Abstract
There is much interest in the potential of dietary antioxidants to attenuate in vivo oxidative stress, but little characterization of the time course of plasma effects exists. Culinary spices have demonstrated potent in vitro antioxidant properties. The objective of this study was to examine whether adding 14 g of a high antioxidant spice blend to a 5060-kJ (1200 kcal) meal exerted significant postprandial effects on markers of plasma antioxidant status and metabolism. Healthy overweight men (n = 6) consumed a control and spiced meal in a randomized crossover design with 1 wk between testing sessions. Blood was sampled prior to the meal and at 30-min intervals for 3.5 h (total of 8 samples). Mixed linear models demonstrated a treatment × time interaction (P < 0.05) for insulin and TG, corresponding with 21 and 31% reductions in postprandial levels with the spiced meal, respectively. Adding spices to the meal significantly increased the ferric reducing antioxidant power, such that postprandial increases following the spiced meal were 2-fold greater than after the control meal (P = 0.009). The hydrophilic oxygen radical absorbance capacity (ORAC) of plasma also was increased by spices (P = 0.02). There were no treatment differences in glucose, total thiols, lipophilic ORAC, or total ORAC. The incorporation of spices into the diet may help normalize postprandial insulin and TG and enhance antioxidant defenses.
Introduction
Oxidative stress is an underlying cause of many chronic diseases (1). In addition to direct oxidative modifications of proteins, lipids, and DNA, amplified oxidative stress promotes inflammation (2, 3) and metabolic derangements (4). Thus, the USDA has generated extensive databases of the antioxidant activity of foods, more specifically, the oxygen radical absorbance capacity (ORAC)8 (5, 6). Because antioxidant values derived from in vitro assays do not consider absorption, metabolism, disposition, and excretion, more research is warranted to examine the in vivo bioactivity of antioxidant-rich foods.
Based on the USDA ORAC database, spices have among the highest ORAC values on a per-gram basis (5) and are potent sources of phenolic antioxidants (7). Most high antioxidant spices also have demonstrated potential benefits on glucose, lipid, and inflammatory homeostasis (8–10), which adds to their appeal as a source of dietary antioxidants. If the increased intake of spice antioxidants translates to in vivo effects, this would have important implications for nutrition recommendations. In fact, recent research has demonstrated that spices can attenuate the production of lipid peroxidation products in vitro and in vivo when they are added to ground beef (11), supporting the hypothesis that in vitro effects translate to demonstrable benefits in humans.
Studies examining the acute in vivo plasma antioxidant effects of foods have been performed with tea (12), fruit and fruit juices (13–15), and nuts (16). Results are limited by lack of time course information and absence of control conditions in some cases. However, these studies have demonstrated that much of the antioxidant potential of plant foods is derived from phenolic compounds rather than micronutrient vitamins and that combining these compounds results in synergistic increases in plasma antioxidant status (17, 18). Spices typically are consumed as blends, and some spice compounds increase the plasma concentrations of others (19), making spice blends well suited for plasma antioxidant studies.
Therefore, the purpose of this study was to examine the postprandial plasma changes caused by consuming a single large dose (14 g) of a high antioxidant spice blend incorporated into a standardized test meal relative to that same meal without spices. Our aims were to characterize the time course of plasma changes on multiple measures of in vivo plasma antioxidant measures [ORAC, ferric reducing antioxidant power (FRAP), and total thiol] and metabolism (glucose and lipid homeostasis) and to evaluate palatability and potential gastrointestinal effects of the experimental meal. Similar to previous research in this area, we chose to study a small, homogeneous sample and use a crossover design to minimize between-participant variability in characterizing these effects (11, 13, 20). We employed intensive sampling (every 30 min) to provide a detailed time course of postprandial plasma changes.
Participants and Methods
Study population
Men (n = 6) aged 30–65 y who were free of any serious illness, did not use tobacco products, and were not taking any medications or nutritional supplements were recruited for the study. Other inclusion characteristics were BMI (in kg/m2) of 25–27, resting blood pressure < 160/100 mm Hg, and general good health (by self-reported medical history and screening blood work). Healthy, overweight men were selected as the study cohort in an effort to minimize between-participant variability while still allowing a chance to observe metabolic benefits should they exist. A complete blood count and standard chemistry panel were obtained at screening to rule out the presence of illness (autoimmune disease, cancer, and immunodeficiency). Blood pressure was measured according to Joint National Committee 7 guidelines (21).
Recruitment and ethical aspects
Participants were recruited through fliers in the community and campus E-mail lists. Potential participants called to indicate interest in participating in the study. They were given information about the study (including a description of the large dose of spices) and, if interested, were asked a series of medical and lifestyle questions. Qualified respondents were scheduled for clinic screening at the Penn State General Clinical Research Center. After written informed consent was provided, a blood sample was drawn for a complete blood count and general health profile (lipid panel, glucose, liver and kidney function). Body weight and height were measured to calculate BMI. A balanced randomization scheme was developed in advance and participants were assigned to a treatment sequence at enrollment. The study protocol was approved by the Institutional Review Board of the Pennsylvania State University.
Design and intervention
A randomized, controlled, 2-period crossover study with 1 wk separation between testing sessions was conducted. The 2 test conditions were a control meal (Table 1) and the control meal with added spice (Table 2). The 5060-kJ (1200 kcal) control meal consisted of a coconut chicken and white rice dish, cheese bread, and dessert biscuit (Table 1). Spices were obtained from the McCormick Science Institute Characterized Samples Program (McCormick Science Institute) and weighed with a balance accurate to 1/100 of a gram (Mettler Toledo). For the spiced test meal, the 14 g of spices were added to these meal items to create a chicken curry, Italian herb bread, and cinnamon biscuit (Table 2). The meal components were weighed in advance and prepared fresh on each day of testing. The spice blend (Table 2) was modified from the blend used by Li et al. (11), who provided 11.3 g spice in a 250-g burger patty and had reduced plasma and urine lipid oxidation products (i.e. malondialdehyde). The dose was increased by providing additional meal items (bread and dessert).
TABLE 1.
Control meal composition
| Energy, kJ | Fat, g | Carbohydrate, g | Protein, g | Sodium, mg | |
| Dessert biscuit | 960 | 10 | 31 | 4 | 604 |
| Coconut chicken | 2270 | 22 | 48 | 38 | 752 |
| Cheese bread | 1830 | 17 | 50 | 21 | 865 |
| Total | 5060 | 49 | 128 | 63 | 2221 |
TABLE 2.
Characteristics of the spice blend that was added to create the spiced meal
| Spice | Dessert biscuit, g | Coconut chicken, g | Cheese bread, g | Total dose, g | H-ORAC contribution,1μmol TE | L-ORAC contribution,1μmol TE | Phenolic contribution,2mg GAE |
| Black pepper | 0.45 | 0.45 | 0.91 | 130 | 122 | 8 | |
| Cinnamon | 0.38 | 0.23 | 0.61 | 1611 | 21 | 64 | |
| Cloves | 0.30 | 0.31 | 0.61 | 935 | 983 | 133 | |
| Garlic powder | 0.91 | 0.90 | 1.81 | 118 | 3 | NA3 | |
| Ginger | 0.38 | 0.75 | 1.51 | 105 | 330 | 15 | |
| Oregano (Mediterranean) | 1.13 | 1.13 | 2.26 | 4139 | 384 | 168 | |
| Paprika | 1.43 | 1.42 | 2.85 | 459 | 52 | 41 | |
| Rosemary | 0.61 | 0.61 | NA | NA | 30 | ||
| Turmeric | 2.09 | 0.70 | 2.79 | 1110 | 3330 | 95 | |
| Total | 14 | 8610 | 5220 | 554 |
ORAC values (in vitro) are from the USDA 2007 Report (5). Values for rosemary were not available in this report. Abbreviations: H-ORAC, hydrophilic ORAC; L-ORAC, lipophilic ORAC.
Total phenolic compounds were evaluated by the McCormick Science Institute under the characterized sample program. Values for garlic were not available. Contribution of ORAC and phenolic compounds was calculated by weight using the values reported in the table.
NA, not available.
Participants were instructed to avoid high antioxidant foods (including all spices) for 48 h prior to testing and kept a dietary record for these 48 h (Supplemental Table 1). Four hours prior to testing, the men consumed a low antioxidant control meal (that was provided) and water ad libitum at home. The controlled low antioxidant breakfast was a commercially available, vanilla-flavored toaster pastry providing 1700 kJ (400 kcal) and 12 g fat, primarily as enriched flour, corn syrup, and vegetable oil. Participants were instructed not to consume any other foods or beverages (besides water) for 12 h prior to testing. The test meal was not provided after a 12-h fast for practical reasons: the amount of food and strong flavors are not typical of breakfast items and this study was designed to inform future research in which the intervention would be provided at midday.
Participants reported to the clinic around noon to have their dietary records reviewed. The records were reviewed to verify compliance with the low antioxidant diet restrictions prior to testing. At the first visit, participants were provided a photocopy of their diet records and instructed to mimic their food intake in preparation for the second visit. Following verification of compliance and absence of acute illness (bacterial or viral infection), an i.v. catheter was inserted and a baseline blood sample obtained.
Participants were allowed 30 min to consume the meal and afterwards completed a questionnaire about the palatability of the meal. The 4-item questionnaire asked participants to rate fullness, satisfaction, enjoyment, and flavors on a scale of 1–10. Participants also were asked open-ended questions about what they liked and did not like about the meal so that it could be improved for future studies.
The first postprandial blood sample was collected at the end of the 30-min eating period. Subsequently, blood was collected every 30 min until a total of 8 samples were collected. For each sample, blood was centrifuged and portions of serum and plasma were saved for analysis.
Assay methods
Serum measures.
Whole blood was drawn into serum separator tubes, allowed to clot for 30 min, and centrifuged according to guidelines. Total cholesterol (C) and TG were measured by enzymatic procedures (Quest Diagnostics; CV < 2% for both). HDL-C was estimated according to the modified heparin-manganese procedure (CV < 2%). LDL-C was not reported, because calculated values are not accurate during postprandial conditions. Insulin was measured by RIA (Quest Diagnostics). Glucose was determined by an immobilized enzyme biosensor using the YSI 2300 STAT Plus Glucose and Lactate Analyzer (Yellow Springs Instruments). Serum high-sensitivity C-reactive protein (hs-CRP) was measured by latex-enhanced immunonephelometry (Quest Diagnostics; assay CV < 8%).
Plasma hydrophilic and lipophilic ORAC.
Plasma samples were analyzed for hydrophilic, lipophilic, and total ORAC according to previously published methods (Brunswick Labs) (22–25).
FRAP assay.
The FRAP assay was used to determine the reducing ability of plasma in a redox-linked colorimetric reaction using methods modified from Benzie and Strain (26). Plasma was incubated at room temperature with the FRAP reagent and the absorbance recorded after 1 h, and Trolox was used to construct a standard curve to calculate the FRAP value of the unknown samples (27).
Total thiol levels in plasma.
Total thiol levels in plasma were determined according to the method of Hu (28). Briefly, an aliquot of EDTA plasma (0.20 mL) was mixed with 0.6 mL of the Tris-EDTA buffer followed by addition of 40 μL of 10 mmol/L 2,2-dithiobisnitrobenzoic acid and 3.16 mL of absolute methanol. After incubation at room temperature for 15 min and centrifugation at 3000 g for 10 min at room temperature, the absorbance of the supernatant was measured at 412 nm. The final result is expressed as mmol/L.
Statistical analyses
Statistical analyses were performed using SAS (version 9.2; SAS Institute). The mixed models procedure (PROC MIXED) was used to test the effects of treatment, time point, and their interaction on changes in outcomes following the meal, and α was set at 0.05 for all tests. Model selection was based on optimizing fit statistics (evaluated as lowest Bayesian information criterion). Means are reported as least-squares means ± SEM. For metabolic endpoints, outcomes were modeled as doubly repeated measures with unstructured by compound symmetry for time point and visits, respectively. For antioxidant endpoints, we imposed a compound symmetry structure by designating participant as random effect. Baseline values were included as covariates. Change scores were calculated by subtracting premeal baseline values from each time point. AUC values were calculated using the trapezoidal rule using the premeal baseline values as the line of reference (29). Sample size was selected based on earlier studies (13, 22). Because we could not identify any previous studies that measured changes in plasma ORAC after consumption of spices, we based sample size calculations on plasma ORAC changes reported after consumption of blueberries, a food that has high in vitro ORAC values. Prior et al. (22) fed 6 healthy participants 89 g blueberries and reported a 150-μmol/L TE increase in hydrophilic-ORAC. Based on comparisons of in vitro ORAC values for spices, we anticipated that the in vivo antioxidant effect of the spice blend would be equal or superior to the response to blueberries. A sample size of 6 was estimated to provide >80% power to detect a difference of 150 μmol/L TE hydrophilic-ORAC with a SD of 100 and 2-tailed α = 0.05.
Results
The screening and visit baseline characteristics of the participants are presented in Tables 3 and 4, respectively. All participants completed both visits and were able to consume the meal in <30 min. The meals were well tolerated (no incidence of gastrointestinal effects) and participants rated the control meal and spiced meals similarly in response to the meal questionnaire about fullness, satiety, enjoyment, and flavors (Table 5).
TABLE 3.
Anthropometrics, blood pressure, and serum biochemistry of the men who participated in the study1,2
| Variable | |
| Age, y | 44 ± 4.9 |
| Height, m | 1.8 ± 0.03 |
| Weight, kg | 85.7 ± 3.5 |
| BMI, kg/m2 | 26.4 ± 0.4 |
| SBP, mm Hg | 115 ± 3.4 |
| DBP, mm Hg | 78.5 ± 3.5 |
| TC, mmol/L | 5.03 ± 0.4 |
| HDL-C, mmol/L | 1.20 ± 0.06 |
| LDL-C, mmol/L | 3.08 ± 0.3 |
| TG, mmol/L | 1.63 ± 0.2 |
| Glucose, mmol/L | 5.22 ± 0.2 |
Values are presented as means ± SEM, = 6.
Blood was drawn after a 12-h fast.
TABLE 4.
Circulating lipids, glucose, insulin, and indicators of antioxidant status in the men prior to consuming each meal12
| Control | Spice | |
| TC, mmol/L | 5.0 ± 0.4 | 5.1 ± 0.4 |
| HDL-C, mmol/L | 1.2 ± 0.1 | 1.1 ± 0.1 |
| LDL-C, mmol/L | 3.1 ± 0.3 | 3.1 ± 0.3 |
| TG, mmol/L | 1.6 ± 0.4 | 2.0 ± 0.4 |
| Glucose, mmol/L | 5.1 ± 0.3 | 4.8 ± 0.3 |
| Insulin, pmol/L | 54 ± 20 | 39 ± 20 |
| Hs-CRP, mg/L | 1.6 ± 0.5 | 1.3 ± 0.5 |
| Total ORAC,3TE | 9930 ± 915 | 10900 ± 915 |
| Lipophilic ORAC, TE | 321 ± 138 | 620 ± 138 |
| Hydrophilic ORAC, TE | 4010 ± 403 | 4340 ± 403 |
| FRAP, μmol TE/L | 482 ± 28 | 480 ± 28 |
| Total thiol, mmol/L | 330 ± 17 | 317 ± 17 |
Values are presented as means ± SEM, = 6.
Premeal baseline values did not significantly differ between treatments.
TE, Trolox equivalents.
TABLE 5.
| Full | Satisfied | Enjoy | Flavors | |
| Control | 8.2 ± 0.5 | 7.3 ± 0.9 | 6.5 ± 0.7 | 5.7 ± 0.9 |
| Spice | 7.7 ± 0.5 | 7.8 ± 0.9 | 6.7 ± 0.7 | 6.5 ± 0.9 |
Values are mean ± SEM, = 6. All comparisons between the treatments were nonsignificant.
Following the meal, participants were asked to rate the meal on a scale of 1–10 (most) in response to the following questions: How full are you? How satisfied are you? How much did you enjoy the meal? How much did you like the flavors of the meal?
Postprandial metabolism and CRP.
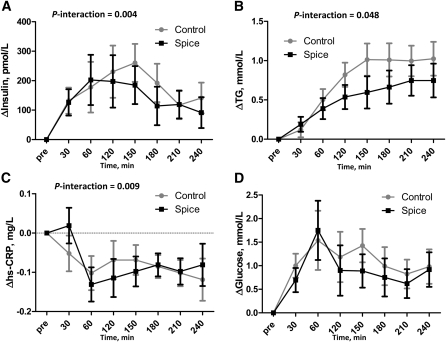
The addition of spices significantly attenuated postprandial insulin and TG responses to the meal (Fig. 1A,B); however, there were no effects on glucose (Fig. 1D). The significant treatment × time point interaction resulted in a 21% reduction in postprandial insulin AUC and a 31% reduction in TG AUC for the spiced meal relative to control. Total and HDL-C concentrations were not affected by treatment (Supplemental Fig. 1).
FIGURE 1.
Changes in serum concentrations of insulin (A), TG (B), hs-CRP (C), and glucose (D) after consumption of control and spice test meals in healthy men. Means are reported as least-squares means ± SEM, n = 6. Change scores were calculated by subtracting premeal baseline values from each time point. In graphs with no statistical annotations, there were no significant effects of treatment × time point or treatment as a main effect. No post hoc comparisons at individual time points were significant after Bonferroni adjustment for multiple comparisons.
There was a significant treatment × time point interaction for hs-CRP that appeared to be due to an increase immediately after the spice meal and then decrease at later time points (Fig. 1C). In fact, the overall postprandial means for serum hs-CRP were the same after the 2 meals (1.36 and 1.36 ± 0.03 mg/L) and concentrations did not differ at any time point.
Plasma antioxidant potential.
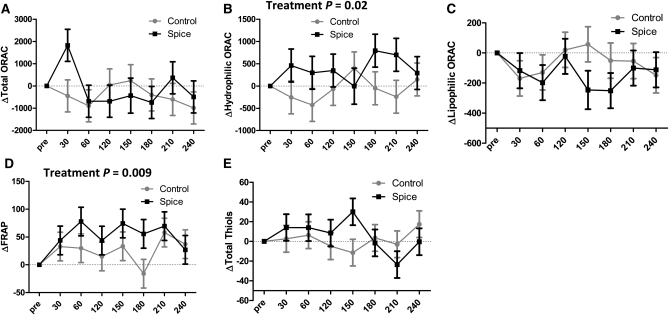
Hydrophilic ORAC levels were 13% higher across time points following the spice meal (P = 0.02), whereas lipophilic and total ORAC were not changed overall (Fig. 2A–C). Total ORAC appeared to increase immediately following the meal and then returned to baseline (Fig. 2A). Our a priori statistical plan was not designed to test this individual time point, but an unadjusted, exploratory t test demonstrated that 22% difference was significant (P = 0.02). However, this effect was also observed for total C concentrations for this first postprandial sample (unadjusted P = 0.006) (Supplemental Fig. 1), suggesting that nonspecific changes in plasma volume may be driving these effects. Postprandial increases in FRAP were 2-fold higher following the spiced meal (P = 0.009) (Fig. 2D). Plasma total thiol concentrations were unaffected by treatment (Fig. 2E).
FIGURE 2.
Changes in plasma antioxidant status as measured by ORAC (A–C), FRAP (D), and total thiol (E) after consumption of control and spice test meals in healthy men. Means are reported as least-squares means ± SEM, n = 6. Change scores were calculated by subtracting premeal baseline values from each time point. In graphs with no statistical annotations, there were no significant effects of treatment by time point or treatment as a main effect. No post hoc comparisons at individual time points were significant after Bonferroni adjustment for multiple comparisons.
Discussion
This study was designed primarily to observe time course changes in plasma antioxidant measures, and we found significant increases in FRAP and hydrophilic ORAC following spice consumption. We also observed unexpected reductions in postprandial TG and insulin (treatment × time P-interaction < 0.05) without concurrent effects on glucose concentrations.
These significant effects were likely a result of the high concentration of phenolic antioxidants in spices. It has been estimated that mean daily intake of polyphenols is ~1 g for most people (30, 31). The spice dose that we examined provided an acute dose of polyphenols (554 gallic acid equivalents) that is similar to 150 mL (5 oz) red wine (32), 240 mL (8 oz) blueberry or acai juice (32), or 40 g dark chocolate (typical commercial candy bar size) (33), and the results may be hypothesis-generating for research on these products as well.
We are not aware of prior reports on spices and postprandial TG, but our findings are consistent for effects observed with tea (34, 35). Potential mechanisms include delayed gastric emptying and direct inhibition of pancreatic lipases (36). Future studies will explore this finding.
We observed an attenuation of insulin responses consistent with earlier studies of cinnamon (37), but we did not see a reduction in glucose (38). Polyphenols may improve insulin sensitivity (39–41). Our participants had large insulin and TG responses to the meal, but their glucose responses were relatively low. Peak postprandial glucose values were <6.8 mmol/L (122 mg/dL). Mean fasting values were 5.2 mmol/L (94 mg/dL), so this is a relatively small glycemic response to the meal. In participants who have developed insulin resistance or type 2 diabetes, the effects may be different (42).
We employed multiple assays to determine changes in antioxidant activity in plasma and detected significant increases in FRAP and hydrophilic ORAC (P < 0.05). The ORAC assay assesses the hydrogen atom-donating capability of antioxidants, the FRAP assay evaluates the electron-transferring capability of antioxidants (43), and the total thiol assay determines antioxidants with sulfhydryl groups. An increase in hydrophilic ORAC was observed, reflecting polyphenolic activity in this plasma fraction, but plasma lipophilic ORAC and total ORAC values were not improved by spices. FRAP increased after the meal, but total thiol levels were unaffected. The increased plasma antioxidant capacity (FRAP and hydrophilic ORAC) following consumption of spices is consistent with the results of similarly powered studies of fruits (13, 14, 22, 44) and tea (12, 45), but we did not observe an increase in lipophilic ORAC that has been detected 2 h following blueberry or pecan consumption (16, 22).
A strength of this study is the use of the control meal to directly compare postprandial responses with and without spices added to the meal and the use of intensive time course sampling during the postprandial period. We incorporated the spice blend into food items and did not use capsules, which enables direct, real world application of the results and a more translatable dietary message. The multiple time course measures that we used to characterize the postprandial state fill a gap in the literature, because there has been very little human data reported in this area.
However, an important limitation was our small sample size (n = 6), consistent with prior studies (11, 13–15). Follow-up work is needed to characterize these effects in larger sample sizes and more diverse populations, including people with cardiovascular risk factors. We did not incorporate cell-based protection assays (e.g. cell-based antioxidant protection in erythrocytes) or markers of lipid oxidation (e.g. TBARS, malondialdehyde, or oxidized LDL), which would have added further insight to the findings. The decision to test the spices as a blend limits interpretation and the observed effects cannot be attributed to any individual spice. Also, it was not possible to have participants blinded to the intervention during testing, although we are not aware of psychological effects for these variables. Further, FRAP, and potentially other plasma antioxidant measures, is affected by plasma concentrations of uric acid, vitamin C, and other confounding factors that we did not measure. However, the use of a crossover design may minimize these effects.
There were significant improvements in postprandial TG, insulin, hydrophilic ORAC, and FRAP following a meal containing 14 g of high antioxidant spices. This very high dose of spices was used for proof of concept. Future studies should assess whether lower doses also result in changes in these endpoints. Additional work may clarify whether certain spices within the blend are more potent than others with respect to these outcomes.
Studies also should examine whether postprandial antioxidant effects result in changes in intracellular gene expression. This would be relevant especially in response to oxidative and inflammatory challenge, because activation of the inflammatory transcription factor NF-κB is redox sensitive (3, 46–48).
In conclusion, dietary surveys typically do not assess intake of culinary spices and thus there are few epidemiological data on spice consumption and incidence of chronic disease. However, we have demonstrated that spices significantly decrease the magnitude of postprandial increases in circulating TG and insulin with favorable effects on plasma antioxidant status (hydrophilic ORAC and FRAP). Therefore, the incorporation of spices into the daily diet may help normalize postprandial disturbances in glucose and lipid homeostasis while enhancing antioxidant defense.
Supplementary Material
Acknowledgments
The meal used to deliver the spice blend was developed and prepared by Amy Ciccarella, Mary Lou Kiel, and Sami Heim. Excellent statistical consultation was provided by Trent Gaugler of the Penn State Statistical Consulting Center. We are grateful for use of the General Clinical Research Center of the Pennsylvania State University. A.C.S-R., P.M.K-E., S.G.W., and J.P.V.H. designed the research; A.C.S-R. and D.L.T. conducted the research; C-Y.O.C. selected and performed the FRAP and thiol measures; A.C.S-R. and S.G.W. performed the statistical analysis; and A.C.S-R .and S.G.W. wrote the manuscript. All authors read and approved the final manuscript.
Footnotes
Supported by the McCormick Science Institute, which had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. The General Clinical Research Center of the Pennsylvania State University was supported by NIH grant M01 RR 10732.
Supplemental Table 1 and Figure 1 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at jn.nutrition.org.
Abbreviations used: C, cholesterol; FRAP, ferric reducing antioxidant potential; hs-CRP, high-sensitivity C-reactive protein; ORAC, oxygen radical absorbance capacity.
Literature Cited
- 1.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84 [DOI] [PubMed] [Google Scholar]
- 2.Yoon JH, Baek SJ. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Med J. 2005;46:585–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Berg R, Haenen GR, van den Berg H, Bast A. Transcription factor NF-kappaB as a potential biomarker for oxidative stress. Br J Nutr. 2001;86 Suppl 1:S121–7 [DOI] [PubMed] [Google Scholar]
- 4.Biesalski HK. Polyphenols and inflammation: basic interactions. Curr Opin Clin Nutr Metab Care. 2007;10:724–8 [DOI] [PubMed] [Google Scholar]
- 5.USDA NDL Oxygen radical absorbance capacity (ORAC) of selected foods 2007. Beltsville (MD): Beltsville Human Nutrition Research Center; 2007 [Google Scholar]
- 6.Halvorsen BL, Carlsen MH, Phillips KM, Bohn SK, Holte K, Jacobs DR, Jr, Blomhoff R. Content of redox-active compounds (ie, antioxidants) in foods consumed in the United States. Am J Clin Nutr. 2006;84:95–135 [DOI] [PubMed] [Google Scholar]
- 7.Shan B, Cai YZ, Sun M, Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agric Food Chem. 2005;53:7749–59 [DOI] [PubMed] [Google Scholar]
- 8.Jagetia GC, Aggarwal BB. "Spicing up" of the immune system by curcumin. J Clin Immunol. 2007;27:19–35 [DOI] [PubMed] [Google Scholar]
- 9.Tapsell LC, Hemphill I, Cobiac L, Patch CS, Sullivan DR, Fenech M, Roodenrys S, Keogh JB, Clifton PM, et al. Health benefits of herbs and spices: the past, the present, the future. Med J Aust. 2006;185:S4–24 [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal B, Van Kuiken ME, Iyer LH, Harikumar KB, Sung B. Molecular targets of nutraceuticals derived from dietary spices: potential role in suppression of inflammation and tumorigenesis. Exp Biol Med (Maywood). 2009;234:825–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Henning SM, Zhang Y, Zerlin A, Li L, Gao K, Lee RP, Karp H, Thames G, et al. Antioxidant-rich spice added to hamburger meat during cooking results in reduced meat, plasma, and urine malondialdehyde concentrations. Am J Clin Nutr. 2010;91:1180–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rietveld A, Wiseman S. Antioxidant effects of tea: evidence from human clinical trials. J Nutr. 2003;133:S3285–92 [DOI] [PubMed] [Google Scholar]
- 13.Prior RL, Gu L, Wu X, Jacob RA, Sotoudeh G, Kader AA, Cook RA. Plasma antioxidant capacity changes following a meal as a measure of the ability of a food to alter in vivo antioxidant status. J Am Coll Nutr. 2007;26:170–81 [DOI] [PubMed] [Google Scholar]
- 14.Mazza G, Kay CD, Cottrell T, Holub BJ. Absorption of anthocyanins from blueberries and serum antioxidant status in human subjects. J Agric Food Chem. 2002;50:7731–7 [DOI] [PubMed] [Google Scholar]
- 15.Vinson JA, Bose P, Proch J, Al Kharrat H, Samman N. Cranberries and cranberry products: powerful in vitro, ex vivo, and in vivo sources of antioxidants. J Agric Food Chem. 2008;56:5884–91 [DOI] [PubMed] [Google Scholar]
- 16.Hudthagosol C, Haddad EH, McCarthy K, Wang P, Oda K, Sabate J. Pecans acutely increase plasma postprandial antioxidant capacity and catechins and decrease LDL oxidation in humans. J Nutr. 2011;141:56–62 [DOI] [PubMed] [Google Scholar]
- 17.Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003;78:S517–20 [DOI] [PubMed] [Google Scholar]
- 18.Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr. 2004;134:S3479–85 [DOI] [PubMed] [Google Scholar]
- 19.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–18 [DOI] [PubMed] [Google Scholar]
- 20.Cao G, Booth SL, Sadowski JA, Prior RL. Increases in human plasma antioxidant capacity after consumption of controlled diets high in fruit and vegetables. Am J Clin Nutr. 1998;68:1081–7 [DOI] [PubMed] [Google Scholar]
- 21.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72 [DOI] [PubMed] [Google Scholar]
- 22.Prior RL, Hoang H, Gu L, Wu X, Bacchiocca M, Howard L, Hampsch-Woodill M, Huang D, Ou B, et al. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC(FL))) of plasma and other biological and food samples. J Agric Food Chem. 2003;51:3273–9 [DOI] [PubMed] [Google Scholar]
- 23.Cao G, Alessio HM, Cutler RG. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic Biol Med. 1993;14:303–11 [DOI] [PubMed] [Google Scholar]
- 24.Cao G, Prior RL. Measurement of oxygen radical absorbance capacity in biological samples. Methods Enzymol. 1999;299:50–62 [DOI] [PubMed] [Google Scholar]
- 25.Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001;49:4619–26 [DOI] [PubMed] [Google Scholar]
- 26.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996;239:70–6 [DOI] [PubMed] [Google Scholar]
- 27.Chen CY, Blumberg JB. In vitro activity of almond skin polyphenols for scavenging free radicals and inducing quinone reductase. J Agric Food Chem. 2008;56:4427–34 [DOI] [PubMed] [Google Scholar]
- 28.Hu ML. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994;233:380–5 [DOI] [PubMed] [Google Scholar]
- 29.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhnau J. The flavonoids. A class of semi-essential food components: their role in human nutrition. World Rev Nutr Diet. 1976;24:117–91 [PubMed] [Google Scholar]
- 31.Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130:S2073–85 [DOI] [PubMed] [Google Scholar]
- 32.Seeram NP, Aviram M, Zhang Y, Henning SM, Feng L, Dreher M, Heber D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J Agric Food Chem. 2008;56:1415–22 [DOI] [PubMed] [Google Scholar]
- 33.Miller KB, Stuart DA, Smith NL, Lee CY, McHale NL, Flanagan JA, Ou B, Hurst WJ. Antioxidant activity and polyphenol and procyanidin contents of selected commercially available cocoa-containing and chocolate products in the United States. J Agric Food Chem. 2006;54:4062–8 [DOI] [PubMed] [Google Scholar]
- 34.Hsu TF, Kusumoto A, Abe K, Hosoda K, Kiso Y, Wang MF, Yamamoto S. Polyphenol-enriched oolong tea increases fecal lipid excretion. Eur J Clin Nutr. 2006;60:1330–6 [DOI] [PubMed] [Google Scholar]
- 35.Kurihara H, Shibata H, Fukui Y, Kiso Y, Xu JK, Yao XS, Fukami H. Evaluation of the hypolipemic property of Camellia sinensisVar. ptilophylla on postprandial hypertriglyceridemia. J Agric Food Chem. 2006;54:4977–81 [DOI] [PubMed] [Google Scholar]
- 36.Wang S, Noh SK, Koo SI. Green tea catechins inhibit pancreatic phospholipase A(2) and intestinal absorption of lipids in ovariectomized rats. J Nutr Biochem. 2006;17:492–8 [DOI] [PubMed] [Google Scholar]
- 37.Hlebowicz J, Hlebowicz A, Lindstedt S, Bjorgell O, Hoglund P, Holst JJ, Darwiche G, Almer LO. Effects of 1 and 3 g cinnamon on gastric emptying, satiety, and postprandial blood glucose, insulin, glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1, and ghrelin concentrations in healthy subjects. Am J Clin Nutr. 2009;89:815–21 [DOI] [PubMed] [Google Scholar]
- 38.O'Keefe JH, Gheewala NM, O'Keefe JO. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J Am Coll Cardiol. 2008;51:249–55 [DOI] [PubMed] [Google Scholar]
- 39.Stote KS, Baer DJ. Tea consumption may improve biomarkers of insulin sensitivity and risk factors for diabetes. J Nutr. 2008;138:S1584–8 [DOI] [PubMed] [Google Scholar]
- 40.Couturier K, Batandier C, Awada M, Hininger-Favier I, Canini F, Anderson RA, Leverve X, Roussel AM. Cinnamon improves insulin sensitivity and alters the body composition in an animal model of the metabolic syndrome. Arch Biochem Biophys. 2010;501:158–61 [DOI] [PubMed] [Google Scholar]
- 41.Solomon TP, Blannin AK. Changes in glucose tolerance and insulin sensitivity following 2 weeks of daily cinnamon ingestion in healthy humans. Eur J Appl Physiol. 2009;105:969–76 [DOI] [PubMed] [Google Scholar]
- 42.Mang B, Wolters M, Schmitt B, Kelb K, Lichtinghagen R, Stichtenoth DO, Hahn A. Effects of a cinnamon extract on plasma glucose, HbA, and serum lipids in diabetes mellitus type 2. Eur J Clin Invest. 2006;36:340–4 [DOI] [PubMed] [Google Scholar]
- 43.Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–56 [DOI] [PubMed] [Google Scholar]
- 44.Mertens-Talcott SU, Rios J, Jilma-Stohlawetz P, Pacheco-Palencia LA, Meibohm B, Talcott ST, Derendorf H. Pharmacokinetics of anthocyanins and antioxidant effects after the consumption of anthocyanin-rich acai juice and pulp (Euterpe oleracea Mart.) in human healthy volunteers. J Agric Food Chem. 2008;56:7796–802 [DOI] [PubMed] [Google Scholar]
- 45.Pecorari M, Villano D, Testa MF, Schmid M, Serafini M. Biomarkers of antioxidant status following ingestion of green teas at different polyphenol concentrations and antioxidant capacity in human volunteers. Mol Nutr Food Res. 2010;54 Suppl 2:S278–83 [DOI] [PubMed] [Google Scholar]
- 46.Haddad JJ. Pharmaco-redox regulation of cytokine-related pathways: from receptor signaling to pharmacogenomics. Free Radic Biol Med. 2002;33:907–26 [DOI] [PubMed] [Google Scholar]
- 47.Haddad JJ, Olver RE, Land SC. Antioxidant/pro-oxidant equilibrium regulates HIF-1alpha and NF-kappa B redox sensitivity. Evidence for inhibition by glutathione oxidation in alveolar epithelial cells. J Biol Chem. 2000;275:21130–9 [DOI] [PubMed] [Google Scholar]
- 48.Ma Q, Kinneer K, Ye J, Chen BJ. Inhibition of nuclear factor kappaB by phenolic antioxidants: interplay between antioxidant signaling and inflammatory cytokine expression. Mol Pharmacol. 2003;64:211–9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.