Abstract
Although the recent historical period is usually treated as a temporal base-line for understanding patterns of mammal extinction, mammalian biodiversity loss has also taken place throughout the Late Quaternary. We explore the spatial, taxonomic and phylogenetic patterns of 241 mammal species extinctions known to have occurred during the Holocene up to the present day. To assess whether our understanding of mammalian threat processes has been affected by excluding these taxa, we incorporate extinct species data into analyses of the impact of body mass on extinction risk. We find that Holocene extinctions have been phylogenetically and spatially concentrated in specific taxa and geographical regions, which are often not congruent with those disproportionately at risk today. Large-bodied mammals have also been more extinction-prone in most geographical regions across the Holocene. Our data support the extinction filter hypothesis, whereby regional faunas from which susceptible species have already become extinct now appear less threatened; they may also suggest that different processes are responsible for driving past and present extinctions. We also find overall incompleteness and inter-regional biases in extinction data from the recent fossil record. Although direct use of fossil data in future projections of extinction risk is therefore not straightforward, insights into extinction processes from the Holocene record are still useful in understanding mammalian threat.
Keywords: body mass, extinction filter, fossil record, Late Quaternary, supertree, threat
1. Introduction
Analysis of global patterns of threat across major taxonomic groups has become an important tool for understanding the ecological patterns, dynamics and magnitude of the current-day biodiversity crisis, and constitutes a key step for prioritizing management actions and allocation of finite resources in systematic conservation planning [1]. Comprehensive assessment of the conservation status of the 5487 wild mammal species recognized as extant since AD 1500 has shown that 25 per cent of all species for which adequate data are available are currently threatened with extinction, and that the distribution of these threatened species is geographically biased [2]. A range of other studies have used large-scale datasets of life-history parameters and phylogenetic ‘supertrees’ for threatened and non-threatened mammals [3,4] to identify intrinsic and extrinsic correlates of extinction risk in different mammal groups [5–10], and to predict future changes in the geographical distribution of threatened species [11].
These studies all treat the recent 500-year historical period, an interval which broadly corresponds with the concerted dispersal of European explorers, traders and colonists around the globe, as the temporal base-line for understanding mammalian threat and extinction. However, although human impacts on global mammal diversity have been documented relatively well for this recent interval [12,13], mammalian biodiversity loss has also occurred throughout much of the Late Quaternary in association with older historical and prehistoric human migration, population expansion and environmental modification. The best-studied series of prehistoric Late Quaternary extinctions is the loss of at least 97 genera of megafaunal vertebrates (more than 44 kg; sensu [14]), mostly mammals but also some birds and reptiles, across the world's continents and some island systems during the Late Pleistocene Epoch without subsequent ecological replacement [15]. A further extensive series of mammalian species extinctions, population extirpations and range contractions continued across insular and continental regions into the Holocene Epoch, the current geological interval, through recent prehistory and into the historical period [16].
The staggered timing of Late Pleistocene mammal extinctions and their close temporal association with first human colonization of different continental regions is increasingly well understood for North America [17,18] and Australia [19–22], and direct or indirect human involvement in these events is now widely accepted by most palaeontologists. Meaningful biological and life-history data are also available for these extinct regional mammal faunas [23–25]. However, the Late Pleistocene was also characterized by large-scale climatic and associated vegetational shifts during the transition from glacial to interglacial conditions, and the nature and extent of human involvement even in these relatively well-studied regional extinctions remains the subject of considerable debate, with non-anthropogenic environmental factors often advocated as primary extinction drivers on the basis of climatic and vegetation models and archaeological data [26–32]. On a regional level, the relationship between prehistoric human activity, climate change and Late Pleistocene mammal extinction in northern Eurasia is apparently more complex than previously thought [33,34]. Late Pleistocene mammalian extinction chronologies and faunal turnover are more poorly resolved for South America, Africa, the insular Mediterranean, eastern and southeast Asia and Australasia, constraining our ability to understand the extent of human involvement in these regional extinction events [16,35,36].
In contrast, mammal extinctions during the Holocene occurred in an interval of modest or minimal climatic variation, and under broadly ‘modern’ climatic and environmental boundary conditions [37]. Human involvement in these events is much less controversial, and only an extremely small proportion of Holocene species or population losses (e.g. disappearance of the mammoth population on St Paul Island; [38]) can even questionably be interpreted as non-anthropogenic events [16]. Although high-resolution geographical data on Holocene range change and population-level extirpation are still only known for a few well-studied mammal species (e.g. [39]), a global dataset of all mammal species currently known or suspected to have died out during the Holocene is now available [16]. However, the relatively well-preserved recent fossil record is still substantially incomplete, and although new insights may be gained through the use of novel methodological approaches [40–42], constraining extinction chronologies and identifying causative extinction drivers remains a major challenge [16].
Despite these potential concerns, the fossil and archaeological records are increasingly recognized as important sources of data for evidence-based conservation, as they can provide a unique temporal perspective on past patterns of ecosystem diversity and function and how global systems respond to changing climatic and anthropogenic drivers [43–46]. However, past human-caused extinctions have primarily been studied by palaeontologists and archaeologists rather than in the context of current mammal extinction risk. Although some studies have investigated biological correlates of extinction-proneness in extinct Late Quaternary mammals, these have only incorporated data for well-studied regional components of the Late Pleistocene megafauna [47–49]. Existing studies that have investigated patterns and correlates of current extinction risk in mammals have typically excluded the great majority of the most susceptible species that have already become extinct as a result of human activities (e.g. [7,8]). Even from such a dataset of extant mammal species, it has been recently suggested that an ‘extinction filter’ [50] has acted throughout the Holocene: large mammals are primarily threatened today in tropical areas, where agricultural impacts in the past have been relatively low, implying that large mammals in temperate regions have already been reduced to small but currently stable populations or driven extinct [51]. Patterns of reduced modern-day species loss in areas that have the longest records of human occupation, and for which large-scale undocumented past extinctions can be assumed, have also been suggested for other taxonomic groups [52]. The possible existence of such an extinction filter implies that the findings of studies based exclusively on extant species may be incomplete, and highlights the need for a more inclusive assessment of human impacts on global mammal faunas over time.
Here, we explore the spatial, taxonomic and phylogenetic patterns of all known Holocene mammal extinctions, to assess whether our understanding of mammalian threat processes has been affected by excluding this category of taxa. Throughout this study, the Holocene is defined as the interval from approximately 11 500 years ago up to the present day (i.e. also including the recent historical period). In order to investigate whether data from the past can provide useful new insights for understanding current mammal extinctions, we incorporate extinct Holocene mammal species into analyses resembling the current comparative literature on extinction risk correlates in extant mammals, adding 241 extinct species to the latest global studies. We also assess the proposed extinction filter effect by investigating whether these Holocene extinctions have been selective with regard to large body mass. Both analyses assume that the fossil record correctly reflects past extinction events; to assess the validity of this assumption, the last part of this paper investigates the extent to which the incompleteness of the recent fossil record constrains our ability to identify extinction drivers and use past extinction data for informing present-day conservation efforts. We therefore use our expanded dataset to investigate whether past and present mammal extinction risks display phylogenetically, ecologically and geographically congruent patterns.
2. Methods
Our dataset of all mammal species known or suspected to have become extinct during the Holocene is based on Turvey [16], and updated following recent taxonomic revisions and new species descriptions [53–56]. This provided a total of 241 mammal species which went extinct after the Pleistocene–Holocene transition (see the electronic supplementary material, table S1, for a full dataset). We were able to incorporate 210 of these species into an existing supertree [3,51], either using published taxon-specific phylogenies or on the basis of taxonomy [57] if phylogenies were unavailable, creating polytomies if necessary (see the electronic supplementary material, table S1). We did not run new dating analyses as for the last taxonomic update [51] because sequence data do not yet exist for most extinct species. We were mostly interested in topological placement of species to assess the phylogenetic signal of extinctions and to control for phylogeny in comparative analyses; we therefore used known divergence dates where available in our references (see the electronic supplementary material, table S1), and otherwise placed new nodes temporally halfway between existing ones. The new supertree contained 5210 species, 210 of which were extinct, and was 51 per cent resolved. To assess the impact of our ad hoc dating, we also ran all phylogenetic analyses on the basis of topology alone, with branch lengths set to equal [58].
Body mass estimates were obtained for 220 of the extinct species using four different methods. (i) A small number of body masses for extinct mammals were already available in the PanTHERIA database [4]. (ii) Body masses for species with extant or extinct congeners of known body mass were calculated as the genus-level mean, largely using the PanTHERIA database. Body masses for species with no extant congeners were either (iii) collated from published estimates that were calculated using predictive regression equations based on skeletal measurement parameters, or (iv) were newly calculated for this study using published regression equations for different taxonomic groups [59–62] and published or newly measured skeletal morphometric data (see the electronic supplementary material, table S1).
Geographical distributions for extinct species were only possible to record at the country level, because a large proportion of these species are poorly known and only recorded from a very limited number of point localities. This precluded accurate reconstruction of their past geographical ranges, so range size was unavailable for extinct species and not used in our analyses. Our country-level occurrences represented only Holocene records; we excluded additional Pleistocene records that are available for many species because climate-led environmental changes between these two time intervals are known to have driven major geographical range shifts in many taxa [33,63,64]. Species from islands that consist of more than one modern geopolitical country (e.g. Hispaniola, New Guinea) were interpreted as having occurred in all of the countries on the island, because in most cases it remains difficult to test whether proposed regions of intra-island endemism in extinct mammals (e.g. [65]) represent genuine biogeographical patterns or artefacts of limited fossil sampling.
Country occurrence and IUCN (International Union for Conservation of Nature) Red List status for extant mammals were taken from the Global Mammal Assessment [66]. We modified their species range maps according to the taxonomy of [57], and excluded historical, introduced and questionable ranges. We also excluded marine species (cetaceans, sirenians, pinnipeds, and the polar bear, sea otter and marine otter) from our spatial analyses. Our final dataset contained 5564 species, including 128 marine species; country occurrence was known for 5380 non-marine species. Of these species, 236 were extinct, two were extinct in the wild, and 768 were ranked as Data Deficient (DD) [66]. Body mass data for 3801 extant mammal species were taken from the PanTHERIA database [4]. A recent study showed that this dataset is biased in that more large-bodied species have known body mass values [67]; to avoid this bias, we used their method to impute body mass data for an additional 1143 extant species as the value of their closest relative on the basis of the near-complete mammalian phylogeny. These imputed data were only used in the randomization when testing for size selectivity of extinction, not in the extinction risk correlate analyses.
Last-occurrence dates for extinct mammals and dates for regional first human and European arrival were largely taken from Refs. [16,68,69] (see the electronic supplementary material, table S2). A new Holocene radiometric last-occurrence date for the extinct Hispaniolan sloth Neocnus dousman was calculated for this study as 7141 ± 35 yr BP full size from newly collected fossil material (Oxford Radiocarbon Accelerator Unit, OxA-19 725).
(a). Geographical and phylogenetic patterns of past and present extinction risk
We used R v. 2.10.1 for all analyses [70]. We initially explored patterns of Holocene extinctions and current extinction risk by mapping the number and proportion of extinct and threatened species within countries. Proportions of extinct species were defined as: (number of extinct species)/(number of extant + number of extinct species); proportions of threatened species as: (number of species in any of the three threatened categories (Vulnerable, Endangered, or Critically Endangered; [66]))/(number of extant species − number of species classified as DD by IUCN). Throughout our analyses, we classified the two mammal species listed as Extinct in the Wild (Elaphurus davidianus and Oryx dammah; [66]) as being extinct. Congruence of spatial patterns was assessed with Pearson correlation coefficients; we did not perform statistical tests for their significance as these would be biased by spatial autocorrelation, but the estimates of the coefficients themselves are thought to be unbiased [71].
To investigate taxonomic patterns in past extinctions and present risk, we calculated the number and proportion of extinct and threatened species for each mammalian order, and tested for equal proportions with Pearson's χ2. We also tested each order's proportion against its expectation given a binomial distribution, with the probability of extinction or risk set as the proportion of all mammals extinct or threatened. These analyses were run on a dataset of 4454 extant and 241 extinct species (including marine but excluding DD species). Phylogenetic signal in extinction and risk was assessed using the D statistic, which tests whether extinct or threatened species are significantly more clustered than expected under phylogenetically random extinction or threat (D < 1) [72]. Additionally, D tests whether the observed phylogenetic signal is as strong as expected if the tested binary trait (e.g. extinct versus extant) was generated by ranking species according to values of a continuous trait evolved under a Brownian motion model. For example, if the largest species were always threatened, and body mass evolved according to a Brownian model, D for threat would not be different from zero (its expectation under a Brownian threshold model). Sample size for phylogenetic signal was the 5210 species in the supertree, 210 of which were extinct in the Holocene (again including marine species).
(b). Effects of body size on extinction and risk
To assess the ‘extinction filter’ effect of extinct species on modelling body mass as a correlate of extinction risk, we fitted models within countries including and excluding extinct species. The response variable was the IUCN Red List categories converted to a numerical scale (Least Concern = 0, Near Threatened = 1, Vulnerable = 2, Endangered = 3, Critically Endangered = 4, Extinct in the Wild or Extinct = 5) [5]; log10-transformed body mass was the explanatory variable. The dataset therefore contained the 3410 non-marine species which were in the phylogeny, not ranked as DD, and for which non-imputed body mass estimates were available; 217 of these were extinct. Previous studies have excluded species ranked as threatened under criteria other than A (population decline; [66]) to avoid circularity arising from inclusion of geographical range size as an explanatory variable for species that have been ranked as threatened due to small range size [5,7,8]. This circularity did not arise for our analysis, as we were unable to use geographical range size as a predictor; therefore, we present analyses for all species.
Models were fitted only for countries with more than 10 species with all data. We used phylogenetic comparative analysis with the CAIC (Comparative Analysis using Independent Contrasts) package for R [73] to control for the fact that related species will be more similar in evolved traits such as body size, but also in extinction risk rating given its strong phylogenetic signal [58,74]. Assuming our branch lengths for extinct species are acceptable estimates, we ran phylogenetic generalized least-squares (PGLS) regression, which controls for variable amounts of phylogenetic covariation within the country datasets [75]. In order to assess effects of branch lengths, we also performed the analysis with phylogenetically independent contrasts setting all branch lengths to equal [76].
We simulated random extinction within countries to investigate whether extinct species were unusually large. The number of extinct species within each country was drawn at random 1000 times from the country's pool of species with body mass values, including those extinct during the Holocene. We then assessed whether the observed median body mass of extinct species within each country was as expected under this random-extinction scenario within countries with a one-tailed significance test. The dataset for this procedure included the extant species for which body mass was imputed (see above), so the global dataset contained 5115 species, 217 of these extinct.
(c). Resolution and bias of the Holocene fossil record
Finally, we examined the resolution of the recent fossil record and the robustness of our understanding of mammalian extinction chronologies and extinction drivers by analysing the temporal pattern of last-occurrence dates in relation to the earliest known dates of prehistoric human arrival on each island. This was conducted for the diverse extinct endemic mammal faunas from Madagascar, Cuba and Hispaniola, the three large islands that have experienced a disproportionately high number of known Holocene mammal extinctions [16].
3. Results and discussion
(a). Geographical and phylogenetic patterns of past and present extinction risk
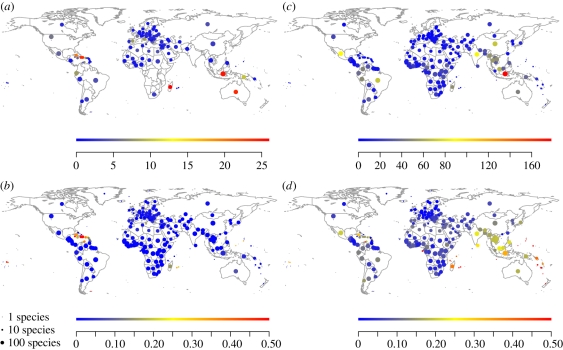
Most recorded Holocene mammal species extinctions have occurred on islands, whereas a high proportion of currently threatened species are also found on continents (figure 1). Australia, Indonesia, Madagascar and the larger Caribbean islands have the highest overall number of extinct species (figure 1a), while only the Caribbean and some Pacific islands stand out for their proportion of extinct species (figure 1b). Most threatened species are concentrated in Indonesia, other regions of Asia (India, China, peninsular southeast Asia), and the Neotropics (Mexico, Brazil; figure 1c). Globally, the proportions of threatened species within countries are much higher than that of documented Holocene species extinctions, especially for peninsular southeast Asia, Indonesia, Madagascar, and some other tropical islands (figure 1d). Congruence between the numbers of extinct and threatened species across countries was relatively weak (Pearson correlation coefficient = 0.37).
Figure 1.
Global patterns of Holocene species extinctions and currently threatened species. (a) Number and (b) proportion of extinct species; (c) number and (d) proportion of threatened species. Countries without extinct or threatened species in our dataset are not plotted in (a) and (c). Plots in (b) and (d) use the same colour scale; proportions above 0.5 were set to 0.5. The size of the plotted circles reflects the total number of species (extant + extinct) in (a) and (b), and the number of extant species in (c) and (d), on a logarithmic scale (see size key).
Across our whole mammal dataset, 4.3 per cent (241/5564) of known species are extinct and 24.3 per cent (1083/4454) are threatened with extinction (excluding DD species), but proportions differ between orders (table 1). Past extinction and current threat are not distributed randomly across mammalian orders (proportion of extinct species: χ2 = 183.95, d.f. = 29, p < 0.001; proportion of threatened species: χ2 = 169.47, d.f. = 27, p < 0.001). Assuming the binomial distribution, artiodactyls, bandicoots and bilbies, bibymalagasians, proboscideans and xenarthrans have significantly more extinct species than expected, and bats have significantly fewer (table 1). Conversely, significantly more species of artiodactyls, perissodactyls, primates and sirenians are threatened, while bats, opossums and rodents have fewer threatened species than expected.
Table 1.
Taxonomic patterns of Holocene mammal extinction and current threat, by order. thr., threatened, DD, Data Deficient [66].
| order | number of species |
|||||||
|---|---|---|---|---|---|---|---|---|
| total | extinct | thr. | DD | % extinct | χ2extinct | % thr. | χ2threat | |
| Afrosoricida | 52 | 1 | 15 | 4 | 1.9 | 0.3 | 31.9 | 0.9 |
| Artiodactyla | 253 | 20 | 92 | 24 | 7.9 | 7.0** | 44.0 | 30.4*** |
| Bibymalagasia | 2 | 2 | 0 | 0 | 100.0 | 24.1*** | ||
| Carnivora | 288 | 6 | 68 | 25 | 2.1 | 3.0§ | 26.5 | 0.3 |
| Cetacea | 84 | 0 | 13 | 44 | 0.0 | 2.8§ | 32.5 | 1.0 |
| Chiroptera | 1128 | 23 | 168 | 197 | 2.0 | 13.8*** | 18.5 | 19.5*** |
| Cingulata | 21 | 0 | 4 | 3 | 0.0 | 0.2 | 22.2 | 0.0 |
| Dasyuromorphia | 72 | 2 | 11 | 3 | 2.8 | 0.1 | 16.4 | 2.2 |
| Dermoptera | 2 | 0 | 0 | 0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Didelphimorphia | 87 | 1 | 7 | 15 | 1.1 | 1.4 | 9.9 | 7.6** |
| Diprotodontia | 148 | 11 | 41 | 4 | 7.4 | 2.7 | 30.8 | 1.1 |
| Erinaceomorpha | 24 | 0 | 3 | 1 | 0.0 | 0.3 | 13.0 | 1.0 |
| Hyracoidea | 4 | 0 | 0 | 0 | 0.0 | 0.0 | 0.0 | 0.3 |
| Lagomorpha | 93 | 2 | 17 | 8 | 2.2 | 0.6 | 20.5 | 0.6 |
| Macroscelidea | 15 | 0 | 2 | 3 | 0.0 | 0.0 | 16.7 | 0.1 |
| Microbiotheria | 1 | 0 | 0 | 0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Monotremata | 5 | 0 | 3 | 0 | 0.0 | 0.0 | 60.0 | 1.8 |
| Notoryctemorphia | 2 | 0 | 0 | 2 | 0.0 | 0.0 | ||
| Paucituberculata | 6 | 0 | 2 | 0 | 0.0 | 0.0 | 33.3 | 0.0 |
| Peramelemorphia | 23 | 5 | 6 | 2 | 21.7 | 12.9*** | 37.5 | 0.0 |
| Perissodactyla | 18 | 1 | 13 | 1 | 5.6 | 0.0 | 81.2 | 22.4*** |
| Pholidota | 8 | 0 | 2 | 0 | 0.0 | 0.0 | 25.0 | 0.0 |
| Pilosa | 23 | 13 | 2 | 0 | 56.5 | 138.9*** | 20.0 | 2.3 |
| Primates | 399 | 23 | 182 | 45 | 5.8 | 1.6 | 55.0 | 139.8*** |
| Proboscidea | 5 | 2 | 1 | 1 | 40.0 | 8.0** | 50.0 | 0.0 |
| Rodentia | 2344 | 115 | 347 | 403 | 4.9 | 1.7 | 19.0 | 43.4*** |
| Scandentia | 20 | 0 | 2 | 3 | 0.0 | 0.2 | 11.8 | 0.9 |
| Sirenia | 5 | 1 | 4 | 0 | 20.0 | 0.4 | 100.0 | 5.7* |
| Soricomorpha | 431 | 13 | 78 | 81 | 3.0 | 1.5 | 23.1 | 0.7 |
| Tubulidentata | 1 | 0 | 0 | 0 | 0.0 | 0.0 | 0.0 | 0.0 |
***p < 0.001, **p < 0.01, *0.01 < p < 0.05, §0.05 < p < 0.1.
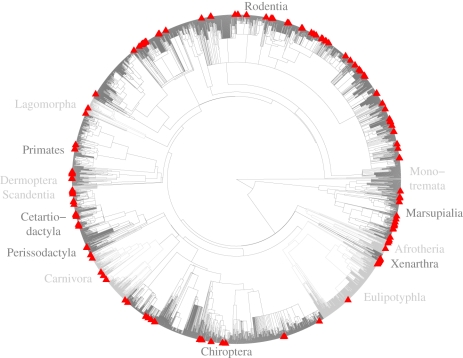
Past extinctions on the mammal supertree show significant phylogenetic signal (figure 2), with extinct species being significantly phylogenetically clumped whether branch lengths are used (D = 0.28, p < 0.001) or not (D = 0.35, p < 0.001). Using branch lengths, D is not quite significantly different from the expectation under the Brownian motion threshold model (p = 0.06), but when branch lengths are discarded the difference becomes significant (p < 0.01).
Figure 2.
The phylogenetic pattern of Holocene extinctions on the mammal supertree. Extinct species are marked by red triangles at the tips; major groups are alternately coloured dark and light grey to visually set them apart. The tree contains 5000 extant and 210 extinct species.
Overall, these results confirm the hypothesis that looking exclusively at current extinction risk patterns may distort our understanding of mammalian extinction: past extinctions throughout the Holocene have affected some areas and taxa disproportionately, and these are often not the areas and taxa that are disproportionately at risk today. The low spatial congruence of within-country past extinction and current extinction risk provides some support for the extinction filter hypothesis, whereby the faunas of countries in which many susceptible species have already become extinct now appear less threatened [50,51]. Similarly, some higher-order taxa containing high proportions of extinct species (e.g. bandicoots and bilbies, xenarthrans) now show average levels of current extinction risk.
However, these results could also be interpreted as indicating that different extinction processes are responsible for driving past versus present species losses. This alternative hypothesis may also be probable given the unprecedented and escalating level of modern-day anthropogenic habitat destruction [77]. In birds, the effects of introduced species as the primary global extinction driver have recently been superseded in importance by habitat loss [78]. Although prehistoric human-driven habitat modification is considered likely to be responsible for some Holocene mammal species losses in regions such as Madagascar [79], many past mammalian island extinctions have been linked to direct overexploitation by early colonists or the effects of invasive species, rather than to direct habitat clearance [16,80].
Different implications of our analyses provide some support for both of these hypotheses. Firstly, the taxonomic and phylogenetic clustering shown by both past extinctions and current extinction risk suggests that conserved biological traits make some taxa or clades consistently more susceptible to extinction than others [81]. Although some higher-order taxa differ in their past and current risk, as described above, this overall common pattern may indicate similar extinction processes operating over time for certain mammal groups. For example, bats are consistently less threatened than expected from the mammalian average, while artiodactyls are consistently more threatened (see [82]). An alternative explanation for this observation is consistent knowledge bias, as we may know more about risk status in artiodactyls than bats; in fact, DD status among extant mammals shows a phylogenetic signal, indicating taxonomic bias in our knowledge of whether mammal species are threatened or not [72]. Additionally, the fact that closely related species tend to live in the same geographical regions and therefore experience similar threats could inflate the global taxonomic and phylogenetic signal [83].
Secondly, while taxonomic clustering of Holocene extinctions has previously been demonstrated [84], as have taxonomic and phylogenetic clustering of currently threatened species [72,81,82,85], our analyses enable a direct comparison to be made between past and present extinction risk. Phylogenetic signal as measured by D is much stronger for extinct species (this study) than for all threatened ones (D = 0.64) [72]. Our results using phylogenetic branch lengths show that phylogenetic clumping of extinctions is indistinguishable from that caused by extinguishing the top cohort of species for an evolved trait such as body mass; this provides further support for the extinction filter hypothesis.
Thirdly, the amount of phylogenetic signal in extinction risk has previously been shown to differ depending on the underlying threat process [72]. Extant species threatened by overexploitation show a very strong phylogenetic signal, presumably due to strong body size selectivity, while species threatened by habitat loss show a much lower phylogenetic signal, as the impact of this threat process is less dependent on evolutionarily conserved biological traits. Species threatened by introduced species show an intermediate level of phylogenetic signal. Comparing our D values for extinct species to D values for extant species [72] shows that phylogenetic signal in extinct species is not as strong as for species threatened by overexploitation (D = 0.08), and is slightly stronger than for those threatened by introduced species (D = 0.46), but it is much stronger than for extant species threatened by habitat loss (D = 0.60). This comparison may suggest that Holocene mammal extinctions up to date have been largely driven by a combination of overexploitation and invasive species rather than habitat loss.
(b). Effects of body size on extinction and risk
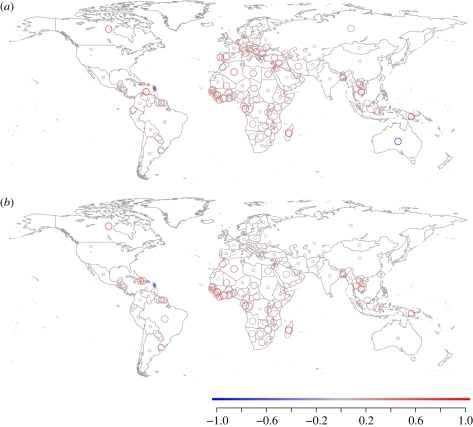
Including extinct species, body mass is significantly correlated with extinction risk in 79 mostly tropical countries, out of the 174 countries for which we have models (figure 3a). Large body mass is almost exclusively associated with high extinction risk (negative slope in only 17 countries); only Australia shows a significant negative relationship between body mass and risk. Results excluding extinct species differ slightly, particularly in areas with many extinct species (figure 3b), with slope estimates for several Caribbean countries and Madagascar decreasing by large amounts. Excluding extinct species makes the relationship between large size and high risk non-significant in 22 countries, most notably in Russia, southern Europe and the Middle East, for which the smaller numbers of documented terrestrial Holocene species extinctions have only involved megafaunal mammals (e.g. Bison priscus, Bos primigenius, Equus hydruntinus, Mammuthus primigenius, Megaloceros giganteus). Interestingly, in Hispaniola, Brazil, Saudi Arabia and South Africa, extinction risk excluding extinct species is significantly related to large body mass, but not when including the extinct species, which were mainly either mesoherbivores (e.g. Antidorcas bondi, Gazella saudiya) or comprised both small-bodied and large-bodied sympatric taxa (e.g. nesophontid island-shrews, heteropsomyine and capromyid rodents, and megalonychid sloths on Hispaniola).
Figure 3.
The relationship between extinction risk and body mass within countries, (a) including and (b) excluding extinct species. Circle colour shows the estimated slope of the relationship from a phylogenetic GLS regression, and circle size the significance for this slope (small circle, p > 0.05; big circle, p < 0.05). Models were fitted for countries with more than 10 species with all data and in the phylogeny. Slope values more than 1 were set to 1.
Results using phylogenetically independent contrasts on the phylogeny with branch lengths set to equal give very similar results to PGLS (see the electronic supplementary material, figure S1), and the spatial congruence across slope estimates between the two methods is high (Pearson correlation coefficient = 0.81 including extinct species, 0.74 excluding extinct species). Only a few countries differ in whether their slope estimates are significant or not, and these tend to have low sample sizes. This high congruence supports the assumption that exact dating of phylogenetic splits for extinct species is not an important factor for our analyses, so we only discuss PGLS results below.
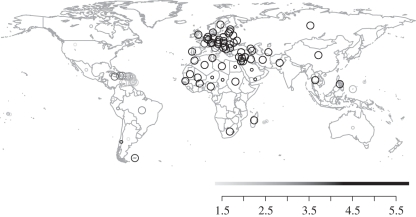
In most countries, Holocene extinctions have been selective with respect to body size (figure 4): median body size of extinct species is larger than expected under random extinction nearly everywhere. Notable exceptions are the USA, Australia, and some Mediterranean and Latin American countries; most of these include both continental regions and also offshore islands (e.g. Hawaii and the California Channel Islands; Corsica and Sardinia), with the islands having been the focus of disproportionately high numbers of extinctions of comparatively small-bodied insular species (typically rodents). On average, extinct species were largest in continental Europe and Asia, but even the smaller-bodied extinct species in insular regions such as the Caribbean, Indonesia and New Guinea constituted a biased sample in terms of body size for these countries.
Figure 4.
The median body mass of extinct species and its departure from expectation under random extinction within countries. Circles are only plotted for countries with at least one extinct species. Circle shading shows the median log10-transformed body mass of extinct species; circle size indicates the results of a one-tailed significance test of this observed median extinct body mass against that expected under random extinction of the same number of species within countries (small circle, p > 0.05; big circle, p < 0.05).
Overall, our results support previous studies in that large body size is an important correlate of high extinction risk [7], and that its effect varies spatially [8,51]. Large extant species are currently at risk almost exclusively in the tropics, but including extinct species in our analyses provides further evidence that there may have been an extinction filter acting throughout the Holocene. This filter seems to have removed large species during the Holocene from many geographical regions, such as southern Europe, Russia, the Caribbean, Madagascar and Indonesia. Additional evidence for a Holocene body-size extinction filter is provided by our simulations of random extinctions within countries, which reveal size selectivity of past extinction across much of the globe. Whereas the increased vulnerability of large mammals to extinction during the Late Pleistocene has been well established [14,49], and the early loss of large-bodied species has been proposed for specific regional mammal faunas during the Holocene (e.g. the Caribbean; [16,69]), increased extinction-proneness of large mammals has not previously been demonstrated for global mammal faunas across the Holocene.
On the other hand, we found a consistently negative relationship between body mass and extinction risk in Australia. It has been suggested that Australian mammals of intermediate body mass (within a proposed ‘critical weight range’ of 35–5500 g) have an elevated risk of extinction due to region-specific interactions between mammal ecology and human threat factors [86,87]. Since we did not fit nonlinear terms, the many more available body mass values for intermediate and large species may drive an apparent negative relationship in Australia, while the true relationship would be hump-shaped. Furthermore, even our inclusion of extinct Holocene species reveals no relationship between body size and extinction risk in the USA, Mexico, several South American countries, and most of central and eastern Asia, which could also be taken as evidence against a general extinction filter hypothesis. However, the non-significant simulation results for countries comprising both continental regions and offshore islands may reflect a mixture of different past extinction processes, notably the higher impacts of invasive species on small island mammals versus anthropogenic overexploitation of larger mammals on continents [16]. It is also probable that older extinction filters have significantly affected our analyses for continental regions such as the Americas, Australia and much of Asia, where many large susceptible mammals were already removed during the Late Pleistocene through glacial cycling or earlier human agency. It would be interesting to test this hypothesis by incorporating country-level data on Late Pleistocene mammal extinctions into similar analyses once more information becomes available on these faunas.
(c). Resolution and bias of the Holocene fossil record
Although we are dependent upon the recent fossil record as the primary source of information on past mammalian species losses, extinction data derived from the fossil record for all three of the major island systems that have experienced particularly high levels of Holocene extinctions (Madagascar, Cuba and Hispaniola) are still demonstrably unresolved. All three islands have incomplete extinction chronologies for their endemic mammal faunas: known radiometric last-occurrence dates (either directly or indirectly dated) are only available for 70 per cent of the extinct species known from the recent fossil record on Madagascar (18/26), 45 per cent on Cuba (9/20) and 42 per cent on Hispaniola (10/24). In addition, a higher proportion of these last-occurrence dates fall completely within the period of human occupancy for Madagascar (16/18) and Hispaniola (9/10) compared with Cuba (2/9).
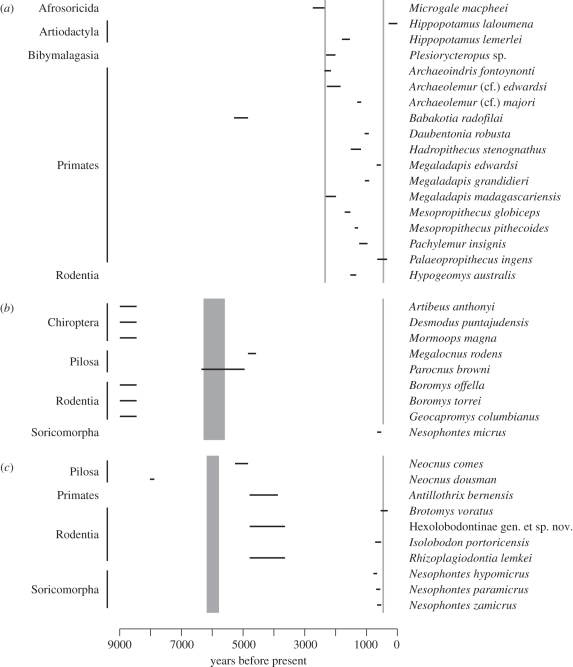
Investigation of the completeness of extinct species last-occurrence dates, and the degree to which these can be temporally correlated with first regional human arrival or other major human-caused environmental impacts, should indicate whether past mammal extinction data can be of use for identifying causative threat factors in modern faunas. The available radiometric dates demonstrate that representatives of all of the major mammal clades present in the Late Quaternary fossil record of Madagascar, Cuba and Hispaniola persisted through the Pleistocene and into the climatically stable Holocene, the interval when humans first reached each of these island systems (figure 5). It is therefore probable that most or all Late Quaternary mammal species losses on all three islands were driven by some form of prehistoric or historical-era human activities, rather than by non-anthropogenic environmental processes [16,69,88]. However, it is only possible to demonstrate evidence for a temporal overlap between extinct species and humans for 62 per cent of the extinct Late Quaternary mammal fauna of Madagascar, 38 per cent of Hispaniola and 10 per cent of Cuba. Extinction events may vary considerably in duration from rapid disappearance to gradual population attrition and decline in response to different drivers and ecologies [89], complicating any retrospective attempt to identify cause and effect in such events. For the remainder of the extinct mammal faunas of each island, in the absence of a meaningful timeline of last-occurrence dates we cannot therefore attempt to identify possible extinction drivers with any confidence, if humans were indeed responsible for these events.
Figure 5.
Available radiometric last-occurrence dates of extinct mammal species in relation to timing of first human arrival and European arrival for (a) Madagascar, (b) Cuba, and (c) Hispaniola. Black lines represent 1σ radiometric confidence limits for extinct mammal species last-occurrence dates; shaded areas represent 1σ radiometric confidence limits for first regional prehistoric evidence of human presence; shaded line represents known calendar date of first regional European arrival.
Furthermore, the resolution of available data indicating temporal overlap between humans and extinct mammals differs significantly between the three islands, demonstrating inter-regional biases in fossil data quality as well as overall incompleteness of fossil data (χ2 = 7.796, d.f. = 2, p < 0.05). This difference probably represents varying levels of past research effort in different regions, rather than intrinsic preservational differences in the regional fossil record for these large islands; the higher resolution of last-occurrence dates available for Madagascar matches the greater amount of historical research that has been carried out on this island's Holocene mammal fauna, due in large part to long-term anthropological interest in extinct lemurs [68], in contrast to the relatively limited radiometric investigations conducted to date for extinct Caribbean mammal faunas. Similar biases are likely to extend to other regional Holocene faunas, in particular for the Indonesia-New Guinea region, for which only 38 per cent (11/29) of the mammal species interpreted as having become extinct during the Holocene have even been formally described (see the electronic supplementary material, table S1). These inter-regional patterns of incompleteness in our understanding of Holocene extinctions therefore present further constraints to identifying causative processes in past extinction events, and further restrict the usefulness of the recent fossil record for informing current-day conservation of phylogenetically or ecologically similar taxa. They also emphasize caution when interpreting the conclusions we have drawn in the last two sections, as our analyses of Holocene mammal extinctions are largely based on fossil data and are therefore probably highly incomplete for many regions.
4. Conclusions
Our results demonstrate that mammal extinctions across the Holocene have been phylogenetically and spatially concentrated in specific taxa and geographical areas, and are generally consistent with the hypothesis that large mammals have been disproportionately vulnerable to extinction since the end of the last glaciation. An extinction filter operating for much of the Late Quaternary has successively removed susceptible large-bodied species from extinction-prone regions. These have tended to be either island systems with naive mammal faunas, such as Madagascar, the Caribbean or southeast Asia, or continental regions with long Holocene histories of high human population density and agricultural activity. The size selectivity of extinction risk throughout the Holocene has led to current mammalian threat hotspots being located in tropical regions with more recent histories of anthropogenic intensification, such as southern Asia and the Neotropics. However, phylogenetic and spatial patterns of past extinction and current risk also support the idea that extinction processes have changed through time, with the focus shifting from islands to continents and from introduced species and overexploitation to widespread habitat loss and fragmentation. Finally, data on the quality of the Holocene fossil record emphasize that there are still substantial limitations to describing the dynamics of mammal extinctions even in the recent past.
Given the complexity of these results, can the past provide useful new insights for understanding current mammal extinctions and developing predictive models for the future? Overall, our results seem to caution against using Holocene extinction data at face value to make oversimplified predictions; although there are consistent similarities in the body-size selectivity of past extinction and current risk, it may be difficult to predict risk for particular countries and taxa, with past extinctions only able to inform us about current risk at a relatively broad scale. Further research is required to investigate whether mammal faunas that have survived historical extinction filters are now threatened by different anthropogenic threats, which may therefore alter the phylogenetic, ecological or geographical pattern of extinction-proneness traits. Data biases and incompleteness pose an additional barrier to the usefulness of past extinction data for understanding long-term patterns or trends in anthropogenic impacts on regional mammal faunas, especially for hotspots of mammalian diversity such as southeast Asia, which is currently experiencing extremely high levels of mammalian extinction risk [2]. Although the direct use of fossil data in future projections of extinction risk is currently not straightforward, additional insights into extinction processes gained from this unique source of data in more locally and taxonomically restricted, high-resolution studies will still be useful in understanding current species extinction risk.
Acknowledgements
This study was funded by a NERC Postdoctoral Fellowship (NE/D009456/1) and a Royal Society University Research Fellowship (UF080320) to STT, a Marie Curie Fellowship from the European Commission to SAF (FP6 Early-Stage Training Network ‘Understanding and Conserving Earth's Biodiversity Hotspots’), and funding from the Danish National Research Foundation to the Centre for Macroecology, Evolution and Climate. We thank Jennifer Crees for data on Holocene distributions of extinct European mammal species, Katie Marske and anonymous reviewers for helpful comments on the manuscript, and William Turvey for technical assistance.
References
- 1.Collen B., McRae L., Deinet S., De Palma A., Carranza T., Cooper N., Loh J., Baillie J. E. M. 2011. Predicting how populations decline to extinction. Phil. Trans. R. Soc. B 366, 2577–2586 10.1098/rstb.2011.0015 (doi:10.1098/rstb.2011.0015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schipper J., et al. 2008. The status of the world's land and marine mammals: diversity, threat, and knowledge. Science 322, 225–230 10.1126/science.1165115 (doi:10.1126/science.1165115) [DOI] [PubMed] [Google Scholar]
- 3.Bininda-Emonds O. R. P., et al. 2007. The delayed rise of present-day mammals. Nature 446, 507–512 [Corrigendum in Nature 2008 456, 274] 10.1038/nature05634 (doi:10.1038/nature05634) [DOI] [PubMed] [Google Scholar]
- 4.Jones K. E., et al. 2009. PanTHERIA: a species-level database of life history, ecology and geography of extant and recently extinct mammals. Ecology 90, 2648. 10.1890/08-1494.1 (doi:10.1890/08-1494.1) [DOI] [Google Scholar]
- 5.Purvis A., Gittleman J. L., Cowlishaw G., Mace G. M. 2000. Predicting extinction risk in declining species. Proc. R. Soc. Lond. B 267, 1947–1952 10.1098/rspb.2000.1234 (doi:10.1098/rspb.2000.1234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher D. O., Owens I. P. F. 2004. The comparative method in conservation biology. Trends Ecol. Evol. 19, 391–398 10.1016/j.tree.2004.05.004 (doi:10.1016/j.tree.2004.05.004) [DOI] [PubMed] [Google Scholar]
- 7.Cardillo M., Mace G. M., Jones K. E., Bielby J., Bininda-Emonds O. R. P., Sechrest W., Orme C. D. L., Purvis A. 2005. Multiple causes of high extinction risk in large mammal species. Science 309, 1239–1241 10.1126/science.1116030 (doi:10.1126/science.1116030) [DOI] [PubMed] [Google Scholar]
- 8.Cardillo M., Mace G. M., Gittleman J. L., Jones K. E., Bielby J., Purvis A. 2008. The predictability of extinction: biological and external correlates of decline in mammals. Proc. R. Soc. B 275, 1441–1448 10.1098/rspb.2008.0179 (doi:10.1098/rspb.2008.0179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purvis A. 2008. Phylogenetic approaches to the study of extinction. Annu. Rev. Ecol. Evol. Syst. 39, 301–319 10.1146/annurev-ecolsys-063008-102010 (doi:10.1146/annurev-ecolsys-063008-102010) [DOI] [Google Scholar]
- 10.Davidson A. D., Hamilton M. J., Boyer A. G., Brown J. H., Ceballos G. 2009. Multiple ecological pathways to extinction. Proc. Natl Acad. Sci. USA 106, 10 702–10 705 10.1073/pnas.0901956106 (doi:10.1073/pnas.0901956106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardillo M., Mace G. M., Gittleman J. L., Purvis A. 2006. Latent extinction risk and the future battlegrounds of mammal conservation. Proc. Natl Acad. Sci. USA 103, 4157–4161 10.1073/pnas.0510541103 (doi:10.1073/pnas.0510541103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacPhee R. D. E., Flemming C. 1999. Requiem æternam: the last five hundred years of mammalian species extinctions. In Extinctions in near time: causes, contexts, and consequences (ed. MacPhee R. D. E.), pp. 333–371 New York, NY: Kluwer Academic/Plenum [Google Scholar]
- 13.Morrison J. C., Sechrest W., Dinerstein E., Wilcove D. S., Lamoreux J. F. 2007. Persistence of large mammal faunas as indicators of global human impacts. J. Mammal. 88, 1363–1380 10.1644/06-MAMM-A-124R2.1 (doi:10.1644/06-MAMM-A-124R2.1) [DOI] [Google Scholar]
- 14.Martin P. S. 1984. Prehistoric overkill: the global model. In Quaternary extinctions: a prehistoric revolution (eds Martin P. S., Klein R. G.), pp. 354–403 Tucson, AZ: Arizona University Press [Google Scholar]
- 15.Barnosky A. D., Koch P. L., Feranec R. S., Wing S. L., Shabel A. B. 2004. Assessing the causes of Late Pleistocene extinctions on the continents. Science 306, 70–75 10.1126/science.1101476 (doi:10.1126/science.1101476) [DOI] [PubMed] [Google Scholar]
- 16.Turvey S. T. 2009. Holocene extinctions. Oxford, UK: Oxford University Press [Google Scholar]
- 17.Gill J. L., Williams J. W., Jackson S. T., Lininger K. B., Robinson G. S. 2009. Pleistocene megafaunal collapse, novel plant communities, and enhanced fire regimes in North America. Science 326, 1100–1103 10.1126/science.1179504 (doi:10.1126/science.1179504) [DOI] [PubMed] [Google Scholar]
- 18.Haynes G. 2009. American megafaunal extinctions at the end of the Pleistocene. Dordrecht, The Netherlands: Springer [Google Scholar]
- 19.Roberts R. G., et al. 2001. New ages for the last Australian megafauna: continent-wide extinction about 46,000 years ago. Science 292, 1888–1892 10.1126/science.1060264 (doi:10.1126/science.1060264) [DOI] [PubMed] [Google Scholar]
- 20.Miller G. H., Fogel M. L., Magee J. W., Gagan M. K., Clarke S. J., Johnson B. J. 2005. Ecosystem collapse in Pleistocene Australia and a human role in megafaunal extinction. Science 309, 287–290 10.1126/science.1111288 (doi:10.1126/science.1111288) [DOI] [PubMed] [Google Scholar]
- 21.Turney C. S. M., et al. 2008. Late-surviving megafauna in Tasmania, Australia, implicate human involvement in their extinction. Proc. Natl Acad. Sci. USA 105, 12 150–12 153 10.1073/pnas.0801360105 (doi:10.1073/pnas.0801360105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grün R., Eggins S., Aubert M., Spooner N., Pike A. W. G., Müller W. 2010. ESR and U-series dating of faunal material from Cuddie Springs, NSW, Australia: implications for the timing of the extinction of the Australian megafauna. Quater. Sci. Rev. 29, 596–610 10.1016/j.quascirev.2009.11.004 (doi:10.1016/j.quascirev.2009.11.004) [DOI] [Google Scholar]
- 23.Alroy J. 2001. Putting North America's end-Pleistocene megafaunal extinction in context: large-scale analyses of spatial patterns, extinction rates, and size distributions. In Extinctions in near time: causes, contexts, and consequences (ed. MacPhee R. D. E.), pp. 105–143 New York, NY: Kluwer Academic/Plenum [Google Scholar]
- 24.Tyndale-Biscoe H. 2005. Life of marsupials. Collingwood, Australia: CSIRO Publishing [Google Scholar]
- 25.Johnson C. 2006. Australia's mammal extinctions: a 50 000 year history. Cambridge, UK: Cambridge University Press [Google Scholar]
- 26.Grayson D. K., Meltzer D. J. 2002. Clovis hunting and large mammal extinction: a critical review of the evidence. J. World Prehistory 16, 313–359 10.1023/A:1022912030020 (doi:10.1023/A:1022912030020) [DOI] [Google Scholar]
- 27.Grayson D. K., Meltzer D. J. 2003. A requiem for North American overkill. J. Archaeol. Sci. 30, 585–593 10.1016/S0305-4403(02)00205-4 (doi:10.1016/S0305-4403(02)00205-4) [DOI] [Google Scholar]
- 28.Grayson D. K., Meltzer D. J. 2004. North American overkill continued? J. Archaeol. Sci. 31, 133–136 10.1016/j.jas.2003.09.001 (doi:10.1016/j.jas.2003.09.001) [DOI] [Google Scholar]
- 29.Wroe S., Field J., Fullagar R., Jermiin L. S. 2004. Megafaunal extinction in the late Quaternary and the global overkill hypothesis. Alcheringa 28, 291–331 10.1080/03115510408619286 (doi:10.1080/03115510408619286) [DOI] [Google Scholar]
- 30.Allen J. R. M., Hickler T., Singarayer J. S., Sykes M. T., Valdes P. J., Huntley B. 2010. Last glacial vegetation of northern Eurasia. Quater. Sci. Rev. 29, 2604–2618 10.1016/j.quascirev.2010.05.031 (doi:10.1016/j.quascirev.2010.05.031) [DOI] [Google Scholar]
- 31.Nogués-Bravo D., Ohlemüller R., Batra P., Araújo M. B. 2010. Climate predictors of Late Quaternary extinctions. Evolution 64, 2442–2449 10.1111/j.1558-5646.2010.01009.x (doi:10.1111/j.1558-5646.2010.01009.x) [DOI] [PubMed] [Google Scholar]
- 32.Davies T. J., Buckley L. B., Grenyer R., Gittleman J. L. 2011. The influence of past and present climate on the biogeography of modern mammal diversity. Phil. Trans. R. Soc. B 366, 2526–2535 10.1098/rstb.2011.0018 (doi:10.1098/rstb.2011.0018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lister A. M., Stuart A. J. 2008. The impact of climate change on large mammal distribution and extinction: evidence from the last glacial/interglacial transition. C. R. Geosci. 340, 615–620 10.1016/j.crte.2008.04.001 (doi:10.1016/j.crte.2008.04.001) [DOI] [Google Scholar]
- 34.Nogués-Bravo D., Rodríguez J., Hortal J., Batra P., Araújo M. B. 2008. Climate change, humans, and the extinction of the woolly mammoth. PLoS Biol. 6, e79. 10.1371/journal.pbio.0060079 (doi:10.1371/journal.pbio.0060079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters J., Gautier A., Brink J. S., Haenen W. 1994. Late Quaternary extinction of ungulates in sub-Saharan Africa: a reductionist's approach. J. Archaeol. Sci. 21, 17–28 10.1006/jasc.1994.1004 (doi:10.1006/jasc.1994.1004) [DOI] [Google Scholar]
- 36.Barnosky A. D., Lindsey E. L. 2009. Timing of Quaternary megafaunal extinction in South America in relation to human arrival and climate change. Quater. Int. 217, 10–29 10.1016/j.quaint.2009.11.017 (doi:10.1016/j.quaint.2009.11.017) [DOI] [Google Scholar]
- 37.Roberts N. 1998. The Holocene: an environmental history, 2nd edn. Oxford, UK: Blackwells [Google Scholar]
- 38.Guthrie R. D. 2004. Radiocarbon evidence of mid-Holocene mammoths stranded on an Alaskan Bering Sea island. Nature 429, 746–749 10.1038/nature02612 (doi:10.1038/nature02612) [DOI] [PubMed] [Google Scholar]
- 39.Sommer R. S., Benecke N. 2006. Late Pleistocene and Holocene development of the felid fauna (Felidae) of Europe: a review. J. Zool. 269, 7–19 10.1111/j.1469-7998.2005.00040.x (doi:10.1111/j.1469-7998.2005.00040.x) [DOI] [Google Scholar]
- 40.Solow A. R., Roberts D. L., Robbirt K. M. 2006. On the Pleistocene extinctions of Alaskan mammoths and horses. Proc. Natl Acad. Sci. USA 103, 7351–7353 10.1073/pnas.0509480103 (doi:10.1073/pnas.0509480103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buck C. E., Bard E. 2007. A calendar chronology for Pleistocene mammoth and horse extinction in North America based on Bayesian radiocarbon calibration. Quater. Sci. Rev. 26, 2031–2035 10.1016/j.quascirev.2007.06.013 (doi:10.1016/j.quascirev.2007.06.013) [DOI] [Google Scholar]
- 42.Haile J., et al. 2009. Ancient DNA reveals late survival of mammoth and horse in interior Alaska. Proc. Natl Acad. Sci. USA 106, 22 352–22 357 10.1073/pnas.0912510106 (doi:10.1073/pnas.0912510106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delcourt P. A., Delcourt H. R. 1998. Paleoecological insights on conservation of biodiversity: a focus on species, ecosystems and landscapes. Ecol. Appl. 8, 921–934 [Google Scholar]
- 44.Sutherland W. J., Pullin A. S., Dolman P. M., Knight T. M. 2004. The need for evidence-based conservation. Trends Ecol. Evol. 19, 305–308 10.1016/j.tree.2004.03.018 (doi:10.1016/j.tree.2004.03.018) [DOI] [PubMed] [Google Scholar]
- 45.Lauwerier R. C. G. M., Plug I. 2004. The future from the past: archaeozoology in wildlife conservation and heritage management. Oxford, UK: Oxbow Books [Google Scholar]
- 46.Jackson S. T., Hobbs R. J. 2009. Ecological restoration in the light of ecological history. Science 325, 567–569 10.1126/science.1172977 (doi:10.1126/science.1172977) [DOI] [PubMed] [Google Scholar]
- 47.Johnson C. N. 2002. Determinants of loss of mammal species during the Late Quaternary ‘megafauna’ extinctions: life history and ecology, but not body size. Proc. R. Soc. B 269, 2221–2227 10.1098/rspb.2002.2130 (doi:10.1098/rspb.2002.2130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brook B. W., Bowman D. M. J. S. 2004. The uncertain blitzkrieg of Pleistocene megafauna. J. Biogeogr. 31, 517–523 10.1046/j.1365-2699.2003.01028.x (doi:10.1046/j.1365-2699.2003.01028.x) [DOI] [Google Scholar]
- 49.Lyons S. K., Smith F. A., Brown J. H. 2004. Of mice, mastodons and men: human-mediated extinctions on four continents. Evol. Ecol. Res. 6, 339–358 [Google Scholar]
- 50.Balmford A. 1996. Extinction filters and current resilience: the significance of past selection pressures for conservation biology. Trends Ecol. Evol. 11, 193–196 10.1016/0169-5347(96)10026-4 (doi:10.1016/0169-5347(96)10026-4) [DOI] [PubMed] [Google Scholar]
- 51.Fritz S. A., Bininda-Emonds O. R. P., Purvis A. 2009. Geographical variation in predictors of mammalian extinction risk: big is bad, but only in the tropics. Ecol. Lett. 12, 538–549 10.1111/j.1461-0248.2009.01307.x (doi:10.1111/j.1461-0248.2009.01307.x) [DOI] [PubMed] [Google Scholar]
- 52.Greuter W. 1995. Extinctions in Mediterranean areas. In Extinction rates (eds Lawton J. H., May R. M.), pp. 88–97 Oxford, UK: Oxford University Press [Google Scholar]
- 53.van Weers D. J. 2005. A taxonomic revision of the Pleistocene Hystrix (Hystricidae, Rodentia) from Eurasia with notes on the evolution of the family. Contrib. Zool. 74, 301–312 [Google Scholar]
- 54.Silva Taboada G., Suárez Duque W., Díaz Franco S. 2007. Compendio de los mamíferos terrestres autóctonos de Cuba vivientes y extinguidos. La Habana, Cuba: Museo Nacional de Historia Natural [Google Scholar]
- 55.Turvey S. T., Weksler M., Morris E. L., Nokkert M. 2010. Taxonomy, phylogeny and diversity of the extinct Lesser Antillean rice rats (Sigmodontinae: Oryzomyini), with description of a new genus and species. Zool. J. Linn. Soc. 160, 748–772 10.1111/j.1096-3642.2009.00628.x (doi:10.1111/j.1096-3642.2009.00628.x) [DOI] [Google Scholar]
- 56.Cooke S. B., Rosenberger A. L., Turvey S. T. 2011. An extinct monkey from Haiti and the origins of the Greater Antillean primates. Proc. Natl Acad. Sci. USA 108, 2699–2704 10.1073/pnas.1009161108 (doi:10.1073/pnas.1009161108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson D. E., Reeder D. M. 2005. Mammal species of the world: a taxonomic and geographic reference, 3rd edn. Baltimore, MD: John Hopkins University Press [Google Scholar]
- 58.Harvey P. H., Pagel M. D. 1991. The comparative method in evolutionary biology. Oxford, UK: Oxford University Press [Google Scholar]
- 59.Biknevicius A. R., McFarlane D. A., MacPhee R. D. E. 1993. Body size in Amblyrhiza inundata (Rodentia: Caviomorpha), an extinct megafaunal rodent from the Anguilla Bank, West Indies: estimates and implications. Am. Museum Novitates 3079, 1–25 [Google Scholar]
- 60.White J. L. 1993. Indicators of locomotor habits in xenarthrans: evidence for locomotor heterogeneity among fossil sloths. J. Verteb. Paleontol. 13, 230–242 10.1080/02724634.1993.10011502 (doi:10.1080/02724634.1993.10011502) [DOI] [Google Scholar]
- 61.Bloch J. I., Rose K. D., Gingerich P. D. 1998. New species of Batodonoides (Lipotyphla, Geolabididae) from the Early Eocene of Wyoming: smallest known mammal? J. Mammal. 79, 804–827 10.2307/1383090 (doi:10.2307/1383090) [DOI] [Google Scholar]
- 62.Hopkins S. S. B. 2008. Reassessing the mass of exceptionally large rodents using toothrow length and area as proxies for body mass. J. Mammal. 89, 232–243 10.1644/06-MAMM-A-306.1 (doi:10.1644/06-MAMM-A-306.1) [DOI] [Google Scholar]
- 63.Lyons S. K. 2003. A quantitative assessment of the range shifts of Pleistocene mammals. J. Mammal. 84, 385–402 (doi:10.1644/1545-1542(2003)084<0385:AQAOTR>2.0.CO;2) [DOI] [Google Scholar]
- 64.Davies T. J., Purvis A., Gittleman J. L. 2009. Quaternary climate change and the geographic ranges of mammals. Am. Nat. 174, 297–307 10.1086/603614 (doi:10.1086/603614) [DOI] [PubMed] [Google Scholar]
- 65.Woods C. A. 1989. A new capromyid rodent from Haiti: the origin, evolution, and extinction of West Indian rodents, and their bearing on the origin of New World hystricognaths. Nat. Hist. Museum Los Angeles County, Sci. Ser. 33, 59–90 [Google Scholar]
- 66.IUCN 2008. 2008 IUCN Red List of threatened species. Cambridge, UK: International Union for Conservation of Nature (IUCN) http://www.iucnredlist.org/mammals [Google Scholar]
- 67.Fritz S. A., Purvis A. 2010. Phylogenetic diversity does not capture body size variation at risk in the world's mammals. Proc. R. Soc. B 277, 2435–2441 10.1098/rspb.2010.0030 (doi:10.1098/rspb.2010.0030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burney D. A., Burney L. P., Godfrey L. R., Jungers W. L., Goodman S. M., Wright H. T., Jull A. J. T. 2004. A chronology for late prehistoric Madagascar. J. Hum. Evol. 47, 25–63 10.1016/j.jhevol.2004.05.005 (doi:10.1016/j.jhevol.2004.05.005) [DOI] [PubMed] [Google Scholar]
- 69.MacPhee R. D. E. 2009. Insulae infortunatae: establishing a chronology for Late Quaternary mammal extinctions in the West Indies. In American megafaunal extinctions at the end of the Pleistocene (ed. Haynes G.), pp. 169–193 Dordrecht, The Netherlands: Springer [Google Scholar]
- 70.R Development Core Team 2009. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; http://www.R-project.org [Google Scholar]
- 71.Legendre P. 1993. Spatial autocorrelation: trouble or new paradigm? Ecology 74, 1659–1673 10.2307/1939924 (doi:10.2307/1939924) [DOI] [Google Scholar]
- 72.Fritz S. A., Purvis A. 2010. Selectivity in mammalian extinction risk and threat types: a new measure of phylogenetic signal strength in binary traits. Conserv. Biol. 24, 1042–1051 10.1111/j.1523-1739.2010.01455.x (doi:10.1111/j.1523-1739.2010.01455.x) [DOI] [PubMed] [Google Scholar]
- 73.Orme C. D. L., Freckleton R. P., Thomas G. H. 2009. CAIC. Comparative analyses using independent contrasts. R package version 1.0.4. http://r-forge.r-project.org/projects/caic
- 74.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1–15 10.1086/284325 (doi:10.1086/284325) [DOI] [Google Scholar]
- 75.Freckleton R. P., Harvey P. H., Pagel M. 2002. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 160, 712–726 10.1086/343873 (doi:10.1086/343873) [DOI] [PubMed] [Google Scholar]
- 76.Purvis A., Rambaut A. 1995. Comparative analysis by independent contrasts (CAIC): an Apple Macintosh application for analysing comparative data. Comput. Appl. Biosci. 11, 247–251 [DOI] [PubMed] [Google Scholar]
- 77.Millennium Ecosystem Assessment 2005. Ecosystems and human well-being: biodiversity synthesis. Washington, DC: World Resources Institute [Google Scholar]
- 78.Bennett P. M., Owens I. P. F. 2002. Evolutionary ecology of birds. Oxford, UK: Oxford University Press [Google Scholar]
- 79.Burney D. A. 1999. Rates, patterns, and processes of landscape transformation and extinction in Madagascar. In Extinctions in near time: causes, contexts, and consequences (ed. MacPhee R. D. E.), pp. 145–164 New York, NY: Kluwer Academic/Plenum [Google Scholar]
- 80.Alcover J. A., Sans A., Palmer M. 1998. The extent of extinctions of mammals on islands. J. Biogeogr. 25, 913–918 10.1046/j.1365-2699.1998.00246.x (doi:10.1046/j.1365-2699.1998.00246.x) [DOI] [Google Scholar]
- 81.Purvis A., Agapow P.-M., Gittleman J. L., Mace G. M. 2000. Nonrandom extinction and the loss of evolutionary history. Science 288, 328–330 10.1126/science.288.5464.328 (doi:10.1126/science.288.5464.328) [DOI] [PubMed] [Google Scholar]
- 82.Mace G., Balmford A. 2000. Patterns and processes in contemporary mammalian extinction. In Priorities for the conservation of mammalian diversity; has the panda had its day? (eds Entwistle A., Dunstone N.), pp. 27–52 Cambridge, UK: Cambridge University Press [Google Scholar]
- 83.Bielby J., Cunningham A. A., Purvis A. 2006. Taxonomic selectivity in amphibians: ignorance, geography or biology? Anim. Conserv. 9, 135–143 10.1111/j.1469-1795.2005.00013.x (doi:10.1111/j.1469-1795.2005.00013.x) [DOI] [Google Scholar]
- 84.Mooers A. Ø., Goring S. J., Turvey S. T., Kuhn T. S. 2009. Holocene extinctions and the loss of feature diversity. In Holocene extinctions (ed. Turvey S. T.), pp. 263–277 Oxford, UK: Oxford University Press [Google Scholar]
- 85.Russell G. J., Brooks T. M., McKinney M. M., Anderson C. G. 1998. Present and future taxonomic selectivity in bird and mammal extinctions. Conserv. Biol. 12, 1365–1376 10.1046/j.1523-1739.1998.96332.x (doi:10.1046/j.1523-1739.1998.96332.x) [DOI] [Google Scholar]
- 86.Cardillo M., Bromham L. 2001. Body size and risk of extinction in Australian mammals. Conserv. Biol. 15, 1435–1440 10.1046/j.1523-1739.2001.00286.x (doi:10.1046/j.1523-1739.2001.00286.x) [DOI] [Google Scholar]
- 87.Johnson C. N., Isaac J. L. 2009. Body mass and extinction risk in Australian marsupials: the ‘critical weight range' revisited. Austral. Ecol. 34, 35–40 10.1111/j.1442-9993.2008.01878.x (doi:10.1111/j.1442-9993.2008.01878.x) [DOI] [Google Scholar]
- 88.Steadman D. W., Martin P. S., MacPhee R. D. E., Jull A. J. T., McDonald H. G., Woods C. A., Iturralde-Vinent M., Hodgins G. W. L. 2005. Asynchronous extinction of Late Quaternary sloths on islands and continents. Proc. Natl Acad. Sci. USA 102, 11 763–11 768 10.1073/pnas.0502777102 (doi:10.1073/pnas.0502777102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Diamond J. M. 1989. Quaternary megafaunal extinctions: variations on a theme by Paganini. J. Archaeol. Sci. 16, 167–175 10.1016/0305-4403(89)90064-2 (doi:10.1016/0305-4403(89)90064-2) [DOI] [Google Scholar]