Abstract
Purpose
To compare characteristics and outcomes of breast cancer in women with and without a history of radiation therapy (RT) for Hodgkin's lymphoma (HL).
Patients and Methods
Women with breast cancer diagnosed from 1980 to 2006 after RT for HL were identified from eight North American hospitals and were matched three-to-one with patients with sporadic breast cancer by age, race, and year of breast cancer diagnosis. Information on patient, tumor and treatment characteristics, and clinical outcomes was abstracted from medical records.
Results
A total of 253 patients with breast cancer with a history of RT for HL were matched with 741 patients with sporadic breast cancer. Median time from HL to breast cancer diagnosis was 18 years. Median age at breast cancer diagnosis was 42 years. Breast cancer after RT for HL was more likely to be detected by screening, was more likely to be diagnosed at an earlier stage, and was more likely to be bilateral at diagnosis. HL survivors had an increased risk of metachronous contralateral breast cancer (adjusted hazard ratio [HR], 4.3; 95% CI, 1.7 to 11.0) and death as a result of any cause (adjusted HR, 1.9; 95% CI, 1.1 to 3.3). Breast cancer–specific mortality was also elevated, but this difference was not statistically significant (adjusted HR, 1.6; 95% CI, 0.7 to 3.4).
Conclusion
In women with a history of RT for HL, breast cancer is diagnosed at an earlier stage, but these women are at greater risk for bilateral disease and are more likely to die as a result of causes other than breast cancer. Our findings support close follow-up for contralateral tumors in these patients and ongoing primary care to manage comorbid conditions.
INTRODUCTION
Scientific and clinical advances have brought dramatic improvements in the treatment and outcomes of Hodgkin's lymphoma (HL), with 10-year relative survival now exceeding 80%.1 However, the radiotherapy and chemotherapy regimens responsible for these improvements are themselves carcinogenic. Consequently, second malignancies are now the leading cause of death in long-term HL survivors.2 Among female HL survivors, breast cancer is the most commonly diagnosed solid tumor.3 For women treated for HL before age 30 years, the risk of developing breast cancer is six times greater than in the general population, with an absolute excess risk of 20 to 40 occurrences per 10,000 annually.4,5 Most of this excess risk is attributed to irradiation of the axillae and mediastinum in HL, with relative risks varying by age at radiation, radiation dose, extent of radiation field, and receipt of chemotherapy.3–10
Although the increased incidence of breast cancer in HL survivors is well documented, less is known about the characteristics, treatment, and outcomes of these cancers. Most available information is from relatively small, single-institution cohorts and lacks comparison to sporadic breast cancer controls.11,12 Because early breast cancer screening is advocated in HL survivors, it could potentially result in earlier detection and, therefore, more favorable prognosis.13,14 Yet, because of their often bilateral radiation exposure, women with a history of radiation for HL may be at increased risk of synchronous or metachronous bilateral breast cancer,11,15 and they may not be candidates for breast-conserving therapy.16 Many HL survivors may also have received chemotherapy, at initial HL diagnosis or for HL recurrence, limiting their options for systemic adjuvant therapy after a breast cancer diagnosis. Thus, local control, prevention of metastases, and prevention of contralateral breast tumors are of particular concern for women who develop breast cancer after radiation for HL. The objectives of this study were to identify the unique patient and breast tumor characteristics in women with a history of radiation for HL and to compare clinical outcomes with those of women with sporadic breast cancer.
PATIENTS AND METHODS
Study Cohort
Women with a history of radiation for HL who were diagnosed with breast cancer between 1980 and 2006 were identified from eight medical centers in North America. Patients were excluded from this cohort if their medical records made no mention of supradiaphragmatic radiation as a component of treatment for primary or recurrent HL.
Each patient in the HL survivor cohort was matched with three patients with breast cancer diagnosed at the same institution who had no history of HL. Patients with sporadic breast cancer, identified from an institutional registry or breast cancer database at each site, were matched to the HL survivors by race, year of breast cancer diagnosis, and age at breast cancer diagnosis. For HL survivors with fewer than three exact matches, the criteria were relaxed to allow matching within 2 years of breast cancer diagnosis and 2 years of age at breast cancer diagnosis.
Patient and Disease Characteristics
We collected information about breast cancer characteristics and treatment from each patient's medical record. Family history was categorized as breast cancer in a first-degree relative, no breast cancer in a first-degree relative, or unknown. Menopausal status at breast cancer diagnosis was defined as premenopausal, peri- or postmenopausal, or unknown. Breast cancer stage at diagnosis was classified according to the American Joint Committee on Cancer Staging Manual, sixth edition. Other tumor characteristics included laterality, histology, hormone receptor status, human epidermal growth factor receptor 2 (HER2) status, and method of detection. We identified treatment modalities for breast cancer, including radiation therapy, adjuvant chemotherapy, and hormonal therapy, and type of surgery. For HL survivors, we also collected information regarding stage and treatment of HL, including radiation dose and field.
Breast Cancer Outcomes
From medical records, we identified local and regional failures, distant recurrences, contralateral breast tumors, and deaths. Cause of death was categorized as breast cancer, other, or unknown. Any tumor found in the contralateral breast more than 1 month after initial breast cancer diagnosis was classified as a metachronous contralateral breast cancer.
Statistical Analysis
Associations between patient characteristics and cohort membership (HL survivors v patients with sporadic breast cancer) were assessed by χ2 statistics, accounting for the matched cohort design. We estimated time to local or regional failure, metastatic failure, metachronous contralateral breast cancer, breast cancer death, any breast cancer event, and death as a result of any cause by using Kaplan-Meier survival estimation. The unadjusted impact of a history of radiotherapy for HL on each end point was assessed by using a competing risk framework, with death as a result of non–breast cancer causes treated as a competing risk and with observations censored at last follow-up. Cumulative incidence functions estimated in the competing-risk analysis were compared using Gray's test.17 We used multivariable Cox proportional hazards regression to estimate the impact of a history of radiation for HL on the risk of each outcome, controlling for patient, tumor, and treatment characteristics. Women who had synchronous bilateral disease at diagnosis or prophylactic contralateral mastectomy were excluded from analysis of metachronous contralateral cancer. Patient race, age at breast cancer diagnosis, and year of breast cancer diagnosis were not included as covariates, because the cohorts were matched on these characteristics. Standard errors were adjusted for matching by using a proportional hazards model stratified by matched groups, a standard method for analysis of matched censored data.18
RESULTS
We identified 253 women with a history of radiotherapy for HL diagnosed with breast cancer between 1980 and 2006. HL survivors were matched with 741 women who had breast cancer and no history of HL. Matching was complete for 94% of HL survivors; others were matched with fewer than three patients with sporadic breast cancer.
HL Characteristics and Treatment in Survivor Cohort
Median age at HL diagnosis was 23 years (range, 11 to 67 years), and the median interval from HL to first breast cancer diagnosis was 18 years (range, 1 to 42 years; Table 1). Slightly more than half of the cohort was diagnosed with HL before 1980, 38% were diagnosed in 1980 to 1989, and 8% were diagnosed in 1990 or later. A majority of HL survivors were diagnosed with stage I or II HL, and 62% had no “B” symptoms at diagnosis. The median cumulative radiation dose for HL was 39 Gy (range, 10 to 50 Gy), and 90% of patients received more than 30 Gy. Approximately one third of the cohort received chemotherapy for HL.
Table 1.
Demographic and Clinical Characteristics of HL in Survivor Cohort
| Characteristic | Patients |
|
|---|---|---|
| No. (N = 253) | % | |
| Year of HL diagnosis | ||
| Before 1980 | 136 | 54 |
| 1980-1989 | 97 | 38 |
| ≥ 1990 | 20 | 8 |
| HL stage at diagnosis | ||
| I | 38 | 15 |
| II | 155 | 61 |
| III | 27 | 11 |
| IV | 13 | 5 |
| Unknown | 20 | 8 |
| “B” symptoms at diagnosis | ||
| No | 158 | 62 |
| Yes | 63 | 25 |
| Unknown | 32 | 13 |
| Lymph node involvement | ||
| Mediastinal hilar | 164 | 65 |
| Supraclavicular/infraclavicular | 134 | 53 |
| Cervical | 126 | 50 |
| Axillary | 38 | 15 |
| Abdominal | 14 | 6 |
| Pelvic | 3 | 1 |
| Other | 34 | 13 |
| Chemotherapy | ||
| No | 156 | 62 |
| Yes | 88 | 35 |
| Unknown | 9 | 4 |
| Radiation dose, Gy | ||
| Median | 39 | |
| Range | 10-50 | |
| Age at HL diagnosis, years | ||
| Median | 23 | |
| Range | 11-67 | |
| Age at BC diagnosis, years | ||
| Median | 42 | |
| Range | 24-84 | |
| Interval from HL to BC, years | ||
| Median | 18 | |
| Range | 1-42 | |
Abbreviations: HL, Hodgkin's lymphoma; BC, breast cancer.
Breast Cancer Characteristics and Treatment: HL Survivors and Patients With Sporadic Breast Cancer
Both cohorts had a median age of 42 years at breast cancer diagnosis (range, 24 to 85 years), 94% were white and non-Hispanic, and 58% were diagnosed with breast cancer before 2000. Compared with patients who had sporadic breast cancer, HL survivors were more likely to have breast cancer detected by screening mammography (40% v 33%), were more likely to be diagnosed at an earlier stage (ie, ductal carcinoma in situ or stage I; 61% v 42%), were less likely to have axillary lymph node involvement (25% v 39%), and were more likely to present with bilateral disease (6% v 2%; Table 2). HL survivors were also less likely to be premenopausal at diagnosis (49% v 69%), perhaps as a result of early menopause associated with HL treatment. HL survivors were less likely than women with sporadic breast cancer to have a lumpectomy (23% v 55%), and they were less likely to receive radiation as part of their breast cancer treatment (8% v 61%).
Table 2.
BC Characteristics and Treatment in HL Survivors and Matched Patients With Sporadic BC
| Characteristic | HL Survivors |
Sporadic BC |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Stage | |||||
| 0 | 43 | 17 | 92 | 12 | < .001 |
| I | 112 | 44 | 222 | 30 | |
| II | 61 | 24 | 201 | 27 | |
| III | 28 | 11 | 116 | 16 | |
| IV | 4 | 2 | 33 | 4 | |
| Unknown | 5 | 2 | 77 | 10 | |
| No. of positive lymph nodes | |||||
| 0 | 147 | 58 | 384 | 52 | < .001 |
| 1-3 | 37 | 15 | 163 | 22 | |
| 4-9 | 17 | 7 | 73 | 10 | |
| ≥ 10 | 7 | 3 | 50 | 7 | |
| Unknown | 45 | 18 | 71 | 10 | |
| Laterality at diagnosis | |||||
| Unilateral | 234 | 92 | 717 | 97 | < .01 |
| Bilateral | 14 | 6 | 15 | 2 | |
| Unknown | 5 | 2 | 9 | 1 | |
| Histology | |||||
| Ductal carcinoma in situ | 43 | 17 | 92 | 12 | NS |
| Invasive ductal carcinoma | 179 | 71 | 558 | 75 | |
| Invasive lobular carcinoma | 14 | 5 | 39 | 5 | |
| Other/unknown | 17 | 7 | 52 | 7 | |
| Extensive intraductal component | |||||
| No | 125 | 49 | 348 | 47 | NS |
| Yes | 44 | 17 | 108 | 15 | |
| Unknown | 84 | 33 | 285 | 38 | |
| Multifocal disease | |||||
| No | 115 | 45 | 358 | 48 | NS |
| Yes | 52 | 21 | 179 | 24 | |
| Unknown | 87 | 34 | 204 | 28 | |
| Type of surgery | |||||
| Lumpectomy | 59 | 23 | 406 | 55 | < .001 |
| Unilateral mastectomy | 120 | 47 | 212 | 29 | |
| Bilateral mastectomy | 60 | 24 | 29 | 4 | |
| Other/no surgery/unknown | 14 | 6 | 94 | 13 | |
| Surgical margins | |||||
| Negative: > 3 mm | 174 | 69 | 473 | 64 | < .05 |
| Close: 1-3 mm | 32 | 13 | 74 | 10 | |
| Positive: < 1 mm | 4 | 2 | 39 | 5 | |
| Unknown | 43 | 17 | 155 | 21 | |
| Hormone receptor status | |||||
| Negative | 72 | 28 | 155 | 21 | NS |
| Positive | 112 | 44 | 374 | 50 | |
| Unknown | 69 | 27 | 212 | 29 | |
| HER2 status | |||||
| Negative | 71 | 28 | 206 | 28 | NS |
| Positive | 21 | 8 | 95 | 13 | |
| Unknown | 161 | 64 | 440 | 59 | |
| Location | |||||
| Upper outer quadrant | 108 | 43 | 254 | 34 | < .001 |
| Upper inner quadrant | 26 | 10 | 54 | 7 | |
| Lower outer quadrant | 10 | 4 | 63 | 9 | |
| Lower inner quadrant | 10 | 4 | 29 | 4 | |
| Central | 20 | 8 | 33 | 4 | |
| Multiple quadrants | 16 | 6 | 109 | 15 | |
| Other/unknown | 63 | 25 | 99 | 7 | |
| Method of detection | |||||
| Patient detected | 103 | 41 | 404 | 55 | < .01 |
| Clinical exam | 23 | 9 | 42 | 6 | |
| Screening mammogram | 102 | 40 | 242 | 33 | |
| Other or unknown | 25 | 10 | 53 | 7 | |
| Menopausal status | |||||
| Premenopausal | 124 | 49 | 508 | 69 | < .001 |
| Peri- or postmenopausal | 91 | 36 | 188 | 25 | |
| Unknown | 38 | 15 | 45 | 6 | |
| Family history of BC | |||||
| None | 173 | 68 | 545 | 74 | < .001 |
| First-degree relative | 43 | 17 | 147 | 20 | |
| Unknown | 37 | 15 | 49 | 7 | |
| Any chemotherapy* | |||||
| Yes | 119 | 47 | 447 | 60 | < .001 |
| No | 113 | 45 | 260 | 35 | |
| Unknown | 21 | 8 | 34 | 5 | |
| Any hormonal therapy* | |||||
| Yes | 89 | 35 | 348 | 47 | < .01 |
| No | 125 | 49 | 309 | 42 | |
| Unknown | 39 | 15 | 84 | 11 | |
| Any radiation therapy* | |||||
| Yes | 20 | 8 | 454 | 61 | < .001 |
| No | 218 | 86 | 241 | 33 | |
| Unknown | 15 | 6 | 46 | 6 | |
Abbreviations: BC, breast cancer; HER2, human epidermal growth factor receptor 2; HL, Hodgkin's lymphoma; NS, not statistically significant.
Chemotherapy, hormonal therapy, or radiation therapy for treatment of breast cancer.
Breast Cancer Outcomes
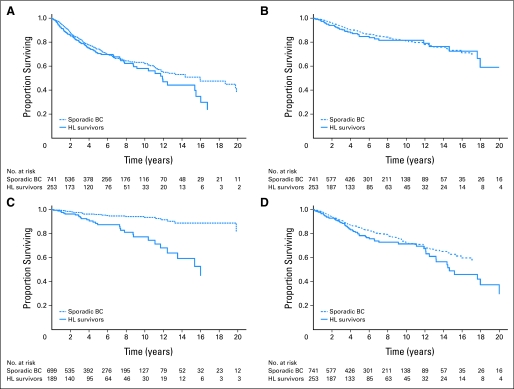
Median follow-up after breast cancer diagnosis was 4.6 years for HL survivors and was 5.2 years for the matched patients with sporadic breast cancer. Breast cancer event–free survival (Fig 1A) and breast cancer–specific survival (Fig 1B) were similar in the two groups. The cumulative incidence functions for these events were not significantly different between groups, although rates of the competing event—nonbreast cancer death—were significantly higher in HL survivors (Gray's test P < .05). In adjusted analysis, rates of local/regional and metastatic failure and breast cancer mortality were elevated, but these differences were not statistically significant (Table 3).
Fig 1.
Breast cancer (BC) outcomes in Hodgkin's lymphoma (HL) survivors and matched patients with sporadic breast cancer. Plots depict the proportion of each cohort (ie, patients with breast cancer [BC] who have a history of radiation for Hodgkin's lymphoma[HL] and matched patients with sporadic BC) surviving free of (A) any BC event, (B) death as a result of BC, (C) metachronous contralateral BC, and (D) death as a result of any cause. BC events include local/regional failure, metastatic failure, metachronous contralateral cancer, and BC death.
Table 3.
BC Outcomes in HL Survivors and Matched Patients With Sporadic BC
| Event | No. at Risk | No. With Event | Adjusted HR* | 95% CI | P |
|---|---|---|---|---|---|
| Local/regional failure | |||||
| HL survivors | 248 | 32 | 0.94 | 0.51 to 1.74 | NS |
| Patients with sporadic BC | 708 | 86 | Reference | ||
| Metastatic failure | |||||
| HL survivors | 248 | 45 | 1.49 | 0.82 to 2.71 | NS |
| Patients with sporadic BC | 708 | 137 | Reference | ||
| Metachronous contralateral tumor | |||||
| HL survivors | 189 | 29 | 4.31 | 1.69 to 10.99 | < .01 |
| Patients with sporadic BC | 699 | 33 | Reference | ||
| Death as a result of BC | |||||
| HL survivors | 253 | 36 | 1.61 | 0.76 to 3.42 | NS |
| Patients with sporadic BC | 741 | 95 | Reference | ||
| Death as a result of any cause | |||||
| HL survivors | 253 | 61 | 1.90 | 1.09 to 3.32 | < .05 |
| Patients with sporadic BC | 741 | 137 | Reference |
NOTE. Local-regional failure included ipsilateral, new primary tumors. Analysis of local-regional failure and analysis of metastatic failure excluded women who presented with distant disease at or within 14 days of initial diagnoses. Analysis of metachronous contralateral disease excluded women who had synchronous bilateral disease at BC diagnosis or prophylactic contralateral mastectomy.
Abbreviations: BC, breast cancer; HL, Hodgkin's lymphoma; HR, hazard ratio; NS, not statistically significant.
HRs were adjusted for BC stage at diagnosis, axillary lymph node involvement, laterality at diagnosis, type of surgery, surgical margin status, menopausal status, family history of BC in a first-degree relative, whether BC was screen detected, receipt of radiation therapy for BC, receipt of chemotherapy for BC, and receipt of hormonal therapy for BC.
HL survivors were more likely than patients with sporadic breast cancer to develop a metachronous contralateral breast cancer (Fig 1C). The 5-year cumulative risk of metachronous contralateral cancer was 18% in the HL survivor cohort and was 6% in the sporadic breast cancer cohort. Controlling for patient and tumor characteristics and breast cancer treatment, the rate of metachronous contralateral tumors was more than four times greater in the HL survivors, compared with the matched patients with sporadic breast cancer (adjusted hazard ratio [HR], 4.3; 95% CI, 1.7 to 11.0; P < .01). In multivariable analysis, only type of surgery and family history were also significantly associated with metachronous contralateral breast cancer (Table 4).
Table 4.
Predictors of Metachronous Contralateral BC and Death As a Result of Any Cause
| Characteristic | Contralateral BC |
Death As a Result of Any Cause |
||||
|---|---|---|---|---|---|---|
| Adjusted HR | 95% CI | P | Adjusted HR | 95% CI | P | |
| HL history | ||||||
| HL survivor | 4.3 | 1.7 to 11.0 | < .01 | 1.9 | 1.1 to 3.3 | < .05 |
| Sporadic BC | Reference | Reference | ||||
| Stage | ||||||
| DCIS or stage I | Reference | Reference | ||||
| Stages IIA-IV | 0.8 | 0.3 to 2.6 | NS | 4.0 | 2.1 to 7.6 | < .001 |
| Unknown | 1.1 | 0.2 to 6.5 | NS | 1.7 | 0.5 to 6.5 | NS |
| Regional lymph node involvement | ||||||
| Negative | Reference | Reference | ||||
| Positive | 1.2 | 0.4 to 3.8 | NS | 1.1 | 0.6 to 2.0 | NS |
| Unknown | 1.4 | 0.5 to 4.2 | NS | 2.7 | 1.2 to 5.9 | < .05 |
| Type of surgery | ||||||
| Lumpectomy | Reference | Reference | ||||
| Unilateral mastectomy | 0.2 | 0.1 to 0.7 | < .01 | 1.2 | 0.7 to 2.1 | NS |
| Bilateral mastectomy | — | — | — | 1.2 | 0.4 to 3.1 | NS |
| Other/none/unknown | 2.2 | 0.4 to 11.7 | NS | 3.5 | 1.0 to 12.2 | < .05 |
| Surgical margins | ||||||
| Negative | Reference | Reference | ||||
| Positive | 1.6 | 0.5 to 5.0 | NS | 2.4 | 1.3 to 4.4 | < .01 |
| Unknown | 0.9 | 0.3 to 3.0 | NS | 1.5 | 0.8 to 2.9 | NS |
| Any chemotherapy* | ||||||
| No | Reference | Reference | ||||
| Yes | 1.0 | 0.4 to 2.6 | NS | 1.6 | 0.8 to 2.8 | NS |
| Any hormonal therapy* | ||||||
| No | Reference | Reference | ||||
| Yes | 1.2 | 0.5 to 3.2 | NS | 0.8 | 0.5 to 1.3 | NS |
| Any radiation* | ||||||
| No | Reference | Reference | ||||
| Yes | 0.7 | 0.3 to 1.9 | NS | 0.9 | 0.5 to 1.5 | NS |
| Method of detection | ||||||
| Screening mammogram | 1.2 | 0.5 to 2.9 | NS | 0.5 | 0.3 to 0.8 | < .05 |
| Other | Reference | Reference | ||||
| Menopausal status | ||||||
| Premenopausal | Reference | Reference | ||||
| Peri- or postmenopausal | 0.7 | 0.2 to 2.7 | NS | 2.8 | 1.2 to 6.4 | < .05 |
| Unknown | 5.2 | 0.8 to 35.8 | NS | 1.6 | 0.8 to 3.4 | NS |
| Family history of breast cancer | ||||||
| None | Reference | Reference | ||||
| First-degree relative | 2.7 | 1.1 to 6.4 | < .05 | 0.9 | 0.5 to 1.6 | NS |
| Unknown | 2.0 | 0.5 to 7.2 | NS | 1.1 | 0.5 to 2.2 | NS |
Abbreviations: BC, breast cancer; DCIS, ductal carcinoma in situ; HL, Hodgkin's lymphoma; HR, hazard ratio; NS, not statistically significant.
Chemotherapy, hormonal therapy, or radiation therapy for treatment of breast cancer.
Overall survival (Fig 1D) was poorer in HL survivors than in patients with sporadic breast cancer. Controlling for patient and disease characteristics and breast cancer treatment, HL survivors had almost twice the hazard of death as a result of any cause (adjusted HR, 1.9; 95% CI, 1.1 to 3.3; P < .05). Independent of HL history, the risk of death as a result of any cause was greater in women with stage II or more advanced disease, those with unknown lymph node status and type of primary surgery, those with positive surgical margins, and those who were peri- or postmenopausal at diagnosis (Table 4). Women with screen-detected breast cancers had almost half the risk of death of women whose cancers were patient- or clinician-detected as a result of symptoms (adjusted HR, 0.5; 95% CI, 0.3 to 0.8; P < .05).
DISCUSSION
In this cohort of 253 HL survivors who were treated with radiotherapy to the upper torso and later developed breast cancer, several breast cancer characteristics and outcomes differed significantly from those of a matched cohort of 741 patients with sporadic breast cancer. Notably, HL survivors had a greater risk of bilateral breast cancer, both synchronous and metachronous; their breast cancers were typically diagnosed at an earlier stage and were more likely to be screen detected. Although HR survivors had a greater risk of death as a result of any cause, rates of local/regional failure, metastatic failure, and death as a result of breast cancer did not differ significantly between the two groups.
Several findings are consistent with some small, single-institution studies of HL survivors that did not include comparison cohorts.11,12,16,19,20 However, the authors of a recent systematic review of these and other studies of women treated with chest irradiation for childhood, adolescent, or young adult cancer concluded that the characteristics of breast cancer in women treated with chest irradiation and the outcomes after diagnosis are similar to those of women in the general population.21 Our results do not support that conclusion.
By comparing HL survivors with patients who had sporadic breast cancer matched on race, age, and year of diagnosis, we were able to control for these characteristics, all of which are associated with HL diagnosis and with breast cancer characteristics and outcomes. For example, young age at breast cancer diagnosis has been well established in HL survivors with prior chest irradiation.7,11,12,16,19–21 The median age at breast cancer diagnosis in our cohort—42 years—is almost 20 years younger than the median age of 61 years of patients with breast cancer in the general population.21 Breast cancer in young patients is likely to display a more aggressive phenotype, to be hormone receptor negative, and to exhibit more vascular and lymphatic invasion and pathologic grade 3 features.22 Age younger than 40 years may be an independent adverse prognostic factor for time to relapse, time to distant failure, and overall survival.23,24 For similar reasons, we also controlled for race25 and year of breast cancer diagnosis,26 thereby minimizing possible bias introduced by these important confounders.
The results of our matched-cohort analysis support uncontrolled prior observations of a high incidence of both synchronous and metachronous bilateral breast cancer in patients who received radiotherapy for HL.11,12,16,20,27,28 Those series showed a bilaterality rate of 12.8% (5.5% synchronous and 7.3% metachronous).21 Among the HL survivors in our study, the rate of bilaterality at diagnosis was 6%; among those with a breast at risk, the actuarial rate of metachronous contralateral breast cancer was 18% at 5 years. In multivariable analysis, history of radiation for HL had a far greater impact on risk of metachronous contralateral breast cancer than did other patient and disease characteristics.
Although bilateral breast cancer has previously been associated with a greater risk for local recurrence and distant metastasis,29,30 we did not see higher rates of these events in our HL survivor cohort compared with the matched patients with sporadic breast cancer. However, the increased risk of developing a metachronous contralateral cancer and the potential for poorer prognosis after a contralateral cancer both support the need for close surveillance of the contralateral breast. Our findings provide important information for discussions of the option of prophylactic contralateral mastectomy in patients who had significant radiation exposure of both breasts at a young age.
Several prognostic characteristics and tumor features were more favorable in the HL survivors than in their matched peers with sporadic breast cancer. Although a family history of breast cancer has been shown to increase the risk of developing breast cancer in patients who received radiotherapy for HL,27,31 we found that breast cancer in a first-degree relative was less common in the HL survivors who developed breast cancer than in the patients with sporadic breast cancer. The HL survivors were also diagnosed at an earlier stage (stage 0 or 1) and were less likely to have lymph node involvement. This difference in stage distribution is likely associated with the greater frequency of screen-detected cancers in the HL survivor cohort. Since the association between radiotherapy for HL and the increased risk of breast cancer was clearly established,7,11,32 awareness and guidelines for early detection, primarily with initiation of early routine mammograms, have been promoted for HL survivors. Most breast tumors that develop after HL are detectable by mammography,13,14 and implementation of routine screening has increased the proportion of patients diagnosed at earlier stages.16
Despite the greater frequency of screen-detected tumors and earlier stage at diagnosis in our HL survivor cohort, these women did not have better breast cancer outcomes than their peers with sporadic breast cancer. Rates of local failure and metastatic failure were similar in the two groups, controlling for patient and disease characteristics and treatment. The rate of death from breast cancer was somewhat elevated in the HL survivors, but this difference was not statistically significant. In addition to the disease characteristics available in our study, breast cancer outcomes may be associated with tumor genes and other markers that differ between radiation-induced and sporadic breast cancers, predisposing the former to more aggressive disease.33
In the HL survivors, breast cancer treatment options were undoubtedly constrained by prior exposure to chest irradiation and, in some, to systemic chemotherapy. In a small, retrospective study comparing women with breast cancer after either HL or non-Hodgkin's lymphoma with women with sporadic breast cancer matched for age, stage, and year of diagnosis, 5-year disease-free survival was only 54% in the lymphoma survivors compared with 91% in the comparison group.34 The investigators speculated that this disparity in outcome was associated with differences in treatment and, specifically, with the underuse of anthracycline-based chemotherapy in HL survivors.
All-cause mortality and the rate of non–breast cancer death were significantly greater in HL survivors than in their matched peers with sporadic breast cancer. These results reinforce prior evidence that young cancer survivors—specifically women who received chest irradiation for HL—are at an elevated risk of death from other second tumors and from noncancer causes.2,35 Survivors of HL face an increased risk of cardiac death, most commonly related to acceleration of coronary disease after mediastinal irradiation, particularly in the presence of other coronary risk factors.36 Treatment of HL with anthracycline-containing chemotherapy may also contribute to the risk of heart disease.37 A recent registry-based comparison of patients with breast cancer with and without a history of radiation for HL found that women in the former group had a seven-fold greater risk of death as a result of other cancers and an elevated risk of death from heart disease.38
More than half of the HL survivors in our study were diagnosed with HL before 1980; therefore, they were likely exposed to the most radical attempts to cure HL with radiation alone by maximizing both the dose and the volume of radiation. It is encouraging that the modern approach to the cure of HL utilizes significantly lower doses of radiation, and most treatments to the upper body lymph nodes now avoid most or all of the breast.8,39 Several studies demonstrate that avoiding treatment of the axillae significantly reduces the risk of breast cancer in HL survivors.6,8 Contemporary, effective treatment regimens for HL have markedly reduced the amount of both chemotherapy and radiation administered to patients.40
Several limitations of our analysis warrant mention. The study cohorts were identified from selected tertiary academic medical centers that see a high volume of both HL and breast cancer patients. If women seen in community-based settings differ with regard to personal factors, disease characteristics, and the treatment they receive, the generalizability of our findings may be limited. Although we were able to identify deaths as a result of breast cancer, we had limited information about other causes of death. Thus, we could not clearly distinguish deaths that were a result of noncancer causes from deaths that were a result of secondary malignancies other than breast cancer, such as lung cancer and non-Hodgkin's lymphoma, both of which are seen at an elevated rate in HL survivors.3
Our results are mostly relevant to patients treated for HL in the era when radiation therapy alone was the dominant form of curative therapy and was used with high radiation doses that almost always included both breasts and the heart. This practice has changed radically, and the reduced exposure of these organs is likely to change the risk profile for long-term complications. Thus, it may be inappropriate to alarm patients who are scheduled for modern, reduced radiation with the experience from radical radiotherapy of 3 to almost 5 decades past. However, our findings are informative for the thousands of HL survivors who remain at risk of long-term complications from their original treatment, underscoring the importance of regular screening for breast cancer and for comorbid conditions. Healthy lifestyle, cessation of smoking, early detection, and treatment of high blood pressure and hyperlipidemia, along with regular breast imaging, should all be part of standard education and follow-up for HL survivors.
Footnotes
Supported by the Lymphoma Foundation and the Sports Foundation Against Cancer (J.Y.); by mentored career development award No. 1 K07 CA118189-01A2 from the National Cancer Institute (E.B.E.); and by the Dr. Mortimer J. Lacher, MD, fellowship at Memorial Sloan-Kettering Cancer Center (M.L.K.).
Presented in part at the 45th Annual Meeting of the American Society of Clinical Oncology, May 29-June 2, 2009, Orlando, FL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The authors indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Elena B. Elkin, Michelle L. Klem,Joachim Yahalom
Financial support: Joachim Yahalom
Administrative support: Joachim Yahalom
Provision of study materials or patients: David Hodgson, Andrea K. Ng, Lawrence B. Marks, Joanne Weidhaas, Gary M. Freedman, Robert C. Miller, Louis S. Constine, Sten Myrehaug, Joachim Yahalom
Collection and assembly of data: Elena B. Elkin, Michelle L. Klem, Anne Marie Gonzales, Nicole M. Ishill, David Hodgson, Andrea K. Ng, Lawrence B. Marks, Joanne Weidhaas, Gary M. Freedman, Robert C. Miller, Louis S. Constine, Sten Myrehaug, Joachim Yahalom
Data analysis and interpretation: Elena B. Elkin, Michelle L. Klem, Anne Marie Gonzales, Nicole M. Ishill, Joachim Yahalom
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Brenner H, Gondos A, Pulte D. Ongoing improvement in long-term survival of patients with Hodgkin disease at all ages and recent catch-up of older patients. Blood . 2008;111:2977–2983. doi: 10.1182/blood-2007-10-115493. [DOI] [PubMed] [Google Scholar]
- 2.Aleman BM, van den Belt-Dusebout AW, Klokman WJ, et al. Long-term cause-specific mortality of patients treated for Hodgkin's disease. J Clin Oncol . 2003;21:3431–3439. doi: 10.1200/JCO.2003.07.131. [DOI] [PubMed] [Google Scholar]
- 3.Dores GM, Metayer C, Curtis RE, et al. Second malignant neoplasms among long-term survivors of Hodgkin's disease: A population-based evaluation over 25 years. J Clin Oncol . 2002;20:3484–3494. doi: 10.1200/JCO.2002.09.038. [DOI] [PubMed] [Google Scholar]
- 4.Hodgson DC, Gilbert ES, Dores GM, et al. Long-term solid cancer risk among 5-year survivors of Hodgkin's lymphoma. J Clin Oncol . 2007;25:1489–1497. doi: 10.1200/JCO.2006.09.0936. [DOI] [PubMed] [Google Scholar]
- 5.Horwich A, Swerdlow AJ. Second primary breast cancer after Hodgkin's disease. Br J Cancer . 2004;90:294–298. doi: 10.1038/sj.bjc.6601499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodgson DC, Koh ES, Tran TH, et al. Individualized estimates of second cancer risks after contemporary radiation therapy for Hodgkin lymphoma. Cancer . 2007;110:2576–2586. doi: 10.1002/cncr.23081. [DOI] [PubMed] [Google Scholar]
- 7.Hancock SL, Tucker MA, Hoppe RT. Breast cancer after treatment of Hodgkin's disease. J Natl Cancer Inst . 1993;85:25–31. doi: 10.1093/jnci/85.1.25. [DOI] [PubMed] [Google Scholar]
- 8.De Bruin ML, Sparidans J, van't Veer MB, et al. Breast cancer risk in female survivors of Hodgkin's lymphoma: Lower risk after smaller radiation volumes. J Clin Oncol . 2009;27:4239–4246. doi: 10.1200/JCO.2008.19.9174. [DOI] [PubMed] [Google Scholar]
- 9.Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA . 2003;290:465–475. doi: 10.1001/jama.290.4.465. [DOI] [PubMed] [Google Scholar]
- 10.van Leeuwen FE, Klokman WJ, Stovall M, et al. Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin's disease. J Natl Cancer Inst . 2003;95:971–980. doi: 10.1093/jnci/95.13.971. [DOI] [PubMed] [Google Scholar]
- 11.Yahalom J, Petrek JA, Biddinger PW, et al. Breast cancer in patients irradiated for Hodgkin's disease: A clinical and pathologic analysis of 45 events in 37 patients. J Clin Oncol . 1992;10:1674–1681. doi: 10.1200/JCO.1992.10.11.1674. [DOI] [PubMed] [Google Scholar]
- 12.Cutuli B, Borel C, Dhermain F, et al. Breast cancer occurred after treatment for Hodgkin's disease: Analysis of 133 cases. Radiother Oncol . 2001;59:247–255. doi: 10.1016/s0167-8140(01)00337-1. [DOI] [PubMed] [Google Scholar]
- 13.Dershaw DD, Yahalom J, Petrek JA. Breast carcinoma in women previously treated for Hodgkin disease: Mammographic evaluation. Radiology . 1992;184:421–423. doi: 10.1148/radiology.184.2.1320281. [DOI] [PubMed] [Google Scholar]
- 14.Diller L, Medeiros Nancarrow C, Shaffer K, et al. Breast cancer screening in women previously treated for Hodgkin's disease: A prospective cohort study. J Clin Oncol. 2002;20:2085–2091. doi: 10.1200/JCO.2002.08.031. [DOI] [PubMed] [Google Scholar]
- 15.Basu SK, Schwartz C, Fisher SG, et al. Unilateral and bilateral breast cancer in women surviving pediatric Hodgkin's disease. Int J Radiat Oncol Biol Phys . 2008;72:34–40. doi: 10.1016/j.ijrobp.2008.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolden SL, Hancock SL, Carlson RW, et al. Management of breast cancer after Hodgkin's disease. J Clin Oncol . 2000;18:765–772. doi: 10.1200/JCO.2000.18.4.765. [DOI] [PubMed] [Google Scholar]
- 17.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat . 1988;16:1141–1154. [Google Scholar]
- 18.Kalbfleisch JD, Prentice RL. Hoboken, NJ: Wiley; 2002. The Statistical Analysis of Failure Time Data (ed 2) [Google Scholar]
- 19.Wahner-Roedler DL, Nelson DF, Croghan IT, et al. Risk of breast cancer and breast cancer characteristics in women treated with supradiaphragmatic radiation for Hodgkin lymphoma: Mayo Clinic experience. Mayo Clin Proc . 2003;78:708–715. doi: 10.4065/78.6.708. [DOI] [PubMed] [Google Scholar]
- 20.Gervais-Fagnou DD, Girouard C, Laperriere N, et al. Breast cancer in women following supradiaphragmatic irradiation for Hodgkin's disease. Oncology . 1999;57:224–231. doi: 10.1159/000012035. [DOI] [PubMed] [Google Scholar]
- 21.Henderson TO, Amsterdam A, Bhatia S, et al. Systematic review: Surveillance for breast cancer in women treated with chest radiation for childhood, adolescent, or young adult cancer. Ann Intern Med . 2010;152:444–455. doi: 10.1059/0003-4819-152-7-201004060-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anders CK, Johnson R, Litton J, et al. Breast cancer before age 40 years. Semin Oncol . 2009;36:237–249. doi: 10.1053/j.seminoncol.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nixon AJ, Neuberg D, Hayes DF, et al. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II breast cancer. J Clin Oncol . 1994;12:888–894. doi: 10.1200/JCO.1994.12.5.888. [DOI] [PubMed] [Google Scholar]
- 24.Gnerlich JL, Deshpande AD, Jeffe DB, et al. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg . 2009;208:341–347. doi: 10.1016/j.jamcollsurg.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deshpande AD, Jeffe DB, Gnerlich J, et al. Racial disparities in breast cancer survival: An analysis by age and stage. J Surg Res . 2009;153:105–113. doi: 10.1016/j.jss.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeSantis C, Jemal A, Ward E, et al. Temporal trends in breast cancer mortality by state and race. Cancer Causes Control . 2008;19:537–545. doi: 10.1007/s10552-008-9113-1. [DOI] [PubMed] [Google Scholar]
- 27.Kenney LB, Yasui Y, Inskip PD, et al. Breast cancer after childhood cancer: A report from the Childhood Cancer Survivor Study. Ann Intern Med . 2004;141:590–597. doi: 10.7326/0003-4819-141-8-200410190-00006. [DOI] [PubMed] [Google Scholar]
- 28.Taylor AJ, Winter DL, Stiller CA, et al. Risk of breast cancer in female survivors of childhood Hodgkin's disease in Britain: A population-based study. Int J Cancer . 2007;120:384–391. doi: 10.1002/ijc.22261. [DOI] [PubMed] [Google Scholar]
- 29.Heron DE, Komarnicky LT, Hyslop T, et al. Bilateral breast carcinoma: Risk factors and outcomes for patients with synchronous and metachronous disease. Cancer . 2000;88:2739–2750. [PubMed] [Google Scholar]
- 30.Kheirelseid EA, Jumustafa H, Miller N, et al. Bilateral breast cancer: Analysis of incidence, outcome, survival and disease characteristics. Breast Cancer Res Treat . 2011;126:131–140. doi: 10.1007/s10549-010-1057-y. [DOI] [PubMed] [Google Scholar]
- 31.Hill DA, Gilbert E, Dores GM, et al. Breast cancer risk following radiotherapy for Hodgkin lymphoma: Modification by other risk factors. Blood . 2005;106:3358–3365. doi: 10.1182/blood-2005-04-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boice JD., Jr Second cancer after Hodgkin's disease: The price of success? J Natl Cancer Inst . 1993;85:4–5. doi: 10.1093/jnci/85.1.4. [DOI] [PubMed] [Google Scholar]
- 33.Broeks A, Braaf LM, Wessels LF, et al. Radiation-associated breast tumors display a distinct gene expression profile. Int J Radiat Oncol Biol Phys . 2010;76:540–547. doi: 10.1016/j.ijrobp.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Sanna G, Lorizzo K, Rotmensz N, et al. Breast cancer in Hodgkin's disease and non-Hodgkin's lymphoma survivors. Ann Oncol . 2007;18:288–292. doi: 10.1093/annonc/mdl399. [DOI] [PubMed] [Google Scholar]
- 35.Ng AK, Bernardo MP, Weller E, et al. Long-term survival and competing causes of death in patients with early-stage Hodgkin's disease treated at age 50 or younger. J Clin Oncol . 2002;20:2101–2108. doi: 10.1200/JCO.2002.08.021. [DOI] [PubMed] [Google Scholar]
- 36.Aleman BM, van den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109:1878–1886. doi: 10.1182/blood-2006-07-034405. [DOI] [PubMed] [Google Scholar]
- 37.Swerdlow AJ, Higgins CD, Smith P, et al. Myocardial infarction mortality risk after treatment for Hodgkin disease: A collaborative British cohort study. J Natl Cancer Inst . 2007;99:206–214. doi: 10.1093/jnci/djk029. [DOI] [PubMed] [Google Scholar]
- 38.Milano MT, Li H, Gail MH, et al. Long-term survival among patients with Hodgkin's lymphoma who developed breast cancer: A population-based study. J Clin Oncol . 2010;28:5088–5096. doi: 10.1200/JCO.2010.29.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yahalom J. Breast cancer after Hodgkin disease: Hope for a safer cure. JAMA . 2003;290:529–531. doi: 10.1001/jama.290.4.529. [DOI] [PubMed] [Google Scholar]
- 40.Engert A, Plütschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin's lymphoma. N Engl J Med. 2010;363:640–652. doi: 10.1056/NEJMoa1000067. [DOI] [PubMed] [Google Scholar]