Abstract
Lactocin 160 is a vaginal probiotic-derived bacteriocin shown to selectively inhibit the growth of Gardenerella vaginalis and some other pathogens commonly associated with bacterial vaginosis. The natural origin of this peptide, its safety, and selective antimicrobial properties make it a promising candidate for successful treatment and prophylaxis of bacterial vaginosis (BV). This study evaluated interactions between lactocin 160 and four other natural antimicrobials in the ability to inhibit G. vaginalis. We report that zinc lactate and soapnut extract act synergistically with lactocin 160 against this pathogen and therefore have a potential to be successfully used as the components of the multiple-hurdle antimicrobial formulation for the treatment of BV.
Keywords: Bacteriocin, Natural antimicrobial, Antimicrobial synergy
Introduction
Bacterial vaginosis is a complex multispecies infection of the lower genital tract, which affects millions of women each year [1]. Aside from having a dramatic negative impact on the quality of a woman’s life, this condition is notorious for causing serious gynecological and obstetric complications. Less than satisfactory results produced by the conventional antibiotic treatments for BV have prompted researchers to look for natural alternatives to antibiotics, particularly among the antimicrobial products of healthy vaginal lactobacilli. The topical application of these lactobacillus-derived antimicrobials, especially if followed by probiotic treatment, can potentially be used to restore a healthy microbial balance in BV-affected individuals [2]. Lactocin 160, a bacteriocin-like compound produced by a vaginal isolate of Lactobacillus rhamnosus 160, is a promising alternative treatment for BV. This antimicrobial peptide selectively inhibits some BV-associated pathogens, including Gardnerella vaginalis, without affecting the commensal vaginal lactobacilli [3]. Moreover, the safety of lactocin 160 for topical applications has been demonstrated using both in vivo and in vitro vaginal models [4].
The emergence of bacterial resistant strains to current antibiotics is a rapidly advancing problem in clinical microbiology [5]. The widespread resistance of G. vaginalis to metronidazole and clindamycin, commonly used for treatment of BV, has already been reported [6, 7]. Moreover, the ability of G. vaginalis to uptake DNA from other vaginal microorganisms drastically increases its chances of developing antimicrobial resistance [8]. One of the most effective ways to minimize the chance of developing resistance is by using multiple antimicrobial hurdles, with each having a different mode of action [9].
The multiple-hurdle approach relies on the use of multiple stress factors that simultaneously deplete various resources of a target cell, making the microbial adaptation processes more challenging. Generally, stressors with different molecular targets are used as hurdles because they tend to act synergistically when used in combination [9]. Multiple-hurdle technology has been utilized for many years to control microorganisms in clinical settings and in food preservation [9]. A secondary advantage of this practice is its cost-effectiveness; synergistically acting components of the antimicrobial formulation can be used in lower concentrations. In addition, the activity of an antimicrobial preparation can be modulated to a desired specificity by using a multicomponent formulation [9, 10]. In this study, we evaluated interactions between lactocin 160 and other natural antimicrobials in an effort to control the growth of G. vaginalis so the data can ultimately be used for the design of an effective multiple-hurdle treatment for BV.
Turovskiy et al. [11] demonstrated that lactocin 160 targets the cytoplasmic membranes of G. vaginalis cells, ultimately dissipating both components of the proton motive force and causing depletion of the cellular ATP content. The exact molecular mechanism of action is yet to be determined, but there is evidence that lactocin 160 facilitates formation of transient pores across the cytoplasmic membranes of G. vaginalis, thereby triggering transmembrane traffic of ions and molecules [11, unpublished data].
The four natural antimicrobials selected for the synergy study were zinc lactate, soapnut extract, poly-l-lysine, and lauric arginate. These substances were chosen because their antimicrobial mode of action is likely to differ from the mode of action of bacteriocins and because they have previously been approved for human use.
Zinc lactate is a salt of lactic acid, which is a major fermentative product of lactic acid bacteria (LAB), including vaginal lactobacilli. As a result, lactates are prevalent in the lower genital tract of healthy women [12]. Lactic acid is a crucial defense factor of healthy vaginal microbiota; thus, a number of feminine hygiene products, including those designed for treatment of BV, contain this compound [13]. Additionally, lactates are used as food preservatives. Several mechanisms are responsible for the antimicrobial properties of lactic acid and its salts. In an acidic environment, these antimicrobials act as ionophores, which drop the intracellular pH in bacteria [14, 15]. Lactates also create a hostile environment for the proliferation of microorganisms by decreasing the water activity [15]. Zinc salt of lactic acid was selected for this study over other lactate species because (1) zinc ions were shown to enhance the activity of the bacteriocin nisin against Listeria monocytogenes [15], and (2) there are reports of ionic zinc having both antiviral and spermicidal properties [16, 17]—two effects desirable in a feminine hygiene product.
Epsilon-poly-l-lysine (poly-l-lysine) is a secondary metabolite secreted by various Streptomycetaceae bacteria. This antimicrobial is a cationic polypeptide that consists of 25–35 l-lysine residues connected by amide bonds between ε-amino and α-carboxyl groups [18]. Commercially, poly-l-lysine is produced through a fermentation process involving Streptomyces albulus [18]. Numerous in vivo studies demonstrated that poly-l-lysine is safe for consumption [18, 19]. This antimicrobial is currently on the commercial market in Japan as a food preservative [20]. The antimicrobial activity of poly-l-lysine is related to its electrostatic adsorption to a cell’s surface. The exact mechanism of its action is largely unknown, although it has been proposed that this ionic adsorption strips the outer membrane in Gram-negative cells [20, 21].
Saponins are steroid or triterpenoid glycosides produced by a variety of plants and by some marine organisms. This group of natural detergents is very common in both human and animal diets [22]. Plant extracts containing saponins are commonly used as food additives. For example, Quillaja saponaria extract is widely used in both the food and beverage industries without any reported toxicity [23]. Saponins derived from the fruit pericarp of Sapindus mukorossi (soapnut) are of particular interest for this study because they have only an insignificant effect on the proliferation of vaginal lactobacilli. Additionally, Sapindus saponins have spermicidal properties and are used as active ingredients in the contraceptive cream CONSAP [24]. Finally, there are some reports of saponins inhibiting the replication of the HIV-1 virus [22, 25]. Although it is clear that the antimicrobial activity of saponins is related to their detergent-like properties, the exact mechanism of action is still unknown. Studies involving liposomes, however, suggest that saponins permanently damage cytoplasmic membranes, making them permeable to macromolecules [22].
Lauramide arginine ethyl ester (LAE) is a derivative of lauric acid, l-arginine, and ethanol. This antimicrobial is generally recognized as safe (GRAS) status for use in meat, poultry, and other food products (GRAS Notice No. GRN 000164). The antimicrobial activity of LAE is thought to be related to the compound’s surfactant properties [26]. Studies conducted by Rodriguez et al. [26] using transmission electron microscopy (TEM) indicated that LAE induced swelling of the outer membrane in a Gram-negative bacterium, Salmonella typhimurium. In contrast, LAE induced the formation of white spots and clear zones in the cytoplasmic membranes of Staphylococcus aureus, a Gram-positive bacterium. In both strains, these alterations induced the flux of potassium ions across the cytoplasmic membrane [26].
In this manuscript, we demonstrate that zinc lactate and soapnut extract act synergistically with lactocin 160 against G. vaginalis. This synergistic interaction indicates a possibility of these natural antimicrobials being successfully used as the components of a multiple-hurdle approach for control of BV-related pathogens.
Materials and Methods
Bacterial Strains and Growth Conditions
Frozen stocks of G. vaginalis ATCC 14018 were maintained at −70 °C in Brain Heart Infusion (BHI) broth (Difco, Sparks, MD) containing 3% horse serum (JRH Biosciences, KS), mixed with 15% glycerol. BHI containing 3% horse serum was also used to propagate the culture, which was passed through the medium overnight at least twice prior to being used in experiments. The cells were always inoculated into fresh medium contained in 50-mL centrifuge tubes (1% v/v) that were pre-incubated with a loosened cap, under anaerobic conditions, to minimize the stress effect; this way, the microorganism was directly transferred into a warm, anaerobic environment. The culture was then incubated under anaerobic conditions at 37 °C.
Preparation of Antimicrobial Solutions
The partially purified preparation of lactocin 160 was produced at the Cell Production and Recovery Facility (Waksman Institute, Rutgers University, NJ) using the method previously described by Aroutcheva et al. [3, 11]. The 10 AU mL−1 stock solution was prepared by dissolving 300 mg of this preparation in 1 mL of double distilled water. The total protein concentration in the stock solution was quantified by using Micro BCA™ Protein Assay Kit (Thermo Scientific, Rockford, IL), and its specific activity was determined to be 10.4 AU mg−1 protein.
The other four antimicrobials were generous gifts provided to us by their manufacturers. The sample of zinc lactate (PURAMEX ZN) was given to us by Purac America (Lincolnshire, IL). We received soapnut extract (SAPINDIN) from Sabinsa Corp. (Piscataway, NJ). Poly-l-lysine (250 mg mL−1) was sent to us by Chisso America, Inc. (Rye, NY), and lauramide arginine ethyl ester (100 mg mL−1, MIRENAT-CF) was a gift from Vedeqsa Corp. (Barcelona, Spain). Prior to being used in the experiments, the aqueous solutions of all the antimicrobials were filter-sterilized through 0.2-µm syringe filters (NALGENE, Rochester, NY).
Determination of the Minimal Inhibitory Concentrations (MICs) of the Antimicrobials
The minimal inhibitory concentrations (MICs) of all the antimicrobials were determined using the method reported by Badaoui Najjar et al. [27] with the some modifications. A separate microplate assay was conducted for each antimicrobial. Aqueous solutions covering a wide range of antimicrobial concentrations were prepared using sterile double distilled water. One hundred microliters of each dilution was then placed into a 96-well plate (Corning, Inc., Corning, NY), in duplicate, immediately followed by 100 µL of the newly inoculated G. vaginalis culture. Sterile, double distilled water was used as a negative control to reveal the growth patterns of G. vaginalis without any antimicrobial restraint. Subsequently, the surface of the wells was covered with sterilized mineral oil for prevention of condensation during the assay. The assay was conducted at 37 °C under anaerobic conditions. The OD595 readings were taken every 2 h for 48 h using an automated microplate reader (Model 550, Bio-Rad Laboratories, Hercules, CA). Some natural antimicrobials used in this study are only available in the partially purified form; therefore, we decided to express the activity of all the antimicrobials against G. vaginalis in arbitrary units (AU), defined as the lowest dilution of the partially purified preparation causing the full inhibition of the microorganism. One AU mL−1 of zinc lactate, soapnut extract, poly-l-lysine, and lauric arginate corresponded to 150, 200, 125, and 1,000 µg mL−1 of these preparations, respectively. The subsequent interaction analysis was conducted using arbitrary units of activity.
The Checkerboard Assay
The interactions between two antimicrobials were investigated using a microplate reader-based checkerboard assay described by Asok et al. [28] and Badaoui Najjar et al. [27]. Briefly, the aqueous solutions of each pair of antimicrobials were prepared separately. The solutions were then mixed in various proportions using a 96-well plate to produce antimicrobial mixtures containing a wide range of concentrations (the highest concentration of each antimicrobial in the mixture was above the antimicrobial’s MIC). In total, each well of the microplate contained 100 µL of the antimicrobial mixture (the composition of which systematically varied throughout the plate) and 100 µL of G. vaginalis culture. The cells used in the assay were prepared by diluting an overnight culture of G. vaginalis 100 times with fresh growth medium that was incubated overnight under anaerobic conditions to rid it of oxygen. The cells were then added to the wells of the microplate containing the antimicrobials (100 µL of diluted culture per well). Changes in turbidity were monitored under anaerobic conditions for 48 h using an automated microplate reader (Model 550, Bio-Rad Laboratories, Hercules, CA). The readings at OD595 were taken every 2 h while the plate was incubated at 37 °C. The ultimate goal of this assay is to determine the MICs of the tested antimicrobials when they are used in various combinations.
Analysis of the Checkerboard Assay Data
The nature of interactions between lactocin 160 and other natural antimicrobials was analyzed using isobolograms. This analytical method relies on the comparison of the MICs of two antimicrobials when they are used individually to their MICs when used in combination. The isobologram method is based on a visual comparison of these values when plotted on the same coordinate axis. Initially, the individual MICs of two antimicrobials are plotted on the x- and y-axes with the coordinates (0, x) and (y, 0). These two points are then connected by a perforated interaction line. The fractional inhibitory concentrations (FICs), defined as the ratio of the compound’s MIC when used in combination with the second antimicrobial to its MIC when used individually, of each compound are then plotted as the x and y coordinates of a single point.
Statistics
All experiments were prepared at least twice in duplicates.
Results
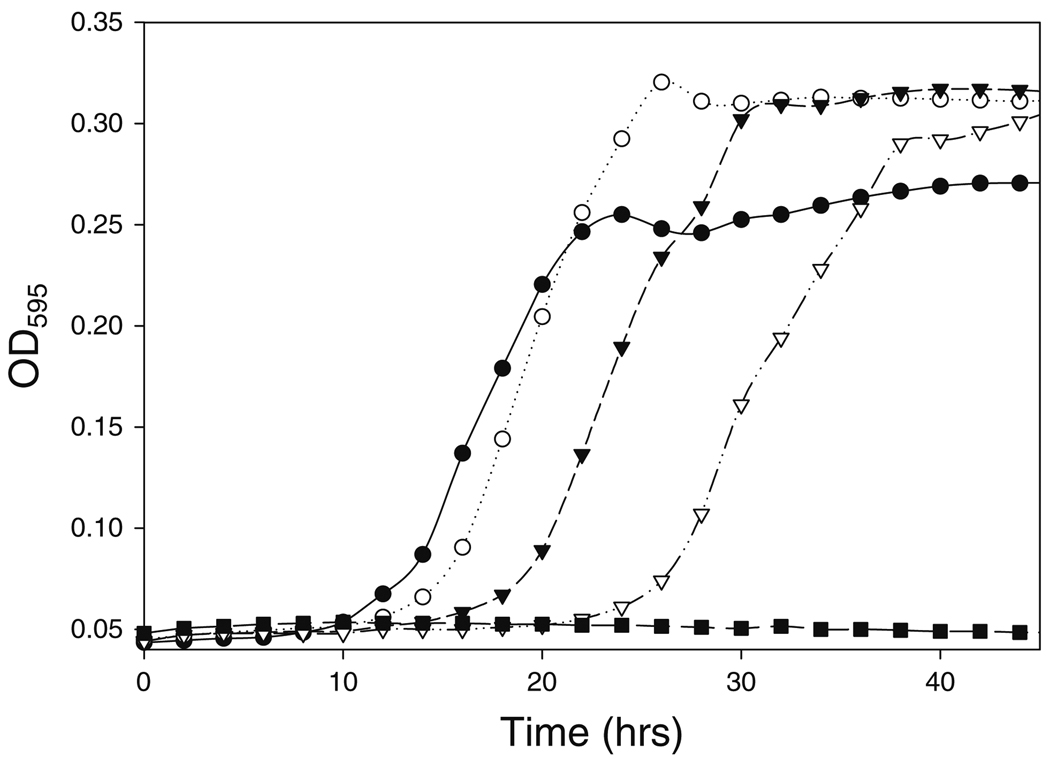
All antimicrobials used in this study had a similar dose-dependent effect on growth kinetics of G. vaginalis as illustrated in Fig. 1, using poly-l-lysine as an example. The checkerboard assay allowed for simultaneous evaluation of multiple concentrations of a two-component mixture [27] to identify antimicrobials that act synergistically with lactocin 160.
Fig. 1.
Growth kinetics of G. vaginalis in the presence of poly-l-lysine. The figure illustrates a typical effect of antimicrobial agents at their subinhibitory concentrations on growth kinetics of a microorganism. G. vaginalis grown at 0.25 AU mL−1 (open circles), 0.5 AU ml−1 (closed reverse triangles), and 0.75 AU ml−1 (open reverse triangles) of poly-l-lysine have a prolonged lag phase compared with the cells grown without the antimicrobial (closed circles). These effects are usually due to cells being stressed and/or due to some portion of the bacterial population being killed by the antimicrobial. By definition, 1 AU ml−1 (closed squares) is fully inhibitory to the bacterium. All the concentrations were tested in duplicates; however, the replicates of the OD values were averaged to simplify the figure
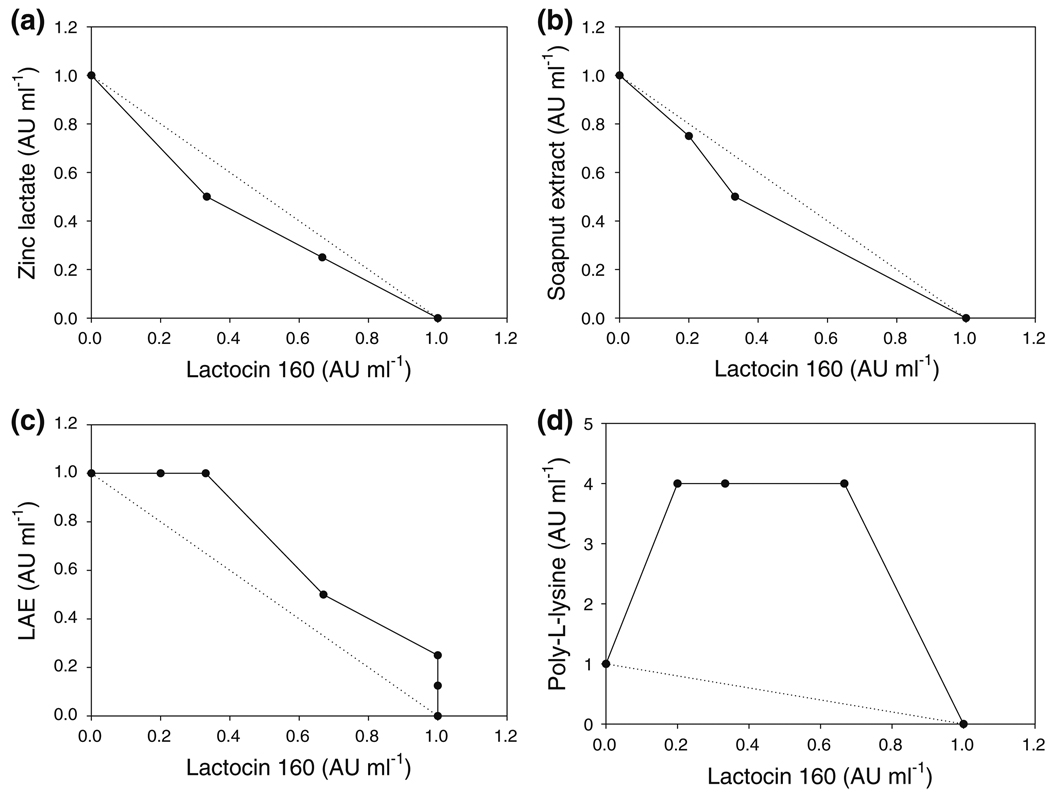
According to the isobologram model, the concentration combinations located below the perforated interaction line signify synergistic interactions. Conversely, the combinations located above the interaction line indicate antagonism, while the ones located along the interaction line show an additive effect between the tested antimicrobials. Finally, the lack of microbial growth inhibition by a combination of two antimicrobials at their subinhibitory concentrations indicates the lack of interaction, i.e., the antimicrobials have no effect on each other’s inhibitory activity [27].
Accordingly, several points representing concentration combinations of lactocin 160 and zinc lactate appeared below the interaction line on the isobologram, indicating synergy between these two antimicrobials (Fig. 2a). Similarly, some synergistic effect was noticed between lactocin 160 and soapnut extract (Fig. 2b). However, we did not observe any interactions between lactocin 160 and LAE; various combinations of these antimicrobials at their subinhibitory concentrations did not inhibit the growth of G. vaginalis (Fig. 2c). Finally, when applied in combination, lactocin 160 and poly-l-lysine had an antagonistic effect (Fig. 2d).
Fig. 2.
Isobolograms of interactions between lactocin 160 and four natural antimicrobials against the vaginal pathogen G. vaginalis. Lactocin 160 synergizes with zinc lactate (a) and soapnut extract (b). For the most part, there are no interactions between lactocin 160 and the LAE preparation (c). Finally, there is a marked antagonism between lactocin 160 and poly-l-lysine (d)
Discussion
Originally, we anticipated to see synergy among all four antimicrobial combinations tested in this study because of the pronounced mode of action differences between the components of each combination. Although poly-l-lysine, LAE, and soapnut extract, much like lactocin 160, target bacterial cytoplasmic membranes, the damage caused by these three antimicrobials is presumably permanent, in contrast to transient pores created by bacteriocin-like substances such as lactocin 160. However, out of the four tested antimicrobials, only zinc lactate and soapnut extract synergized with lactocin 160 against G. vaginalis.
Lactates may act as ionophores at pH values close to and below the pKa of lactic acid (3.86 at 25 °C). However, under the conditions of the checkerboard assay (pH close to neutral), the antimicrobial activity of these salts is mainly due to a decrease in water activity within the bacterial environment. In contrast, lactocin 160 inhibits G. vaginalis’ growth by depleting its various transmembrane gradients [11]; therefore, synergy between these antimicrobials with very different inhibition mechanisms is not surprising. Soapnut extract acts as a detergent, inducing immense, permanent membrane damage that is significantly different from the transient channels formed by bacteriocins.
The antimicrobial activity of LAE is also thought to be related to the surfactant properties of this compound. Much like lactocin 160, LAE makes bacterial cytoplasmic membranes permeable to potassium ions, although little is known about the nature of the perturbance caused by this antimicrobial. The lack of interactions between these two antimicrobials can possibly be explained by a similar mode of action.
The reasons for antagonism between lactocin 160 and poly-l-lysine are unclear. It is possible that the electrostatic interactions between these two peptides reduce their activity against a target cell. The second possibility is that lactocin 160 adsorbed to the cell surface may hinder interactions between poly-l-lysine and its cellular targets. Finally, it is possible that a subinhibitory concentration of the one antimicrobial triggers some adaptive response in the target cell, making it resistant to the second antimicrobial. Interestingly, the antagonism effect was only observed at subinhibitory concentrations of lactocin 160; the effect was not evident at the inhibitory concentrations. For that reason, poly-l-lysine can, theoretically, still be included in an antimicrobial formulation involving lactocin 160, as long as both these antimicrobials are used at concentrations at or above their MICs. However, due to their synergy with lactocin 160, zinc lactate and soapnut extract are the most promising candidates for a lactocin 160-based multiple-hurdle preparation for control of G. vaginalis. Ultimately, BV has a complex polymicrobial nature with a significant role being played by vaginal lactobacilli; therefore, in future studies, we will also elucidate the effect of the selected antimicrobial combinations on the healthy vaginal microbiota and on the BV-related pathogens other than G. vaginalis.
The pH of the system used in this study was around neutral, resembling the elevated vaginal pH characteristic of BV. This system is reflective for the use of lactocin 160-based formulations for treatment of BV. However, these antimicrobials can also be potentially used to prevent recurrence of BV by suppressing the growth of G. vaginalis in successfully treated patients. The healthy vaginal environment has an acidic pH (<4.5). Therefore, if the formulations were used for prophylaxis of recurrent BV, the interactions between antimicrobials would take place in acidic conditions. We were unable to grow G. vaginalis in vitro under acidic conditions; thus, we can only speculate about the antimicrobial interactions in these conditions. Theoretically, the acidic environment should enhance the bactericidal properties of the antimicrobials, because this environment provides an additional stress for the microorganism. This is especially true for antimicrobials such as lactocin 160 and zinc lactate, which act as ionophores, making the bacterial cytoplasmic membranes permeable to traffic of hydrogen ions. However, it is also possible that the acid tolerance response (ATR) of G. vaginalis would induce resistance to other antimicrobials. For instance, induction of ATR in Listeria monocytogenes through exposure to lactic acid increased this bacterium’s tolerance to the bacteriocin nisin [29, 30]. Therefore, the experimental approach is ultimately unavoidable. Accordingly, future studies can evaluate the effectiveness of the selected antimicrobial combinations against biofilms of G. vaginalis, which are known to have an inherent tolerance of lactic acid [31]. The biofilms can be grown to maturity at neutral pH and then can be resuspended in acidic media to test the antimicrobial interactions under acidic conditions resembling a healthy vaginal environment.
Acknowledgments
This research was sponsored by NIH Grant “Natural antimicrobials against bacterial vaginosis” NCCAM NIH R21AT002897-01. The authors thank Dr. Katia Sutyak Noll for the editorial work.
References
- 1.Reid G. Probiotic agents to protect the urogenital tract against infection. Am J Clin Nutr. 2001;73:437S–443S. doi: 10.1093/ajcn/73.2.437s. [DOI] [PubMed] [Google Scholar]
- 2.Dover SE, Aroutcheva AA, Faro S, Chikindas ML. Natural antimicrobials and their role in vaginal health: a short review. Int J Probiot Prebiot. 2008;3:219–230. [PMC free article] [PubMed] [Google Scholar]
- 3.Aroutcheva AA, Simoes JA, Faro S. Antimicrobial protein produced by vaginal Lactobacillus acidophilus that inhibits Gardnerella vaginalis. Infect Dis Obstet Gynecol. 2001;9:33–39. doi: 10.1155/S1064744901000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dover SE, Aroutcheva AA, Faro S, Chikindas ML. Safety study of an antimicrobial peptide lactocin 160, produced by the vaginal Lactobacillus rhamnosus. Infect Dis Obstet Gynecol. 2007;2007:78248. doi: 10.1155/2007/78248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kant S, Maurya AK, Kushwaha RA, Nag VL, Prasad R. Multi-drug resistant tuberculosis: an iatrogenic problem. Biosci Trends. 2010;4:48–55. [PubMed] [Google Scholar]
- 6.McLean NW, McGroarty JA. Growth inhibition of metronidazole-susceptible and metronidazole-resistant strains of Gardnerella vaginalis by lactobacilli in vitro. Appl Environ Microbiol. 1996;62:1089–1092. doi: 10.1128/aem.62.3.1089-1092.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagaraja P. Antibiotic resistance of Gardnerella vaginalis in recurrent bacterial vaginosis. Indian J Med Microbiol. 2008;26:155–157. doi: 10.4103/0255-0857.40531. [DOI] [PubMed] [Google Scholar]
- 8.Harwich MD, Jr, Alves JM, Buck GA, Strauss JF, III, Patterson JL, Oki AT, et al. Drawing the line between commensal and pathogenic Gardnerella vaginalis through genome analysis and virulence studies. BMC Genomics. 2010;11:375. doi: 10.1186/1471-2164-11-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leistner L. Basic aspects of food preservation by hurdle technology. Int J Food Microbiol. 2000;55:181–186. doi: 10.1016/s0168-1605(00)00161-6. [DOI] [PubMed] [Google Scholar]
- 10.Curtis GD, Lee WH. Culture media and methods for the isolation of Listeria monocytogenes. Int J Food Microbiol. 1995;26:1–13. doi: 10.1016/0168-1605(93)e0027-o. [DOI] [PubMed] [Google Scholar]
- 11.Turovskiy Y, Ludescher RD, Aroutcheva AA, Faro S, Chikindas ML. Lactocin 160, a bacteriocin produced by vaginal Lactobacillus rhamnosus, targets cytoplasmic membranes of the vaginal pathogen, Gardnerella vaginalis. Probiot Antimicrob Proteins. 2009;1:67–74. doi: 10.1007/s12602-008-9003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aroutcheva A, Gariti D, Simon M, Shott S, Faro J, Simoes JA, et al. Defense factors of vaginal lactobacilli. Am J Obstet Gynecol. 2001;185:375–379. doi: 10.1067/mob.2001.115867. [DOI] [PubMed] [Google Scholar]
- 13.Andersch B, Forssman L, Lincoln K, Torstensson P. Treatment of bacterial vaginosis with an acid cream: a comparison between the effect of lactate-gel and metronidazole. Gynecol Obstet Invest. 1986;21:19–25. doi: 10.1159/000298923. [DOI] [PubMed] [Google Scholar]
- 14.Cherrington CA, Hinton M, Mead GC, Chopra I. Organic acids: chemistry, antibacterial activity and practical applications. Adv Microb Physiol. 1991;32:87–108. doi: 10.1016/s0065-2911(08)60006-5. [DOI] [PubMed] [Google Scholar]
- 15.McEntire JC, Montville TJ, Chikindas ML. Synergy between nisin and select lactates against Listeria monocytogenes is due to the metal cations. J Food Prot. 2003;66:1631–1636. doi: 10.4315/0362-028x-66.9.1631. [DOI] [PubMed] [Google Scholar]
- 16.Bourne N, Stegall R, Montano R, Meador M, Stanberry LR, Milligan GN. Efficacy and toxicity of zinc salts as candidate topical microbicides against vaginal herpes simplex virus type 2 infection. Antimicrob Agents Chemother. 2005;49:1181–1183. doi: 10.1128/AAC.49.3.1181-1183.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chvapil M, Droegemueller W, Betts K, Heine W, Weinstein L. Preliminary testing of the contraceptive collagen sponge. Obstet Gynecol. 1980;56:503–506. [PubMed] [Google Scholar]
- 18.Nishikawa M, Ogawa K. Inhibition of epsilon-poly-l-lysine biosynthesis in Streptomycetaceae bacteria by short-chain polyols. Appl Environ Microbiol. 2006;72:2306–2312. doi: 10.1128/AEM.72.4.2306-2312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiraki J, Ichikawa T, Ninomiya S, Seki H, Uohama K, Seki H, et al. Use of ADME studies to confirm the safety of epsilon-polylysine as a preservative in food. Regul Toxicol Pharmacol. 2003;37:328–340. doi: 10.1016/s0273-2300(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida T, Nagasawa T. epsilon-Poly-l-lysine: microbial production, biodegradation and application potential. Appl Microbiol Biotechnol. 2003;62:21–26. doi: 10.1007/s00253-003-1312-9. [DOI] [PubMed] [Google Scholar]
- 21.Shima S, Matsuoka H, Iwamoto T, Sakai H. Antimicrobial action of epsilon-poly-l-lysine. J Antibiot (Tokyo) 1984;37:1449–1455. doi: 10.7164/antibiotics.37.1449. [DOI] [PubMed] [Google Scholar]
- 22.Francis G, Kerem Z, Makkar HP, Becker K. The biological action of saponins in animal systems: a review. Br J Nutr. 2002;88:587–605. doi: 10.1079/BJN2002725. [DOI] [PubMed] [Google Scholar]
- 23.Kirk DD, Rempel R, Pinkhasov J, Walmsley AM. Application of Quillaja saponaria extracts as oral adjuvants for plant-made vaccines. Expert Opin Biol Ther. 2004;4:947–958. doi: 10.1517/14712598.4.6.947. [DOI] [PubMed] [Google Scholar]
- 24.Ojha P, Maikhuri JP, Gupta G. Effect of spermicides on Lactobacillus acidophilus in vitro-nonoxynol-9 vs. Sapindus saponins. Contraception. 2003;68:135–138. doi: 10.1016/s0010-7824(03)00138-0. [DOI] [PubMed] [Google Scholar]
- 25.Mengoni F, Lichtner M, Battinelli L, Marzi M, Mastroianni CM, Vullo V, et al. In vitro anti-HIV activity of oleanolic acid on infected human mononuclear cells. Planta Med. 2002;68:111–114. doi: 10.1055/s-2002-20256. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez E, Seguer J, Rocabayera X, Manresa A. Cellular effects of monohydrochloride of l-arginine, N-lauroyl ethylester (LAE) on exposure to Salmonella typhimurium and Staphylococcus aureus. J Appl Microbiol. 2004;96:903–912. doi: 10.1111/j.1365-2672.2004.02207.x. [DOI] [PubMed] [Google Scholar]
- 27.Badaoui Najjar M, Kashtanov D, Chikindas ML. Epsilon-poly-l-lysine and nisin A act synergistically against Gram-positive food-borne pathogens Bacillus cereus and Listeria monocytogenes. Lett Appl Microbiol. 2007;45:13–18. doi: 10.1111/j.1472-765X.2007.02157.x. [DOI] [PubMed] [Google Scholar]
- 28.Asok KK, Mazumdar K, Dutta NK, Karak P, Dastidar SG, Ray R. Evaluation of synergism between the aminoglycoside antibiotic streptomycin and the cardiovascular agent amlodipine. Biol Pharm Bull. 2004;27:1116–1120. doi: 10.1248/bpb.27.1116. [DOI] [PubMed] [Google Scholar]
- 29.Bonnet M, Rafi MM, Chikindas ML, Montville TJ. Bioenergetic mechanism for nisin resistance, induced by the acid tolerance response of Listeria monocytogenes. Appl Environ Microbiol. 2006;72:2556–2563. doi: 10.1128/AEM.72.4.2556-2563.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonnet M, Montville TJ. Acid-tolerant Listeria monocytogenes persist in a model food system fermented with nisin-producing bacteria. Lett Appl Microbiol. 2005;40:237–242. doi: 10.1111/j.1472-765X.2005.01661.x. [DOI] [PubMed] [Google Scholar]
- 31.Patterson JL, Girerd PH, Karjane NW, Jefferson KK. Effect of biofilm phenotype on resistance of Gardnerella vaginalis to hydrogen peroxide and lactic acid. Am J Obstet Gynecol. 2007;197:170–177. doi: 10.1016/j.ajog.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]