Abstract
The small effect size of most individual risk factors for psychiatric disorders likely reflects biological heterogeneity and diagnostic imprecision, which has encouraged genetic studies of intermediate biologic phenotypes that are closer to the molecular effects of risk genes than are the clinical symptoms. Neuroimaging-based intermediate phenotypes have emerged as particularly promising because they map risk associated gene effects onto physiological processes in brain that are altered in patients and in their healthy relatives. Recent evidence using this approach has elucidated discrete, dissociable biological mechanisms of risk genes at the level of neural circuitries, and their related cognitive functions. This approach may greatly contribute to our understanding of the genetics and pathophysiology of psychiatric disorders.
Risk genes and psychiatric disorders
Most cases of psychiatric disorders are thought to result from complex interactions between multiple genes of mostly small to modest effect and the environment [1]. The identification of these genes and their function has proven to be an extraordinarily challenging endeavor, even using the popular and biologically agnostic genome-wide association (GWA) approach, which results in the potential identification of genes whose mechanisms of risk association are almost always unknown. This approach, as with earlier linkage and candidate gene approaches, has not produced incontrovertible evidence of association of common genetic variation with clinical diagnosis, though a few promising loci have been found. As psychiatric disorders are syndromal, analogous to most common medical illnesses, it is rational to assume that genetic association is stronger at the level of biological substrates related to syndromal risk. This is analogous to evidence that genes for common medical syndromal disorders show much stronger association to the biological substrates that contribute to risk. Examples include lipid levels and risk for heart disease [2], sodium homeostasis and risk for hypertension [3], and body mass index (BMI) and risk for diabetes [4].
In an attempt to investigate the relevant functions of genes implicated in psychiatric illness, the study of biological substrates has become of increasing interest. In particular, the application of so-called neuroimaging genetics – a technique based on in vivo brain measures - has illustrated how risk genes can modulate specific neural processes, translating gene effects on brain function and structure in a more meaningful way than the clinical association approach alone [5, 6]. Investigators, in general, have favored the study of effects of risk associated genotypes in the brains of healthy individuals [7–11], to circumvent the potential contamination of signal from non-genetic and/or illness-related factors (e.g. treatment, symptoms, smoking, general health issues) that make it difficult to interpret results in patients alone. On the other hand, studying “healthy volunteers” exclusively runs the risk of identifying a genetic effect in brain that has little or no relationship to the disorder itself.
Intermediate phenotypes
Genes have pleiotropic biologic effects and can be expected to have diverse effects on the development and function of the brain. The role that genes play in increasing risk for a psychiatric disorder presumably reflects a particular effect of that gene on neural systems that impact on the biology of susceptibility. A crucial point in identifying the mechanisms through which genes confer risk for psychiatric disorders is to define whether the brain phenotype that the risk gene modulates is a biological mechanism implicated in the psychiatric disorder and in risk for the psychiatric disorder. Finding an association between a gene and a brain function does not mean that that association is related to the mechanism of risk for the clinical illness. The analogous conundrum in clinical medicine would be to find that a newly identified risk gene for diabetes also affects BMI, without having evidence that BMI itself is a risk factor for diabetes (which it clearly is).
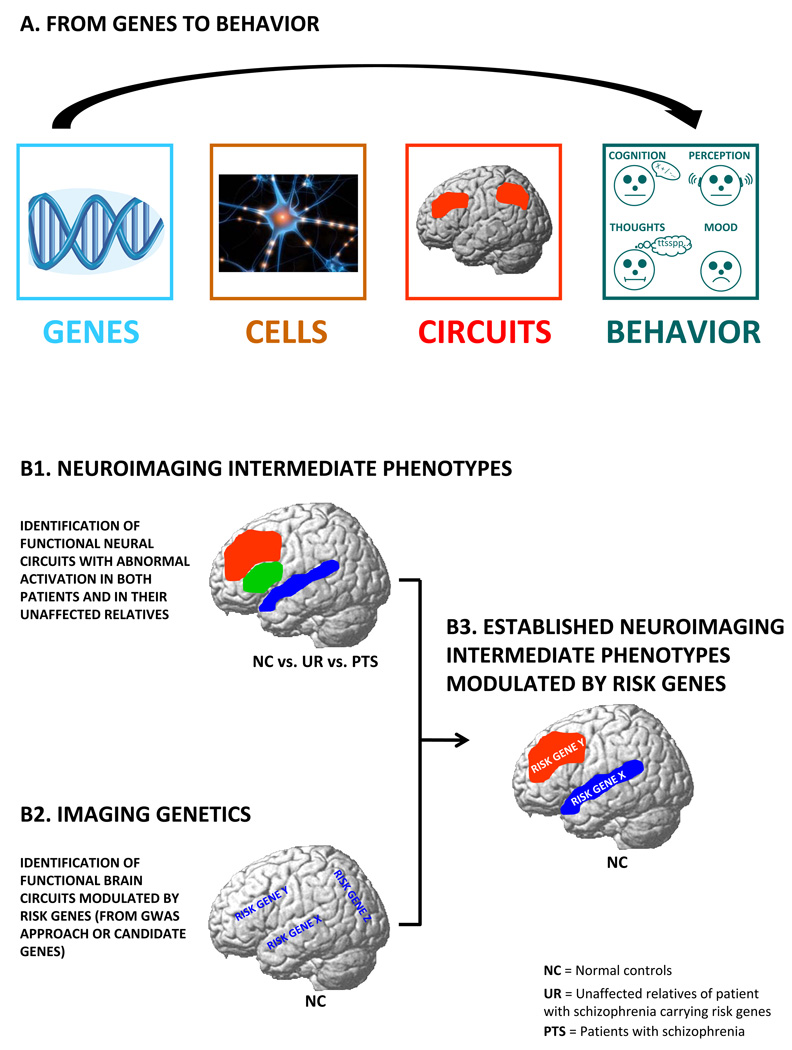
To link a gene effect in brain to the gene effect on risk for the syndromal diagnosis, it is necessary to show that the brain effect is a biological substrate also linked to illness risk, a so called intermediate phenotype. An intermediate phenotype related to mental illness is a heritable trait that is located in the path of pathogenesis from genetic predisposition to psychopathology [12]. The path goes from relatively simple effects in cells, to more complex effects in neural circuits in the brain, to much more complex effects on the emergent phenomenology of these simpler effects, i.e. behavior and psychiatric syndromes (see Figure 1A).
Figure 1.
A. From genes to behavior. Genes encode for molecules, not specific symptoms. The abnormal behaviors observed in psychiatric disorders (such as delusions, hallucinations and cognitive deficits in schizophrenia) are the product of intermediate steps that occur between genes and behavior, such as cell activity and neural circuits. An intermediate phenotype is a heritable trait that is located on the pathogenesis path from genetic predisposition to psychopathology and is likely associated with a more basic and proximal etiological process and therefore more amenable to genetic investigation. B. Genetic risk on vulnerable brain circuits. B1. Identification of neuroimaging intermediate phenotypes – which are alterations in neural circuit functions in patients with psychiatric disorders as well as in high genetic risk subjects (i.e. unaffected relatives). B2. Imaging genetics defines neural systems that are modulated by genetic variations, including genetic variations that have been associated with increased risk for psychiatric disorders. B3. To increase the probability that the observed biological modulation by the risk genetic variation is the mechanism through which that gene increases the risk for a psychiatric disorder, it is important to demonstrate that the gene modulates a neuroimaging intermediate phenotype.
The search for intermediate phenotypes is best conducted in specific populations that carry risk genes for the disorder without confounding factors related to the state of the disease (e.g. treatment, smoking, etc). Unaffected relatives of patients with psychiatric disorders, ideally healthy cotwins or siblings, fulfill both criteria: they are enriched in risk genes and are healthy. Many aspects of human brain function, behavior and physiology have been studied in such populations with evidence that a variety of such measures are heritable and enriched in relatives [13]. Phenotype studies of relatives of patients with schizophrenia, however, have potentially serious methodological problems which should be appreciated. Relatives may share environmental or behavioral characteristics (e.g. drug or tobacco use, temperament) that can impact on measures of brain function. Moreover, comparisons of relatives across generations are especially problematic because they involve age and life experience factors that are difficult to control.
The literature has many examples of abnormalities in relatives of patients with schizophrenia, from simple tests of processing speed to more complex cognitive operations that mirror those found in patients, implicating a number of potential intermediate phenotypes of interest. Because many of these will turn out to be redundant, it will be important to clarify which are independent, not only for the unnecessary repetition of information that a lack of independency could represent, but most importantly for studies of risk genes. Indeed, it is expected that some risk genes will map onto some intermediate phenotypes and not others; thus, the overlap or autonomy of different intermediate phenotypes is a crucial aspect in dissecting neural mechanisms of genetic risk.
In this article, we review neuroimaging intermediate phenotype findings related to schizophrenia and evidence of genes associated with increased risk for schizophrenia that modulate these intermediate phenotypes (Figure 1B 1–3). We will focus on schizophrenia and fMRI as an example, but the model can be extended to other psychiatric disorders and other neuroimaging techniques. Based on previous reviews [14–16], studies have been grouped into five different cognitive domains whose circuits have been consistently reported altered in patients with schizophrenia.
Neuroimaging intermediate phenotypes related to working memory and the impact of selected risk genes
Altered fMRI-based activation of prefrontal-parietal circuitry during working memory, the cognitive process to maintain and manipulate information for a short period of time, has been consistently reported in patients with schizophrenia. For the most part, unaffected relatives of patients with schizophrenia show qualitatively similar abnormal engagement of prefrontal cortex (PFC), thalamus, hippocampus-parahippocampus formation (HF) and inferior parietal lobule – especially in the right hemisphere [14, 16, 17–20 ··] (Supplementary Table 1.A). Activation of these regions and of the circuit involving them has been studied using the “imaging genetic” approach for several putative schizophrenia associated genes [8–11, 21–34, 30 ··] (Supplementary Table 2.A). Interestingly, the first GWA positive gene, ZNF804A, has not shown association with this phenotype per se [10] (but see below).
Genetic modulation of PFC coupling between different areas implicated in working memory circuits has also been explored in imaging genetics paradigms with interesting results (e.g. modulation of DLPFC-HF and DLPFC-PFC coupling by ZNF804A [10 ··]; DLPFC or VLPFC coupling with parietal cortex by COMT-GRM3 epistasis [31]). These circuit based associations involve more complex and likely realistic measures of brain function, but PFC coupling with other regions as an intermediate phenotype related to risk for schizophrenia has not been demonstrated yet and requires further exploration. Moreover, in some reports, engagement of the prefrontal cortex may be modulated in different ways by genes based on diagnosis (patients with schizophrenia versus normal controls) [29, 32]. These findings are not easily interpreted, because while they suggest complex gene by disease modulation, they can also be driven by confounders related to disease-state factors, such as gene by medication interactions.
In summary, prefrontal-parietal activation during working memory tasks seems to be a robust intermediate phenotype, consistently reported as abnormal (mostly increased engagement referred to as “inefficiency” or increase noise) in healthy relatives of patients with schizophrenia. Genetic exploration of this circuit suggests a relatively selective modulation of the circuit by some risk genes but not by others (Figure 2.A).
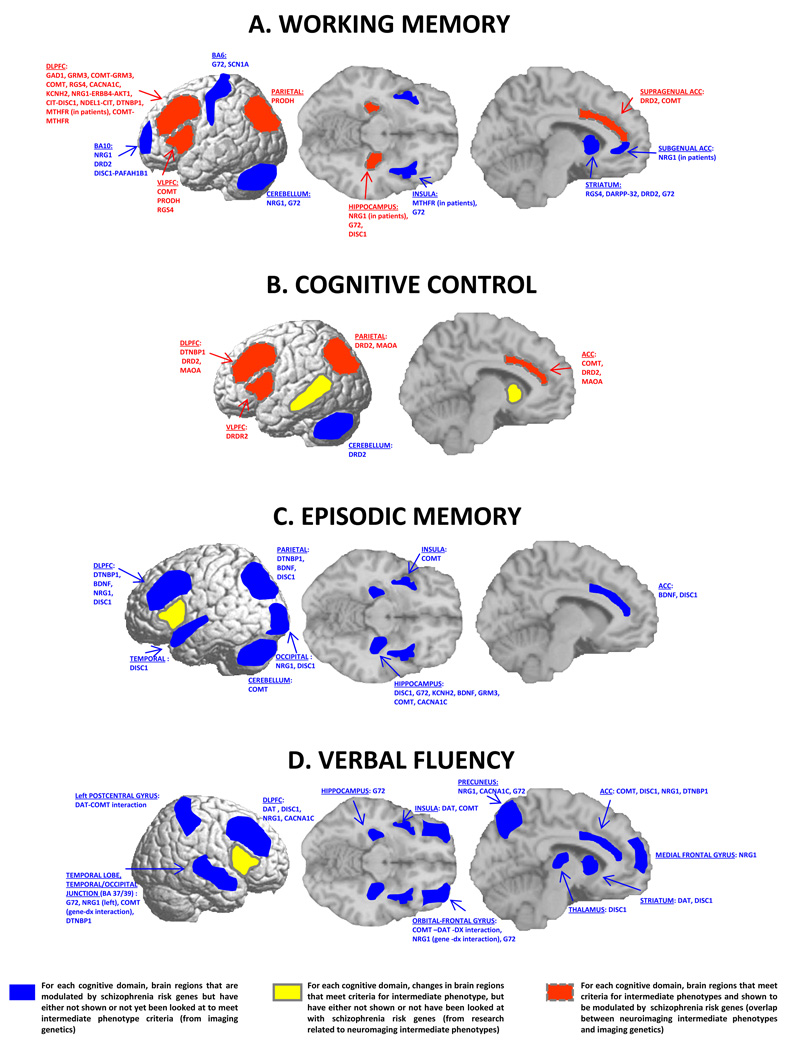
Figure 2. Genetic modulation on vulnerable circuits.
A. Working memory. Most brain areas reported altered in patients and their healthy relatives during working memory task are also modulated by a number of risk genes explored with the same paradigm (red fields with square dots) (DLPFC, VLPFC, ACC, parietal cortex, and HF). Many other effects of genes during working memory paradigms have not been show to be intermediate phenotypes (striatum, basal ganglia, subgenual ACC, insula, BA10, BA 4/6, cerebellum) (blue fields). B. Cognitive control circuit. Several brain areas within the cognitive control circuit have been reported to be modulated by risk genes during cognitive control processing (PFC, especially ACC, superior temporal gyrus, parietal cortex, and cerebellum). Among these, only PFC (DLPFC, VLPFC and ACC) and parietal cortex have been consistently reported being altered in patients with schizophrenia and their unaffected relatives with cognitive control paradigms (red fields with square dots). Striatum and middle temporal gyrus (BA 21) have been reported altered in patients with schizophrenia and their healthy relatives during cognitive control, although none of the risk genes studied so far have shown modulation of these regions (yellow fields with solid line). C. Episodic memory circuit. Studies of potential intermediate phenotypes during episodic memory paradigms are very few and the only area consistently reported altered in patients and in their unaffected relatives is the VLPFC, a region that has not been shown to be modulated by risk genes so far explored with this paradigm (yellow field with solid line). On the other hand, several risk genes have been reported to modulate hippocampal activity during episodic memory, as well as DLPFC, ACC, insula, cerebellum, temporal, parietal, and occipital cortices, all regions whose role as intermediate phenotypes during episodic memory in schizophrenia has not been convincingly demonstrated (blue fields). Thus, there are no brain regions yet that show overlap between the two areas of research (no red fields). D. Verbal fluency circuit. Right IFG has been reported altered in patients with schizophrenia and their healthy relatives during verbal fluency paradigms. This same area has not been shown to be modulated by risk genes (yellow field with solid line). Many other regions have been reported to be modulated by risk genes during verbal fluency related paradigms, but their role as intermediate phenotypes has not been consistently established (blue fields).
For working memory and cognitive control, only brain areas that were reported consistently altered in at least three studies are presented as potential intermediate phenotypes (from Supplementary Table 1). For episodic memory and verbal fluency, given the paucity of studies, only brain areas with at least one replicated result are reported as intermediate phenotypes (from Supplementary Table 1). For list of genes showing modulation on each circuit, refer to Supplementary Table 2.
Neuroimaging intermediate phenotypes related to cognitive control/attention and the impact of selected genes
Cognitive control is an executive function that refers to the ability to direct behavior toward a goal in the presence of conflict and is an integral process of many different cognitive paradigms. The inferior lateral frontal and anterior cingulate (ACC) cortices are key regions implicated in cognitive control [35] and consistently reported altered in patients with schizophrenia [36]. Several studies have explored dysfunction of ACC activation in the context of cognitive control paradigms in unaffected relatives of patients with schizophrenia [14, 16, 37, 38] with somewhat variable results, including decrease [37 ·], increase [38], or no difference [14, 16] in ACC activity (Supplementary Table 1.B). In contrast, altered PFC activation has been consistently demonstrated in healthy relatives during cognitive control tasks, although its independence from other cognitive domains, such as working memory (and vice versa) is unclear since this question has not been explicitly explored. Recently, investigators have started to study the abnormal coupling in cognitive control circuits as a potential intermediate phenotype and preliminary results suggest a decrease of the coupling within prefrontal regions in healthy relatives of patients with schizophrenia [39]. Few studies so far have explored the modulation of risk genes on cognitive control circuits, reporting increase [40], decrease [41] or no effect [42] on ACC activity (Supplementary Table 2.B; Figure 2.B).
Neuroimaging intermediate phenotypes related to episodic memory and the impact of selected genes
The hippocampal formation (hippocampus proper and the parahippocampal cortex) plays a fundamental role in episodic memory - the ability to learn, store and retrieve information [43] - and hippocampus dysfunction has been consistently reported in schizophrenia [44]. Surprisingly, episodic memory studies in healthy relatives of patients with schizophrenia so far have failed to report abnormal hippocampal activation [14], though one study reported abnormal parahippocampal activity [45 ·] (Supplementary Table 1.C). Many studies have explored the role of risk genes in hippocampus modulation during episodic memory tasks in healthy volunteers [8, 9, 11, 28, 30, 46–50] (Supplementary Table 2.C) (Figure 2.C). Since the status of hippocampus function during episodic memory as an intermediate phenotype related to risk for schizophrenia has not been convincingly demonstrated, the link between the neurophysiological effect of these genes on HF and their mechanism for increasing the risk of schizophrenia is unclear.
Neuroimaging intermediate phenotypes related to verbal fluency and the impact of selected genes
Verbal fluency is a classic test of language production that requires the subject to generate words, beginning with a particular letter or within a particular semantic category. Functional MRI studies have reported disturbed patterns of left hemisphere dominance of language processing in verbal fluency in patients with schizophrenia [51], showing increased activity in right hemisphere, with bilateral activation of Broca’s area during word generation tasks. The results in healthy relatives are similar, but few studies have looked at this (Supplementary Table 1.D). Two studies [52, 53 ·], using verb and word generation tasks, reported increased right VLPFC activation in healthy twins discordant for schizophrenia compared to normal control twins, reproducing the same pattern observed in patients. Despite several reports suggesting a modulation of verbal fluency circuits by schizophrenia risk genes [54–62], none of these studies show genetic modulation of right VLPFC activation, which has most consistently been reported as an intermediate phenotype during verbal fluency paradigms (Supplementary Table 2.D; Figure 2.D). An exception was found with NRG1 [55], although it is impossible to exclude a medication effect on gene modulation since the gene effect was only found in patients with schizophrenia.
Using a different verbal fluency fMRI paradigm, a sentence completion task, Whalley et al. found decreased medial frontal and cerebellar activation [63] and aberrant coupling between Broca's area and parietal cortex in subjects at high genetic risk [64]. The effects of three risk genes were explored with this paradigm (G72, NRG1, COMT) (Supplementary Table 2.D) [65–67] and only NRG1 was reported to modulate the medial frontal gyrus [66], although the relationship of this area with verbal fluency processing is not clear.
In conclusion, so far, abnormal activation of the right VLPFC has potential as an intermediate phenotype related to genetic risk for schizophrenia, but risk genes that show effects in other paradigms do not seem to modulate this response.
Neuroimaging intermediate phenotypes related to faces/emotion processing and the impact of genes
Amygdala reactivity to threatening stimuli appears to be abnormal in schizophrenia [68]. Only three studies have examined the genetic liability of faces/emotion processing in subjects at increased genetic risk for schizophrenia [18, 69, 70], with inconsistent results (Supplementary Table 1.E). Indeed, the largest study [18 ·] found no evidence of an abnormality in healthy sibs. This circuit has been extensively explored in imaging genetics, and results seem to suggest that it is vulnerable to modulation by genes increasing the risk for affective disorders [10, 71], while genes associated with risk for schizophrenia seem to be protective (e.g val/val subjects in the COMT functional val/met genotype have reduced amygdala reactivity to threatening stimuli [7, 72] (Supplementary Table 2.E). In conclusion, genes impacting this circuit, if associated with risk for schizophrenia, likely increase the risk for the disorder through a mechanism different from their effect on amygdala reactivity.
Conclusions
The study of brain-based intermediate phenotypes in psychiatry is conceptually appealing and biologically compelling. It is a “no brainer” that genes do not encode for psychiatric symptoms but for simpler molecular processing in cells and information processing in brain. However, the intermediate phenotype approach has to be undertaken with considerable caution and attention to detail. A number of caveats need to be recognized in the literature as it currently exists, including inconsistencies in directionality of findings (hypo- or hyper-activation), biases in criteria for selection of relatives (offspring versus siblings or parents, and the presence of other psychiatric diagnoses in the relatives but not in the comparison groups), small sample sizes not powered to detect small effect sizes, and the relative independency of the different intermediate phenotypes, still not explored for any of them.
These important limitations notwithstanding, the evidence is growing of genetic vulnerability maps of the brain and the way in which they are modulated by risk genes in psychiatry. To date, a consistent observation in unaffected relatives of patients with schizophrenia is abnormal PFC and inferior parietal lobule activation, across different executive cognitive paradigms, and an abnormal lateralization of prefrontal-temporal areas during verbal fluency. These neuroimaging phenotypes have been most frequently studied in relation to schizophrenia-risk genes. Interestingly, there appear to be specific effects of genes on some neuroimaging intermediate phenotypes but not on others, suggesting discrete biological mechanisms of risk in some but not all brain areas/circuits and their related cognitive functions. On the other hand, there are still important gaps between the data on “imaging genetics” and the data on intermediate phenotypes. Many schizophrenia risk genes have been shown to modulate circuits that have not yet been demonstrated to be intermediate phenotypes (e.g. genes modulating prefrontal cortex coupling during working memory tasks or genes modulating hippocampus activity during episodic memory), and not all reported neuroimaging intermediate phenotypes have been systematically investigated with risk genes, despite clear evidence of their being enriched in healthy relatives (e.g., cognitive control). Connecting these two different lines of investigation could greatly contribute to the field's progress in understanding the complex pathophysiology of psychiatric disorders.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health. We thank Venkata S. Mattay for helpful discussion.
Footnotes
Financial Disclosures
None of the authors have conflicts of interest to disclose.
References
- 1.Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354(12):1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 3.Ji W, Foo JN, O'Roak BJ, Zhao H, Larson MG, Simon DB, Newton-Cheh C, State MW, Levy D, Lifton RP. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet. 2008;40(5):592–599. doi: 10.1038/ng.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walters RG, Jacquemont S, Valsesia A, de Smith AJ, Martinet D, Andersson J, Falchi M, Chen F, Andrieux J, Lobbens S, et al. A new highly penetrant form of obesity due to deletions on chromosome 16p11.2. Nature. 2010;463(7281):671–675. doi: 10.1038/nature08727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hariri AR, Weinberger DR. Imaging genomics. Br Med Bull. 2003;65:259–270. doi: 10.1093/bmb/65.1.259. [DOI] [PubMed] [Google Scholar]
- 6.Bigos KL, Weinberger DR. Imaging genetics--days of future past. Neuroimage. 2010;53(3):804–809. doi: 10.1016/j.neuroimage.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 7.Drabant EM, Hariri AR, Meyer-Lindenberg A, Munoz KE, Mattay VS, Kolachana BS, Egan MF, Weinberger DR. Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Arch Gen Psychiatry. 2006;63(12):1396–1406. doi: 10.1001/archpsyc.63.12.1396. [DOI] [PubMed] [Google Scholar]
- 8.Egan MF, Straub RE, Goldberg TE, Yakub I, Callicott JH, Hariri AR, Mattay VS, Bertolino A, Hyde TM, Shannon-Weickert C, et al. Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. Proc Natl Acad Sci U S A. 2004;101(34):12604–12609. doi: 10.1073/pnas.0405077101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callicott JH, Straub RE, Pezawas L, Egan MF, Mattay VS, Hariri AR, Verchinski BA, Meyer-Lindenberg A, Balkissoon R, Kolachana B, et al. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc Natl Acad Sci U S A. 2005;102(24):8627–8632. doi: 10.1073/pnas.0500515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Esslinger C, Walter H, Kirsch P, Erk S, Schnell K, Arnold C, Haddad L, Mier D, Opitz von Boberfeld C, Raab K, et al. Neural mechanisms of a genome-wide supported psychosis variant. Science. 2009;324(5927):605. doi: 10.1126/science.1167768. ··This study tested the effect of the first GWAS positive schizophrenia risk SNP (rs1344706 ZNF804A) on a complex brain imaging phenotype. They reported that this SNP modulates DLPFC-hippocampus coupling but not DLPFC activation in a sample of normal controls.
- 11.Bigos KL, Mattay VS, Callicott JH, Straub RE, Vakkalanka R, Kolachana B, Hyde TM, Lipska BK, Kleinman JE, Weinberger DR. Genetic variation in CACNA1C affects brain circuitries related to mental illness. Arch Gen Psychiatry. 2010;67(9):939–945. doi: 10.1001/archgenpsychiatry.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 13.Egan MF, Cannon T. Intermediate phenotypes and genetic origins. In: Weinberger DR, Harrison P, editors. Schizophrenia. Third Edition. Oxford, UK: Wiley-Blackwell; 2011. [Google Scholar]
- 14.MacDonald AW, 3rd, Thermenos HW, Barch DM, Seidman LJ. Imaging genetic liability to schizophrenia: systematic review of FMRI studies of patients' nonpsychotic relatives. Schizophr Bull. 2009;35(6):1142–1162. doi: 10.1093/schbul/sbn053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7(10):818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- 16.Goghari VM. Executive functioning-related brain abnormalities associated with the genetic liability for schizophrenia: an activation likelihood estimation meta-analysis. Psychol Med. 2010;14:1–14. doi: 10.1017/S0033291710001972. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106(4):1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rasetti R, Mattay VS, Wiedholz LM, Kolachana BS, Hariri AR, Callicott JH, Meyer-Lindenberg A, Weinberger DR. Evidence that altered amygdala activity in schizophrenia is related to clinical state and not genetic risk. Am J Psychiatry. 2009;166(2):216–225. doi: 10.1176/appi.ajp.2008.08020261. ·This study reported reduced amygdala reactivity to threatening stimuli in patients with schizophrenia and modulation of amygdala coupling by antipsychotic drugs. No amygdala differences were detected between healthy siblings and normal controls, although this study used the largest sample size compared to similar studies.
- 19.Karch S, Leicht G, Giegling I, Lutz J, Kunz J, Buselmeier M, Hey P, Sporl A, Jager L, Meindl T, et al. Inefficient neural activity in patients with schizophrenia and nonpsychotic relatives of schizophrenic patients: evidence from a working memory task. J Psychiatr Res. 2009;43(15):1185–1194. doi: 10.1016/j.jpsychires.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 20. Callicott JH, Egan MF, Mattay VS, Bertolino A, Bone AD, Verchinksi B, Weinberger DR. Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am J Psychiatry. 2003;160(4):709–719. doi: 10.1176/appi.ajp.160.4.709. ··In this pioneering study, two different cohorts of non-psychotic siblings of patients with schizophrenia were investigated. Non-psychotic siblings manifested exaggerated activation response at the dorso-lateral prefrontal and parietal cortices, despite similar accuracy in n-back task. This is interpreted as “inefficiency” of DLPFC, since the increase in the fMRI response was observed for the same level of accuracy across groups.
- 21.Nicodemus KK, Callicott JH, Higier RG, Luna A, Nixon DC, Lipska BK, Vakkalanka R, Giegling I, Rujescu D, Clair DS, et al. Evidence of statistical epistasis between DISC1, CIT and NDEL1 impacting risk for schizophrenia: biological validation with functional neuroimaging. Hum Genet. 2010 Jan 19; doi: 10.1007/s00439-009-0782-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Straub RE, Lipska BK, Egan MF, Goldberg TE, Callicott JH, Mayhew MB, Vakkalanka RK, Kolachana BS, Kleinman JE, Weinberger DR. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12(9):854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- 23.Krug A, Markov V, Eggermann T, Krach S, Zerres K, Stocker T, Shah NJ, Schneider F, Nothen MM, Treutlein J, et al. Genetic variation in the schizophrenia-risk gene neuregulin1 correlates with differences in frontal brain activation in a working memory task in healthy individuals. Neuroimage. 2008;42(4):1569–1576. doi: 10.1016/j.neuroimage.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 24.Buckholtz JW, Meyer-Lindenberg A, Honea RA, Straub RE, Pezawas L, Egan MF, Vakkalanka R, Kolachana B, Verchinski BA, Sust S, et al. Allelic variation in RGS4 impacts functional and structural connectivity in the human brain. J Neurosci. 2007;27(7):1584–1593. doi: 10.1523/JNEUROSCI.5112-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kircher T, Thienel R, Wagner M, Reske M, Habel U, Kellermann T, Frommann I, Schwab S, Wolwer W, von Wilmsdorf M, et al. Neuregulin 1 ICE-single nucleotide polymorphism in first episode schizophrenia correlates with cerebral activation in fronto-temporal areas. Eur Arch Psychiatry Clin Neurosci. 2009;259(2):72–79. doi: 10.1007/s00406-008-0837-4. [DOI] [PubMed] [Google Scholar]
- 26.Kempf L, Nicodemus KK, Kolachana B, Vakkalanka R, Verchinski BA, Egan MF, Straub RE, Mattay VA, Callicott JH, Weinberger DR, Meyer-Lindenberg A. Functional polymorphisms in PRODH are associated with risk and protection for schizophrenia and fronto-striatal structure and function. PLoS Genet. 2008;4(11):e1000252. doi: 10.1371/journal.pgen.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldberg TE, Straub RE, Callicott JH, Hariri A, Mattay VS, Bigelow L, Coppola R, Egan MF, Weinberger DR. The G72/G30 gene complex and cognitive abnormalities in schizophrenia. Neuropsychopharmacology. 2006;31(9):2022–2032. doi: 10.1038/sj.npp.1301049. [DOI] [PubMed] [Google Scholar]
- 29.Bertolino A, Fazio L, Caforio G, Blasi G, Rampino A, Romano R, Di Giorgio A, Taurisano P, Papp A, Pinsonneault J, et al. Functional variants of the dopamine receptor D2 gene modulate prefronto-striatal phenotypes in schizophrenia. Brain. 2009;132(Pt 2):417–425. doi: 10.1093/brain/awn248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huffaker SJ, Chen J, Nicodemus KK, Sambataro F, Yang F, Mattay V, Lipska BK, Hyde TM, Song J, Rujescu D, et al. A primate-specific, brain isoform of KCNH2 affects cortical physiology, cognition, neuronal repolarization and risk of schizophrenia. Nat Med. 2009;15(5):509–518. doi: 10.1038/nm.1962. ··A new brain-specific isoform of the ether- à -go-go-related K channel KCNH2 was identified. This isoform was highly expressed in hippocampus of subjects with schizophrenia and low expressed in heart and highly associated with a single nucleotide polymorphism in KCNH2 that showed association with schizophrenia in a meta-analysis of five clinical data sets and with abnormal hippocampus activation during an episodic memory task. Given that the binding of some antipsychotics with KCNH2 is likely responsible for the cardiac side effects of some antipsychotics (abnormal QT interval, sudden cardiac failure), the discovery of a brain-specific isoform has potential therapeutic implications.
- 31.Tan HY, Chen Q, Sust S, Buckholtz JW, Meyers JD, Egan MF, Mattay VS, Meyer-Lindenberg A, Weinberger DR, Callicott JH. Epistasis between catechol-O-methyltransferase and type II metabotropic glutamate receptor 3 genes on working memory brain function. Proc Natl Acad Sci U S A. 2007;104(30):12536–12541. doi: 10.1073/pnas.0610125104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roffman JL, Weiss AP, Deckersbach T, Freudenreich O, Henderson DC, Wong DH, Halsted CH, Goff DC. Interactive effects of COMT Val108/158Met and MTHFR C677T on executive function in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(6):990–995. doi: 10.1002/ajmg.b.30684. [DOI] [PubMed] [Google Scholar]
- 33.Nicodemus KK, Law AJ, Radulescu E, Luna A, Kolachana B, Vakkalanka R, Rujescu D, Giegling I, Straub RE, McGee K, et al. Biological validation of increased schizophrenia risk with NRG1, ERBB4, and AKT1 epistasis via functional neuroimaging in healthy controls. Arch Gen Psychiatry. 2010;67(10):991–1001. doi: 10.1001/archgenpsychiatry.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jansen A, Krach S, Krug A, Markov V, Eggermann T, Zerres K, Stocker T, Shah NJ, Nothen MM, Treutlein J, et al. A putative high risk diplotype of the G72 gene is in healthy individuals associated with better performance in working memory functions and altered brain activity in the medial temporal lobe. Neuroimage. 2009;45(3):1002–1008. doi: 10.1016/j.neuroimage.2008.12.054. [DOI] [PubMed] [Google Scholar]
- 35.Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7(4):367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- 36.Eisenberg DP, Berman KF. Executive function, neural circuitry, and genetic mechanisms in schizophrenia. Neuropsychopharmacology. 2010;35(1):258–277. doi: 10.1038/npp.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sepede G, Ferretti A, Perrucci MG, Gambi F, Di Donato F, Nuccetelli F, Del Gratta C, Tartaro A, Salerno RM, Ferro FM, Romani GL. Altered brain response without behavioral attention deficits in healthy siblings of schizophrenic patients: an event-related fMRI study. Neuroimage. 2010;49(1):1080–1090. doi: 10.1016/j.neuroimage.2009.07.053. ·This recent work is the only example of cognitive control study that reported decreased AAC activation in relatives compared to normal controls after controlling for performance similarity between groups.
- 38.McAllindon DP, Wilman AH, Purdon SE, Tibbo PG. Functional magnetic resonance imaging of choice reaction time in chronic schizophrenia and first-degree relatives. Schizophr Res. 2010;120(1–3):232–233. doi: 10.1016/j.schres.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Woodward ND, Waldie B, Rogers B, Tibbo P, Seres P, Purdon SE. Abnormal prefrontal cortical activity and connectivity during response selection in first episode psychosis, chronic schizophrenia, and unaffected siblings of individuals with schizophrenia. Schizophr Res. 2009;109(1–3):182–190. doi: 10.1016/j.schres.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 40.Blasi G, Mattay VS, Bertolino A, Elvevag B, Callicott JH, Das S, Kolachana BS, Egan MF, Goldberg TE, Weinberger DR. Effect of catechol-O-methyltransferase val158met genotype on attentional control. J Neurosci. 2005;25(20):5038–5045. doi: 10.1523/JNEUROSCI.0476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan J, Fossella J, Sommer T, Wu Y, Posner MI. Mapping the genetic variation of executive attention onto brain activity. Proc Natl Acad Sci U S A. 2003;100(12):7406–7411. doi: 10.1073/pnas.0732088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thimm M, Krug A, Kellermann T, Markov V, Krach S, Jansen A, Zerres K, Eggermann T, Stocker T, Shah NJ, et al. The effects of a DTNBP1 gene variant on attention networks: an fMRI study. Behav Brain Funct. 2010;6:54. doi: 10.1186/1744-9081-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dickerson BC, Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology. 2010;35(1):86–104. doi: 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinberger DR. Cell biology of the hippocampal formation in schizophrenia. Biol Psychiatry. 1999;45(4):395–402. doi: 10.1016/s0006-3223(98)00331-x. [DOI] [PubMed] [Google Scholar]
- 45. Thermenos HW, Seidman LJ, Poldrack RA, Peace NK, Koch JK, Faraone SV, Tsuang MT. Elaborative verbal encoding and altered anterior parahippocampal activation in adolescents and young adults at genetic risk for schizophrenia using FMRI. Biol Psychiatry. 2007;61(4):564–574. doi: 10.1016/j.biopsych.2006.04.044. ·This is the only study showing differences in HF activation between relatives of patients with schizophrenia and normal controls during an episodic memory task.
- 46.Thimm M, Krug A, Markov V, Krach S, Jansen A, Zerres K, Eggermann T, Stöcker T, Shah NJ, Nöthen MM, et al. The impact of dystrobrevin-binding protein 1 (DTNBP1) on neural correlates of episodic memory encoding and retrieval. Hum Brain Mapp. 2010;31(2):203–209. doi: 10.1002/hbm.20857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krug A, Markov V, Krach S, Jansen A, Zerres K, Eggermann T, Stocker T, Shah NJ, Nothen MM, Treutlein J, et al. The effect of Neuregulin 1 on neural correlates of episodic memory encoding and retrieval. Neuroimage. 2010;53(3):985–991. doi: 10.1016/j.neuroimage.2009.12.062. [DOI] [PubMed] [Google Scholar]
- 48.Jansen A, Krach S, Krug A, Markov V, Thimm M, Paulus FM, Zerres K, Stocker T, Shah NJ, Nothen MM, et al. The effect of G72 genotype on neural correlates of memory encoding and retrieval. Neuroimage. 2010;53(3):1001–1006. doi: 10.1016/j.neuroimage.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 49.Di Giorgio A, Blasi G, Sambataro F, Rampino A, Papazacharias A, Gambi F, Romano R, Caforio G, Rizzo M, Latorre V, et al. Association of the SerCys DISC1 polymorphism with human hippocampal formation gray matter and function during memory encoding. Eur J Neurosci. 2008;28(10):2129–2136. doi: 10.1111/j.1460-9568.2008.06482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krach S, Jansen A, Krug A, Markov V, Thimm M, Sheldrick AJ, Eggermann T, Zerres K, Stocker T, Shah NJ, et al. COMT genotype and its role on hippocampal-prefrontal regions in declarative memory. Neuroimage. 2010;53(3):978–984. doi: 10.1016/j.neuroimage.2009.12.090. [DOI] [PubMed] [Google Scholar]
- 51.Li X, Branch CA, DeLisi LE. Language pathway abnormalities in schizophrenia: a review of fMRI and other imaging studies. Curr Opin Psychiatry. 2009;22(2):131–139. doi: 10.1097/YCO.0b013e328324bc43. [DOI] [PubMed] [Google Scholar]
- 52.Sommer IE, Ramsey NF, Mandl RC, van Oel CJ, Kahn RS. Language activation in monozygotic twins discordant for schizophrenia. Br J Psychiatry. 2004;184:128–135. doi: 10.1192/bjp.184.2.128. [DOI] [PubMed] [Google Scholar]
- 53. Costafreda SG, Fu CH, Picchioni M, Kane F, McDonald C, Prata DP, Kalidindi S, Walshe M, Curtis V, Bramon E, et al. Increased inferior frontal activation during word generation: a marker of genetic risk for schizophrenia but not bipolar disorder? Hum Brain Mapp. 2009;30(10):3287–3298. doi: 10.1002/hbm.20749. ·During a verbal fluency task, patients with schizophrenia and their unaffected twins showed increased activation of right inferior frontal cortex relative to healthy controls and patients with bipolar disorder, suggesting abnormal left language lateralization as a marker of genetic risk for schizophrenia, but not bipolar disorder.
- 54.Prata DP, Mechelli A, Fu CH, Picchioni M, Toulopoulou T, Bramon E, Walshe M, Murray RM, Collier DA, McGuire P. Epistasis between the DAT 3' UTR VNTR and the COMT Val158Met SNP on cortical function in healthy subjects and patients with schizophrenia. Proc Natl Acad Sci U S A. 2009;106(32):13600–13605. doi: 10.1073/pnas.0903007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mechelli A, Prata DP, Fu CH, Picchioni M, Kane F, Kalidindi S, McDonald C, Demjaha A, Kravariti E, Toulopoulou T, et al. The effects of neuregulin1 on brain function in controls and patients with schizophrenia and bipolar disorder. Neuroimage. 2008;42(2):817–826. doi: 10.1016/j.neuroimage.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 56.Markov V, Krug A, Krach S, Whitney C, Eggermann T, Zerres K, Stocker T, Shah NJ, Nothen MM, Treutlein J, et al. Genetic variation in schizophrenia-risk-gene dysbindin 1 modulates brain activation in anterior cingulate cortex and right temporal gyrus during language production in healthy individuals. Neuroimage. 2009;47(4):2016–2022. doi: 10.1016/j.neuroimage.2009.05.067. [DOI] [PubMed] [Google Scholar]
- 57.Nieratschker V, Markov V, Krach S, Jansen A, Zerres K, Eggermann T, Stocker T, Shah NJ, Treutlein J, et al. Effect of CACNA1C rs1006737 on neural correlates of verbal fluency in healthy individuals. Neuroimage. 2010;49(2):1831–1836. doi: 10.1016/j.neuroimage.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 58.Kircher T, Krug A, Markov V, Whitney C, Krach S, Zerres K, Eggermann T, Stocker T, Shah NJ, Treutlein J, et al. Genetic variation in the schizophrenia-risk gene neuregulin 1 correlates with brain activation and impaired speech production in a verbal fluency task in healthy individuals. Hum Brain Mapp. 2009;30(10):3406–3416. doi: 10.1002/hbm.20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prata DP, Mechelli A, Fu CH, Picchioni M, Kane F, Kalidindi S, McDonald C, Howes O, Kravariti E, Demjaha A, et al. Opposite effects of catechol-O-methyltransferase Val158Met on cortical function in healthy subjects and patients with schizophrenia. Biol Psychiatry. 2009;65(6):473–480. doi: 10.1016/j.biopsych.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 60.Prata DP, Mechelli A, Picchioni MM, Fu CH, Toulopoulou T, Bramon E, Walshe M, Murray RM, Collier DA, McGuire P. Altered effect of dopamine transporter 3'UTR VNTR genotype on prefrontal and striatal function in schizophrenia. Arch Gen Psychiatry. 2009;66(11):1162–1172. doi: 10.1001/archgenpsychiatry.2009.147. [DOI] [PubMed] [Google Scholar]
- 61.Prata DP, Mechelli A, Fu CH, Picchioni M, Kane F, Kalidindi S, McDonald C, Kravariti E, Toulopoulou T, Miorelli A, et al. Effect of disrupted-in-schizophrenia-1 on pre-frontal cortical function. Mol Psychiatry. 2008;13(10):915–917. doi: 10.1038/mp.2008.76. 909. [DOI] [PubMed] [Google Scholar]
- 62.Krug A, Markov V, Krach S, Jansen A, Zerres K, Eggermann T, Stocker T, Shah NJ, Nothen MM, Georgi A, et al. Genetic variation in G72 correlates with brain activation in the right middle temporal gyrus in a verbal fluency task in healthy individuals. Hum Brain Mapp. 2011;32(1):118–126. doi: 10.1002/hbm.21005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whalley HC, Simonotto E, Flett S, Marshall I, Ebmeier KP, Owens DG, Goddard NH, Johnstone EC, Lawrie SM. fMRI correlates of state and trait effects in subjects at genetically enhanced risk of schizophrenia. Brain. 2004;127(Pt 3):478–490. doi: 10.1093/brain/awh070. [DOI] [PubMed] [Google Scholar]
- 64.Whalley HC, Simonotto E, Marshall I, Owens DG, Goddard NH, Johnstone EC, Lawrie SM. Functional disconnectivity in subjects at high genetic risk of schizophrenia. Brain. 2005;128(Pt 9):2097–2108. doi: 10.1093/brain/awh556. [DOI] [PubMed] [Google Scholar]
- 65.Hall J, Whalley HC, Moorhead TW, Baig BJ, McIntosh AM, Job DE, Owens DG, Lawrie SM, Johnstone EC. Genetic variation in the DAOA (G72) gene modulates hippocampal function in subjects at high risk of schizophrenia. Biol Psychiatry. 2008;64(5):428–433. doi: 10.1016/j.biopsych.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 66.Hall J, Whalley HC, Job DE, Baig BJ, McIntosh AM, Evans KL, Thomson PA, Porteous DJ, Cunningham-Owens DG, Johnstone EC, et al. A neuregulin 1 variant associated with abnormal cortical function and psychotic symptoms. Nat Neurosci. 2006;9(12):1477–1478. doi: 10.1038/nn1795. [DOI] [PubMed] [Google Scholar]
- 67.McIntosh AM, Baig BJ, Hall J, Job D, Whalley HC, Lymer GK, Moorhead TW, Owens DG, Miller P, Porteous D, et al. Relationship of catechol-O-methyltransferase variants to brain structure and function in a population at high risk of psychosis. Biol Psychiatry. 2007;61(10):1127–1134. doi: 10.1016/j.biopsych.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 68.Marwick K, Hall J. Social cognition in schizophrenia: a review of face processing. Br Med Bull. 2008;88(1):43–58. doi: 10.1093/bmb/ldn035. [DOI] [PubMed] [Google Scholar]
- 69.Barbour T, Murphy E, Pruitt P, Eickhoff SB, Keshavan MS, Rajan U, Zajac-Benitez C, Diwadkar VA. Reduced intra-amygdala activity to positively valenced faces in adolescent schizophrenia offspring. Schizophr Res. 2010;123(2–3):126–136. doi: 10.1016/j.schres.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Habel U, Klein M, Shah NJ, Toni I, Zilles K, Falkai P, Schneider F. Genetic load on amygdala hypofunction during sadness in nonaffected brothers of schizophrenia patients. Am J Psychiatry. 2004;161(10):1806–1813. doi: 10.1176/ajp.161.10.1806. [DOI] [PubMed] [Google Scholar]
- 71.Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008;63(9):852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smolka MN, Schumann G, Wrase J, Grusser SM, Flor H, Mann K, Braus DF, Goldman D, Buchel C, Heinz A. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci. 2005;25(4):836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.