Abstract
Purpose
Clinicians are increasingly willing to treat prostate cancer within the primary site in the presence of regional lymph node or even limited distant metastases. However, no formal study on the merits of this approach has been reported. We used a preoperative clinical discovery platform to prioritize pathways for assessment as therapeutic targets and to test the hypothesis that the primary site harbors potentially lethal tumors after aggressive treatment.
Patients and Methods
Patients with locally advanced or lymph node–metastatic prostate cancer underwent 1 year of androgen ablation and three cycles of docetaxel therapy, followed by prostatectomy. All specimens were characterized for stage by accepted criteria. Expression of select molecular markers implicated in disease progression and therapy resistance was determined immunohistochemically and compared with that in 30 archived specimens from untreated patients with high-grade prostate cancer. Marker expression was divided into three groups: intracellular signaling pathways, stromal-epithelial interaction pathways, and angiogenesis.
Results
Forty patients were enrolled, 30 (75%) of whom underwent prostatectomy and two (5%) who underwent cystoprostatectomy. Twenty-nine specimens contained sufficient residual tumor for inclusion in a tissue microarray. Immunohistochemical analysis showed increased epithelial and stromal expression of CYP17, SRD5A1, and Hedgehog pathway components, and modulations of the insulin-like growth factor I pathway.
Conclusion
A network of molecular pathways reportedly linked to prostate cancer progression is activated after 1 year of therapy; biomarker expression suggests that potentially lethal cancers persist in the primary tumor and may contribute to progression.
INTRODUCTION
Androgen deprivation is the mainstay of therapy for patients with metastatic and high-risk localized prostate cancer.1 However, despite any initial response, castration resistance eventually develops.2 Various therapeutic modalities have been used to manage castration-resistant prostate cancer (CRPC). Among them, docetaxel has shown antitumor activity in metastatic CRPC3,4; its preoperative administration has yielded decreases of more than 50% in prostate-specific antigen (PSA) concentration and modestly decreased tumor volume.5 As a result, docetaxel has also been explored for use in multimodality approaches for the treatment of high-risk localized prostate cancer.6,7
Unlike the approaches commonly taken to treat other solid tumors, the primary site is usually ignored in metastatic prostate cancer. This derives from the commonly held view that efforts to control the primary site will be futile or unnecessary in patients with established metastases because the major risk to the length and quality of survival is attributed to the pre-existing metastases. However, increasing evidence suggests that there is a survival benefit when local treatment is added to systemic therapy.8,9
The investigational strategy of using preoperative therapy followed by prostatectomy allows assessment of the effects of candidate therapies on the primary tumor.10,11 We designed such a preoperative clinical-discovery platform to identify pathways activated under the selective pressure of therapy (and thus implicated in castration resistance), to prioritize them for further study, and to determine whether the primary site harbors potentially lethal tumors despite aggressive systemic treatment.
In this study of 1 year of preoperative androgen ablation (AA) and docetaxel treatment, followed by prostatectomy, we analyzed the pathologic and molecular features of the residual tumor in the prostatectomy specimens from the enrolled patients for the expression of pathways that may lead to lethal progression of the cancer. Similar approaches have been used for studying patients treated for shorter durations, ranging from 3 to 9 months.12–14 We speculated, however, that longer preoperative treatment in patients with unresectable or metastatic prostate cancer would better reflect truly therapy- resistant cancer.
We and others have hypothesized that pathways linked to prostate carcinogenesis are also implicated in the development of resistance to AA.15 Thus, we explored the expression of molecular markers involved in intracellular signaling (ie, ki67, bcl2, p53, chromogranin, synaptophysin, insulin-like growth factor [IGF] pathway components, including IGF-I and IGF-I receptor B [IGF-IRB]); stromal-epithelial interaction, including androgen receptor (AR), phosphorylated AR, the steroid-synthetic enzymes CYP17 and 5-α-steroid 4-dehydrogenase 1 (SRD5A1), and Hh pathway components (Sonic Hh [Shh], Patched [Ptch], Smoothened, Gli1, Gli2); and angiogenesis (ie, vascular endothelial growth factor [VEGF], CD31) in the patients' prostatectomy specimens and compared their expression with that in archived tissues with high-grade prostate cancer from untreated control subjects.
Our results add to the evidence that cancers with complex active biologic networks persist within the primary site after 1 year of combined AA treatment and chemotherapy. Previous studies showed that the presence and extent of residual tumor in prostate biopsy specimens after radiotherapy are the most important prognostic factors of disease-free survival in patients with intermediate- and high-risk prostate cancer.16,17 These data provide the rationale for developing an integrated treatment strategy for patients with prostate cancer and justify a re-examination of the roles of surgery and radiation in patients with lymph node involvement, which may be extended to select patients with other sites of metastasis.
PATIENTS AND METHODS
Patients
Patients were enrolled prospectively in this phase II study; eligibility criteria were the presence of histologically confirmed prostate cancer, presence or high probability of lymph node metastasis, and no evidence of bone or visceral metastasis. Patients were required to have one or more of the following: biopsy-proven lymph node metastasis; pelvic or retroperitoneal lymphadenopathy ≥ 2.0 cm visualized on computed tomography scanning; primary tumor Gleason score ≥ 8 and serum PSA concentration ≥ 25 ng/mL; primary tumor clinical stage T3 and Gleason score ≥ 7; and primary tumor clinical stage T4 that is potentially resectable after neoadjuvant treatment.
Exclusion criteria were small-cell or sarcomatoid histologic features, prior chemotherapy, severe comorbidities, or inability to give written informed consent.
All enrolled patients provided written informed consent to participate in the study, the protocol for which was approved by the institutional review board of The University of Texas MD Anderson Cancer Center.
Treatment Regimen
Patients received three cycles of intravenous docetaxel (35 mg/m2 administered on days 1, 8, 15, and 22 every 6 weeks), plus AA therapy with intramuscular leuprolide for 1 year, followed by prostatectomy. Docetaxel was given at the beginning of the 1-year preoperative period.
Tissue Specimens
The entire radical prostatectomy specimens were submitted for histologic evaluation using a standard method.18 Additional details are provided in the Appendix (online only). The number of tumor foci per case was recorded, and the volume of each focus was determined as previously described.19 No Gleason score was assigned to the specimens in the treated group, although the proposed classification for treated tumors20 was used to record the histologic patterns present.
In addition, archived radical prostatectomy specimens with high-grade prostate cancer from 30 previously untreated patients who had undergone surgery from 1998 through 2007 were retrieved from the files of the tissue bank of the Department of Pathology, MD Anderson, and included in the study. Because needle biopsies have inherent sampling bias, the control specimens were selected on the basis of the Gleason score of the prostatectomy specimen.
Tissue Microarrays and Immunohistochemical Analysis
Hematoxylin and eosin–stained slides from available specimen blocks were reviewed, and areas representative of the different histologic patterns20 in each case were selected for inclusion in the tissue microarrays (TMAs). Two TMAs, one for the specimens from the treated patients and one for the archived control specimens, containing 0.6 mm–diameter cores were made. Serial 4-μm sections from both TMAs were cut and subjected to immunohistochemical analysis using an autostainer (Dako North America Inc, Carpinteria, CA), as previously described,11 with antibodies against various intracellular signaling, stromal-epithelial interaction, and angiogenesis-related markers (Table 1). Additional details are provided in the Appendix (online only).
Table 1.
Antibodies Used Against Markers of Epithelial Function, Stromal-Epithelial Interaction, and Angiogenesis
| Type of Marker | Dilution | Supplier |
|---|---|---|
| Intracellular signaling pathways | ||
| ki67 | 1:50 | Dako North America, Carpinteria, CA |
| bcl2 | 1:200 | Dako North America |
| p53 | 1:1000 | Dako North America |
| Chromogranin | 1:200 | Dako North America |
| Synaptophysin | 1:25 | Dako North America |
| IGF-I | 1:200 | Abcam, Cambridge, MA |
| IGF-IRB | 1:50 | Cell Signaling Technology, Danvers, MA |
| Stromal-epithelial interaction pathways | ||
| AR | 1:50 | Dako North America |
| pAR | 1:20 | Imgenex Corp, San Diego, CA |
| CYP17 | 1:100 | Novus Biologicals, Littleton, CO |
| SRD5A1 | 1:400 | Novus Biologicals |
| Sonic hedgehog | 1:100 | Santa Cruz Biotechnology, Santa Cruz, CA |
| Ptch (patched) | 1:350 | Strategic Diagnostics, Newark, DE |
| Smo (smoothened) | 1:80 | Abcam |
| Gli1 | 1:300 | Novus |
| Gli2 | 1:600 | Abcam |
| Angiogenesis | ||
| VEGF | prediluted | Abcam |
| CD31 | 1:30 | Dako North America |
Abbreviations: IGF-I, insulin-like growth factor I; IGF-IRB, IGF-I receptor B; AR, androgen receptor; pAR, phosphorylated androgen receptor; VEGF, vascular endothelial growth factor.
Statistical Analyses
The patients' characteristics and biomarker data were summarized using descriptive statistics and exploratory data analysis. Continuously scaled measures were summarized with descriptive statistical measures (ie, mean with standard deviation [SD]), and categorical data were described with the use of contingency tables. Fisher's exact test was used to assess the association between categorical variables, and a two-sample t test was used to assess the mean between-group differences in continuous variables, including biomarker expression. Spearman's correlation coefficient testing was used to assess correlation in expression between biomarkers. Mixed-effects models were fitted to incorporate multiple observations (ie, from multiple tissue cores from an individual patient), allowing us to estimate variability both between patients and within individual patients.
All P value determinations were two sided, at a significance level of .05. A Bonferroni correction was applied to adjust for multiple comparisons. Analyses were performed with the use of SAS for Windows (1999-2000; version 8.1; SAS Institute Inc, Cary, NC) and S-PLUS 2000 (Insightful Corporation, Seattle, WA) software.
RESULTS
Patients and Specimens
In total, 40 patients were enrolled in the study from April 21, 2005, through April 1, 2008. Their ages ranged from 38 to 75 years (mean, 60; SD, 7), and their mean ± SD PSA level at the time of enrollment was 11 ± 18 ng/mL. According to the results of prostate biopsies performed before treatment began, three patients had a Gleason score of 7 (4 + 3), six had a Gleason score of 8 (4 + 4), 25 had a Gleason score of 9 (4 + 5), four had a Gleason score of 9 (5 + 4), and two had a Gleason score of 10 (5 + 5).
At the end of the treatment period, 32 patients underwent surgery: 30 underwent radical prostatectomy, and two underwent cystoprostatectomy because of gross bladder infiltration by the tumor. The remaining eight patients did not undergo prostatectomy for the following reasons: patient's decision (four patients), disease progression during the preoperative period (two patients), and inability to complete the surgery because of dense pelvic adhesions or intraoperative hypoxemia (one patient each). The primary clinical end point of the trial was the postoperative 1-year survival rate without biochemical progression; the full results of the trial will be reported in a separate publication.
The pathologic characteristics of the pretreatment biopsies and surgical specimens from the treated and control patients are summarized in Table 2. Further details are given in the Appendix (online only).
Table 2.
Pathologic Characteristics of the Specimens From Treated Patients (n = 32) and the Archived Specimens From Untreated Control Patients (n = 30)
| Characteristic | Treated |
Control |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Biopsy Gleason score | ||||
| 7 (4 + 3, 3 + 4) | 3 | 9 | 19 | 63 |
| 8 (4 + 4) | 5 | 16 | 6 | 20 |
| 9 (4 + 5) | 19 | 59 | 4 | 13 |
| 9 (5 + 4) | 4 | 13 | 0 | 0 |
| 10 (5 + 5) | 1 | 3 | 1 | 4 |
| Prostatectomy Gleason score | ||||
| 7 (4 + 3) | NA | 10 | 33 | |
| 8 (4 + 4, 3 + 5) | 2 | 7 | ||
| 9 (4 + 5) | 17 | 57 | ||
| 9 (5 + 4) | 1 | 3 | ||
| pT stage | ||||
| T0* | 2 | 6 | 0 | 0 |
| T2 | 4 | 13 | 3 | 10 |
| T3a | 2 | 6 | 7 | 23 |
| T3b | 21 | 66 | 20 | 67 |
| T4 | 3 | 9 | 0 | 0 |
| pN stage | ||||
| N0 | 11 | 34 | 17 | 57 |
| N1 | 21 | 66 | 13 | 43 |
One specimen with a cluster of tumor cells within a vascular space.
Twenty-nine of 32 specimens from the treated patients were included in the TMA analysis. Tissues from three patients were not included because of lack of available blocks, poor block quality, and loss of cohesion between the cores and the TMA block (one case each). In total, 762 cores and 169 blocks were included in the TMAs. The TMA from the treated specimens consisted of 492 cores and that from the controls, 270 cores. Each patient's case was represented by a median of nine cores (mean, 13; SD, 4; range, 6 to 18 cores).
Biomarker Expression
The expression levels of the biomarkers are presented in Table 3. No correlation was noted between the expression of the biomarkers and the pathologic pT and pN stages or the tumor volume.
Table 3.
Expression Levels (and SD) of Biomarkers in Treated and Untreated Tumors
| Pathway and Localization | Marker | Untreated | Treated | SD Between Patients | SD Within Patients | P |
|---|---|---|---|---|---|---|
| Proliferation | ||||||
| Epithelial cells | ki67 | 0.1229 | 0.3753 | 0.4017 | 0.4059 | .0322 |
| Apoptosis | ||||||
| Epithelial cells | bcl2 | 0.3713 | 1.1905 | 1.6116 | 1.387 | .076 |
| P53 | 0.2569 | 0.2968 | 0.6964 | 0.4009 | .8345 | |
| Neuroendocrine differentiation | ||||||
| Epithelial cells | Chromogranin | 0.2153 | 0.878 | 1.5373 | 0.9122 | .1205 |
| Synaptophysin | 0.2115 | 0.2632 | 0.7101 | 0.6398 | .7988 | |
| IGF pathway | ||||||
| Epithelial cells | IGF-I | 5.886 | 8.0576 | 2.0296 | 1.9409 | .0004 |
| IGF-IRB | 7.0423 | 3.7132 | 2.2382 | 2.0792 | < .001 | |
| Stromal cells | IGF-I | 0.0716 | 0.1302 | 0.1 | 0.3471 | .1985 |
| IGF-IRB | 1.098 | 0.8596 | 0.5097 | 0.7283 | .1323 | |
| AR signaling | ||||||
| Epithelial cells | Nuclear AR | 8.0269 | 7.6484 | 1.6355 | 2.0728 | .4529 |
| Nuclear pAR | 4.951 | 5.4951 | 3.0589 | 2.0803 | .5227 | |
| Stromal cells | AR | 1.2174 | 2.4065 | 1.0148 | 1.7593 | .0009 |
| pAR | 0.5688 | 0.224 | 0.4844 | 0.6169 | .0199 | |
| Androgen synthesis | ||||||
| Epithelial cells | CYP17 | 6.1911 | 9.5164 | 2.2763 | 1.3346 | < .001 |
| Nuclear SRD5A1 | 2.0913 | 9.7256 | 1.1971 | 0.811 | < .001 | |
| Cytoplasmic SRD5A1 | 4.6126 | 3.6022 | 2.6371 | 2.1421 | .1749 | |
| Stromal cells | CYP17 | 1.9681 | 5.8481 | 1.8947 | 1.6957 | < .001 |
| Nuclear SRD5A1 | 1.4343 | 7.579 | 0.6886 | 1.4322 | < .001 | |
| Cytoplasmic SRD5A1 | 2.1902 | 1.4882 | 0.8689 | 1.0357 | .0072 | |
| Hedgehog pathway | ||||||
| Epithelial cells | Sonic hedgehog | 7.2084 | 8.1113 | 2.1773 | 1.7655 | .145 |
| Patched | 3.2668 | 6.4566 | 2.4571 | 1.8316 | < .001 | |
| Smoothened | 9.0207 | 6.3242 | 1.5433 | 1.8994 | .02 | |
| Nuclear Gli1 | 6.0929 | 8.7449 | 2.6566 | 1.5882 | .0006 | |
| Nuclear Gli2 | 8.5177 | 9.9294 | 1.8614 | 0.5667 | .0064 | |
| Stromal cells | Sonic hedgehog | 1.0862 | 1.0277 | 0.5131 | 0.7557 | .7112 |
| Patched | 7.9661 | 7.3036 | 1.011 | 1.5056 | .0357 | |
| Smoothened | 4.5403 | 3.5715 | 1.8185 | 1.8724 | .0688 | |
| Gli1 | 2.6042 | 4.8577 | 1.6994 | 1.7779 | < .001 | |
| Gli2 | 4.5415 | 7.9119 | 1.4999 | 1.5875 | < .001 | |
| Angiogenesis | ||||||
| Epithelial cells | VEGF | 8.2946 | 5.3617 | 1.9072 | 2.0047 | < .001 |
| Stromal cells | VEGF | 2.6674 | 2.8619 | 1.1734 | 1.6383 | .5841 |
| Stroma | CD31 | 2.3786 | 2.6459 | 0.9542 | 0.8633 | .3274 |
NOTE. The percentage of positively stained cells was recorded on a scale from 0 (lowest) to 10 (highest); details are given in the Appendix (online only). Bold type indicates statistically significant P values after Bonferroni correction.
Abbreviations: SD, standard deviation; IGF, insulin-like growth factor; IGF-I, insulin-like growth factor I; IGF-IRB, IGF-I receptor B; AR, androgen receptor; pAR, phosphorylated androgen receptor; VEGF, vascular endothelial growth factor.
Markers of Intracellular Signaling
No significant differences in the expression of markers related to proliferation (ie, ki67), apoptosis (ie, bcl2, p53), or neuroendocrine differentiation (ie, chromogranin, synaptophysin) were noted between treated and untreated specimens. As expected, chromogranin expression correlated with that of synaptophysin (P = .001; r = 0.617).
IGF-I was expressed almost exclusively in the epithelial cells, and its expression increased after treatment (P = .0004). IGF-IRB was also expressed primarily in the epithelial cells, but its expression decreased after treatment (P < .001).
Markers of Stromal-Epithelial Interaction
Nuclear expression of the AR was high in epithelial cells of both treated and untreated patients. Its stromal expression increased after treatment (P = .0009). Phosphorylated AR was expressed primarily in the nuclei of the epithelial cells, with low expression levels in the stroma; its expression in the epithelial cells correlated with AR and ki67 expression (P < .001; r = 0.715, and P = .039; r = 0.4, respectively). No difference in phosphorylated AR expression was noted between treated and control specimens.
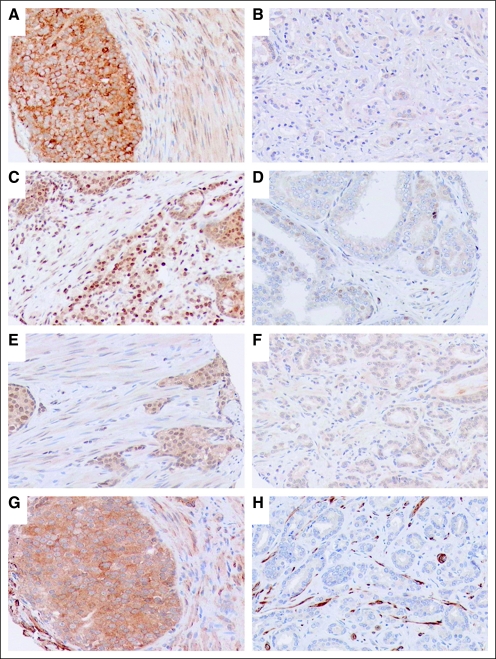
Expression of the steroid-synthetic enzymes CYP17 and SRD5A1 was enhanced in both epithelial and stromal cells of the treated specimens relative to that in the untreated ones (Fig 1; P < .001). SRD5A1 was expressed primarily in the nuclei, and its expression in epithelial cells correlated with the epithelial expression of CYP17 (P = .01; r = 0.482).
Fig 1.
Representative images of expression of (A,B) CYP17, (C,D) SRD5A1, (E,F) Gli1, and (G,H) Ptch. Expression is greater in the (A,C,E,G) treated tumors than in the (B,D,F,H) untreated tumors. Original magnification, ×200.
Epithelial Hh signaling was coordinately upregulated in epithelial cells of treated specimens, as shown by enhanced expression of nuclear Gli1 (P = .0006) and cytoplasmic Ptch (P < .001; Fig 1) and by the correlation between Shh and Ptch (P < .001; r = 0.697), Gli1 (P = .049; r = 0.368), and Gli2 (P = .025; r = 0.430) expression. Stromal Hh signaling also showed evidence of enhancement after treatment: both Gli1 and Gli2 expression in stromal cells was higher in treated than in untreated specimens (P < .001).
Markers of Angiogenesis
Epithelial expression of VEGF was downregulated in treated tumors relative to that in untreated ones (P < .001). Microvessel density, as determined by measuring CD31 expression, was similar in tumors from treated and untreated patients.
Synchronous Activation of Multiple Pathways After Treatment With AA and Docetaxel
Our results revealed that three major pathways were upregulated after treatment: intratumoral CYP17 and SRD5A1 expression (ie, intratumoral steroidogenesis), Hh signaling, and IGF-I expression. Considering the respective median value of the untreated tumors as a cutoff value (Table 3), we found that 20 of 29 treated tumors upregulated all three pathways, eight upregulated two of the three pathways, and only one upregulated one of the three pathways (Table 4). These findings indicate that more than one pathway is activated simultaneously in the microenvironment of prostate cancer in the context of treatment with AA and docetaxel.
Table 4.
Modulation of Stromal-Epithelial Interacting Pathways in Patients With Regionally Metastatic Prostate Cancer after Androgen Ablation and Docetaxel Treatment
| Patient No. | Intratumoral Steroidogenesis | Hedgehog Signaling | IGF-I Expression |
|---|---|---|---|
| 1 | U | U | U |
| 2 | U | U | U |
| 3 | U | U | U |
| 4 | U | U | U |
| 5 | U | U | U |
| 6 | U | U | U |
| 7 | U | U | Low expression |
| 8 | U | U | U |
| 9 | U | Low expression | Low expression |
| 10 | U | U | U |
| 11 | U | U | U |
| 12 | U | U | U |
| 13 | U | U | Low expression |
| 14 | U | U | U |
| 15 | U | Low expression | U |
| 16 | U | U | U |
| 17 | U | Low expression | U |
| 18 | U | U | U |
| 19 | U | U | U |
| 20 | U | U | U |
| 21 | U | U | U |
| 22 | U | Low expression | U |
| 23 | U | Low expression | U |
| 24 | U | U | U |
| 25 | U | U | U |
| 26 | U | Low expression | U |
| 27 | U | U | U |
| 28 | U | U | U |
| 29 | U | Low expression | U |
Abbreviations: IGF-I, insulin-like growth factor I; U, upregulation.
DISCUSSION
Previous investigators have reported the molecular effects of up to 9 months of AA treatment in patients with localized prostate cancer,12–14 whereas others have analyzed the molecular profile of heavily treated CRPC.21–23 However, the study we report here is the first to our knowledge to have evaluated the molecular alterations noted in the primary tumor site after 1 year of AA and docetaxel therapy in patients with clinically detected lymph node–metastatic cancer. We selectively examined the modulation of pathways that are implicated in the pathogenesis and progression of prostate carcinoma since those have also been associated with the development of therapy resistance.15
Modulated proliferation and apoptosis and increased expression of markers of neuroendocrine differentiation have been associated with AA therapy, with varying results depending on the duration and type of therapy.21–26 Our results revealed for the first time, to our knowledge, that proliferation and apoptosis markers are not statistically significantly different between untreated controls and patients uniformly treated with AA and docetaxel for 1 year. Similarly, no difference in the expression of the neuroendocrine markers chromogranin and synaptophysin was noted. These findings imply that proliferation, apoptosis, and neuroendocrine differentiation–associated pathways are not significantly altered after 1 year of treatment.
IGF-I interacts with androgen signaling and is implicated in castrate-resistant progression mediated by interaction with androgens and the AR.27,28 We found that IGF-I and its receptor, IGF-IRB, were expressed almost exclusively in epithelial cells and that IGF-I expression increased, whereas IGF-IRB expression decreased in the treated tumors. These findings agree with those reported previously,29,30 supporting a role of the IGF pathway in the development of CRPC.
We next focused on stromal–epithelial interaction pathways with well-defined roles in prostate cancer pathogenesis and progression. Among them, androgen signaling is central in CRPC progression.31,32 We did not observe increased nuclear AR expression, as has been reported in later stages of prostate cancer progression.33 If our findings are confirmed, they strongly suggest that persistent androgen signaling as an adaptive response to castration is a delayed phenomenon. Further studies are needed to uncover the temporal heterogeneity of androgen signaling in castration resistance. Understanding this will guide selection of the duration of treatment needed for targeting persistent androgen signaling.
In contrast to that in epithelial cells, AR expression in stromal cells was enhanced in treated tumors. Androgen signaling in stromal cells has been shown to exhibit tumor-promoting effects in a paracrine manner34–37 and to enhance the aggressiveness of prostate cancer.38 These findings, together with our findings of increased stromal expression of AR after therapy, imply that AR signaling plays an important role in stromal cells that warrants further investigation.
Despite castrate levels of circulating androgens during AA, local androgen production in the prostate cancer microenvironment maintains AR transcriptional activity.39,40 Expression of CYP17, the rate-limiting enzyme in androgen synthesis,41 and of SRD5A1, the predominant SRD5A isoform in recurrent prostate cancer, is increased in castrate-resistant metastases.39,40 In line with this, we observed increased epithelial and stromal CYP17 and SRD5A1 expression in treated primary tumors relative to that in untreated ones. These findings implicate activation of the local steroid-synthetic machinery in the primary site as an adaptive response to therapy.
Complex adult solid tumors, such as prostate cancer, are likely driven by multiple pathways, thus subverting efforts to effectively control tumor progression by blocking a single pathway.42 We prioritized the Hh signaling pathway for further study because it is central to prostate and bone development, has been implicated in prostate carcinogenesis, and can be targeted with selective inhibitors.43–45 Of particular interest is the evidence linking AR and Hh signaling to prostate development.46 This reported linkage provides the rationale for studying the response of Hh signaling in prostate cancer progression after AA therapy. In this study, we observed increased epithelial Gli1 and Ptch expression and upregulated stromal Gli1 and Gli2 expression in the treated tumors relative to their expression in untreated controls. These findings complement our previous findings of Hh activation after 4 months of AA and chemotherapy47 and of Hh target gene amplification after castration in a murine model of prostate cancer (unpublished observations).
Interplay between androgen signaling and angiogenesis has been suggested because of evidence that AA reduces VEGF levels in cancer cell lines,48 preclinical models,48,49 and human specimens of prostate cancer,49 and enhances normalization of tumor vasculature.49,50 Similarly, docetaxel treatment has been associated with antiangiogenic effects in vivo and in vitro and with downregulated VEGF levels in vitro.51 Our results demonstrated that 1 year of AA and docetaxel resulted in significantly lower levels of epithelial VEGF expression in human specimens of prostate cancer. No correlation with CD31 expression was noted, probably because of the complexity of angiogenesis regulation in the tumor microenvironment.
Overall, we showed that the enzymes associated with intracrine androgen synthesis, CYP17 and SRD5A1, the Hh pathway, and the IGF-I pathway were upregulated in the microenvironment of prostate cancer treated with AA and docetaxel for 1 year. Whether similar pathways are also activated at the metastatic site, so that therapeutic targeting will be beneficial in patients who develop resistance to therapy, requires further investigation. However, our results provide evidence that these pathways (ie, local steroid-synthetic enzyme production, Hh pathway activation, and growth factor signaling) are relevant in the primary site and suggest a strategy for multitargeted therapy centered on the tumor microenvironment. On the basis of the results of our own studies47 and our unpublished observations, a phase I/II clinical trial of combined Hh signaling inhibition and AA in select patients with prostate cancer has been initiated.
In conclusion, we showed that molecular features associated with potentially lethal prostate cancer remain in tumor cells within the primary site after aggressive treatment, despite a favorable therapeutic response according to traditional criteria (eg, serum PSA concentration). Even though the signaling pathways are present in the primary tumor after 1 year of therapy, we cannot determine with precision the degree of change in this study. In addition, due to the limited follow-up period, we could not correlate the pathways activated with patients' prognosis. Further studies on the role of controlling the primary site in patients with lymph node–metastatic disease treated systemically are warranted.
Acknowledgment
We thank Ina N. Prokhorova, MD, from the Department of Pathology,MD Anderson Cancer Center, for her expert technical assistance with pathologic specimens, and Karen F. Phillips, ELS, from the Department of Genitourinary Medical Oncology,MD Anderson Cancer Center, for editing the manuscript.
Appendix
The cross-sections of the prostate were subdivided into portions to fit standard-size cassettes. For the cystoprostatectomy specimens, the prostate and bladder neck region were extensively sampled (approximately 70% of the area submitted). The specimens were reviewed by two pathologists (V.T., P.T.).
A multitumor TMA composed of small (approximately 1 cm) tissue fragments from a variety of tumors (including breast carcinoma, basal cell carcinoma, melanoma, prostate carcinoma, and colon carcinoma) was used as a positive control. The whole slide for each biomarker was scanned with the Bliss system (Bacus Laboratories Inc, Lombard, IL), viewed with WebSlide Browser 4 (Bacus), and automatically stored for later retrieval. Epithelial and stromal cells were evaluated separately, and the percentage of positively stained cells was determined for each core. Results were scored according to the following 11-point scale: 0 ≤ 1% positive cells; 1 = 1% to 10% positive cells; 2 = 11% to 20%; 3 = 21% to 30%; 4 = 31% to 40%; 5 = 41% to 50%; 6 = 51% to 60%; 7 = 61% to 70%; 8 = 71% to 80%; 9 = 81% to 90%; and 10 = 91% to 100%.
Among the radical prostatectomy specimens from the treated patients, 25 (78%) had one tumor focus, one (3%) had two tumor foci, and two (6%) had three tumor foci. The prostatectomy specimens from the remaining two treated patients were considered pT0: one patient had no residual tumor, and the other's tumor was limited to a single cluster of tumor cells within a vascular space located in the intraprostatic portion of one seminal vesicle. The mean tumor volume was 1.91 cm3 (SD, 2.38; range, 0 to 8.4 cm3).
Footnotes
Supported in part by Grant No. CA016672 from the National Institutes of Health.
Presented at the 46th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 4-8, 2010.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT01076335.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Christopher J. Logothetis, BIND Biosciences (C) Stock Ownership: None Honoraria: Christopher J. Logothetis, Novartis, Bristol-Myers Squibb, Pfizer, sanofi-aventis, GlaxoSmithKline Research Funding: Christopher J. Logothetis, Novartis, Bristol-Myers Squibb, Pfizer, sanofi-aventis, GlaxoSmithKline Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Vassiliki Tzelepi, Eleni Efstathiou, Patricia Troncoso, Curtis A. Pettaway, Louis L. Pisters, Lance C. Pagliaro
Financial support: Christopher J. Logothetis
Administrative support: Christopher J. Logothetis, Lance C. Pagliaro
Provision of study materials or patients: Curtis A. Pettaway, Louis L. Pisters, Christopher J. Logothetis, Lance C. Pagliaro
Collection and assembly of data: Vassiliki Tzelepi, Eleni Efstathiou, Patricia Troncoso, Louis L. Pisters, Anh Hoang, Lance C. Pagliaro
Data analysis and interpretation: Vassiliki Tzelepi, Eleni Efstathiou, Sijin Wen, Patricia Troncoso, Maria Karlou, Curtis A. Pettaway, Louis L. Pisters, Christopher J. Logothetis, Lance C. Pagliaro
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Harris WP, Mostaghel EA, Nelson PS, et al. Androgen deprivation therapy: Progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6:76–85. doi: 10.1038/ncpuro1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonkhoff H, Berges R. From pathogenesis to prevention of castration resistant prostate cancer. Prostate. 2010;70:100–112. doi: 10.1002/pros.21042. [DOI] [PubMed] [Google Scholar]
- 3.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 4.Tannock IF, de Wit R, Berry WR, et al. For the TAX 327 Investigators: Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 5.Febbo PG, Richie JP, George DJ, et al. Neoadjuvant docetaxel before radical prostatectomy in patients with high-risk localized prostate cancer. Clin Cancer Res. 2005;11:5233–5240. doi: 10.1158/1078-0432.CCR-05-0299. [DOI] [PubMed] [Google Scholar]
- 6.Beer TM, El-Geneidi M, Eilers KM. Docetaxel (Taxotere®) in the treatment of prostate cancer. Expert Rev Anticancer Ther. 2003;3:261–268. doi: 10.1586/14737140.3.3.261. [DOI] [PubMed] [Google Scholar]
- 7.Chi KN, Chin JL, Winquist E, et al. Multicenter phase II study of combined neoadjuvant docetaxel and hormone therapy before radical prostatectomy for patients with high risk localized prostate cancer. J Urol. 2008;180:565–570. doi: 10.1016/j.juro.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Gjertson CK, Asher KP, Sclar JD, et al. Local control and long-term disease-free survival for stage D1 (T2–T4N1-N2M0) prostate cancer after radical prostatectomy in the PSA era. Urology. 2007;70:723–727. doi: 10.1016/j.urology.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Verhagen PC, Schröder FH, Collette L, et al. Does local treatment of the prostate in advanced and/or lymph node metastatic disease improve efficacy of androgen-deprivation therapy? A systematic review. Eur Urol. 2010;58:261–269. doi: 10.1016/j.eururo.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 10.Mathew P, Dipaola R. Taxane refractory prostate cancer. J Urol. 2007;178:S36–S41. doi: 10.1016/j.juro.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 11.Efstathiou E, Troncoso P, Wen S, et al. Initial modulation of the tumor microenvironment accounts for thalidomide activity in prostate cancer. Clin Cancer Res. 2007;13:1224–1231. doi: 10.1158/1078-0432.CCR-06-1938. [DOI] [PubMed] [Google Scholar]
- 12.Hellström M, Ranepall P, Wester K, et al. Effect of androgen deprivation on epithelial and mesenchymal tissue components in localized prostate cancer. Br J Urol. 1997;79:421–426. doi: 10.1046/j.1464-410x.1997.02920.x. [DOI] [PubMed] [Google Scholar]
- 13.Scattoni V, Montironi R, Mazzucchelli R, et al. Pathological changes of high-grade prostatic intraepithelial neoplasia and prostate cancer after monotherapy with bicalutamide 150 mg. BJU Int. 2006;98:54–58. doi: 10.1111/j.1464-410X.2006.06204.x. [DOI] [PubMed] [Google Scholar]
- 14.Mostaghel EA, Page ST, Lin DW, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: Therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67:5033–5041. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 15.Efstathiou E, Logothetis CJ. A new therapy paradigm for prostate cancer founded on clinical observations. Clin Cancer Res. 2010;16:1100–1107. doi: 10.1158/1078-0432.CCR-09-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crook JM, Malone S, Perry G, et al. Twenty-four-month postradiation prostate biopsies are strongly predictive of 7-year disease-free survival: Results from a Canadian randomized trial. Cancer. 2009;115:673–679. doi: 10.1002/cncr.24020. [DOI] [PubMed] [Google Scholar]
- 17.Liauw SL, Fricano J, Correa D, et al. Dose-escalated radiation therapy for intermediate-risk prostate cancer: Patient selection for androgen deprivation therapy using percentage of positive cores. Cancer. 2009;115:1784–1790. doi: 10.1002/cncr.24176. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Ortiz RF, Troncoso P, Babaian RJ, et al. African-American men with nonpalpable prostate cancer exhibit greater tumor volume than matched white men. Cancer. 2006;107:75–82. doi: 10.1002/cncr.21954. [DOI] [PubMed] [Google Scholar]
- 19.Chen ME, Johnston D, Reyes AO, et al. A streamlined three-dimensional volume estimation method accurately classifies prostate tumors by volume. Am J Surg Pathol. 2003;27:1291–1301. doi: 10.1097/00000478-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Efstathiou E, Abrahams NA, Tibbs RF, et al. Morphologic characterization of preoperatively treated prostate cancer: Toward a post-therapy histologic classification. Eur Urol. 2010;57:1030–1038. doi: 10.1016/j.eururo.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baretton GB, Klenk U, Diebold J, et al. Proliferation- and apoptosis-associated factors in advanced prostatic carcinomas before and after androgen deprivation therapy: Prognostic significance of p21/WAF1/CIP1 expression. Br J Cancer. 1999;80:546–555. doi: 10.1038/sj.bjc.6690390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Y, Chatterjee SJ, Brands FH, et al. Role of coordinated molecular alterations in the development of androgen-independent prostate cancer: An in vitro model that corroborates clinical observations. BJU Int. 2006;97:170–178. doi: 10.1111/j.1464-410X.2006.05857.x. [DOI] [PubMed] [Google Scholar]
- 23.D'Antonio JM, Ma C, Monzon FA, et al. Longitudinal analysis of androgen deprivation of prostate cancer cells identifies pathways to androgen independence. Prostate. 2008;68:698–714. doi: 10.1002/pros.20677. [DOI] [PubMed] [Google Scholar]
- 24.Frigo DE, McDonnell DP. Differential effects of prostate cancer therapeutics on neuroendocrine transdifferentiation. Mol Cancer Ther. 2008;7:659–669. doi: 10.1158/1535-7163.MCT-07-0480. [DOI] [PubMed] [Google Scholar]
- 25.Ohlson N, Wikström P, Stattin P, et al. Cell proliferation and apoptosis in prostate tumors and adjacent non-malignant prostate tissue in patients at different time-points after castration treatment. Prostate. 2005;62:307–315. doi: 10.1002/pros.20139. [DOI] [PubMed] [Google Scholar]
- 26.Hirano D, Okada Y, Minei S, et al. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. Eur Urol. 2004;45:586–592. doi: 10.1016/j.eururo.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 27.Kojima S, Inahara M, Suzuki H, et al. Implications of insulin-like growth factor-I for prostate cancer therapies. Int J Urol. 2009;16:161–167. doi: 10.1111/j.1442-2042.2008.02224.x. [DOI] [PubMed] [Google Scholar]
- 28.Pandini G, Mineo R, Frasca F, et al. Androgens up-regulate the insulin-like growth factor-I receptor in prostate cancer cells. Cancer Res. 2005;65:1849–1857. doi: 10.1158/0008-5472.CAN-04-1837. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan PJ, Mohan S, Cohen P, et al. The insulin-like growth factor axis and prostate cancer: Lessons from the transgenic adenocarcinoma of mouse prostate (TRAMP) model. Cancer Res. 1999;59:2203–2209. [PubMed] [Google Scholar]
- 30.Chott A, Sun Z, Morganstern D, et al. Tyrosine kinases expressed in vivo by human prostate cancer bone marrow metastases and loss of the type 1 insulin-like growth factor receptor. Am J Pathol. 1999;155:1271–1279. doi: 10.1016/S0002-9440(10)65229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Locke JA, Guns ES, Lubik AA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–6415. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 32.Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst. 2001;93:1687–1697. doi: 10.1093/jnci/93.22.1687. [DOI] [PubMed] [Google Scholar]
- 33.Linja MJ, Savinainen KJ, Saramäki OR, et al. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61:3550–3555. [PubMed] [Google Scholar]
- 34.Planz B, Wang Q, Kirley SD, et al. Androgen responsiveness of stromal cells of the human prostate: Regulation of cell proliferation and keratinocyte growth factor by androgen. J Urol. 1998;160:1850–1855. doi: 10.1016/s0022-5347(01)62431-5. [DOI] [PubMed] [Google Scholar]
- 35.Nakano K, Fukabori Y, Itoh N, et al. Androgen-stimulated human prostate epithelial growth mediated by stromal-derived fibroblast growth factor-10. Endocr J. 1999;46:405–413. doi: 10.1507/endocrj.46.405. [DOI] [PubMed] [Google Scholar]
- 36.Le H, Arnold JT, McFann KK, et al. DHT and testosterone, but not DHEA or E2, differentially modulate IGF-I, IGFBP-2, and IGFBP-3 in human prostatic stromal cells. Am J Physiol Endocrinol Metab. 2006;290:E952–E960. doi: 10.1152/ajpendo.00451.2005. [DOI] [PubMed] [Google Scholar]
- 37.Levine AC, Liu XH, Greenberg PD, et al. Androgens induce the expression of vascular endothelial growth factor in human fetal prostatic fibroblasts. Endocrinology. 1998;139:4672–4678. doi: 10.1210/endo.139.11.6303. [DOI] [PubMed] [Google Scholar]
- 38.Niu Y, Altuwaijri S, Lai KP, et al. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proc Natl Acad Sci U S A. 2008;105:12182–12187. doi: 10.1073/pnas.0804700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 40.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Owen CP. 17α-hydroxylase/17,20-lyase (p45017α) inhibitors in the treatment of prostate cancer: A review. Anticancer Agents Med Chem. 2009;9:613–626. doi: 10.2174/187152009788680046. [DOI] [PubMed] [Google Scholar]
- 42.Taichman RS, Loberg RD, Mehra R, et al. The evolving biology and treatment of prostate cancer. J Clin Invest. 2007;117:2351–2361. doi: 10.1172/JCI31791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prins GS, Putz O. Molecular signaling pathways that regulate prostate gland development. Differentiation. 2008;76:641–659. doi: 10.1111/j.1432-0436.2008.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang L, Xie G, Fan Q, et al. Activation of the hedgehog-signaling pathway in human cancer and the clinical implications. Oncogene. 2010;29:469–481. doi: 10.1038/onc.2009.392. [DOI] [PubMed] [Google Scholar]
- 45.Shaw A, Bushman W. Hedgehog signaling in the prostate. J Urol. 2007;177:832–838. doi: 10.1016/j.juro.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 46.Karhadkar SS, Bova GS, Abdallah N, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–712. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 47.Efstathiou E, Troncoso P, Wen S, et al. Coordinated modulation of sonic hedgehog (Shh) signaling and androgen receptor (AR) in the prostate tumor microenvironment by chemo-hormonal therapy. J Clin Oncol. 2007;25(suppl):251s. abstr 5066. [Google Scholar]
- 48.Stewart RJ, Panigrahy D, Flynn E, et al. Vascular endothelial growth factor expression and tumor angiogenesis are regulated by androgens in hormone responsive human prostate carcinoma: Evidence for androgen dependent destabilization of vascular endothelial growth factor transcripts. J Urol. 2001;165:688–693. doi: 10.1097/00005392-200102000-00095. [DOI] [PubMed] [Google Scholar]
- 49.Jain RK, Safabakhsh N, Sckell A, et al. Endothelial cell death, angiogenesis, and microvascular function after castration in an androgen-dependent tumor: Role of vascular endothelial growth factor. Proc Natl Acad Sci U S A. 1998;95:10820–10825. doi: 10.1073/pnas.95.18.10820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mazzucchelli R, Montironi R, Santinelli A, et al. Vascular endothelial growth factor expression and capillary architecture in high-grade PIN and prostate cancer in untreated and androgen-ablated patients. Prostate. 2000;45:72–79. doi: 10.1002/1097-0045(20000915)45:1<72::aid-pros9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 51.Sweeney CJ, Miller KD, Sissons SE, et al. The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res. 2001;61:3369–3372. [PubMed] [Google Scholar]