Abstract
Within most terrestrial groups of animals, including mammals, species richness varies along two axes of environmental variation, representing energy availability and plant productivity. This relationship has led to a search for mechanistic links between climate and diversity. Explanations have traditionally focused on single mechanisms, such as variation in environmental carrying capacity or evolutionary rates. Consensus, though, has proved difficult to achieve and there is growing appreciation that geographical patterns of species richness are a product of many interacting factors including biogeographic history and biological traits. Here, we review some current hypotheses on the causes of gradients in mammal richness and range sizes since the two quantities are intimately linked. We then present novel analyses using recent datasets to explore the structure of the environment–richness relationship for mammals. Specifically, we consider the impact of glaciation on present day mammalian diversity gradients. We conclude that not only are multiple processes important in structuring diversity gradients, but also that different processes predominate in different places.
Keywords: species richness, range size, climate change, diversity, glaciation
1. Introduction
The search for a universal law explaining spatial variation in species richness is a major focus of biological endeavour: termed ‘The Holy Grail of Ecology’ [1], it is a common feature within listings of the top unanswered questions in science (e.g. [2,3]). At broad geographical scales, species richness among higher taxa covaries closely (e.g. [4]) and correlates with similar climate variables often related to environmental energy and plant productivity [5]. The ubiquity of the latitudinal richness gradient has focused much effort on the search for a common cause, such as latitudinal variation in species carrying capacity [6,7], evolutionary rates [7], times for speciation [8–10], or some combination thereof [11]. However, there is increasing appreciation that geographical patterns of species richness are likely to be a product of many interacting factors, including biological traits, historical and contemporary biogeography, as well as environment (e.g. [12–15]). Consequently, our understanding of the relationship between richness and the increasing number of putative causal factors has become more nuanced.
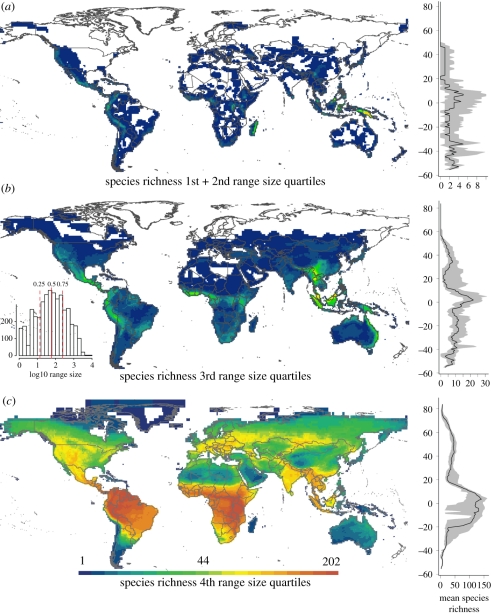
The distribution of mammalian diversity is not a smooth gradient (figure 1), and it has been suggested that different drivers of species richness may apply at different latitudes or biogeographic regions [16–20], indicating the importance of historical contingency (e.g. dispersal events and local speciation and extinction regimes) in mediating the environment–richness relationship [19,21,22]. Ecological and evolutionary responses will vary with the temporal and spatial scales at which historical factors are experienced. At regional scales, disturbance events of long periodicity but with large impacts (e.g. glaciations) might be most important. Here, we focus on these low-frequency high-amplitude climate perturbations: we briefly review their likely evolutionary and ecological effects, and use recent data on mammal species' distributions to consider their importance in shaping present-day patterns of species richness and the environment–richness relationship for mammals.
Figure 1.
The distribution of mammalian species richness for (a) narrow-ranged species in the bottom half of the range-size distribution (inset); (b) species in the third quartile of the range-size distribution; and (c) species in the upper quartile of the range-size distribution. A latitudinal gradient in mean richness (black) is presented to the right of each map, with the interquartile range of richness given by the grey polygon.
2. Climate and glaciation
Glacial history has long been thought important in shaping regional diversity patterns [23,24]. Climate has been highly cyclical during the late Tertiary and the Quaternary, driven by Milankovitch oscillations—periodical shifts in the Earth's orbit and axial tilt that change the amount and distribution of received insolation. Importantly, the strength of Quaternary glacial–interglacial cycles shows a strong latitudinal gradient, being greater away from the tropics [25]. Quaternary climate oscillations might have impacted species richness via two pathways. First, if richness accumulates over time, then communities that persisted through glacial cycles might be older and hence more species rich—the ‘available time theory’ [23]. Second, Quaternary oscillations may have influenced net diversification rates by increasing extinction rates as species failed to disperse or lost their habitat, and reducing speciation rates, with diverging gene pools becoming re-integrated as species tracked shifting climates [25,26].
The impact of oscillations in Quaternary climate on rates of speciation and extinction may be evident in the distribution of species' range sizes. For example, range sizes might be larger where climate oscillations were greater because large range size provided a buffer against extinction. Large-ranged species are stochastically more likely to occupy what become refuge locations or habitats, and may be composed of a greater number of individuals [27]. In addition, the postglacial recolonization process may have favoured good dispersers or habitat generalists, and hence species with a biology that predisposes them to eventual broad geographical ranges [28–31].
We compare here predictions and empirical data on mammal species richness and the distribution of their range sizes to evaluate the influence of past and present environment on contemporary diversity gradients. First, we test for a latitudinal trend in species range extents (Rapoport's rule [32]) and discuss predictors of species geographical range sizes. Second, we evaluate whether the functional relationship between climate and richness—the environment–richness relationship—differs between high- and low-latitude communities.
3. Variations in the geographical range size of species
As is the case for species richness, variation in species' range sizes can be attributed to a variety of mechanisms (e.g. [27,28,33]). The geographical distribution of species' range sizes is highly non-random (figure 1). Terrestrial mammals with the smallest ranges are mostly restricted to the tropics, whereas species with the largest ranges are found across high northern latitudes [34,35]. The tendency for species' latitudinal range extents to increase toward the poles was termed Rapoport's rule [32]. As originally framed, the rule reflects the greater seasonal variability of high latitude environments—species adapted to this greater annual temperature range can occupy a correspondingly broad latitudinal extent [32]. Empirical support for the ‘rule’ is mixed, and it has increasingly been regarded as a regional phenomenon, most pronounced at higher northern latitudes [29,33,36]. Price et al. [31] and Brown [28] suggested that Rapoport's observation was therefore better explained by longer-term climate cycles, consistent with Milankovitch oscillations which demonstrate a similar hemispheric bias, being more pronounced in the North [33].
(a). A test of Rapoport's rule in mammals
We derived latitudinal extents for terrestrial mammals from the database on species distributions published in Grenyer et al. [4]. We compare the empirical distribution of range extents to a null generated by randomly shuffling range locations across latitudes, following the approach implemented in RangeModel [37] using the ‘empirical range size frequency distribution, random midpoints’ option. First, we restricted the maximum and minimum latitudinal extents of the analysis to the union domain of all extant mammals. Second, to evaluate congruence in latitudinal trends among higher taxa, we generated separate null distributions for each of the five most species-rich mammal orders: Rodentia, Chiroptera, a paraphyletic traditional ‘Insectivora’, Carnivora and Artiodactyla. Deviation from expectations in each of the three cases was assessed by contrasting mean range extent for 1000 simulations against the empirical mean value in each latitudinal band.
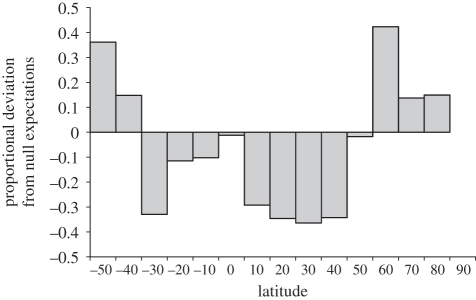
We observe a Rapoport-like pattern for terrestrial mammals, latitudinal range extents are greatest at mid to high latitudes and narrower at more equatorial latitudes (figure 2), but a balance of evidence (compare figures 2 and S1) confirms the ‘rule’ to be most pronounced in the Northern Hemisphere. A similar pattern is evident among each of the major, most species-rich, mammal orders (see the electronic supplementary data, figure S1). While absolute range extents are greatest in the Northern Hemisphere [34,35], at high southern latitudes, where glacial encroachment and desertification was also evident at the last glacial maximum (LGM), range extents are also larger than expectations from domain boundaries. Our results are consistent with the impact of Quaternary climate oscillations on range extents, but they do not provide a direct test. For example, it is possible that geographical variation in range sizes simply reflect the available area or size of biomes [38,39], or the biological traits characterizing the clades that have radiated in different biomes. However, in the absence of comprehensive data on palaeontological distributions (e.g. [40,41]), differentiating among the various putative drivers of range size to evaluate these alternative hypotheses has proved challenging.
Figure 2.
Mean deviation in latitudinal range extent from null expectations generated from 1000 replicates of randomly shuffling species range extents. Bar heights represent the proportional difference between mean predicted range extent and empirical range extents ([empirical–predicted]/whichever was the larger) in 10° latitudinal bands, positive values indicate greater than predicted extents, negative values indicate narrower than predicted extents. Southern Hemisphere represented by negative latitudes.
(b). A short note on studying variation in range size
Dispersal ability is the biological trait most frequently linked to the size of a species' geographical range (e.g. [42,43]). Although we do not currently have detailed data on dispersal for many species, for active dispersers such as mammals dispersal is positively correlated with body size [44]. Because body size covaries closely with phylogeny [45], we might also expect range size to show strong phylogenetic signal. However, current evidence suggests that closely related species are only marginally more likely to share similar range sizes than by chance [46]. If there is an association between body size and range size, why do we observe strong phylogenetic signal in body mass but not range size? Further, if geographical range does not covary with phylogeny, are we then justified in using non-phylogenetic methods to search for predictors of range size?
Because the most common mode of speciation is geographical, with populations diverging in allopatry, daughter species do not simply inherit geographical range size from the ancestor; rather, ancestral area is apportioned between daughter lineages in a manner reflecting the process of speciation. For vicariant speciation, ancestral ranges might be split more or less arbitrarily among daughter lineages [47]. For peripatric speciation (i.e. speciation by peripheral isolates), the newly formed species would have small initial geographical range, and the ancestral range would be little changed—resulting in large asymmetries in range size between sister species [48]. The evolutionary model of range size heritability therefore follows a different trajectory to genetically determined biological traits, such as body size [49]. Nonetheless, range size might still covary with phylogeny if biological traits determine the potential for range expansion, and there has been sufficient time for range size to reach equilibrium [46].
Phylogenetically informed approaches should be considered when there is a phylogenetic signal in any variable not included in the analysis but which might additionally influence species' responses, even when the response (in this case, range size) is not directly heritable [50–52]. Because there remains large uncertainty as to the key traits that influence range size [53], phylogeny should be considered in any search for mechanistic links between traits and range area. In addition, there may be strong phylogenetic conservatism in range location and associated environmental attributes [10,54], which might limit species' range extents. Environment may therefore explain both the mixed signal linking dispersal ability with geographical range [53] and apparent low phylogenetic heritability of range size.
(c). Shifting climate and shifting ranges
In a recent study, Davies et al. [35] used phylogenetic contrasts between mammal sister-species to explore the relationship between Quaternary climate oscillations and range extent. Sister-species contrasts provide a robust method for controlling for phylogenetic non-independence while avoiding pitfalls associated with reconstructing ancestral traits to compare differences between nodes deeper in the phylogenetic tree. Species with larger geographical ranges were found to occupy more habitat types; hence range size cannot be simply explained by variation in areas of suitable habitat. In high northern latitudes, the size of a species' geographical range was best predicted by the magnitude of warming that it had experienced across its distribution since the LGM. However, Davies and colleagues suggest that the relationship between range size and climate variability was direct, and after correction for area, showed that species at high latitudes tended to be more generalist where historical temperature fluctuations were of low amplitude. Biomes that experienced large fluctuations in temperature and/or those that were previously glaciated at the LGM have mammal faunas with, on average, large geographical ranges, because small-ranged species are largely absent from their present-day biotas.
Quaternary oscillations at high latitudes are most evident as changes in temperature and glacial extent. During glacial maxima, small ranged species may have been driven locally or globally extinct [8,55]. Following glacial retreats and global warming, good dispersers and habitat generalist may have been able to rapidly recolonize this competition free space. There is strong supporting evidence for this process in the contemporary distribution of European reptiles and amphibians [56], where the 0°C isotherm at the LGM follows very closely the northern limit of the contemporary distribution of species in the bottom quartile of the European range-size distribution. It is interesting to note the similarity of the northern limit in Europe of the narrow-ranging species in figure 1 with the LGM 0°C isotherm shown by Araújo et al. [56].
Quaternary temperatures were much less variable in the tropics [57]; nonetheless, equatorial biomes were far from constant and experienced large shifts in precipitation due to changes in global ocean currents and atmospheric conditions. For example, during the LGM, significant drying expanded the Saharan and Namib deserts, and reduced tropical forest cover in equatorial Africa and the Amazon [58]. By focusing on species distributed within more equatorial latitudes, Davies et al. [35] were able to show that species' range sizes tend to be larger where Quaternary rainfall patterns had been most stable. Within the tropics, Quaternary oscillations may therefore have influenced species' ranges and community composition via range contractions, but resulted in few extinctions, certainly relative to biotas at higher latitudes, where the majority of the landscape was denuded under many hundreds of metres of ice (the Late Devensian British–Irish ice sheet, for example, was in places over 1 km thick [59]). Following climate reversals, range expansion in the tropics may have been inhibited by biotic factors such as competition, mutualism and parasitism—long thought important in limiting species' abundances and distributions in the tropical biome [60,61]. The rapid postglacial range expansion witnessed at higher latitudes was not possible because there was no equivalent to the competition-free landscapes made available by retreating glaciers. Within more climatically stable tropical environments, species were able to maintain larger geographical ranges, explaining the opposing relationship between range size and climate change observed for high- versus low-latitudes.
We suggest that the differing climatic histories and species' responses at high versus low latitude biomes, evident in the distribution of species' geographical range extents, might also shape the structure of their regional communities. For example, we would predict that tropical communities will be composed of a greater proportion of narrow-ranged habitat specialist, and constraints to local diversity may be imposed by biotic interactions and ecological limits to environmental carrying capacity [60,61]. In contrast, we predict high-latitude communities will be composed of broad-ranged species with wide habitat preferences, and local diversity may be unsaturated because of recent, glacially induced, extinctions [8,55]. Because richness at the local and the regional scale is, at least in part, interdependent [62], we might then also expect concomitant regional variation in the richness–environment relationship.
4. Evaluating the richness–environment relationship
There is an extensive literature supporting a close correlation between species richness and productivity or water-energy dynamics [5,18,19]. A common approach has been to use multiple regression to explore correlation strengths among multiple climate variables, their various transformations and the interaction terms between them, to distinguish between alternative hypotheses (e.g. [18]). An increasingly sophisticated set of statistical models have been developed to help analyse spatial data and account for the spatial autocorrelation inherent within them (see [63]). Nonetheless, even more recent empirical studies that have considered regional or historical effects (e.g. in plants [64]; birds [14,65]; amphibians [21]) assume that spatial autocorrelation and effects of environmental correlates are constant, i.e. spatial stationarity.
Geographically weighted regression (GWR) [66] provides one method to accommodate non-stationarity by allowing parameter values to vary in space, with local coefficients estimated by assigning higher weights to nearby observations than more distant ones. In the search for global mechanisms linking richness and climate, spatial stationarity is implicitly assumed, but when this assumption is not met, interpretation of coefficients might mislead (e.g. [67]). Because, by design, coefficients from GWR vary with location, it has only limited use for discriminating among alternative models, as has been the standard practice in more traditional hypotheses testing [63,68]. However, by exploring the spatial pattern of local coefficients, GWR may help in identifying missing variables, which could then be evaluated within a standard regression framework.
(a). Methods
Here we explore the relationship between terrestrial mammal species richness within equal area (100 × 100 km) grid cells, approximately equivalent to 1 × 1° at equatorial latitudes, and three key environmental predictors: temperature [69], actual evapotranspiration (AET [70]) and annual temperature range (seasonality; calculated as the change in temperature between the warmest and coolest months). We collated digital raster datasets at a 0.5 × 0.5° resolution for mean annual temperature and AET. These two variables, encapsulating environmental energy and water-energy dynamics, might be key predictors if evolutionary speed (sensu Rohde [7]) or productivity set limits to species richness. In addition, we considered one index of historical climatic variability, estimated as the change in mean annual temperature since the LGM (see [71]). The climates of the present interglacial and of the LGM (21 kyr ago) represent near extremes in Milankovitch oscillations, therefore, changes in climate between the present and the LGM can be used as a proxy for spatial patterns for the last millions of years (i.e. the Pleistocene and the Quaternary) [72]. Past and present annual surface air temperatures were derived from the UGAMP [73] general circulation model, at a resolution of 2.8 × 2.8°. We re-sampled the UGAMP data at 1 × 1° resolution using cubic convolution interpolation [74]. Alternative re-sampling algorithms (e.g. nearest neighbour) had negligible influence on estimated cell values (assessed by linear regression, all pairwise r2 > 0.99). Raster data manipulation was performed using the ArcView Spatial Analyst (ESRI 2002, v.2.0a) and Grid Machine [75] extensions.
First, we generated a series of single predictor regression models (table 1) of species richness against each variable in turn, using a generalized linear model with Poisson errors. As an index of model explanatory power, we estimated a ‘pseudo-r2’ as the per cent deviance explained. Second, we constructed a multiple regression model with the three key contemporary environment measures (AET, temperature and seasonality) as predictor variables. Third, we reran the multiple regression model but also including our measure of historical climate change among the predictors.
Table 1.
Single predictor regression models of species richness against temperature, AET and short- and long-term climatic oscillations (seasonality and climate change, respectively) across a grid of 100 × 100 km cells, using generalized linear models with Poisson errors. All models significant at p < 0.01.
| explanatory variable | coefficient | z | pseudo r2 |
|---|---|---|---|
| temperature | 3.523E−03 | 363.5 | 0.31 |
| AET | 1.113E−03 | 558.5 | 0.60 |
| seasonality | −3.811E−03 | −437.9 | 0.46 |
| climate change | −0.0524059 | −320.2 | 0.26 |
Next, we evaluated how estimates of model coefficients might be sensitive to spatial structure in the data by constructing an additional series of regressions using maximum-likelihood spatial autoregressive models (SARs). We implemented error dependence models to account for spatial-autocorrelation in the error term [76]. Longitude and latitude were used to develop neighbourhoods with threshold distances of 2000 and 4000 km. Threshold distances were selected by examining correlograms. Neighbours were weighted a priori using row standardization, such that the weights of all neighbours within the threshold distance sum to 1 [76]. We used 25 per cent of the database for spatial analysis due to memory limitations on size of the covariance matrix, sampling one in every four cells using an evenly spaced lattice. Spatial autocorrelation of the model residuals was evaluated using Moran's I. Both generalized linear and SAR models (R package spdep [77]) were constructed in R (R Development Core Team 2004) and are presented as table 2.
Table 2.
Multiple regressions of species richness against various combinations of environmental predictor variables (see Table 1).
| model | model r2 | explanatory variable | coefficient | z | p-value |
|---|---|---|---|---|---|
| GLM | 0.63 | temperature | 3.800e−04 | 20.58 | <2e−16 |
| AET | 8.429e−04 | 290.73 | <2e−16 | ||
| seasonality | −1.155e−03 | −64.21 | <2e−16 | ||
| SARa | n.a. | temperature | 3.7164e−03 | 8.5498 | <2e−16 |
| AET | 4.5875e−03 | 54.2796 | <2e−16 | ||
| seasonality | 4.1664e−05 | 0.0966 | 0.923 | ||
| GLM | 0.64 | temperature | −8.868e−05 | −4.078 | 4.54e−05 |
| AET | 8.421e−04 | 290.042 | <2e−16 | ||
| seasonality | −1.110e−03 | −61.783 | <2e−16 | ||
| climate change | −1.004e−02 | −40.333 | <2e−16 | ||
| SARa | n.a. | temperature | 2.1932e−03 | 4.1638 | 3.129e−05 |
| AET | 4.5640e−03 | 54.1079 | <2.2e−16 | ||
| seasonality | −1.4365e−05 | −0.0334 | 0.9734 | ||
| climate change | −2.8724e−02 | −5.0825 | 3.725e−07 |
aBecause of computational limitations, SAR models were generated using 25% of the database sampling one in every four cells using an evenly spaced lattice.
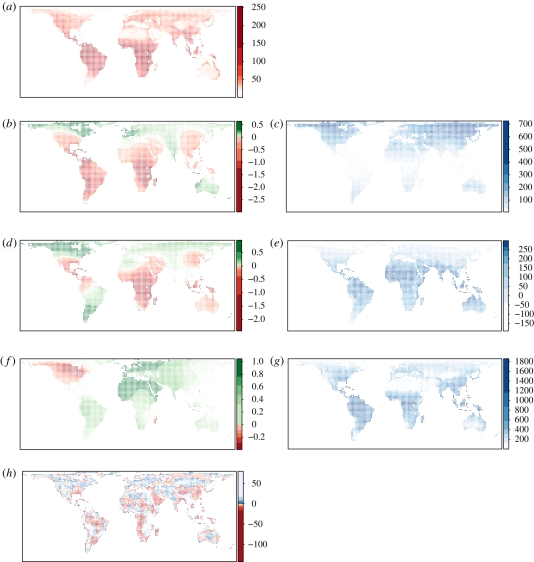
Last, we used GWR to explore the changing relationship between environment and species richness and spatial variation in coefficient estimates from local regressions. Weighted regression models were constructed in SAM (http://www.ecoevol.ufg.br/sam/ [78]) following Svenning et al. [79], using a bi-squared kernel function and the square-root of species richness as the response. Again, a regular sample of 25 per cent of the data was used across the domain. We used a local neighbourhood of 1000 km radius, which we consider reasonable distances given the continental analytical scales (see also SAR model details, above). Extending the local neighbourhood to 2000 km did not qualitatively change spatial trends (not presented). Maps of component variation in model terms are presented as figure 3.
Figure 3.
Geographical variation in regression of standardized coefficients from the geographically weighted regression (GWR) of mammalian species richness (a) on three environmental input variables: (b,c) temperature variation, (d,e) mean temperature and (f,g) AET. (b,d,f) Standardized coefficient of variation (red–green) of the input variable to the right (c,e,g; blue). (h) Residuals in response from the overall GWR (quantile shaded about zero; red–blue).
(b). Results
Species richness was significantly correlated with each of our environmental variables in the single predictor GLMs (table 1). Over 63 per cent of the variation in global species richness could be explained by our simple additive model including AET, temperature and seasonality. Richness was positively correlated with temperature and AET, with the latter the best single predictor (z = 558.5, pseudo-r2 = 0.60; table 1). Species richness was negatively correlated with both long-term (glacial-interglacial) and short-term (seasonal) climate cycles; however, the significant correlation with seasonality was lost after correcting for spatial autocorrelation (table 2). When we include change in temperature since the LGM in the model, we get a much better fit to the data (Δ Akaike Information Criterion (AIC) 1651), but the sign of the relationship with temperature switches in the GLMs so that cooler climates appear more species-rich (table 2). In addition, AIC favours the inclusion of 2-, 3- and 4-way interaction terms in the GLMs (data not shown), suggesting a complex relationship between species richness and environment. This complexity is not easily reconcilable with simple mechanistic explanations linking diversity and climate.
Complex environment–richness relationships are readily explainable if the actual relationship between richness and environment takes a different form in different regions. Non-stationarity provides a good explanation for the apparent complexity of the environment–richness relationship depicted in the GLMs and the sensitivity of coefficient estimate to modelled spatial structure in the data. As shown in figure 3, our GWRs reveal large spatial variation in the relationship between richness and contemporary environment (AIC corrected for small sample size, AICc = 5926 versus 14 661 for ordinary least squares (OLS), and F = 90.86 for the improvement in performance of GWR over OLS, p < 0.01). At high northern latitudes, local slopes for temperature are positive, but at more equatorial latitudes the relationship between temperature and richness differs between the Old and New Worlds. In Africa and Asia, tropical richness is negatively correlated with temperature, whereas at equivalent latitudes in the New World, including the wet tropics of the Amazon basin, local slopes with temperature remain positive. In addition, we observe a striking relationship between AET and richness in North America, where a negative correlation is evident across most of the continent, contrasting with the broadly positive slopes for the rest of the Nearctic, and indeed the world. AET also provides an illustration of the power of GWR to detect local gradients, for example, in Madagascar (the only other part of the world where AET correlates negatively with richness) where mammalian richness peaks in the southern xerophyllic scrubland and spiny forests.
(c). Discussion
The relationship between richness and environment varies spatially, perhaps explaining sensitivity of global models. Hawkins et al. [18] also reported a shift in the relative importance of environmental predictors of animal species richness, with water-energy variables (represented by AET in our analysis) predominant in the tropics, and ambient energy becoming more important towards the poles. Hawkins and colleagues suggested that energetic constraints might be more important in limiting diversity at high latitudes. Although we do not observe a general latitudinal trend in the relationship between richness and productivity, the local slopes for temperature shifts from negative to positive, moving from mid- to high latitudes. We propose that the spatial trend in GWR slopes reflects a shift in the mechanisms linking richness to climate. For example, temperature-dependent dynamics might be more important for regions where richness has been eroded via a history of glacial cycles, whereas ecological processes related to niche partitioning and competition might set limits to diversity in regions which have been more stable in the long term. Further, examination of the mapped residuals from the GWR suggests a strong role for topographic variation in modulating the environmental determination of species richness worldwide.
Why do the Americas depart from the trends observed in the rest of the world? We suggest that the two biogeographical phenomena thought to have been critical in defining New World faunal assemblages might explain the difference: megafaunal extinction, and the Great American Faunal Interchange. Following the LGM, much of the megafauna that populated North America disappeared between 10 000–18 000 years ago (see also [80]). One explanation for these recent extinctions is human range expansion and hunting—the ‘Pleistocene overkill hypothesis’ of Martin [81]. The overkill hypothesis remains controversial (e.g. [82,83]); but, whatever the underlying cause, American megafaunal diversity was greatly reduced and perhaps depressed below the carrying capacity of the environment, decoupling the link between richness and productivity. Because of the relatively recent timing of this extinction event, evolutionary processes may not yet have had time to compensate via increased diversification rates.
The South America mammal fauna might also represent a non-equilibrium biota, but with deeper temporal roots. The formation of the Isthmus of Panama around 3 Ma catalysed the Great American Interchange [84]—providing the opportunity for North American fauna to migrate southwards, and South American fauna to migrate northwards. Although movement of species between these two previously isolated landmasses was initially reciprocal, with only few exceptions (e.g. the Virginia opossum, Didelphis virginiana), neotropical lineages failed to persist and thrive in the Neartic; whereas a number of North American clades radiated successfully in the neotropics (e.g. sigmodontine rodents, canids and cervids) [84]. The neotropical mammal fauna therefore represents a young dynamic biota (see also [34]), with many rapid radiations of recently arrived lineages, reconfiguring the slope of the richness–environment relationship [54].
5. Conclusions
Species range extents tend to be narrower at more tropical latitudes and wider at high latitudes, especially in the Northern Hemisphere, demonstrating a clear ‘Rapoport-like’ pattern. We compared the empirical distribution of species range extents with an expected null assuming that species with large distributions, approaching the size of a bounded domain, are constrained to have their centre point near the centre of the domain [85]. Deviation from null expectations may be sensitive to how a domain is delimited; however, we show that the relationship between latitude and range extent has arisen independently among multiple taxa occupying different parts of the world and assuming different domain boundaries. Phylogenetic analysis reveals range size at high latitudes to correlate strongly with the magnitude of Quaternary temperature oscillations, indicating that Rapoport's rule may be a product of long-term climate cycles (e.g. [33]) rather than seasonal variability.
Our analysis indicates that the processes regulating species richness also vary spatially, reflecting both the biogeographical and climatic histories of regions. We explored the species richness–environment relationship using four key variables: present day temperature, AET, seasonality and historical climate change, selected to represent the influences of environmental energy, productivity, short- and long-term climatic oscillations, respectively. At high northern latitudes, species richness correlates strongly and positively with temperature and seasonality. However, the environment–richness relationship is quite different where Quaternary temperatures were less variable. We suggest that at high latitudes, Quaternary climate oscillations may have depressed species numbers below the carrying capacity of the environment (e.g. via range contractions and the extinction of small-ranged species during glaciations). At these latitudes, temperature-dependent dynamics or energetic constraints might be more important in setting limits to diversity [16,18]. In contrast, the greater climatic stability and higher productivity of tropical latitudes may have allowed for persistence of small-ranged specialists and greater regional species diversity in the face of increasing local saturation of niche space and competition [55,60].
We show that single environment-richness models are probably insufficient to explain diversity gradients across regions that differ in their climatic and biogeographical histories. It remains possible, and is in fact probable, that multiple additional environmental and historical factors not included in our models are important in explaining regional variation. For example, we suggest that New World mammal biodiversity retains the imprint of biogeographical processes over the past few millions of years. In addition, we note that the spatial distribution of phylogenetic and functional diversity is therefore likely to demonstrate an equally heterogeneous relationship with environment, but not necessarily congruent with that for species richness [86]. Current global environment-richness models do not adequately capture this complex regional variation in the relationship between diversity and climate.
Acknowledgements
We thank Kate Jones and Kamran Safi for the invitation to contribute to this volume, and two anonymous reviewers for comments on an earlier version of this manuscript.
References
- 1.Huston M. A. 1994. Biological diversity: the coexistence of species on changing landscapes. Cambridge, UK: Cambridge University Press [Google Scholar]
- 2.Kennedy D. 2005. 125. Science 309, 19. 10.1126/science.1115951 (doi:10.1126/science.1115951) [DOI] [PubMed] [Google Scholar]
- 3.Adams J. 2009. Species richness: patterns in the diversity of life. Chichester, UK: Springer [Google Scholar]
- 4.Grenyer R., et al. 2006. Global distribution and conservation of rare and threatened vertebrates. Nature 444, 93–96 10.1038/nature05237 (doi:10.1038/nature05237) [DOI] [PubMed] [Google Scholar]
- 5.Currie D. J., et al. 2004. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett. 7, 1121–1134 10.1111/j.1461-0248.2004.00671.x (doi:10.1111/j.1461-0248.2004.00671.x) [DOI] [Google Scholar]
- 6.Hutchinson G. E. 1959. Homage to Santa Rosalina or why are there so many kinds of animals? Am. Nat. 93, 145–159 10.1086/282070 (doi:10.1086/282070) [DOI] [Google Scholar]
- 7.Rohde K. 1992. Latitudinal gradients in species-diversity— the search for the primary cause. Oikos 65, 514–527 10.2307/3545569 (doi:10.2307/3545569) [DOI] [Google Scholar]
- 8.Pianka E. R. 1966. Latitudinal gradients in species diversity: a review of the concepts. Am. Nat. 100, 33–46 10.1086/282398 (doi:10.1086/282398) [DOI] [Google Scholar]
- 9.Pianka E. R. 1989. Latitudinal gradients in species diversity. Trends Ecol. Evol. 4, 223. 10.1016/0169-5347(89)90163-8 (doi:10.1016/0169-5347(89)90163-8) [DOI] [Google Scholar]
- 10.Wiens J. J., Donoghue M. J. 2004. Historical biogeography, ecology, and species richness. Trends Ecol. Evol. 19, 639–644 10.1016/j.tree.2004.09.011 (doi:10.1016/j.tree.2004.09.011) [DOI] [PubMed] [Google Scholar]
- 11.Jablonski D., Roy K., Valentine J. W. 2006. Out of the tropics: evolutionary dynamics of the latitudinal diversity gradient. Science 314, 102–106 10.1126/science.1130880 (doi:10.1126/science.1130880) [DOI] [PubMed] [Google Scholar]
- 12.Whittaker R. J., Willis K. J., Field R. 2001. Scale and species richness: towards a general, hierarchical theory of species diversity. J. Biogeogr. 28, 453–470 10.1046/j.1365-2699.2001.00563.x (doi:10.1046/j.1365-2699.2001.00563.x) [DOI] [Google Scholar]
- 13.Willig M. R., Kaufman D. M., Stevens R. D. 2003. Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 34, 273–309 10.1146/annurev.ecolsys.34.012103.144032 (doi:10.1146/annurev.ecolsys.34.012103.144032) [DOI] [Google Scholar]
- 14.Hawkins B. A., Diniz-Filho J. A. F., Jaramillo C. A., Soeller S. A. 2007. Climate, niche conservatism, and the global bird diversity gradient. Am. Nat. 170, 16–27 10.1086/519009 (doi:10.1086/519009) [DOI] [PubMed] [Google Scholar]
- 15.Mittelbach G. G., et al. 2007. Evolution and the latitudinal diversity gradient: speciation, extinction and biogeography. Ecol. Lett. 10, 315–331 10.1111/j.1461-0248.2007.01020.x (doi:10.1111/j.1461-0248.2007.01020.x) [DOI] [PubMed] [Google Scholar]
- 16.Kerr J. T., Packer L. 1997. Habitat heterogeneity as a determinant of mammal species richness in high-energy regions. Nature 385, 252–254 10.1038/385252a0 (doi:10.1038/385252a0) [DOI] [Google Scholar]
- 17.Kerr J. T. 1999. Weak links: ‘Rapoport's rule’ and large-scale species richness patterns. Global Ecol. Biogeogr. 8, 47–54 10.1046/j.1365-2699.1999.00315.x (doi:10.1046/j.1365-2699.1999.00315.x) [DOI] [Google Scholar]
- 18.Hawkins B. A., et al. 2003. Energy, water, and broad-scale geographic patterns of species richness. Ecology 84, 3105–3117 10.1890/03-8006 (doi:10.1890/03-8006) [DOI] [Google Scholar]
- 19.Hawkins B. A., Porter E. E., Diniz-Filho J. A. F. 2003. Productivity and history as predictors of the latitudinal diversity gradient of terrestrial birds. Ecology 84, 1608–1623 10.1890/0012-9658(2003)084[1608:PAHAPO]2.0.CO;2 (doi:10.1890/0012-9658(2003)084[1608:PAHAPO]2.0.CO;2) [DOI] [Google Scholar]
- 20.Kisel Y., McInnes L., Toomey N. H., Orme C. D. L. 2011. How diversification rates and diversity limits combine to create large-scale species–area relationships. Phil. Trans. R. Soc. B 366, 2514–2525 10.1098/rstb.2011.0022 (doi:10.1098/rstb.2011.0022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buckley L. B., Jetz W. 2007. Environmental and historical constraints on global patterns of amphibian richness. Proc. R. Soc. B 274, 1167–1173 10.1098/rspb.2006.0436 (doi:10.1098/rspb.2006.0436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian H. 2009. Global tests of regional effect on species richness of vascular plants and terrestrial vertebrates. Ecography 32, 553–560 10.1111/j.1600-0587.2008.05755.x (doi:10.1111/j.1600-0587.2008.05755.x) [DOI] [Google Scholar]
- 23.Baker H. G. 1960. Evolution in the tropics. Biotropica 2, 101–111 10.2307/2989767 (doi:10.2307/2989767) [DOI] [Google Scholar]
- 24.Whittaker R. H. 1965. Dominance and diversity in land plant communities. Science 147, 250–260 10.1126/science.147.3655.250 (doi:10.1126/science.147.3655.250) [DOI] [PubMed] [Google Scholar]
- 25.Dynesius M., Jansson R. 2000. Evolutionary consequences of changes in species' geographical distributions driven by Milankovitch climate oscillations. Proc. Natl Acad. Sci. USA 97, 9115–9120 10.1073/pnas.97.16.9115 (doi:10.1073/pnas.97.16.9115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jansson R., Dynesius M. 2002. The fate of clades in a world of recurrent climatic change: Milankovitch oscillations and evolution. Annu. Rev. Ecol. Syst. 33, 741–777 10.1146/annurev.ecolsys.33.010802.150520 (doi:10.1146/annurev.ecolsys.33.010802.150520) [DOI] [Google Scholar]
- 27.Gaston K. J. 2003. The structure and dynamics of geographic ranges. Oxford, UK: Oxford University Press [Google Scholar]
- 28.Brown J. H. 1995. Macroecology. Chicago, IL: University of Chicago Press [Google Scholar]
- 29.Rohde K. 1996. Rapoport's rule is a local phenomenon and cannot explain latitudinal gradients in species diversity. Biodiv. Lett. 3, 10–13 10.2307/2999704 (doi:10.2307/2999704) [DOI] [Google Scholar]
- 30.Rohde K., Heap M. 1996. Latitudinal ranges of teleost fish in the Atlantic and Indo-Pacific oceans. Am. Nat. 147, 659–665 10.1086/285873 (doi:10.1086/285873) [DOI] [Google Scholar]
- 31.Price T. D., Helbig A. J., Richman A. D. 1997. Evolution of breeding distributions in the Old World leaf warblers (genus Phylloscopus). Evolution 51, 552–561 10.2307/2411127 (doi:10.2307/2411127) [DOI] [PubMed] [Google Scholar]
- 32.Stevens G. C. 1989. The latitudinal gradient in geographical range—how so many species coexist in the tropics. Am. Nat. 133, 240–256 10.1086/284913 (doi:10.1086/284913) [DOI] [Google Scholar]
- 33.Gaston K. J., Blackburn T. M., Spicer J. I. 1998. Rapoport's rule: time for an epitaph? Trends Ecol. Evol. 13, 70–74 10.1016/S0169-5347(97)01236-6 (doi:10.1016/S0169-5347(97)01236-6) [DOI] [PubMed] [Google Scholar]
- 34.Davies T. J., et al. 2008. Phylogenetic trees and the future of mammalian biodiversity. Proc. Natl Acad. Sci. USA 105, 11 556–11 563 10.1073/pnas.0801917105 (doi:10.1073/pnas.0801917105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies T. J., Purvis A., Gittleman J. L. 2009. Quaternary climate change and the geographic ranges of mammals. Am. Nat. 174, 297–307 10.1086/603614 (doi:10.1086/603614) [DOI] [PubMed] [Google Scholar]
- 36.Gaston K. J., Chown S. L. 1999. Why Rapoport's rule does not generalise. Oikos 84, 309–312 10.2307/3546727 (doi:10.2307/3546727) [DOI] [Google Scholar]
- 37.Colwell R. K. 2000. Range model: a Monte Carlo simulation tool for assessing geometric constraints on species richness. Version 3. Available at: http://viceroy.eeb.uconn.edu/rangemodel. [Google Scholar]
- 38.Brown J. H., Lomolino M. V. 1998. Biogeography. Sunderland, MA: Sinauer Associates [Google Scholar]
- 39.Gaston K. J. 1998. Species-range size distributions: products of speciation, extinction and transformation. Phil. Trans. R. Soc. Lond. B 353, 219–230 10.1098/rstb.1998.0204 (doi:10.1098/rstb.1998.0204) [DOI] [Google Scholar]
- 40.Lyons S. K. 2003. A quantitative assessment of the range shifts of Pleistocene mammals. J Mammal. 84, 385–402 (doi:10.1644/1545-1542(2003)084<0385:AQAOTR>2.0.CO;2) [DOI] [Google Scholar]
- 41.Lyons S. K. 2005. A quantitative model assessing the community dynamics of Pleistocene mammals. Am. Nat. 165, E168–E185 10.1086/429699 (doi:10.1086/429699) [DOI] [PubMed] [Google Scholar]
- 42.Brown J. H., Stevens G. C., Kaufman D. M. 1996. The geographic range: size, shape, boundaries, and internal structure. Annu. Rev. Ecol. Syst. 27, 597–623 10.1146/annurev.ecolsys.27.1.597 (doi:10.1146/annurev.ecolsys.27.1.597) [DOI] [Google Scholar]
- 43.Gaston K. J. 1996. Species-range-size distributions: patterns, mechanisms and implications. Trends Ecol. Evol. 11, 197–201 10.1016/0169-5347(96)10027-6 (doi:10.1016/0169-5347(96)10027-6) [DOI] [PubMed] [Google Scholar]
- 44.Jenkins D. G., et al. 2007. Does size matter for dispersal distance? Global Ecol. Biogeogr. 16, 415–425 10.1111/j.1466-8238.2007.00312.x (doi:10.1111/j.1466-8238.2007.00312.x) [DOI] [Google Scholar]
- 45.Gittleman J. L., Anderson C. G., Kot M., Luh K. 1996. Phylogenetic lability and rates of evolution: a comparison of behavioral, morphological and life history traits. In Phylogenies and the comparative method in animal behavior. (ed. Martins E. P.), pp. 166–205 Oxford, UK: Oxford University Press [Google Scholar]
- 46.Jones K. E., Sechrest W., Gittleman J. L. 2005. Age and area revisited: identifying global patterns and implications for conservation. In Phylogeny and Conservation. (eds Purvis A., Gittleman J. L., Brooks T. M.), pp. 141–165 Cambridge, UK: Cambridge University Press [Google Scholar]
- 47.Waldron A. 2007. Null models of geographic range size evolution reaffirm its heritability. Am. Nat. 170, 221–231 10.1086/518963 (doi:10.1086/518963) [DOI] [PubMed] [Google Scholar]
- 48.Barraclough T. G., Vogler A. P. 2000. Detecting the geographical pattern of speciation from species-level phylogenies. Am. Nat. 155, 419–434 10.1086/303332 (doi:10.1086/303332) [DOI] [PubMed] [Google Scholar]
- 49.Stephens P. R., Gittleman J. L. Submitted Pattern and process underlying phylogenetic signal in geographic range area. [Google Scholar]
- 50.Grafen A. 1989. The phylogenetic regression. Phil. Trans. R. Soc. Lond. B 326, 119–157 10.1098/rstb.1989.0106 (doi:10.1098/rstb.1989.0106) [DOI] [PubMed] [Google Scholar]
- 51.Freckleton R. P., Harvey P. H., Pagel M. 2002. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 160, 712–726 10.1086/343873 (doi:10.1086/343873) [DOI] [PubMed] [Google Scholar]
- 52.Purvis A. 2008. Phylogenetic approaches to the study of extinction. Annu. Rev. Ecol. Evol. Syst. 39, 301–319 10.1146/annurev.ecolsys.063008.102010 (doi:10.1146/annurev.ecolsys.063008.102010) [DOI] [Google Scholar]
- 53.Lester S. E., Ruttenberg B. I., Gaines S. D., Kinlan B. P. 2007. The relationship between dispersal ability and geographic range size. Ecol. Lett. 10, 745–758 10.1111/j.1461-0248.2007.01070.x (doi:10.1111/j.1461-0248.2007.01070.x) [DOI] [PubMed] [Google Scholar]
- 54.Buckley L. B., et al. 2010. Phylogeny, niche conservatism and the latitudinal diversity gradient in mammals. Proc. R. Soc. B 277, 131–2138 10.1098/rspb.2010.0179 (doi:10.1098/rspb.2010.0179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fischer A. G. 1960. Latitudinal variation in organic diversity. Evolution 14, 64–81 10.2307/2405923 (doi:10.2307/2405923) [DOI] [Google Scholar]
- 56.Araújo M. B., Nogués-Bravo D., Diniz-Filho J. A. F., Haywood A. M., Valdes P. J., Rahbek C. 2008. Quaternary climate changes explain diversity among reptiles and amphibians. Ecography 31, 8–15 10.1111/j.2007.0906-7590.05318.x (doi:10.1111/j.2007.0906-7590.05318.x) [DOI] [Google Scholar]
- 57.Denton G. H., Hughes T. H. 1981. The last great ice sheets. New York, NY: Wiley-Interscience [Google Scholar]
- 58.Ray N., Adams J. M. 2001. A GIS-based vegetation map of the world at the last glacial maximum (25 000–15 000 BP). Internet Archaeol. 11, 1–44 [Google Scholar]
- 59.Fretwell P. T., Smith D. E., Harrison S. 2008. The last glacial maximum British–Irish ice sheet: a reconstruction using digital terrain mapping. J. Quat. Sci. 23, 241–248 10.1002/jqs.1143 (doi:10.1002/jqs.1143) [DOI] [Google Scholar]
- 60.Dobzhansky T. 1950. Evolution in the tropics. Am. Nat. 38, 209–221 [Google Scholar]
- 61.MacArthur R. H. 1969. Patterns of communities in the tropics. Biol. J. Linn. Soc. 1, 19–30 10.1111/j.1095-8312.1969.tb01809.x (doi:10.1111/j.1095-8312.1969.tb01809.x) [DOI] [Google Scholar]
- 62.Ricklefs R. E., Schluter D. 1993. Species diversity in ecological communities. Chicago, IL: Chicago University Press [Google Scholar]
- 63.Dormann C. F., et al. 2006. Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30, 609–628 10.1111/j.2007.0906-7590.05171.x (doi:10.1111/j.2007.0906-7590.05171.x) [DOI] [Google Scholar]
- 64.Kreft H., Jetz W. 2007. Global patterns and determinants of vascular plant diversity. Proc. Natl Acad. Sci. USA 104, 5925–5930 10.1073/pnas.0608361104 (doi:10.1073/pnas.0608361104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davies R. G., et al. 2007. Topography, energy and the global distribution of bird species richness. Proc. R. Soc. B 274, 1189–1197 10.1098/rspb.2006.0061 (doi:10.1098/rspb.2006.0061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fotheringham A. S., Brunsdon C., Charlton M. 2002. Geographically weighted regression: the analysis of spatially varying relationships. Chichester, UK: Wiley [Google Scholar]
- 67.Cassemiro F. A. S., Barreto B., Rangel T. F. L. V. B., Diniz-Filho J. A. F. 2007. Non-stationarity, diversity gradients and the metabolic theory of ecology. Global Ecol. Biogeogr. 16, 820–822 10.1111/j.1466-8238.2007.00332.x (doi:10.1111/j.1466-8238.2007.00332.x) [DOI] [Google Scholar]
- 68.Jetz W., Rahbek C., Lichstein J. W. 2005. Local and global approaches to spatial data analysis in ecology. Global Ecol. Biogeogr. 14, 97–98 10.1111/j.1466-822X.2004.00129.x (doi:10.1111/j.1466-822X.2004.00129.x) [DOI] [Google Scholar]
- 69.Leemans R., Cramer W. P. 1991. The IIASA database for mean monthly values of temperature, precipitation, and cloudiness of a global terrestrial grid. Global Ecosystems Database Version 2.0, dataset AO3. See http://www.ngdc.noaa.gov/ecosys/ged.shtml [Google Scholar]
- 70.Ahn C.-H., Tateishi R. 2000. Monthly potential and Actual Evapotranspiration and water balance. Global Ecosystems Database (GED) and UNEP/GRID, Geneva. See http://www.grid.unep.ch/data/data.php, dataset GNV183. [Google Scholar]
- 71.Jansson R., Davies T. J. 2008. Global variation in diversification rates of flowering plants: energy versus climate change. Ecol. Lett. 11, 173–183 10.1111/j.1461-0248.2007.01138.x (doi:10.1111/j.1461-0248.2007.01138.x) [DOI] [PubMed] [Google Scholar]
- 72.Jansson R. 2003. Global patterns in endemism explained by past climate change. Proc. R. Soc. Lond. B 270, 583–590 10.1098/rspb.2002.2283 (doi:10.1098/rspb.2002.2283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dong B., Valdes P. J. 1998. Simulations of the Last Glacial Maximum climates using a general circulation model: prescribed versus computed sea surface temperatures. Clim. Dyn. 14, 571–591 10.1007/s003820050242 (doi:10.1007/s003820050242) [DOI] [Google Scholar]
- 74.Keys R. G. 1981. Cubic convolutiuon interpolation for digital image processing. IEEE Trans. Acoustic Speech Signal Process. 29, 1153–1160 10.1109/TASSP.1981.1163711 (doi:10.1109/TASSP.1981.1163711) [DOI] [Google Scholar]
- 75.Weigel J. 2009. Grid Machine 6.81. (http://www.ecogis.de/gridmachine.htm)
- 76.Haining R. H. 2003. Spatial data analysis: theory and practice. Cambridge, UK: Cambridge University Press [Google Scholar]
- 77.Bivand R. 2005. The Spdep package. Comprehensive R Archive Network, 0.3-13. Available at: http://www.cran.r-project.org [Google Scholar]
- 78.Rangel T. F. L. V. B., Diniz-Filho J. A. F., Bini L. M. 2010. SAM: a comprehensive application for spatial analysis in macroecology. Ecography 33, 46–50 10.1111/j.1600-0587.2009.06299.x (doi:10.1111/j.1600-0587.2009.06299.x) [DOI] [Google Scholar]
- 79.Svenning J.-C., Normand S., Skov F. 2009. Plio-Pleistocene climate change and geographic heterogeneity in plant diversity–environment relationships. Ecography 32, 13–21 10.1111/j.1600-0587.2008.05732.x (doi:10.1111/j.1600-0587.2008.05732.x) [DOI] [Google Scholar]
- 80.Turvey S. T., Fritz S. A. The ghosts of mammals past: biological and geographical patterns of global mammalian extinction across the Holocene. Phil. Trans. R. Soc. 366, 2564–2576 10.1098/rstb.2011.0020 (doi:10.1098/rstb.2011.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martin P. S. 1984. Prehistoric overkill: the global model. In Quaternary extinctions: a prehistoric revolution. (eds Martin P. S., Klein R. G.), pp. 354–403 Tucson, AZ: University of Arizona Press [Google Scholar]
- 82.Grayson D., Meltzer D. 2003. Requiem for North American overkill. J. Archaeol. Sci. 30, 585–593 10.1016/S0305-4403(02)00205-4 (doi:10.1016/S0305-4403(02)00205-4) [DOI] [Google Scholar]
- 83.Wroe S., Field J., Fullagar R., Jermin L. S. 2004. Megafaunal extinction in the late Quaternary and the global overkill hypothesis. Alcheringa: Australasian J. Palaeont. 28, 291–331 10.1080/03115510408619286 (doi:10.1080/03115510408619286) [DOI] [Google Scholar]
- 84.Webb S. D. 1976. Mammalian faunal dynamics of the Great American Interchange. Paleobiology 2, 220–234 [Google Scholar]
- 85.Colwell R. K., Hurt G. C. 1994. Nonbiological gradients in species richness and a spurious Rapoport effect. Am. Nat. 144, 570–595 10.1086/285695 (doi:10.1086/285695) [DOI] [Google Scholar]
- 86.Safi K., Cianciaruso M. V., Loyola R. D., Brito D., Armour-Marshall K., Diniz-Filho J. A. 2011. Understanding global patterns of mammalian functional and phylogenetic diversity. Phil. Trans. R. Soc. B 366, 2536–2544 10.1098/rstb.2011.0024 (doi:10.1098/rstb.2011.0024) [DOI] [PMC free article] [PubMed] [Google Scholar]