Abstract
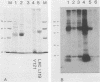
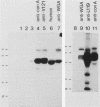
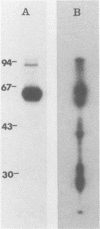
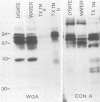
The total integral membrane proteins of promastigotes of Leishmania major were extracted by using the Triton X-114 phase separation technique and were characterized by immunoprecipitation, Western blotting (immunoblotting), and lectin chromatography. Of the 40 or more proteins which partitioned into the detergent phase, only about 10 proteins could be surface radioiodinated on live promastigotes, suggesting their surface orientation. The abundance of the gp58-63 antigen varied markedly between two strains of L. major. Sera from patients with visceral leishmaniasis caused by Leishmania donovani chagasi recognized the gp58-63 complex and an additional Mr-42,000 polypeptide shared between L. major and L. donovani chagasi. A subpopulation of six surface proteins, including the abundant gp58-63 antigen and a group of proteins of Mr 81,000 to 105,000, were glycoproteins recognized by antiserum to wheat germ agglutinin- or concanavalin A-binding proteins. The membrane proteins of the LRC-L119 isolate of L. major could successfully vaccinate genetically susceptible mice, thus opening the way for a molecularly defined subunit vaccine composed of glycolipid and membrane protein antigens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balber A. E., Ho L. M. Trypanosoma brucei gambiense: partitioning of glycopeptides of bloodstream and procyclic forms in Triton X-114. Exp Parasitol. 1988 Apr;65(2):290–293. doi: 10.1016/0014-4894(88)90134-8. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bordier C. The promastigote surface protease of Leishmania. Parasitol Today. 1987 May;3(5):151–153. doi: 10.1016/0169-4758(87)90199-2. [DOI] [PubMed] [Google Scholar]
- Bouvier J., Etges R. J., Bordier C. Identification and purification of membrane and soluble forms of the major surface protein of Leishmania promastigotes. J Biol Chem. 1985 Dec 15;260(29):15504–15509. [PubMed] [Google Scholar]
- Bouvier J., Etges R., Bordier C. Identification of the promastigote surface protease in seven species of Leishmania. Mol Biochem Parasitol. 1987 May;24(1):73–79. doi: 10.1016/0166-6851(87)90117-4. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Button L. L., McMaster W. R. Molecular cloning of the major surface antigen of leishmania. J Exp Med. 1988 Feb 1;167(2):724–729. doi: 10.1084/jem.167.2.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Colomer-Gould V., Glvao Quintao L., Keithly J., Nogueira N. A common major surface antigen on amastigotes and promastigotes of Leishmania species. J Exp Med. 1985 Sep 1;162(3):902–916. doi: 10.1084/jem.162.3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross G. A. Eukaryotic protein modification and membrane attachment via phosphatidylinositol. Cell. 1987 Jan 30;48(2):179–181. doi: 10.1016/0092-8674(87)90419-3. [DOI] [PubMed] [Google Scholar]
- Etges R. J., Bouvier J., Hoffman R., Bordier C. Evidence that the major surface proteins of three Leishmania species are structurally related. Mol Biochem Parasitol. 1985 Feb;14(2):141–149. doi: 10.1016/0166-6851(85)90033-7. [DOI] [PubMed] [Google Scholar]
- Etges R., Bouvier J., Bordier C. The major surface protein of Leishmania promastigotes is anchored in the membrane by a myristic acid-labeled phospholipid. EMBO J. 1986 Mar;5(3):597–601. doi: 10.1002/j.1460-2075.1986.tb04252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner P. R., Jaffe C. L., Dwyer D. M. Identification of cross-reactive promastigote cell surface antigens of some leishmanial stocks by 125I labeling and immunoprecipitation. Infect Immun. 1984 Feb;43(2):637–643. doi: 10.1128/iai.43.2.637-643.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Goding J. W. The Leishmania receptor for macrophages is a lipid-containing glycoconjugate. EMBO J. 1985 Feb;4(2):329–336. doi: 10.1002/j.1460-2075.1985.tb03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Hocking R. E., Mitchell G. F., Spithill T. W. Isolation and characterization of infective and non-infective clones of Leishmania tropica. Mol Biochem Parasitol. 1983 Feb;7(2):111–126. doi: 10.1016/0166-6851(83)90039-7. [DOI] [PubMed] [Google Scholar]
- Handman E., Mitchell G. F., Goding J. W. Identification and characterization of protein antigens of Leishmania tropica isolates. J Immunol. 1981 Feb;126(2):508–512. [PubMed] [Google Scholar]
- Handman E., Mitchell G. F. Immunization with Leishmania receptor for macrophages protects mice against cutaneous leishmaniasis. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5910–5914. doi: 10.1073/pnas.82.17.5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Schnur L. F., Spithill T. W., Mitchell G. F. Passive transfer of Leishmania lipopolysaccharide confers parasite survival in macrophages. J Immunol. 1986 Dec 1;137(11):3608–3613. [PubMed] [Google Scholar]
- Heath S., Chance M. L., Hommel M., Crampton J. M. Cloning of a gene encoding the immunodominant surface antigen of Leishmania donovani promastigotes. Mol Biochem Parasitol. 1987 Apr;23(3):211–222. doi: 10.1016/0166-6851(87)90028-4. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Use of protein A-bearing staphylococci for the immunoprecipitation and isolation of antigens from cells. Methods Enzymol. 1981;73(Pt B):442–459. doi: 10.1016/0076-6879(81)73084-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lepay D. A., Nogueira N., Cohn Z. Surface antigens of Leishmania donovani promastigotes. J Exp Med. 1983 May 1;157(5):1562–1572. doi: 10.1084/jem.157.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville M. J., Bacic A. A family of glycoinositol phospholipids from Leishmania major. Isolation, characterization, and antigenicity. J Biol Chem. 1989 Jan 15;264(2):757–766. [PubMed] [Google Scholar]
- McConville M. J., Bacic A., Mitchell G. F., Handman E. Lipophosphoglycan of Leishmania major that vaccinates against cutaneous leishmaniasis contains an alkylglycerophosphoinositol lipid anchor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8941–8945. doi: 10.1073/pnas.84.24.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. F., Handman E. Heterologous protection in murine cutaneous leishmaniasis. Immunol Cell Biol. 1987 Oct;65(Pt 5):387–392. doi: 10.1038/icb.1987.44. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Handman E., Spithill T. W. Examination of variables in the vaccination of mice against cutaneous leishmaniasis using living avirulent cloned lines and killed promastigotes of Leishmania major. Int J Parasitol. 1985 Dec;15(6):677–684. doi: 10.1016/0020-7519(85)90015-3. [DOI] [PubMed] [Google Scholar]
- Orlandi P. A., Jr, Turco S. J. Structure of the lipid moiety of the Leishmania donovani lipophosphoglycan. J Biol Chem. 1987 Jul 25;262(21):10384–10391. [PubMed] [Google Scholar]
- Rogers M. V., Davern K. M., Smythe J. A., Mitchell G. F. Immunoblotting analysis of the major integral membrane protein antigens of Schistosoma japonicum. Mol Biochem Parasitol. 1988 May;29(1):77–87. doi: 10.1016/0166-6851(88)90122-3. [DOI] [PubMed] [Google Scholar]
- Russell D. G., Alexander J. Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J Immunol. 1988 Feb 15;140(4):1274–1279. [PubMed] [Google Scholar]
- Smythe J. A., Coppel R. L., Brown G. V., Ramasamy R., Kemp D. J., Anders R. F. Identification of two integral membrane proteins of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5195–5199. doi: 10.1073/pnas.85.14.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]