ABSTRACT
BACKGROUND
Four population-based studies of screening for CRC with fecal occult blood testing (FOBT) have shown that mortality can be significantly reduced. However, nearly half of all positive screening tests are not appropriately evaluated.
OBJECTIVES
We evaluated whether an electronic record intervention improved the follow-up of patients with a positive FOBT (FOBT+) result.
DESIGN
We conducted a cluster randomized trial involving four Veteran’s Affairs (VA) medical centers pair-matched by colonoscopy volume and randomized within the pair to receive the electronic intervention or usual care.
PARTICIPANTS
All patients with FOBT+ results at participating facilities during a matched pre- and post-intervention time period.
INTERVENTIONS
In the two intervention sites, an electronic consult that imported relevant clinical information was automatically submitted to the gastroenterology (GI) clinic for all FOBT+ patients at the time the result was recorded in the laboratory. In both intervention and control sites (usual care), PCPs continued to be notified of FOBT+ results in the usual manner
MEASURES
Pre- and post-intervention changes in the proportion of FOBT+ patients having: (1) a GI consult or (2) a GI consult plus complete diagnostic evaluation (CDE) of the colon within 30, 90 and 180 days were compared across intervention and control sites. Log rank tests were used to determine statistical significance.
RESULTS
The 30-, 90- and 180-day GI consult rates improved 21–33 % (p < 0.001) among intervention sites, but did not change in the usual care sites. Thirty-, 90- and 180-day CDE rates improved 9–31% (p < 0.03) in intervention sites, but did not significantly change in the usual care sites. Time to GI consult and CDE decreased significantly over time in the intervention sites (p < 0.001), but remained unchanged in the usual care sites.
CONCLUSIONS
The relatively simple electronic intervention evaluated can significantly improve the follow-up of FOBT+ results. Interventions such as this could improve patient care and may be applicable to other practice settings, as well as other types of tests.
KEY WORDS: colorectal cancer, cancer screening, cancer prevention
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths in the US and accounted for approximately 49,920 deaths in 2009.1,2 Fortunately, four population-based studies of fecal occult blood test (FOBT) screening programs have shown CRC mortality reductions of 15% to 33%, as well as significant reductions in CRC incidence.2–7 In these studies, more than 80% of individuals with positive FOBT (FOBT+) screening tests underwent an evaluation of their colon with either colonoscopy or sigmoidoscopy to identify polyps and/or early CRC. Nationally, CRC incidence and mortality rates have been declining for the last several years; this is believed to be due to the increased incidence of screening, colonoscopy and polyp removal—true cancer prevention.
However, for FOBT screening to be fully effective, positive screening tests must be followed with appropriate evaluation and treatment. Numerous intervention programs have been used to improve initial CRC screening rates, but data indicate that less than half of patients with FOBT+ results undergo further evaluation.8–10 One prior study conducted in the VA 9 identified lack of GI referral as an important barrier to CDE, suggesting that interventions designed to facilitate the referral process might provide an effective approach to improving FOBT+ follow-up rates.
To test a method of improving follow-up of FOBT+ screening, we developed a program that functions within the VA electronic medical record to provide automatic referral to gastroenterology (GI) for patients with FOBT+ results. We report here the findings from a multisite, randomized clinical trial of this program. The primary objective of the study was to improve the follow-up of FOBT+ results and to reduce waiting times for CDE. The long-term objective of the project is to reduce colorectal cancer morbidity and mortality by improving the follow-up of FOBT+ screening tests.
METHODS
Pilot Study
In August 2004, we invited primary care providers (PCPs), GI and programming experts at the Portland VA to discuss and develop strategies for improving FOBT+ follow-up using an electronic intervention. The pilot study had two phases. The first was to create a feasible and effective electronic intervention to improve FOBT+ follow-up that would meet the needs of the PCPs and gastroenterologists who would be reviewing the electronic intervention. The second phase involved testing the intervention locally and meeting with PCPs and gastroenterologists to evaluate and improve the process.
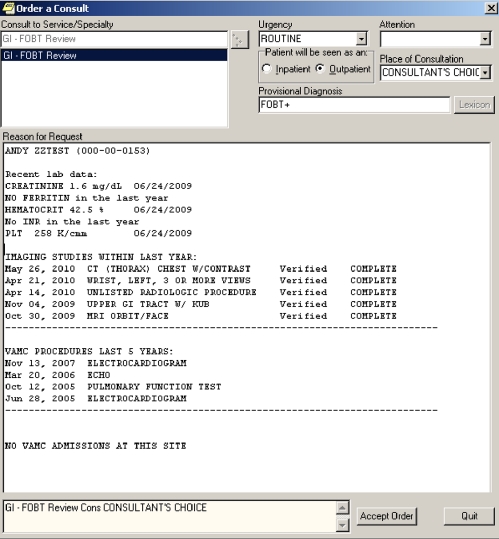
These discussions led to the creation of an automated electronic “consult” that is directly sent to GI providers when a patient has a FOBT+ result recorded in the laboratory. An example of the templated consult is shown in Figure 1. For all FOBT+ screening results, the automated consult imports information commonly used by gastroenterologists to make decisions about appropriate follow-up/colonoscopy for referred patients. This includes age, gender, relevant laboratory data, procedures from the last 5 years (including prior endoscopic procedures), and all imaging procedures and dates of hospital admission over the last year. The goal of the templated consult was to provide a “picture” of the patient and their co-morbidity so a GI provider could quickly make a decision regarding triage of patients with FOBT+ results. For example, a patient with no recent imaging, procedures or admissions could have the colonoscopy discussed and scheduled by phone. More complex patients might be placed in a pre-colonoscopy clinic to facilitate closer discussion of the risks and benefits of further evaluation. Information contained in the consult about prior endoscopic procedures facilitated decisions about appropriate follow-up of positive tests as well. The automated consult was tested in Portland prior to installation at intervention sites beginning in May 2005.
Figure 1.
Example of FOBT+ consult template.
Controlled Trial
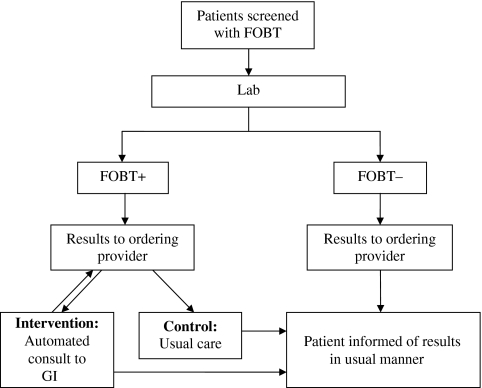
Study Design This multisite cluster randomized trial involved eight VA medical centers recruited based on their interest in CRC and this project. Primary investigators at each site facilitated IRB approval, implementation of the intervention (if they were an intervention site) and data collection. After identifying the eight participating VA sites, pairs were matched by colonoscopy volume and randomized within the pair by random number generator to the control/usual care site or intervention site. Figure 2 illustrates the process and shows the difference between the process of notification of positive results at both control and intervention sites, which is described further below.
Figure 2.
Trial design.
Intervention Sites The centers randomized to the consult intervention received an electronic programming code based on that developed and tested by the study team. The programming code consisted of a relatively simple alteration of the usual process for notifying the PCP of a FOBT+ result. Once an FOBT+ result was recorded in the laboratory, the automatic notification, as described above, was sent to the PCP and to the GI clinic simultaneously. This differed from the usual practice in which a FOBT+ result is sent only to the ordering provider and follow-up must be initiated by the PCP. Further, it allowed for streamlining of the triage process, through auto-population of important decision-making data points (described above). Notification and review of information in the consult by the gastroenterologist then set off a cascade of events that would normally only be triggered by a consult request from the PCP.
Control Sites The centers randomized as control sites received no intervention, and in those sites, PCPs continued to be notified of FOBT+ results in the usual manner—any patient follow-up that was needed was initiated by the PCP. In each arm of the study, patients continued to be notified of their test results by their PCP.
Outcomes
Analysis There were two primary endpoints of interest. The first was the pre- to post-intervention change in the proportion of patients who had a GI consult following a FOBT+ result. Since FOBT+ findings, in appropriate patients, should result in some anatomic evaluation of the GI tract, the second primary endpoint was the rate of complete diagnostic examination (CDE), measured as the change in the percent of patients with GI consults who also underwent an anatomic procedure (endoscopic or radiologic) within 30, 90 and 180 days. The secondary endpoints of interest were the timing of the GI consult and/or CDE and whether the electronic intervention reduced waiting times for follow-up.
Data Sources Outcome data were collected at a single time point; pre-intervention data were collected for 12 months and post-intervention data for 6 months, beginning approximately 2 months following installation of the electronic intervention at each intervention site and its matched control site on all positive FOBTs. Focus groups were conducted with each intervention site before and after implementation of the intervention to assess the acceptability of the intervention to gastroenterologists and PCPs at each intervention site.
Statistical Analysis Statistical analysis was performed using SAS 9.2. chi-square analysis, and t-tests were used to determine whether the pre- and post-intervention patient samples differed by age, gender or the proportion surviving at least 6 months. Because all cases had at least 6 months of follow-up, the data from patients who survived for 6 months were censored at 6 months. Patients who died within 6 months of the FOBT+ finding were censored at the time of death. Because the data set only included time intervals for patients who had an outcome event (consultation, GI procedure or radiology), the censoring status for patients who were missing time interval data for an outcome was set to zero (outcome did not occur).The proportions of patients having either GI consultation and/or CDE within 30, 90 and 180 days were compared between the pre- and post-intervention time periods using chi-square tests. Log rank tests were used to compare the time to clinical follow-up (GI consultation and/or CDE) in the pre- and post-intervention time period. Survival plots graphing the proportion waiting for a GI consult or CDE by time since a FOBT+ result were generated using the Kaplan-Meier method. For all sites, time zero occurred at the time a FOBT+ result was recorded in the laboratory. All statistical tests were conducted separately within each site, as the number of sites with data per group was too small to allow the typical mixed model analysis of a cluster randomized trial that was originally planned.
RESULTS
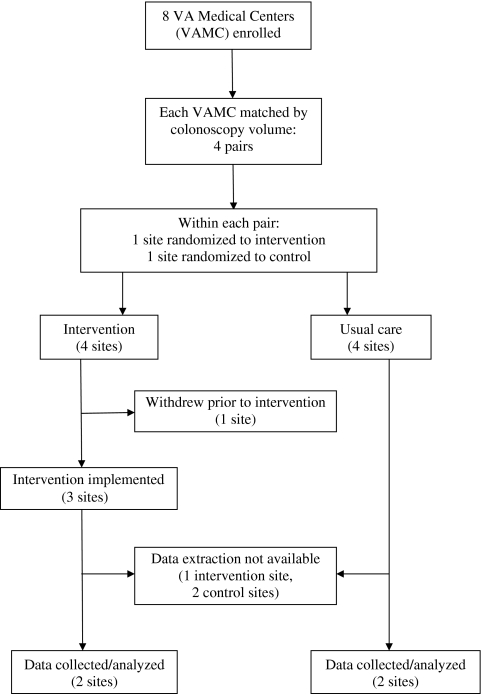
Figure 3 illustrates the flow of VA sites participating in this study. Eight VA sites were initially recruited. However, because of limited local programming resources, one intervention site was unable to participate in the study after recruitment and randomization. An additional three sites that were recruited and randomized (two control and one intervention site that installed the intervention) were unable to extract data from the VA electronic record system because of changes in VA programming and statistical support, and were thus unable to contribute to the results. Thus, four sites completed the study. Fortunately, the four sites completing the study included two matched pairs of control and intervention sites. The number of cases collected for each time interval from each institution is summarized in Table 1. As shown in the table, the randomization provided a balance of case load across the intervention and control sites, and the sites did not differ in the age and gender distribution of patients.
Figure 3.
Flow of participants in the trial.
Table 1.
Number of Patients with Positive FOBT Results
| Pre-intervention time interval | Post-intervention time interval | |||||
|---|---|---|---|---|---|---|
| Site | N FOBT+ | Age mean (SD) | Male N (%) | N FOBT+ | Age mean (SD) | Male N (%) |
| Intervention 1a | 737 | 63 (12) | 702 (95%) | 321 | 63 (11) | 308 (96%) |
| Control 1b | 803 | 69 (11) | 785 (98%) | 409 | 68 (12) | 403 (99%) |
| Intervention 2a | 301 | 68 (11) | 286 (95%) | 138 | 67 (12) | 135 (98%) |
| Control 2b | 433 | 69 (12) | 408 (94%) | 180 | 68 (12) | 175 (97%) |
FOBT+, positive fecal occult blood test; SD, standard deviation
Patient age and gender distributions did not change significantly in the post-intervention period at any clinic
Among the intervention sites, 30-day GI consult rates for evaluation of a FOBT+ result improved from 39% to 68% at site 1a (p < 0.0001) and from 47% to 80% at site 2a (p < 0.001) (Table 2). Ninety-day GI consult rates improved from 46% to 71% (p < 0.0001) at intervention site 1; at intervention site 2, they improved from 50% to 82% (p < 0.0001). After 180 days, the GI consult rate pre-intervention improved from 50% to 74% post-intervention (p < 0.0001) at site 1a and from 55% to 86% at site 2a (p < 0.0001). Rates of GI consultation for a FOBT+ result were essentially unchanged over all of the follow-up periods at both control sites. See Table 2.
Table 2.
Percent of Patients Receiving a GI Consult Within 30, 90 and 180 Days of Positive FOBT Results
| Time interval after positive FOBT | ||||||
|---|---|---|---|---|---|---|
| 30 days | 90 days | 180 days | ||||
| Period | Period | Period | ||||
| Site | Pre | Post | Pre | Post | Pre | Post |
| Intervention 1a | 39 | 68* | 46 | 72* | 50 | 74* |
| Control 1b | 64 | 63† | 70 | 69† | 71 | 72† |
| Intervention 2a | 47 | 80* | 50 | 82* | 55 | 86* |
| Control 2b | 51 | 48† | 56 | 56† | 62 | 59† |
*Significantly different from pre-intervention period at p < 0.0001
†Differences from pre-intervention period not significant
FOBT, fecal occult blood test
We next evaluated rates of CDE among all patients with FOBT+ results (Table 3). Notably, 30-day rates improved from 4% pre- to 30% post-intervention (p < 0.0001) at site 1a and from 12% to 21% at intervention site 2a (p 0.03). CDE rates at control sites either did not change or worsened. Ninety-day follow-up CDE rates also improved in both intervention sites, increasing from 51% to 82% (p < 0.001) at site 1a and from 23% to 39% at site 2a (p 0.009); control sites showed no significant improvement in CDE rates. CDE rates during the 180-day time frame improved in both intervention sites. In site 1a, the percentage of patients receiving a CDE increased from 25% to 51% (p < 0.0001) and from 29% to 42% (p < 0.0114) at site 2a pre- to post-intervention. These proportions also improved in the site 1b control group, although not as dramatically (48% pre-intervention to 54% post-intervention; p 0.053). There was no change in quite low rates of CDE at site 2b (22% at both times).
Table 3.
Percent of Patients Receiving GI Consult Plus Anatomic Workup Within 30, 90 and 180 Days of Positive FOBT Results
| Time interval after positive FOBT | ||||||
|---|---|---|---|---|---|---|
| 30 days | 90 days | 180 days | ||||
| Period | Period | Period | ||||
| Site | Pre | Post | Pre | Post | Pre | Post |
| Intervention 1a | 4 | 30* | 18 | 49* | 25 | 51* |
| Control 1b | 19 | 13‡ | 44 | 50‡ | 48 | 54‡ |
| Intervention 2a | 12 | 21† | 26 | 39† | 29 | 42* |
| Control 2b | 4 | 5‡ | 15 | 17‡ | 22 | 22‡ |
*Significantly different from pre-intervention period at p < 0.0001
†Significantly different from pre-intervention period at p < 0.02
‡Differences from pre-intervention period not significant
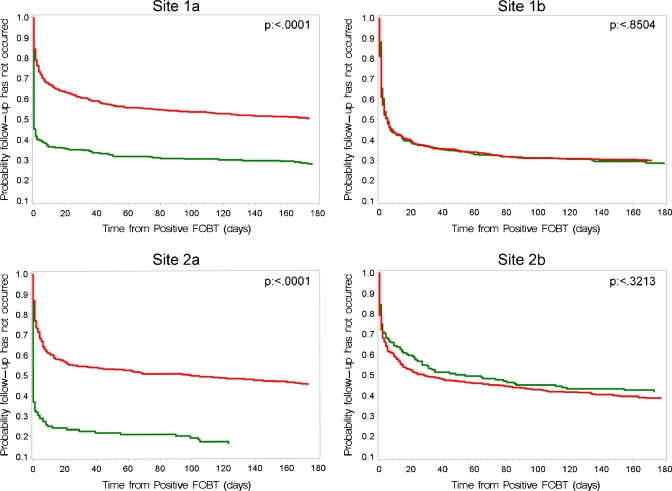
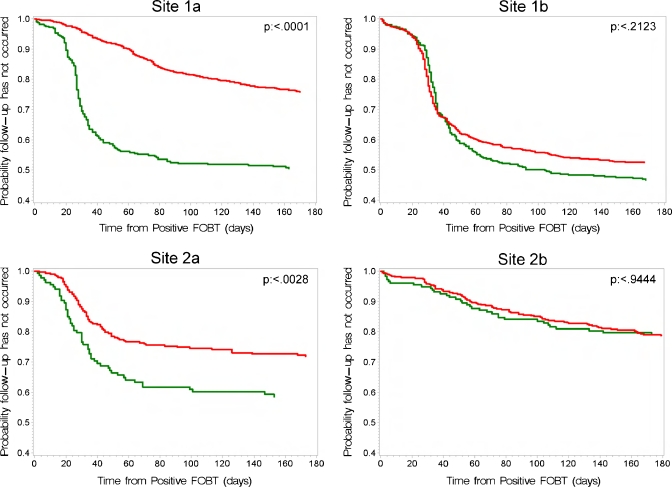
The proportion of patients waiting for GI consultation is shown in Figure 4 for all sites, which shows that the time lag from a FOBT+ result to the scheduled consult is significantly decreased in the post-intervention time period in the intervention sites, but is unchanged in the two usual care VAs. Time to CDE is shown in Figure 5. In both intervention sites, the time to CDE was significantly shortened in the post-intervention period; there was no change in time to CDE in the usual care sites. (log rank test P values are shown in Figures 4 and 5.)
Figure 4.
Log rank analysis of the time to a GI consultation following a positive FOBT.
Figure 5.
Log rank analysis of the time to completion of both a GI consultation and an anatomical assessment following a positive FOBT.
DISCUSSION
We have shown that a simple electronic intervention that involves an automatic GI consult for patients with FOBT+ results improves follow-up and reduces the time between a FOBT+ result and GI evaluation, as well as CDE. Our findings are similar in both intervention sites and are clinically relevant. We do not have data on anatomic or distal health outcomes such as CRC death rates, but believe that our data show a practice change that could only improve the outcomes of CRC screening programs involving FOBTs, given the well-established benefit of FOBT-based CRC screening programs in reducing CRC mortality. The focus groups conducted at each intervention site showed that all providers were uniformly positive about this intervention.
The results achieved in this study compare favorably with results from prior studies evaluating interventions to improve FOBT+ follow-up.11–13 These studies all evaluated multifaceted interventions involving provider education, tracking systems and reminders, and none involved directly notifying GI of FOBT+ results. Our study results suggest that a simple modification of the electronic medical record system that involves directly notifying GI of FOBT+ results can lead to increases in FOBT+ follow-up comparable to those observed in prior studies. This intervention may also be less resource dependent than other interventions to improve follow-up. It is important to note, however, that factors other than referral play a role in patients obtaining appropriate and timely follow-up of FOBT screening tests, including institutional access and patient adherence.
The problem of inadequate follow-up of FOBT+ findings is important and not well recognized. Nationally, only one in three individuals with positive FOBT results undergoes colonoscopy.8 Data from a 120-site study showed that only 59% of patients with a positive FOBT result had any sort of evaluation within 6 months and that the average time until colonoscopy was 252 days after a FOBT+ result.14 Similar results have been shown with Medicare populations.8 Results from our control sites suggest similarly low rates of follow-up in spite of a major national effort in the VA to improve CRC screening and colonoscopy rates. A recent study evaluating 531 patients with newly diagnosed CRC identified 161 patients with at least one missed opportunity to diagnose CRC (or polyps) earlier.12 In addition, they found that FOBT+ results were missed 128 times in 64 patients who waited a median of 146 days after a FOBT+ finding before GI referral. Thus, a program that improves test follow-up has the potential to improve quality of care at the individual as well as the population level.
Failure to follow-up abnormal tests is a major patient safety issue and a source of anxiety for patients and physicians.15,16 One study showed that up to one-third of physicians do not notify patients of abnormal test results.17 Another study showed that only 32% of physicians have a reliable system of test follow-up.17 The Agency for Health Care Research and Quality advises patients that “no news is not good news” and recommends that patients ensure their physicians review laboratory results with them.18
In addition, PCPs are inundated with results. One survey showed that each week, full-time PCPs review 800 laboratory data points, 40 radiology reports and 12 pathology reports.19 This is in the midst of providing ongoing clinical care, including patient phone calls and e-mails. Similar findings were documented in a recent study as well.20 Thus, the opportunity for important test results to be missed is great. Notably, failure to follow up abnormal tests is an important area of medical malpractice and the source of 25% of all malpractice cases.21 The clear advantage of the electronic intervention we are reporting here is in directly notifying the appropriate specialist of a patient’s positive screening results. Effectively, the automatic FOBT+ consult created a redundant system so that positive tests were not missed.
Our study has important limitations. First, half of the sites initially randomized to the study could not be included in the final study because of the logistics of extracting data from the VA record system and limited programming resources. This barrier made the original data analysis plan unusable and limited the possible statistical tests to within site comparisons. This barrier was unexpected and will be important to other investigators who attempt to use the rich data base contained in the electronic medical records of the VA. Second, for unclear reasons, GI referral rates should have been nearly 100%, yet were not. A complicating factor in our study involved the movement of programming and IT supervision from individual VAs to the national VA office in the middle of our study, which decreased local programming flexibility and reduced our ability to interact with programmers at each of the sites. In our pilot test of the intervention in Portland, GI referral rates were above 90% after the intervention was implemented. Third, because of programming limitations, we were not able to evaluate exactly what sort of diagnostic evaluation our patients received, although we were able to date such procedures. We made an assumption that once the patient’s consult was reviewed by a gastroenterologist, the patient’s care would be appropriate for that patient. Finally, in the VA, there is great emphasis on screening patients for CRC, and much of the screening is done at clinic check-in or at a nursing or medical assistant level without careful review of patients’ records. This means that some patients will be screened inappropriately. Thus, even though referred to a gastroenterologist, these patients may be determined by the gastroenterologist or their PCP to be unlikely to benefit from follow-up of a FOBT+ result, which then results in a consult without further colon evaluation. Since the method of identifying patients for FOBT screening varies among VAs and practice settings, the number of inappropriate FOBT screens will vary as well. Such variations may explain differences in rates of follow-up of FOBT+ results at baseline and at follow-up. Because of differences in screening practices at each of the VAs, it was important that PCPs remain “in the loop” and could direct the care of their patients.
We recognize that instituting this intervention in VA medical centers may be more feasible than in non-veteran’s institutions because of our linked medical records and because it is essentially a closed system. However, we believe that other integrated systems and large group practices could create such an intervention. In addition, in these settings, this type of intervention might be more feasible as smaller size might allow more nimble manipulation of the electronic medical record. We also believe that as Accountable Care Organizations are developed, this sort of intervention will be feasible and effective.
In summary, we have shown that an electronic intervention that creates test notification redundancy improves the follow-up of patients with FOBT+ results. We believe that our study provides a model for improving the follow-up of important laboratory tests that is generalizable to many health care systems, as well as to many types of tests. In general, in medical care settings, physicians have relied on creating their own systems of follow-up that typically have relied on diligence and memory, and less often on systematic processes. The process we have created emulates systems in other fields such as the airline industry and anesthesiology, where creation of redundant, “fail-safe” systems improves over-all safety for travelers and patients. It is our belief that as physicians become more inundated with data that might include e-mails, written correspondence and phone calls, as well as results from laboratories, procedures, consultants and imaging, creation of system changes that assure patient safety will become increasingly important “due to man’s limitations as a data processor,” as noted by Clement McDonald in 1976.22
Acknowledgements
This research was funded by VA HSR&D grant number CRT-02-059. This publication was made possible with support from the Oregon Clinical and Translational Research Institute (OCTRI); grant number UL1 RR024140 01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); and NIH Roadmap for Medical Research. The funding organizations had no direct involvement in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. We are grateful for the help of Barbara Genovese, MS; Courtney Maxcy, BS; Paige Farris, MSW; David Pauly, MBA, MSW; David Lieberman, MD; Joan Ash, PhD; Robert Socherman PhD; David Douglas, MD; and Michele Freeman, MPH, in the completion of this study and manuscript. We also acknowledge the help of the many co-investigators and programming staff involved with this study at each of the participating sites.
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Conflict of Interest None disclosed.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328(19):1365–1371. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 4.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348(9040):1472–1477. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 5.Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348(9040):1467–1471. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 6.Jorgensen OD, Kronborg O, Fenger C. A randomised study of screening for colorectal cancer using faecal occult blood testing: results after 13 years and seven biennial screening rounds. Gut. 2002;50(1):29–32. doi: 10.1136/gut.50.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faivre J, Dancourt V, Lejeune C, et al. Reduction in colorectal cancer mortality by fecal occult blood screening in a French controlled study. Gastroenterology. 2004;126(7):1674–1680. doi: 10.1053/j.gastro.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Lurie JD, Welch HG. Diagnostic testing following fecal occult blood screening in the elderly. J Natl Cancer Inst. 1999;91(19):1641–1646. doi: 10.1093/jnci/91.19.1641. [DOI] [PubMed] [Google Scholar]
- 9.Fisher DA, Jeffreys A, Coffman CJ, et al. Barriers to full colon evaluation for a positive fecal occult blood test. Cancer Epidemiol Biomark Prev. 2006;15(6):1232–1235. doi: 10.1158/1055-9965.EPI-05-0916. [DOI] [PubMed] [Google Scholar]
- 10.Rao SK, Schilling TF, Sequist TD. Challenges in the management of positive fecal occult blood tests. J Gen Intern Med. 2009;24(3):356–360. doi: 10.1007/s11606-008-0893-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miglioretti DL, Rutter CM, Bradford SC, et al. Improvement in the diagnostic evaluation of a positive fecal occult blood test in an integrated health care organization. Med Care. 2008;46(9 Suppl 1):S91–S96. doi: 10.1097/MLR.0b013e31817946c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh H, Daci K, Petersen LA, et al. Missed opportunities to initiate endoscopic evaluation for colorectal cancer diagnosis. Am J Gastroenterol. 2009;104(10):2543–2554. doi: 10.1038/ajg.2009.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myers RE, Turner B, Weinberg D, et al. Impact of a physician-oriented intervention on follow-up in colorectal cancer screening. Prev Med. 2004;38(4):375–381. doi: 10.1016/j.ypmed.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Etzioni DA, Yano EM, Rubenstein LV, et al. Measuring the quality of colorectal cancer screening: the importance of follow-up. Dis Colon Rectum. 2006;49(7):1002–1010. doi: 10.1007/s10350-006-0533-2. [DOI] [PubMed] [Google Scholar]
- 15.Meza JP, Webster DS. Patient preferences for laboratory test results notification. Am J Manag Care. 2000;6(12):1297–1300. [PubMed] [Google Scholar]
- 16.Murff HJ, Patel VL, Hripcsak G, Bates DW. Detecting adverse events for patient safety research: a review of current methodologies. J Biomed Inform. 2003;36(1–2):131–143. doi: 10.1016/j.jbi.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Boohaker EA, Ward RE, Uman JE, McCarthy BD. Patient notification and follow-up of abnormal test results. A physician survey. Arch Intern Med. 1996;156(3):327–331. doi: 10.1001/archinte.156.3.327. [DOI] [PubMed] [Google Scholar]
- 18.20 Tips to Help Prevent Medical Errors. Patient Fact Sheet. AHRQ Publication No. 00-PO38, February 2000. Agency for Healthcare Research and Quality, Rockville, MD. http://www.ahrq.gov/consumer/20tips.htm. Accessed January 10, 2011.
- 19.Poon EG, Wang SJ, Gandhi TK, Bates DW, Kuperman GJ. Design and implementation of a comprehensive outpatient results manager. J Biomed Inform. 2003;36(1–2):80–91. doi: 10.1016/S1532-0464(03)00061-3. [DOI] [PubMed] [Google Scholar]
- 20.Baron RJ. What’s keeping us so busy in primary care? A snapshot from one practice. N Engl J Med. 1636;362(17):1632. doi: 10.1056/NEJMon0910793. [DOI] [PubMed] [Google Scholar]
- 21.Kravitz RL, Rolph JE, Petersen L. Omission-related malpractice claims and the limits of defensive medicine. Med Care Res Rev. 1997;54(4):456–471. doi: 10.1177/107755879705400404. [DOI] [PubMed] [Google Scholar]
- 22.McDonald CJ. Protocol-based computer reminders, the quality of care and the non-perfectability of man. N Engl J Med. 1976;295(24):1351–1355. doi: 10.1056/NEJM197612092952405. [DOI] [PubMed] [Google Scholar]