4 count <200 cells/mm3, the proportion of patients with an AIDS-defining opportunistic infection at clinic enrollment was similar during the first two calendar periods (33.0% in 2000–2002 and 32.4% in 2003–2005), and substantially lower during the two more recent periods (20.3% in 2006–2008 and 15.1% in 2009–2010; P < 0.001; Table 1). Time series regression analysis confirmed this pattern, again suggesting that the percentage of patients presenting with an opportunistic infection started to decrease in advance of the release of the revised CDC guidelines (Fig. 2; P < 0.001).
Figure 2.
Temporal trends in the percentage of patients with an opportunistic infection upon initial presentation to outpatient HIV medical care, UAB 1917 HIV Clinic, 2000–2010. Temporal trends in late presentation for outpatient HIV medical care using time series logistic regression analysis without a control group to evaluate the proportion of patients with an opportunistic infection at clinic enrollment. Each point on the figure represents “calendar quarter” as a time unit, representing the proportion of patients with late presentation during each 3-month interval from 2000–2010. To limit a priori assumptions regarding the form of the time trend and when a change in curve slope may have taken place, the calendar quarter variable was modeled using restricted cubic spline with four knots placed at percentiles 5, 35, 65 and 95, of the quarter distribution (2nd quarter 2000, 3rd quarter 2003, 2nd quarter 2006, 3rd quarter 2008).16 To account for possible serial correlation among the observations, standard errors for the parameters of interest were estimated using bootstrap method (1000 replications); bias-corrected and accelerated 95% confidence intervals were also calculated (dashed line).1
Early Mortality
Contingency-table analysis suggested that a significant (P = 0.01) decline in risk of 1-year mortality took place between 2000–2002 (4.2%) and 2006–2008 (1.2%; Table 1). This temporal decline in early-mortality was also observed in time series regression analysis (P = 0.02; Figure not shown). Once again, modeling suggested that the downward trend started prior to 2006. In univariate logistic regression analysis, as expected, baseline CD4 count <200 cells/mm3 (OR, 8.7; 95% CI, 2.6-29.3) and baseline viral load >100,000 c/mL (OR, 3.3; 95% CI, 1.4-7.5) were most strongly associated with 1-year mortality (Data not shown).
DISCUSSION
Between 2000–2010, our clinic observed a significant decline in the proportion of patients presenting with advanced HIV infection, illustrated by dramatically fewer presentations with CD4 counts <200 cells/mm3 and fewer patients with concomitant opportunistic infections at enrollment. Importantly, these trends appear to have translated into improved short-term survival, with significant declines observed in 1-year mortality in more recent years. Our study is among the first to evaluate temporal trends in late presentation for HIV care over the time period incorporating the updated CDC HIV testing guidelines and to further evaluate the implications for short-term mortality. Our results indicate a trend towards earlier presentation for outpatient HIV care which preceded the release of the revised HIV testing recommendations in September 2006, suggesting other factors may have contributed. If mirrored across the country, our findings of earlier presentation for outpatient HIV care and associated declines in short-term mortality in more recent years holds considerable promise for individual health outcomes and may have profound public health implications.
It is noteworthy that downward trends in late presentation for outpatient HIV care preceded the release of the revised CDC HIV testing recommendations in September 2006, and that these trends have continued through 2010. The current study is not able to assess HIV testing practices among primary care providers in Alabama to determine the degree to which changes in testing practices, either prior to or following release of the updated CDC guidelines, may have impacted study findings. Other factors may have contributed to the observed trends towards earlier presentation for HIV medical care. In 2006 the Alabama Department of Public Health launched the Enhanced Referral and Treatment System, designed and implemented to identify persons testing positive for HIV infection and track their entry into care. Additionally, the UAB 1917 Clinic launched Project CONNECT, a systems-level new patient navigation program in January 2007. Following implementation of this program, the proportion of patients who failed to enroll in HIV care decreased from 31% to 18%, resulting in improved linkage to outpatient care at the clinic.19,20 These statewide and clinic-level programs may have contributed to the downward trends in late presentation for HIV care that persisted from 2006–2010. Further, socio-demographic shifts were observed over the observation period with increases in non-white males, MSM, and younger patients. It is unclear to what extent these shifts may have contributed to the observed study findings.
Public health and individual benefits of earlier diagnosis and enrollment into HIV-care include improved quality of health, longevity and early survival. Our data demonstrate that between 2000–2010 the proportion of patients entering HIV care is doing so earlier, with a baseline CD4 count <200 cells/mm3, down 18.1% [2000–2002 (48.7%); 2009–2010 (30.6%)]. It appears similar changes are being observed elsewhere in the US, although data are limited. 21 Prior to opt-out testing recommendations, rates of CD4 counts ≤ 200 cells/mm3 at the time of HIV diagnosis and enrollment in care have been documented as high as 36% (1999–2000) and 49% (2002–2004).4,7 A study at the Johns Hopkins Moore HIV Clinic found no significant difference in CD4 counts among patients enrolling in care between 1990–2006.6 Similarly, during a subset of our study period between 2000–2005, we found that nearly one-half of our patients consistently enrolled into HIV care with CD4 counts <200 cells/mm3. However, between 2006–2010 a significant and clinically meaningful decline in the proportion of patients presenting with advanced immunosuppression was observed in the current study.
The significant improvement in early mortality declining from 4.2% to 1.2% (death with-in 1 year of enrollment), is likely linked to earlier presentation for care, as the strongest factor associated with short-term mortality was an initial CD4 count <200 cells/mm3, which carried an over eightfold increase in odds of death. The 1-year mortality rate of 4.2% in the earliest study period (2000–2002) elucidates the consequences of late diagnosis and enrollment in clinical care. This problem has been identified in other developed nations as well. Studies in Europe demonstrate 1-year mortality with rates as high as 9% in the Mortalité 2000 and 2005 Surveys of France, upwards of 20% in London, and 3.2% in heterosexuals in England and Wales.22–24
Although race was not found to be associated with early mortality in our study, a disparity in the race of the women enrolling into HIV medical care was seen consistently across our 10-year study period. A disproportionate number of non-white females (18.4%) compared to white females (5.2%), entering HIV medical care was seen. Moreover, a significant increase in the absolute number and proportion of new patients accounted for by non-white males was observed during the study period (36% in 2000–2002 and 50.2% in 2009–2010). These findings indicate that emphasis is needed in reaching racial/ethnic minority populations as steps are taken towards implementing the new CDC HIV opt-out testing guidelines.
The diagnosis of HIV early in its course is beneficial for our public health system, shifting the focus from acute/urgent healthcare to prevention. There is clear potential benefit for secondary HIV prevention efforts to decrease HIV transmission in patients unaware of their status. Those unaware of their HIV infection have 3.5 greater odds of sexual transmission compared to those aware of their HIV infection, where an estimated 80% of newly diagnosed HIV infections occur via sexual transmission.10 In a recent meta-analysis, a 68% reduction in the prevalence of unprotected anal vaginal intercourse (UAV) occurred in HIV positive individuals aware of their serostatus compared to those patients unaware of their HIV status.25 These results indicate that changes in behavior occur as a result of knowledge of HIV serostatus, potentially increasing secondary HIV prevention. Marks et al. have estimated that the transmission rate of HIV in patients unaware of their HIV status compared to those aware of their HIV status is 6.9% vs. 2.0%.10 In the setting of expanded testing, with consequential increased self knowledge of HIV positivity, it is estimated that the number of newly acquired HIV infections could decrease by 31%, as recently demonstrated by a CDC mathematical model presented in 2006.10
The findings of our study are highly germane with regards to the recently released National HIV/AIDS Strategy for the United States.26 Three principle tenets of that document are to: 1) reduce new HIV infections, 2) increase access to care and improve health outcomes for people living with HIV, and 3) reduce HIV-related disparities and inequities. As previously noted, trends toward earlier presentation for HIV medical care, as observed in our study, have strong potential to reduce HIV transmission and new infections. Furthermore, more timely presentation and access to HIV medical care in more recent years has been accompanied by improved short-term mortality, an important health outcome. Finally, our study highlights the continued challenge of addressing HIV-related disparities as racial-ethnic minorities continue to be over-represented among newly diagnosed patients enrolling in HIV care.
Limitations and Conclusion
The findings of our study should be interpreted with respect to the limitations in our study design. As we conducted an observational study, associations can be made but causality cannot be proven. We are able to identify significant temporal trends but cannot definitively ascribe these findings to the revised CDC testing guidelines or other factors that may have contributed. Furthermore, we conducted this study at a single site in the Southeastern United States. Therefore our findings may not be applicable to all patient populations or to other geographical regions. Furthermore, our study evaluated those patients who enrolled into care at our clinic and we are unable to account for patients who have been diagnosed but not enrolled into care in our catchment area.
In conclusion, over the time period of 2000–2010 we observed a significant decline in the proportion of patients presenting with advanced HIV infection (CD4 count <200 cells/mm3 and/or OI at clinic enrollment). These findings have translated to a significant decline in 1-year mortality among patients establishing initial HIV care in more recent years. The improved timeliness of HIV diagnosis and linkage to care has clear implications for individual patients and the public health, meriting further evaluation in larger patient populations.
Acknowledgments
Contributors There are no additional contributors to this manuscript.
Funding This study was supported by grant K23MH082641-04 (MJM) from the National Institute of Mental Health. The UAB 1917 HIV/AIDS Clinic Cohort Observational Database project receives financial support from the following: UAB Center for AIDS Research (P30-AI027767), CFAR-Network of Integrated Clinical Systems (R24AI067039), and the Mary Fisher CARE Fund.
Prior Presentations Data presented in part at the National HIV Prevention Conference, Atlanta, Georgia, USA from August 23–26, 2009. “Late diagnosis, early mortality: A call to action for implementation of routine HIV testing (abstract #206T).”
Conflict of Interest P. Seal, D. Jackson, C. Nevin, J. Allison, J. Raper, M. Kempf and J. Schumacher have no conflicts of interest.E. Chamot has received funding from NCI and NIMH.J. Willig has received grant support and/or consultancies from Tibotec, Bristol Myers Squibb and Pfizer.M. Saag has received research support and/or consultancies for Achillion Pharmaceuticals, Avexa, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Merck, Monogram Biosciences, Panacos, Pfizer, Progenics, Roche, Serono, Tanox, Tibotec, Trimeris, and Vertex.M. Mugavero has received grant support and/or consultancies from Bristol-Myers Squibb, Gilead Sciences, Pfizer, and Tibotec.
References
- 1.Campsmith ML, Rhodes PH, Hall HI, Green TA. Undiagnosed HIV Prevalence Among Adults and Adolescents in the United States at the End of 2006. J Acquir Immune Defic Syndr. 2010;53(5):619–624. doi: 10.1097/QAI.0b013e3181bf1c45. [DOI] [PubMed] [Google Scholar]
- 2.Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300(5):520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report 2004. Atlanta: U.S. Department of Health and Human Services, Center for Disease Control and Prevention. 2005.16:1–46.
- 4.Dybul M, Bolan R, Condoluci D, et al. Evaluation of initial CD4+ T cell counts in individuals with newly diagnosed human immunodeficiency virus infection, by sex and race, in urban settings. J Infect Dis. 2002;185(12):1818–1821. doi: 10.1086/340650. [DOI] [PubMed] [Google Scholar]
- 5.Gay CL, Napravnik S, Eron JJ., Jr Advanced immunosuppression at entry to HIV care in the southeastern United States and associated risk factors. AIDS. 2006;20(5):775–778. doi: 10.1097/01.aids.0000216380.30055.4a. [DOI] [PubMed] [Google Scholar]
- 6.Keruly JC, Moore RD. Immune status at presentation to care did not improve among antiretroviral naive persons from 1990 to 2006. Clin Infect Dis. 2007;45(10):1369–1374. doi: 10.1086/522759. [DOI] [PubMed] [Google Scholar]
- 7.Mugavero JM, Castellano C, Edelman D, Hicks C. Late diagnosis of HIV infection: the role of age and sex. Am J Med. 2007;120(4):370–373. doi: 10.1016/j.amjmed.2006.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ulett KB, Willig JH, Lin HY, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDS. 2009;23(1):41–49. doi: 10.1089/apc.2008.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleishman JA, Yehia BR, Moore RD, et al. The Economic burden of late entry into medical care for patients with HIV infection. Med Care. 2010;48(12):1071–1079. doi: 10.1097/MLR.0b013e3181f81c4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marks G, Crepaz N, Janssen R. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS. 2006;20(10):1447–1450. doi: 10.1097/01.aids.0000233579.79714.8d. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention Revised Recommendations for HIV Testing in Adults, Adolescents, and Pregnant Women in Health-Care Settings. MMWR Morb Mortal Wkly Rep. 2006;55(RR14):1–17. [PubMed] [Google Scholar]
- 12.Chen RY, Accortt NA, Westfall AO, et al. Distribution of health care expenditures for HIV-infected patients. Clin Infect Dis. 2006;42(7):1003–1010. doi: 10.1086/500453. [DOI] [PubMed] [Google Scholar]
- 13.Mugavero MJ, Lin HY, Allison JJ, et al. Failure to establish HIV care: characterizing the“no show” phenomenon. Clin Infect Dis. 2007;45(1):127–130. doi: 10.1086/518587. [DOI] [PubMed] [Google Scholar]
- 14.Willig JH, Westfall AO, Allison J, et al. Nucleoside reverse-transcriptase inhibitor dosing errors in an outpatient HIVclinic in the electronic medical record era. Clin Infect Dis. 2007;45(5):658–661. doi: 10.1086/520653. [DOI] [PubMed] [Google Scholar]
- 15.2009 Excellence in Information Integrity Awards Program. The Excellence in Information Integrity Awards. Available at: http://www.eiiaward.org/2007_winners.htm. Accessed March 7, 2011.
- 16.Harrell FE., Jr . Regression modeling strategies with applications to linear models logistic regression, and survival analysis. New York, New York: Springer; 2001. pp. 16–26. [Google Scholar]
- 17.Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6(4):356–365. doi: 10.1097/00001648-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med. 2000;19(9):1141–1164. doi: 10.1002/(SICI)1097-0258(20000515)19:9<1141::AID-SIM479>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 19.Wylie R, Marler M, Lin H, et al. Project CONNECT: overcoming barriers to establishing HIV care. In: Program and abstracts of the 4th International Conference on HIV Treatment Adherence. Miami; 5–7 April 2009.
- 20.Mugavero MJ. Improving engagement in HIV care: what can we do? Top HIV Med. 2008;16:156–161. [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention Expanded HIV Testing and Trends in Diagnoses of HIV Infection—District of Columbia, 2004–2008. MMWR Morb Mortal Wkly Rep. 2010;59(24):737–741. [PubMed] [Google Scholar]
- 22.Sabin CA, Smith CJ, Youle M, et al. Deaths in the era of HAART: contribution of late presentation, treatment exposure, resistance and abnormal laboratory markers. AIDS. 2006;20(1):67–71. doi: 10.1097/01.aids.0000196178.73174.24. [DOI] [PubMed] [Google Scholar]
- 23.Chadborn TR, Delpech VC, Sabin CA, Sinka K, Evans BG. The late diagnosis and consequent short-term mortality of HIV-infected heterosexuals (England and Wales, 2000–2004) AIDS. 2006;20(18):2371–2379. doi: 10.1097/QAD.0b013e32801138f7. [DOI] [PubMed] [Google Scholar]
- 24.Lewden C, Rosenthal E, Burty C, et al. Changes in causes of death among adults infected by HIV between 2000 and 2005: The “Mortalité 2000 and 2005” Surveys (ANRS EN 19 and Mortavic) J Acquir Immune Defic Syndr. 2008;48(5):590–598. doi: 10.1097/QAI.0b013e31817efb54. [DOI] [PubMed] [Google Scholar]
- 25.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39(4):446–453. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 26.Office of National AIDS Policy. 2010 National HIV/AIDS Strategy for the United States. ONAP, The White House. Available at: http://www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf Accessed March 7, 2011.