Abstract
Matrix Gla protein (MGP) is a calcification inhibitor in vascular tissue that must be carboxylated by vitamin K to function. Evidence suggests circulating uncarboxylated MGP (ucMGP) is elevated in persons with disease characterized by vascular calcification. The primary purpose of this study was to determine cross-sectional and longitudinal associations between plasma ucMGP, vitamin K status, and coronary artery calcium (CAC) in older adults without coronary heart disease. Genetic determinants of ucMGP were also explored. Cross-sectional associations among baseline plasma ucMGP, vitamin K status biomarkers [plasma phylloquinone, uncarboxylated prothrombin (PIVKA-II), serum uncarboxylated osteocalcin (%ucOC)], CAC, and plausible genetic polymorphisms were examined in 438 community-dwelling adults (60–80 y, 59% women). The effect of phylloquinone supplementation (500 μg/d) for 3 y on plasma ucMGP was determined among 374 participants. At baseline, plasma phylloquinone was lower and %ucOC and PIVKA-II were greater across higher plasma ucMGP quartiles (all P < 0.001, age-adjusted). Major allele homozygotes for MGP rs1800801 and rs4236 had higher plasma ucMGP than heterozygotes or minor allele homozygotes. (P ≤ 0.004). The decrease in plasma ucMGP was greater in the 190 participants who received phylloquinone (mean ± SD) (−345 ± 251 pmol/L) than in the 184 who did not (−40 ± 196 pmol/L) (P < 0.0001). CAC did not differ according to ucMGP quartile (P = 0.35, age-adjusted). In the phylloquinone-supplemented group, the 3-y change in ucMGP was not associated with the 3-y change in CAC [unstandard β (SE) = −0.02 (0.02); P = 0.44]. Plasma ucMGP was associated with vitamin K status biomarkers and was reduced following phylloquinone supplementation, suggesting it may be a useful marker of vitamin K status in vascular tissue. Plasma ucMGP did not reflect CAC in healthy older adults.
Introduction
Coronary artery calcium (CAC)13 predicts cardiovascular disease morbidity and mortality independent of established risk factors (1, 2). Proteins that inhibit calcification have been identified as important negative regulators of this pathology. Matrix Gla protein (MGP) is a calcification inhibitor found in vascular and other soft tissue (3, 4). In mice, targeted deletion of the MGP gene results in rapid and complete arterial calcification, resulting in death by 6 wk (5). For MGP to function, it must be partially γ-carboxylated, which requires vitamin K. MGP is synthesized in the uncarboxylated form (ucMGP), and without sufficient vitamin K, MGP remains uncarboxylated and does not inhibit calcification (6, 7). The degree of γ-carboxylation required for MGP to inhibit calcification in humans is not known. ucMGP is elevated in human sclerotic arterial tissue and the carboxylated MGP form is more abundant in healthy vascular tissue (8), which provides evidence that a lack of functional MGP increases risk for vascular calcification. In addition to being carboxylated, MGP can also undergo a post-translational phosphorylation, which is also thought to contribute to its functionality (9). The phosphorylated ucMGP accumulates in the vessel wall, whereas the dephosphorylated form is detectable in plasma (10).
Although the role of MGP in vascular calcification has been elucidated in animal models, there have been few human studies. Data are conflicting as to whether total MGP in circulation, which reflects the total pool of MGP regardless of its carboxylation status, differs between patients with known cardiovascular disease and healthy controls (11–13). Distinguishing the uncarboxylated and carboxylated fractions of MGP in the circulation may better clarify the role of the functional forms of MGP in CAC (4). Evidence suggests the amount of ucMGP in the circulation is increased among patient populations characterized by pathologic soft-tissue calcification (9, 10, 14). However, the studies that have examined the association between plasma ucMGP and vascular calcification thus far are limited to case-control comparisons or specific disease populations (9, 10, 14). To evaluate the utility of ucMGP as a predictive marker of CAC, it is necessary to examine its association with CAC in a population free of clinical disease.
In a randomized controlled trial of vitamin K supplementation, we found that older community-dwelling adults who adhered to phylloquinone (vitamin K1) supplementation had less CAC progression over 3 y (15). The assessment of ucMGP from archived baseline and postintervention blood samples from this study provided an opportunity to determine the correlates of circulating ucMGP, the effect of phylloquinone supplementation on plasma ucMGP, and the association between plasma ucMGP and CAC progression after 3 y. We hypothesized that increased circulating ucMGP would be associated with poorer vitamin K status and positively associated with CAC in older, community-dwelling adults.
The impact of MGP on regulation of calcification in humans appears to have a genetic component (16). Genotype data were available for participants in the parent study (16, 17), so the genetic determinants of plasma ucMGP were explored in a secondary analysis.
Methods
Study design and participants.
A total of 452 community-dwelling men and women (age range 60–80 y; 421 whites, 14 blacks, 4 Hispanics, 11 Asians, and 2 Native Americans) participated in a randomized controlled trial designed to determine the effect of vitamin K supplementation (500 μg/d for 3 y) on CAC and age-related bone loss, as previously described (18). Prior to enrollment, all participants completed a detailed medical history questionnaire. Participants were generally in good health and free from clinical cardiovascular disease and laboratory evidence of kidney or liver disease or osteoporosis, and not taking warfarin. All participants provided written informed consent.
Equal numbers of men and women were randomized to receive a daily multi-vitamin with 500 μg of phylloquinone or the same multi-vitamin without phylloquinone. All participants also received a second supplement that contained 600 mg of elemental calcium and 10 μg (400 iu) of cholecalciferol (15, 18). The nutrient composition of all supplements is described in detail elsewhere (15, 18). Supplements were manufactured specifically for this study by Hermes Arzeneimittel. Upon receipt, the phylloquinone content of the phylloquinone-containing supplements was (mean ± SD) 564 ± 77 μg; at 19 mo, the final phylloquinone content was 428 ± 32 μg (18).
Of the 452 participants enrolled, 438 (258 women) had measures of ucMGP and CAC at baseline and were included in cross-sectional analyses. Of these, 374 also had measures of ucMGP and CAC at follow-up and were included in longitudinal analyses (51 did not complete the study and 27 had missing CAC scans at follow-up). The participants excluded from the longitudinal analysis had significantly higher CAC, measured according to agatston score (AS; the standard scoring method for CAC) (19), at baseline [median (IQR) AS = 85 (348) vs. 27 (181); P = 0.007; based on Wilcoxon’s Rank Sum test] and slightly lower HDL cholesterol [(mean ± SD) 1.4 ± 0.3 vs. 1.5 ± 0.4 mmol/L; P = 0.05 (independent samples t test)]. Otherwise, these participants did not differ from the 374 who were included in longitudinal analysis (all P ≥ 0.10).
Biochemical measurements.
All blood samples were drawn after a 12-h fast and dedicated aliquots were stored at −80°C until the time of analysis.
MGP.
Dephosphorylated-ucMGP was measured from stored samples of citrated plasma using a sandwich ELISA, which uses 2 monoclonal antibodies directed against the nonphosphorylated sequence and noncarboxylated amino acid sequences. The reported intra- and inter-assay variability for this assay were 5.6 and 9.9%, respectively (10). Total MGP was measured using RIA, as previously described (13, 20), but does not distinguish the γ-carboxylated from ucMGP.
Vitamin K status.
Plasma phylloquinone was measured using reversed-phase HPLC (18, 21). The serum total and uncarboxylated osteocalcin (ucOC) were measured using the RIA method of Gundberg (18, 22). The uncarboxylated prothrombin, known as PIVKA-II, was measured in citrated plasma using ELISA (American Bioproducts) (17).
Scanning and analysis for CAC.
CAC was measured at baseline and after the 3-y follow-up using an 8-slice multi-detector computed-tomography scan (LightSpeed Ultra, General Electric), as previously described (13, 15). Each scan was assessed for the presence of CAC by a trained radiologist and scored using a modification of the Agatston method (19).
Genotyping.
Of the 452 participants, 406 provided additional informed consent for genotyping, as previously described in detail (16, 17). Genetic variation in the γ-glutamyl carboxylase enzyme (GGCX), which catalyzes the carboxylation of vitamin K-dependent proteins (23), was associated with the carboxylation of osteocalcin in this sample (17). Therefore, we examined the association between plasma ucMGP and the following GGCX single nucleotide polymorphisms (SNPs): rs10187424 (−5718C > T, located upstream −5718 relative to the mRNA start site), rs7568458 (405A > T, located in intron 1), rs699664 [8044A > G, a nonsynonymous (Arg > Gly) polymorphism located in exon 8], and rs2028898 (11310C > T, located in intron 14). We also explored the association of plasma ucMGP with genetic variation in the vitamin K epoxide reductase (VKORC1) enzyme (which reduces vitamin K so that it can carboxylate MGP) and the MGP gene. The VKORC1 SNPs genotyped included: rs17878544 (−1651A > G, located upstream −1651 relative to the mRNA start site), rs8050894 (1768G > C, located in intron 2), and rs7294 (3956G > A, located in the 3′-untranslated region). Two additional VKORC1 SNPs were genotyped (rs17708472 and rs2884737) but were not in Hardy-Weinberg equilibrium, as previously reported (17), so were not included in these analyses. The MGP SNPs genotyped included: rs1800802 (T-138C, located in the promoter region), rs1800801 (G-7A, located in the 5′-untranslated region), and rs4236 (a nonsynonymous polymorphism located at exon 4, causing an alanine to threonine amino acid change) (17). As previously reported, these 3 SNPs were in linkage disequilibrium (P < 0.001) (17).
Covariates.
At baseline and at the y 3 follow-up, information on medical history, medication use, and smoking status was collected. Other measures included blood pressure as well as height and weight, from which BMI was calculated (18). Dietary intakes over the previous year were estimated using the Harvard FFQ, which has been validated for vitamin K intake assessment (24).
Plasma lipid concentrations were measured enzymatically (13). Plasma C-reactive protein (CRP) was measured using high-sensitivity Immunulite CRP kits (Diagnostic Products) and the COBAS MIRA. Plasma IL-6 was measured using a commercially available ELISA (R & D Systems) (25).
Statistical analyses.
To reduce skewness, a natural logarithmic transformation (ln) was applied to ucMGP, CRP, and IL-6 for formal analyses. AS was also transformed as ln(AS+1) to avoid taking the natural log of zero. Pearson correlations were computed to explore the association of ucMGP with baseline measures of vitamin K status and potential determinants. We then determined the crude and age-adjusted associations between ucMGP quartiles and vitamin K status, and potential determinants using ANOVA (proc glm, SAS version 9.1) for continuous measures and logistic regression for categorical measures. We tested for interactions between sex and plasma ucMGP with respect to CAC, measures of vitamin K status, and other potential covariates. There was a significant interaction between sex and ucMGP with respect to BMI (P = 0.003), but no other significant interactions were detected (all P-interaction ≥ 0.06), and the effect of phylloquinone supplementation on plasma ucMGP did not differ by sex (P-interaction = 0.70). Therefore, we did not report sex-specific analyses. A trend test between ucMGP quartile and potential covariates was conducted using a contrast statement. To examine if the association between ucMGP and CAC was nonlinear, we fit a power term (ucMGP2) into the regression models. We did not detect a nonlinear association between ucMGP and AS (a continuous outcome, ucMGP2 P = 0.35) or between ucMGP and prevalent CAC (defined as AS > 0, ucMGP2 P = 0.16). To determine the independent correlates of circulating ucMGP, the following exposures (chosen based on correlation coefficients and biological plausibility) were entered into a stepwise multivariable linear regression: age, sex, total MGP, BMI, TG, plasma phylloquinone, %ucOC, PIVKA-II, vitamin K intake, AS, systolic blood pressure (SBP), CRP, and IL-6. P < 0.20 was prespecified for inclusion. A 2-sided P < 0.05 was considered significant.
ANCOVA was used to explore the association between baseline plasma ucMGP concentrations and polymorphisms in the GGCX, VKORC1, and MGP genes. To be consistent with previous analyses of genetic determinants of vitamin K status in this study (16, 17), an adjustment was made for age, sex, BMI, TG, vitamin K intake, smoking status, and race/ethnicity. The association between MGP polymorphisms and ucMGP was also adjusted for total MGP concentration. Because we explored associations between ucMGP and 10 SNPs, a Bonferroni adjustment was applied so that an overall P-value < 0.005 was considered significant for genetic associations. We subsequently included the genotypes that were independently associated with ucMGP in the stepwise models used to identify independent nongenetic correlates of ucMGP.
To determine the effect of vitamin K supplementation on ucMGP, we compared the 3-y change in ucMGP between the vitamin K supplementation group and the control group using an independent samples t test in an intent-to-treat analysis. ANCOVA (proc glm) was subsequently used to adjust for baseline ucMGP. Because vitamin K supplementation was associated with CAC progression (15) and change in ucMGP, we examined the association between change in ucMGP and change in AS in those who received vitamin K supplementation, using linear regression with change in AS as the outcome and change in ucMGP as the primary exposure. Adjustment was made for age, sex, BMI, statin use, baseline AS, and baseline ucMGP. Because the effect of vitamin K supplementation on CAC progression was significant among those who were adherent to treatment (predefined as ≥85%, determined by direct pill count), we subsequently restricted this analysis to those who were adherent to treatment. A similar approach was used to explore the association between baseline ucMGP and change in AS in the group that did not receive vitamin K supplementation.
Results
Cross-sectional.
The overall plasma ucMGP concentration in the participants [median (IQR)] [466 (277) pmol/L] was comparable to the previously reported concentration of healthy adults of a similar age (mean ± SD) (525 ± 423 pmol/L) (10). In age-adjusted analyses, ucMGP was significantly associated with BMI, SBP, TG, and with the inflammatory markers CRP and IL-6, but not CAC (Table 1). The circulating measures of vitamin K status, as well as vitamin K intake, were also significantly associated with ucMGP. In the stepwise linear regression, total MGP, age, plasma phylloquinone, %ucOC, vitamin K intake, TG, CRP, and sex were significantly associated with plasma ucMGP (Table 2). These exposures, together with BMI (P = 0.076) and SBP, explained 33.4% of the variability in plasma ucMGP in our study sample.
TABLE 1.
Age-adjusted measures of vitamin K status and potential correlates according to ucMGP concentration quartile at baseline in older adults1
| Plasma ucMGP,23 pmol/L | Quartile 1 237 (52–330) | Quartile 2 403 (335–462) | Quartile 3 524 (464–599) | Quartile 4 826 (604–2994) | P-trend4 |
| n | 105 | 111 | 105 | 117 | <0.001 |
| Age, y | 66 ± 0.5a | 68 ± 0.5b | 69 ± 0.5b | 70 ± 0.5c | 0.21 |
| Female,5n (%) | 58 (55) | 62 (56) | 62 (58) | 76 (65) | <0.001 |
| Total MGP,3μg/L | 184 ± 4a | 194 ± 4a,b | 201 ± 4b | 221 ± 4c | <0.001 |
| BMI, kg/m2 | 26.0 ± 0.5a | 27.4 ± 0.5b | 28.8 ± 0.5c | 29.3 ± 0.5c | 0.004 |
| Systolic BP, mm Hg | 126.2 ± 1.6a | 134.1 ± 1.6b | 133.0 ± 1.6b | 133.4 ± 1.5b | 0.18 |
| Diastolic BP, mm Hg | 73.6 ± 0.9 | 76.8 ± 0.9 | 75.5 ± 0.9 | 75.8 ± 0.8 | 0.35 |
| HDL cholesterol,3mmol/L | 1.5 ± 0.02 | 1.5 ± 0.02 | 1.5 ± 0.02 | 1.5 ± 0.02 | 0.26 |
| Total cholesterol,3mmol/L | 5.2 ± 0.1 | 5.2 ± 0.1 | 5.4 ± 0.1 | 5.3 ± 1.0 | <0.001 |
| TG,3mmol/L | 1.1 ± 0.1a | 1.3 ± 0.1a,b | 1.4 ± 0.1b | 1.5 ± 0.1b | <0.001 |
| CRP,36mg/L | 2.2 ± 0.5a | 3.4 ± 0.5a,b | 3.2 ± 0.5b | 4.2 ± 0.5c | 0.008 |
| IL-6,36ng/L | 1.5 ± 0.2a | 2.0 ± 0.2b | 1.8 ± 0.2b | 1.8 ± 0.2b | 0.94 |
| Framingham 10 y risk score6 | 12 ± 1 | 12 ± 1 | 12 ± 1 | 12 ± 1 | 0.28 |
| Diabetes,5n (%) | 6 (6) | 7 (6) | 3 (3) | 11 (9) | 0.81 |
| Current smoker,5 n (%) | 8 (8) | 6 (5) | 4 (4) | 6 (5) | 0.15 |
| Statin use,5n (%) | 17 (16) | 31 (28) | 28 (27) | 33 (28) | |
| CAC | 0.35 | ||||
| AS6 | 149 ± 50 | 263 ± 48 | 243 ± 49 | 235 ± 47 | 0.55 |
| Prevalent CAC4 (AS > 0), n (%) | 61 (58) | 76 (70) | 73 (72) | 83 (71) | 0.55 |
| Vitamin K status | |||||
| %ucOC7 | 34 ± 2a | 39 ± 2b | 44 ± 2c | 45 ± 2c | <0.001 |
| Phylloquinone,36nmol/L | 3.8 ± 0.4c | 2.4 ± 0.4b | 2.4 ± 0.4b | 2.0 ± 0.2a | <0.001 |
| PIVKA-II,36μg/L | 2.2 ± 0.1a | 2.4 ± 0.1b | 2.7 ± 0.1c | 2.5 ± 0.1b,c | 0.01 |
| Vitamin K intake, μg/d | 191 ± 11b | 192 ± 10b | 163 ± 11a,b | 159 ± 10a | 0.01 |
Least-square (LS) mean ± SEM, unless otherwise indicated. Except for analysis of age, LS means are adjusted for age. Values in a row without a common letter differ, < 0.05.
Values are mean (range).
Measured in plasma.
-trend based on ANCOVA followed by a contrast statement, unless indicated otherwise.
Based on logistic regression.
Outcome was ln-transformed for formal analysis; however, LS means ± SEM are shown in original scale.
Measured in serum.
TABLE 2.
Determinants of ucMGP (pmol/L) in older adults (n = 438) based on linear stepwise regression1
| Exposure | Unstandard β | P-value | Partial r2 |
| Total MGP,2μg/L | 0.002 | <0.001 | 0.085 |
| %ucOC3 | 0.005 | <0.001 | 0.077 |
| TG,2mmol/L | 0.001 | 0.001 | 0.048 |
| (ln) Phylloquinone,2nmol/L | −0.290 | <0.001 | 0.047 |
| Age, y | 0.016 | <0.001 | 0.029 |
| BMI, kg/m2 | 0.009 | 0.076 | 0.021 |
| (ln) CRP,2mg/L | 0.066 | 0.037 | 0.008 |
| Vitamin K intake, μg/d | −0.004 | 0.032 | 0.006 |
| Male sex | −0.092 | 0.043 | 0.006 |
| SBP, mm Hg | 0.002 | 0.121 | 0.004 |
| model r2 = 0.334 |
The outcome (ucMGP) was natural log-transformed to improve normality; the following exposures were entered: total MGP, age, TG, sex, SBP, BMI, CRP, IL-6, %ucOC, plasma phylloquinone, PIVKA-II, vitamin K intake, AS, statin use (yes/no); < 0.20 was specified for inclusion.
Measured in plasma.
Measured in serum.
All genotype frequencies used in this analysis were previously reported to be in accordance with Hardy-Weinberg Equilibrium (P > 0.05) (16, 17). Polymorphisms in the GGCX and VKORC1 genes were not associated with baseline ucMGP concentrations. MGP SNPs rs1800801 and rs4236 were both associated with plasma ucMGP (P = 0.003 and P = 0.004, respectively). For rs4236, the major allele homozygotes had significantly higher ucMGP (n = 153; 536 ± 21 pmol/L) compared with heterozygotes (n = 186; 503 ± 19 pmol/L) and minor allele homozygotes (n = 69; 429 ± 32 pmol/L). For rs1800801, the major allele homozygotes had significantly higher ucMGP (n = 164; 536 ± 21 pmol/L) compared with heterozygotes (n = 182; 500 ± 20 pmol/L and minor allele homozygotes (n = 62; 424 ± 34 pmol/L). rs1800801 and rs4236 together explained 2.0% of the variability in plasma ucMGP. Results of genetic determinants of plasma ucMGP are presented in Supplemental Table 1. When rs1800801 and rs4236 genotypes were included in separate backward selection stepwise models that included the same covariates used to identify the nongenetic determinants of plasma ucMGP, each SNP was selected as an independent predictor of ucMGP (rs1800801 partial r2 = 0.017, P = 0.002; rs4236 partial r2 = 0.017, P = 0.003). In these models, vitamin K intake was no longer significantly predictive of ucMGP, but the other nongenetic determinants remained the same and the total percent variability explained by nongenetic and genetic factors was 34.4%. Inclusion of MGP rs1800801 and rs4236 genotypes in the longitudinal analyses did not affect the association between baseline ucMGP and change in CAC in the control group or between change in ucMGP and change in CAC in the vitamin K-treated group. The affect of vitamin K supplementation on ucMGP was also not changed when MGP rs1800801 and rs4236 genotypes were adjusted for.
Longitudinal.
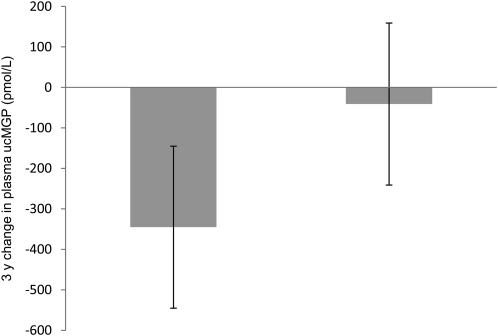
At baseline, participants who received vitamin K supplementation were similar to those who did not (15, 18) (Supplemental Table 2). The baseline plasma ucMGP of the vitamin K supplementation group [median (IQR)] [485 (267) pmol/L] did not differ from the unsupplemented group [450 (281) pmol/L] (P = 0.24). After 3 y, the plasma ucMGP concentration in the vitamin K supplementation group decreased more than in the control group with follow-up concentrations [median (IQR)] of 97 (136) pmol/L in the vitamin K group and 432 (257) pmol/L in controls (P < 0.0001) (Fig. 1). Subsequent adjustment for baseline ucMGP did not appreciably change these results. Among those in the phylloquinone-supplemented group, the change in ucMGP was not associated with the change in AS [adjusted unstandard β (SE) = −0.03 (0.03); P = 0.31]. These results were not appreciably different after exclusion of the 37 nonadherent participants in that treatment group [adjusted unstandard β (SE) = −0.07 (0.05); P = 0.22]. Among participants in the control group, the baseline ucMGP was not associated with the 3-y change in AS [adjusted unstandard β (SE) = 0.01 (0.03); P = 0.75].
FIGURE 1.
Changes in plasma ucMGP concentration in 190 older adults who received 500 μg/d phylloquinone for 3 y and 184 who did not. Values are mean ± SD. *The changes, adjusted for baseline plasma ucMGP, differed, P < 0.0001.
Discussion
In our cross-sectional analysis, plasma ucMGP was positively associated with other measures of uncarboxylated vitamin K-dependent proteins and inversely associated with circulating vitamin K concentrations in older adults. These observations were strengthened by the postintervention analysis, which found a significant reduction in the plasma ucMGP among older adults who received 500 μg/d of phylloquinone for 3 y compared with those who did not receive phylloquinone. The carboxylation of MGP is dependent on the availability of vitamin K, so higher ucMGP would reflect insufficient vitamin K, as has been demonstrated with osteocalcin and prothrombin, 2 other vitamin K-dependent proteins. Because vitamin K supplementation significantly reduced the %ucOC in our sample as well (18), our results provide consistent evidence that ucMGP may be a useful marker of vitamin K status in vascular and other tissues in which MGP functions to inhibit calcification.
However, our data did not show that ucMGP was associated with CAC in older adults free of clinical cardiovascular disease, cross-sectionally and over 3 y of follow-up. These findings are in contrast with results of case-control studies that have reported higher plasma ucMGP (measured using this same assay) among patients with diseases characterized by vascular calcification, including those with aortic valve disease, aortic stenosis, and kidney disease (9, 10, 14). However, the prior studies did not measure CAC directly as we have. Furthermore, the ucMGP may be a marker for other risk factors, such as hyperlipidemia and hypertension. These findings were surprising, because we previously reported that adherent participants in the vitamin K-supplemented group had less CAC progression compared with adherent participants in the control group after 3 y (15). Therefore, we had predicted that ucMGP would have been associated with CAC.
Novel roles for vitamin K are emerging (26) and it is plausible vitamin K may influence cardiovascular health through mechanisms other than MGP. It is also plausible the circulating ucMGP reflects processes of vascular calcification that are not related to atherosclerosis. Although atherosclerotic arterial calcification is localized to the intimal layer, arterial calcification can also occur in the medial layer (known as Monckeberg’s sclerosis) (27). Rats treated with warfarin, a vitamin K antagonist, exhibit extensive medial arterial calcification and an accumulation of ucMGP, which was reversed by high-vitamin K intake (6), providing evidence that the pathology underlying medial calcification is related to the carboxylation of MGP. Intimal and medial calcifications are thought to differ in clinical relevance. Arterial intimal calcification may contribute to plaque rupture and thrombosis, whereas medial calcification is associated with arterial stiffness, hypertension, and diabetic nephropathy (28, 29). Medial arterial calcification is common in renal disease, which may explain why our findings do not agree with a previous study that reported positive associations between ucMGP and aortic calcification in patients with kidney disease (9).
Plasma ucMGP was associated with CAC in unadjusted analysis (data not shown), but the association was attenuated when age was held constant. Increasing age is strongly associated with increasing CAC (30, 31) and is correlated with ucMGP even among the relatively narrow age-range (60–80 y) in our sample (r = 0.23; P < 0.0001). In an earlier cross-sectional analysis of this cohort, the total MGP was modestly positively associated with CAC in women but not men (13). We performed a sex-specific analysis of the association between ucMGP and CAC (data not shown), but ucMGP was not associated with CAC in men or women. In addition to age and vitamin K status, results of our stepwise regression indicate that ucMGP is independently associated with TG, inflammation (CRP), and sex. Future analysis of associations between ucMGP and chronic disease in older adults should consider adjustment for the correlates we have identified.
We also explored the association between ucMGP and SNPs in genes encoding the GGCX and VKOR enzymes and MGP. We previously found genetic variation in the GGCX and VKORC genes to be associated with plasma phylloquinone and %ucOC in this cohort (17). Although none of the GGCX or VKOR SNPs we analyzed were found to be associated with plasma ucMGP, genetic variation in MGP rs4236 and rs1800801 was associated with plasma ucMGP in our cohort, such that homozygous carriers of the major alleles had significantly higher ucMGP relative to heterozygous or homozygous minor allele carriers. These results confirm our previous analysis that found MGP rs4236 and rs1800801 were associated with total MGP (16). To the best of our knowledge, the association between genetic variability and ucMGP has not been reported.
Our study has several notable strengths and certain limitations to consider. Clinically apparent cardiovascular disease was an exclusion criterion for this study, and we did not detect an independent association between plasma ucMGP and CAC in our cohort. Whereas the available studies suggest the association between ucMGP and vascular calcification is stronger in less healthy populations (9, 14), our results may be generalized to healthier older adults. We were able to examine the cross-sectional associations among ucMGP, vitamin K status, and CAC and then determine the effect of phylloquinonone supplementation on ucMGP over 3 y of follow-up in a randomized controlled setting in which CAC was measured using a multi-detector computed-tomography scan at baseline and follow-up. Future studies are warranted in larger samples of older and middle-aged men and women. The assay used in our study measured plasma dephosphorylated-ucMGP. This measure was chosen because it was reported to increase (reflecting a decrease in vitamin K status) in response to treatment with warfarin, a vitamin K antagonist (10). An alternate monoclonal-antibody ELISA that measures ucMGP regardless of its phosphorylation status is available, and this measure was reported to be inversely associated with CAC in patients with kidney disease (32). However, the plasma ucMGP measured using this monoclonal assay did not respond to changes in vitamin K status (10). At this time, much remains to be learned about the biochemistry and physiology of MGP, and the application of the ucMGP measures may be clarified by future laboratory-based and clinical research. No study that we are aware of has explored the nongenetic and genetic determinants of ucMGP in community-dwelling adults. However, in consideration of the modest sample size and our focus on older men and women, our genetic analysis should be considered exploratory and the results need to be confirmed in additional cohorts. Because our sample was primarily Caucasian, our results may not be applicable to other racial or ethnic groups.
In summary, our data suggest plasma ucMGP may be a useful indicator of vitamin K status, but we found no evidence in older adults that it is associated with CAC. Circulating ucMGP is correlated with known risk factors for CAC, which merit consideration in future analyses. Studies in populations at greater risk for nonatherosclerotic arterial calcification may identify novel associations between vitamin K, ucMGP, and pathologic calcification in certain disease populations, such as those with kidney disease or diabetes (29).
Supplementary Material
Acknowledgments
S.L.B. designed the study; S.L.B., C.J.O., C.M.G., M.D.C., J.M.O., and S.B.K. contributed to the design of the analyses and the interpretation of the data; S.L.B., C.J.O., C.M.G., M.D.C., J.M.O., S.B.K., C.V., and E.J.P.M. contributed to the writing of the manuscript; M.K.S. performed the statistical analyses and drafted the manuscript; and C.V. and E.J.P.M. performed laboratory analyses. All authors read and approved the final manuscript.
Footnotes
Supported by the USDA, Agricultural Research Service under Cooperative Agreement No. 58-1950-7-707, the NIH (AG14759, AG23914, HL69272, T32HL69772, and P30AG021332-08), and the AHA (09CRP2070013 and 0515605T). Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA.
This trial was registered at clinicaltrials.gov as NCT00183001.
Supplemental Tables 1 and 2 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at jn.nutrition.org.
Abbreviations used: AS, agatston score; CAC, coronary artery calcium; ln, natural log; SBP, systolic blood pressure; SNP, single nucleotide polymorphism; TG, triglycerides.
Literature Cited
- 1.Church TS, Levine BD, McGuire DK, LaMonte MJ, Fitzgerald SJ, Cheng YJ, Kimball TE, Blair SN, Gibbons LW, et al. Coronary artery calcium score, risk factors, and incident coronary heart disease events. Atherosclerosis. 2007;190:224–31 [DOI] [PubMed] [Google Scholar]
- 2.Pletcher MJ, Tice JA, Pignone M, Browner WS. Using the coronary artery calcium score to predict coronary heart disease events: a systematic review and meta-analysis. Arch Intern Med. 2004;164:1285–92 [DOI] [PubMed] [Google Scholar]
- 3.Proudfoot D, Shanahan CM. Molecular mechanisms mediating vascular calcification: role of matrix Gla protein. Nephrology (Carlton). 2006;11:455–61 [DOI] [PubMed] [Google Scholar]
- 4.Schurgers LJ, Cranenburg EC, Vermeer C. Matrix Gla-protein: the calcification inhibitor in need of vitamin K. Thromb Haemost. 2008;100:593–603 [PubMed] [Google Scholar]
- 5.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81 [DOI] [PubMed] [Google Scholar]
- 6.Schurgers LJ, Spronk HM, Soute BA, Schiffers PM, DeMey JG, Vermeer C. Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin K in rats. Blood. 2007;109:2823–31 [DOI] [PubMed] [Google Scholar]
- 7.Fu X, Wang XD, Mernitz H, Wallin R, Shea MK, Booth SL. 9-Cis retinoic acid reduces 1alpha,25-dihydroxycholecalciferol-induced renal calcification by altering vitamin K-dependent gamma-carboxylation of matrix gamma-carboxyglutamic acid protein in A/J male mice. J Nutr. 2008;138:2337–41 [DOI] [PubMed] [Google Scholar]
- 8.Schurgers LJ, Teunissen KJ, Knapen MH, Kwaijtaal M. van Diest R, Appels A, Reutelingsperger CP, Cleutjens JP, Vermeer C. Novel conformation-specific antibodies against matrix gamma-carboxyglutamic acid (Gla) protein: undercarboxylated matrix Gla protein as marker for vascular calcification. Arterioscler Thromb Vasc Biol. 2005;25:1629–33 [DOI] [PubMed] [Google Scholar]
- 9.Schurgers LJ, Barreto DV, Barreto FC, Liabeuf S, Renard C, Magdeleyns EJ, Vermeer C, Choukroun G, Massy ZA. The circulating inactive form of matrix Gla protein is a surrogate marker for vascular calcification in chronic kidney disease: a preliminary report. Clin J Am Soc Nephrol. 2010;5:568–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cranenburg EC, Koos R, Schurgers LJ, Magdeleyns EJ, Schoonbrood TH, Landewe RB, Brandenburg VM, Bekers O, Vermeer C. Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb Haemost. 2010;104:811–22 [DOI] [PubMed] [Google Scholar]
- 11.Braam LA, Dissel P, Gijsbers BL, Spronk HM, Hamulyak K, Soute BA, Debie W, Vermeer C. Assay for human matrix gla protein in serum: potential applications in the cardiovascular field. Arterioscler Thromb Vasc Biol. 2000;20:1257–61 [DOI] [PubMed] [Google Scholar]
- 12.Jono S, Ikari Y, Vermeer C, Dissel P, Hasegawa K, Shioi A, Taniwaki H, Kizu A, Nishizawa Y, et al. Matrix Gla protein is associated with coronary artery calcification as assessed by electron-beam computed tomography. Thromb Haemost. 2004;91:790–4 [DOI] [PubMed] [Google Scholar]
- 13.O'Donnell CJ, Shea MK, Price PA, Gagnon DR, Wilson PW, Larson MG, Kiel DP, Hoffmann U, Ferencik M, et al. Matrix Gla protein is associated with risk factors for atherosclerosis but not with coronary artery calcification. Arterioscler Thromb Vasc Biol. 2006;26:2769–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueland T, Gullestad L, Dahl CP, Aukrust P, Aakhus S, Solberg OG, Vermeer C, Schurgers LJ. Undercarboxylated matrix Gla protein is associated with indices of heart failure and mortality in symptomatic aortic stenosis. J Intern Med. 2010;268:483–92 [DOI] [PubMed] [Google Scholar]
- 15.Shea MK, O'Donnell CJ, Hoffman U, Dallall GE, Dawson-Hughes B, Ordovas JM, Price PL, Williamson PK, Booth SL. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr. 2009:89:1799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crosier MD, Booth SL, Peter I, Dawson-Hughes B, Price PA, O'Donnell CJ, Hoffmann U, Williamson MK, Ordovas JM. Matrix Gla protein polymorphisms are associated with coronary artery calcification in men. J Nutr Sci Vitaminol (Tokyo). 2009;55:59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crosier MD, Peter I, Booth SL, Bennett G, Dawson-Hughes B, Ordovas JM. Association of sequence variations in vitamin K epoxide reductase and gamma-glutamyl carboxylase genes with biochemical measures of vitamin K status. J Nutr Sci Vitaminol (Tokyo). 2009;55:112–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Booth SL, Dallal G, Shea MK, Gundberg C, Peterson JW, Dawson-Hughes B. Effect of vitamin K supplementation on bone loss in elderly men and women. J Clin Endocrinol Metab. 2008;93:1217–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32 [DOI] [PubMed] [Google Scholar]
- 20.Price PA, Rice JS, Williamson MK. Conserved phosphorylation of serines in the Ser-X-Glu/Ser(P) sequences of the vitamin K-dependent matrix Gla protein from shark, lamb, rat, cow, and human. Protein Sci. 1994;3:822–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadowski JA, Hood SJ, Dallal GE, Garry PJ. Phylloquinone in plasma from elderly and young adults: factors influencing its concentration. Am J Clin Nutr. 1989;50:100–8 [DOI] [PubMed] [Google Scholar]
- 22.Gundberg CM, Nieman SD, Abrams S, Rosen H. Vitamin K status and bone health: an analysis of methods for determination of undercarboxylated osteocalcin. J Clin Endocrinol Metab. 1998;83:3258–66 [DOI] [PubMed] [Google Scholar]
- 23.Berkner KL. The vitamin K-dependent carboxylase. J Nutr. 2000;130:1877–80 [DOI] [PubMed] [Google Scholar]
- 24.McKeown NM, Jacques PF, Gundberg CM, Peterson JW, Tucker KL, Kiel DP, Wilson PW, Booth SL. Dietary and nondietary determinants of vitamin K biochemical measures in men and women. J Nutr. 2002;132:1329–34 [DOI] [PubMed] [Google Scholar]
- 25.Shea MK, Dallal GE, Dawson-Hughes B, Ordovas JM, O'Donnell CJ, Gundberg CM, Peterson JW, Booth SL. Vitamin K, circulating cytokines, and bone mineral density in older men and women. Am J Clin Nutr. 2008;88:356–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCann JC, Ames BN. Vitamin K, an example of triage theory: is micronutrient inadequacy linked to diseases of aging? Am J Clin Nutr. 2009;90:889–907 [DOI] [PubMed] [Google Scholar]
- 27.Amann K. Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1599–605 [DOI] [PubMed] [Google Scholar]
- 28.Jeffcoate WJ, Rasmussen LM, Hofbauer LC, Game FL. Medial arterial calcification in diabetes and its relationship to neuropathy. Diabetologia. 2009;52:2478–88 [DOI] [PubMed] [Google Scholar]
- 29.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–59 [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann U, Massaro JM, Fox CS, Manders E, O'Donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study). Am J Cardiol. 2008;102:1136– 41, 1141.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, Bild DE, Burke GL. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2007;115:2722–30 [DOI] [PubMed] [Google Scholar]
- 32.Cranenburg EC, Brandenburg VM, Vermeer C, Stenger M, Muhlenbruch G, Mahnken AH, Gladziwa U, Ketteler M, Schurgers LJ. Uncarboxylated matrix Gla protein (ucMGP) is associated with coronary artery calcification in haemodialysis patients. Thromb Haemost. 2009;101:359–66 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.