Abstract
Cues in the environment associated with drug use draw the attention of addicts, elicit approach, and motivate drug-seeking and drug-taking behavior, making abstinence difficult. However, preclinical studies have identified large individual differences in the extent to which reward cues acquire these incentive motivational properties. For example, only in some rats does a spatially discrete food cue become attractive, eliciting approach and engagement with it, and acts as an effective conditioned reinforcer. Moreover, a discrete cocaine cue also acquires greater motivational control over behavior in rats prone to attribute incentive salience to a food cue. In this study, we asked whether there is similar individual variation in the extent to which interoceptive cues produced by cocaine itself instigate cocaine-seeking behavior. After quantifying individual variation in the propensity to attribute incentive salience to a food cue, rats were trained to self-administer cocaine in the absence of an explicit conditional stimulus. We then assessed motivation for cocaine by: (1) performance on a progressive ratio schedule, and (2) the degree to which a cocaine ‘prime' reinstated cocaine-seeking following extinction of self-administration behavior. We found that rats prone to attribute incentive salience to a food cue worked harder for cocaine, and showed more robust cocaine-induced reinstatement. We conclude that there is considerable individual variation in the motivational properties of cocaine itself, and this can be predicted by the propensity to attribute incentive salience to reward cues.
Keywords: incentive salience, sign tracking, goal tracking, motivation, reinstatement, cocaine
INTRODUCTION
Cues associated with rewards can act as predictive conditional stimuli (CS), but if they are attributed with Pavlovian incentive motivational value (‘incentive salience') they can also function as incentive stimuli, and thus exert considerable control over motivated behavior (Berridge, 2001; Bindra, 1978; Cardinal et al, 2002; Flagel et al, 2009; Rescorla, 1988; Stewart et al, 1984). Indeed, incentive stimuli can acquire such a strong hold over behavior that some individuals have difficulty resisting them. For example, drug cues, including the places and paraphernalia associated with drug use, powerfully motivate behavior. In addicts, drug cues engage attention more powerfully than other stimuli (Duka and Townshend, 2004; Field and Cox, 2008; Schoenmakers et al, 2008), can provoke craving, and relapse (Ehrman et al, 1992; O'Brien et al, 1992), and even stimulate ‘approach' behavior (Wiers et al, 2009). In non-human animals, drug cues are attractive and ‘wanted,' facilitating approach and self-administration (Arroyo et al, 1998; Caggiula et al, 2001; Schenk and Partridge, 2001; Uslaner et al, 2006), and serve as powerful instigators of reinstatement/relapse of drug seeking (de Wit and Stewart, 1981; Milton and Everitt, 2010; Shaham et al, 2003). It is thought, therefore, that the high prevalence of continued drug use and relapse in addiction is due in part because addicts are hypersensitive to the incentive motivational properties of drug-associated cues (DeJong, 1994; Milton and Everitt, 2010; Robinson and Berridge, 1993; Stewart et al, 1984).
We have recently found, however, that there is considerable individual variation in the extent to which rats attribute incentive salience to reward cues (Flagel et al, 2007; Robinson and Flagel, 2009; see also Boakes, 1977; Zener, 1937). When a localizable cue is associated with the receipt of food reward, for some rats (‘sign trackers', STs), the cue itself becomes attractive, eliciting approach and engagement with it (Hearst and Jenkins, 1974). For these rats, the CS also serves as a potent conditioned reinforcer (ie, STs will work to get it). Thus, in STs the cue comes to exert an effect as an incentive stimulus (Flagel et al, 2009; Robinson and Flagel, 2009). For other rats (‘goal trackers', GTs; Boakes, 1977), the cue is equally predictive of reward (ie, it serves as an effective CS), but they instead learn to approach the location of reward delivery, and for these rats the CS is relatively ineffective as a conditioned reinforcer (Robinson and Flagel, 2009; Yager and Robinson, 2010). This variation in the propensity to attribute incentive salience to food cues has considerable relevance to addiction-like behavior. For example, STs more readily acquire cocaine self-administration behavior (Beckmann et al, 2011). Additionally, the removal of a discrete cocaine cue attenuates self-administration in STs, while having little effect on GTs, and the same discrete cocaine cue robustly reinstates cocaine-seeking behavior in STs but not GTs (Saunders and Robinson, 2010).
Our previous studies focused on spatially discrete drug cues in the environment; however, it is unknown whether individual variation in the propensity to attribute incentive salience to a discrete drug cue (Saunders and Robinson, 2010) generalizes to another type of drug cue—the internal interoceptive cues produced by a drug itself. This is an important question given that the interoceptive effects of drugs also powerfully motivate drug-seeking behavior. Human addicts report that drug craving is most intense in the moments just following drug use (Gawin and Kleber, 1986) and even a small ‘taste' (a prime) of a drug can significantly increase reported drug craving (Jaffe et al, 1989) and future drug intake (de Wit and Chutuape, 1993). Priming doses of drugs also increase attentional bias to drug cues, suggesting that they generally enhance an individual's motivation for drug use (Duka and Townshend, 2004; Schoenmakers et al, 2008). Additionally, in non-human studies, a drug prime is a potent instigator of drug-seeking behavior (de Wit and Stewart, 1981; Shaham et al, 2003; Stretch et al, 1971). We asked, therefore, whether variation in the propensity to attribute incentive salience to a discrete food cue predicts variation in the motivational properties of cocaine itself, as assessed by performance on a progressive ratio (PR) test and cocaine-induced reinstatement.
MATERIALS AND METHODS
Subjects And Housing
A total of 70 male Sprague-Dawley rats (Harlan, IN) weighing 250–300 g on arrival were housed individually on a 12-h light/12-h dark cycle (lights on at 0800) in a climate-controlled colony room. Water and food were available ad libitum. After arrival, rats were given 1 week to acclimate to the colony room before testing began. All procedures were approved by the University of Michigan Committee on the Use and Care of Animals (UCUCA).
Apparatus
Behavioral testing was conducted in standard (22 × 18 × 13 cm) test chambers (Med Associates St Albans, VT, USA) located inside sound-attenuating cabinets. A ventilating fan masked background noise. For Pavlovian training each chamber had a food cup located in the center of one wall, 3 cm above a stainless steel grid floor. Head entries into the food cup were recorded by breaks of an infrared photobeam located inside the magazine. A retractable lever illuminated from behind was located 2.5 cm to the left or right of the food cup, ∼6 cm above the floor. The location of the lever with respect to the food cup was counterbalanced across rats. On the wall opposite the food cup, a red house light remained illuminated throughout all sessions. For self-administration sessions, the food cup and lever were removed and replaced with two nose-poke ports located 3 cm above the floor on the wall opposite the house light. A nose poke into the active port, detected by an infrared photobeam inside the hole, resulted in an intravenous cocaine infusion, delivered by an external pump through a tube connected to the rat's catheter back port. The infusion tube was suspended into the chamber via a swivel mechanism, allowing the rat free movement. Active and inactive nose-poke ports were counterbalanced to control for side bias. All measures were recorded using Med Associates software.
Pavlovian Conditioned Approach Training
Rats were first trained using a Pavlovian approach (‘autoshaping') procedure similar to that described previously (Flagel et al, 2007). For 2 days before the start of training, 10 banana-flavored pellets (45 mg, BioServe, #F0059; Frenchtown, USA) were placed in the home cages to familiarize rats with this food. The following day, rats were placed in the test chambers, with the lever retracted, and trained to retrieve pellets from the food cup by presenting 50 food pellets on a variable time (VT) 30-s schedule. After 2 days of pretraining, Pavlovian training commenced. Each training trial consisted of insertion (and simultaneous illumination) of the lever (CS) into the chamber for 8 s, after which the lever was retracted and a single food pellet (unconditional stimulus, US) was immediately delivered into the food cup. Each of five daily training sessions consisted of 25 response-independent trials, in which CS-US pairing occurred on a variable time (VT) 90-s schedule (the time between CS presentations varied randomly between 30 and 150 s). Lever deflections, food cup entries, latency to lever deflection, and latency to food cup entry during CS presentation were measured.
Following Pavlovian training, rats were assigned to one of three groups based on whether they (1) preferentially interacted with the lever CS (‘sign trackers', STs); (2) preferentially interacted with the food cup during lever-CS presentation (‘goal trackers', GTs); or (3) had no preference for the lever CS or food cup (‘intermediate group', IG). This was quantified using a composite Pavlovian conditioned approach (PCA) index, which was the average of three measures of conditioned approach behavior: (1) the probability of contacting either the lever CS or food cup during the CS period (P(lever)–P(food cup)); (2) the response bias for contacting the lever CS or the food cup during the CS period ((#lever deflections–#food cup entries)/(#lever deflections+#food cup entries)); and (3) the mean latency to deflect the lever or enter the food cup during the CS period ((food cup entry latency–lever deflection latency)/8 (ie, CS duration)). This produces values on a scale ranging from −1.0 to +1.0. A score of +1.0 indicates a rat that on every trial deflected the lever CS and never entered the food cup, a score of −1.0 indicates a rat that entered the food cup and never deflected the lever CS, and a score of 0 indicates a rat that distributed his behavior equally between lever CS and food cup. The average PCA index score for days 4 and 5 of training was used to classify animals. For the purposes of classification, we operationally defined animals as STs (n=16, 23%), if they obtained a PCA index score of +0.5 or greater (meaning they rapidly approached and vigorously engaged the lever CS at least twice as much as to the food cup), and as GTs (n=19, 27%), if they obtained a score of −0.5 or less. The remaining animals within the −0.5/+0.5 range, those rats whose preference vacillated between lever CS and food cup, were labeled as IGs (n=35, 50%). For this study, we were interested in comparing rats that clearly differed in their propensity to attribute incentive salience to reward cues, and therefore, only rats identified as STs or GTs were used in the rest of the study.
Intravenous Catheter Surgery
Next, ST and GT rats were prepared with intravenous catheters as described previously (Crombag et al, 2000) under ketamine hydrochloride (100 mg/kg i.p.) and xylazine (10 mg/kg i.p.) anesthesia. Following surgery, catheters were flushed daily with 0.2 ml sterile saline containing 5 mg/ml gentamicin sulfate (Vedco, MO) to minimize infection and prevent occlusions. Catheter patency was tested weekly by intravenous injection of 0.2 ml sodium thiopental (20 mg/ml in sterile water, Hospira, IL). Only rats that became ataxic within 5–10 s were considered to have patent catheters and included in the analyses.
Self-Administration: Acquisition
Self-administration sessions began 7 days after surgery in chambers outfitted with two nose ports as described above. A nose poke into the active port resulted in an intravenous infusion of cocaine hydrochloride (NIDA) dissolved in 0.9% sterile saline (0.2 mg (weight of the salt) per kg per infusion in 50 μl delivered over 2.6 s) on a fixed ratio (FR) 1 schedule (Carroll and Lac, 1997). Coincident with the start of an infusion was an unsignaled 20-s timeout period, during which nose pokes were recorded, but had no consequences. No discrete cue in the environment (eg, light, or tone) was explicitly paired with drug delivery, to help ensure that behavior was largely controlled by the interoceptive effects of the drug itself. We also wanted all rats to receive exactly the same number of drug injections, and thus an infusion criterion (IC) was imposed on self-administration sessions (ie, session length was determined by how long it took each rat to reach the IC, not by an explicit time limit (Saunders and Robinson, 2010). Rats were initially allowed to take 10 infusions per session, and the IC was increased to 20, 40, and then 80 infusions. Rats were trained at IC 10, 20, and 40 for three consecutive sessions and at IC 80 for five consecutive sessions. A total of 27 rats (ST n=12, GT n=15) completed the self-administration portion of the experiment. Rats were eliminated when they failed to acquire self-administration (ST n=1, GT n=1), or lost catheter patency (ST n=3, GT n=4).
Self-Administration: Progressive Ratio Test
Following five sessions at IC 80 all rats received two consecutive sessions during which the reinforcement schedule was changed from a FR1 to a PR schedule. On the PR schedule, the number of active nose pokes required to produce the next cocaine infusion increased after each infusion according to the following exponential progression: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40…, derived from the formula ((5 × e0.2n)−5), rounded to the nearest integer, where n is the position in the sequence of ratios (Roberts and Richardson, 1992). PR sessions were terminated only after 1 h had lapsed between completed ratios/infusions received. After the two PR sessions, rats were returned to a FR1 reinforcement schedule at IC 80 for three additional sessions to re-stabilize behavior.
Cocaine-Primed Reinstatement Test
After the last self-administration session at IC 80, rats underwent eight 60-min sessions of extinction training. In these sessions an active nose-poke response did not produce a cocaine infusion, although the infusion pump was turned on. The day after the final extinction session, rats were tested for cocaine-induced reinstatement of drug-seeking behavior. On this day, immediately before placement in the testing chamber, each rat received a 15 mg/kg i.p. injection of cocaine. Nose pokes were recorded, but had no consequences. Before testing, rats were habituated to the injection experience by receiving an i.p. injection of saline in their home cages.
Statistical Analysis
Linear mixed-models (LMM) analysis was used for all repeated measures data. The best-fitting model of repeated measures covariance was determined by the lowest Akaike information criterion score (Verbeke, 2009). Depending on the model selected, the degrees of freedom may have been adjusted to a non-integer value. Analysis of variance was used to compare PR and reinstatement responding. Statistical significance was set at p<0.05.
RESULTS
Individual Variation in Pavlovian Conditioned Approach Behavior
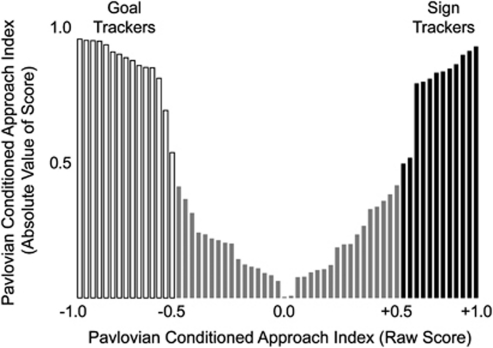
Figure 1 shows the distribution of PCA index scores for all rats. As discussed above, GTs were operationally defined as rats with scores ranging between −1.0 and −0.5, STs between +0.5 and +1.0, and IGs between −0.5 and +0.5. There was some variation in index scores within the ST and GT groups, but most rats clustered near the extreme ends of the potential score range (ie, the rats tested on self-administration showed a strong preference for either the lever CS or the food magazine during PCA training). Figure 2 shows the change in approach behavior as a function of training session in STs and GTs. With experience, STs developed a high probability of rapidly approaching and vigorously engaging the lever CS (Figure 2a–c). In contrast, GTs rarely engaged the lever CS and instead came to rapidly approach the food magazine during lever-CS presentation (Figure 2d–f). Thus, as in our previous studies (Flagel et al, 2007; Saunders and Robinson, 2010), the lever CS developed the ability to evoke a conditioned response (CR) in both STs and GTs—it served as a predictive CS in both—but it was an attractive incentive stimulus only for STs.
Figure 1.
Distribution of Pavlovian conditioned approach (PCA) composite index scores based on behavior during sessions 4 and 5 of PCA training. Rats receiving a raw score of +0.5–+1.0 were designated sign trackers (STs) and rats receiving a raw score of −0.5 to −1.0 were designated goal trackers (GTs). The remaining rats, with scores between −0.5 and +0.5 were designated intermediates (IGs). Raw scores are presented along the x-axis and corresponding absolute values along the y-axis. Index scores for IG rats are included to illustrate the bimodality of the index distribution, but IG rats were excluded from all further testing.
Figure 2.
Behavior directed towards the lever CS (sign tracking) is shown in panels a–c and that directed towards the food cup during CS presentation (goal tracking) is shown in panels d–f. Mean+SEM (a) probability of contacting the lever CS (#trials with a lever CS contact/#trials per session) during the 8-s CS period (b) number of lever CS contacts made during the 8-s CS period, (c) latency to the first lever-CS contact, (d) probability of food cup entry (# trials with a food cup entry/#trials per session) during the 8-s CS period (e) number of food cup entries made during the 8-s CS period, (f) latency to the first food cup entry during the CS period. For all of these measures there was a significant effect of group (ST or GT), session, and a group x session interaction (p<0.001).
Acquisition of Cocaine Self-Administration in STs and GTs
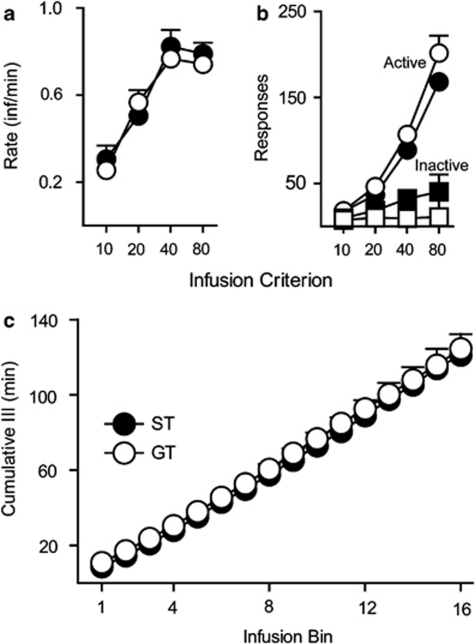
Rats were next trained to nose poke for an IV cocaine infusion under conditions in which no discrete cue (CS) was paired with drug delivery. Given that training sessions were limited to a fixed infusion criterion, group differences in acquisition would be evident in the number of cocaine infusions taken per minute (rate). There were no group differences in rate at any infusion criterion (no effect of group, F(1,38.05)=0.022, p=0.884; Figure 3a). Both groups made the same number of active nose pokes (F(1,22.55)=1.80, p=0.19), which increased across training (effect of session, F(13,19.86)=18.7, p<0.0001), and inactive nose pokes (F(1,27.31)=3.274, p=0.081), which did not increase (no effect of session, F(13,19.26)=1.297, p=0.294; Figure 3b). To further examine baseline self-administration behavior, within-session responding was analyzed during the final two sessions of training at IC 80. The results of this analysis are shown in Figure 3c. The pattern of cocaine intake was the same in STs and GTs. By the end of training, both groups took infusions at a consistent, uniform rate (no group × infusion bin interaction, F(1,44.14)=0.626, p=0.838). Thus, using this procedure, there were no group differences in the acquisition self-administration behavior, as we have reported previously (Saunders and Robinson, 2010). However, using other procedures and doses STs have been reported to acquire cocaine self-administration more readily than GTs (Beckmann et al, 2011).
Figure 3.
Acquisition of self-administration behavior in sign trackers (n=12) and goal trackers (n=15). (a) The mean+SEM number of cocaine infusions per minute for infusion criteria 10, 20, 40, and 80 (0.2 mg/kg/inf). (b) The mean+SEM number of active (circles) and inactive (squares) nose-poke responses at each infusion criterion. (c) The mean+SEM cumulative interinfusion interval (III) during the final two self-administration sessions at IC 80.
STs are More Motivated to Obtain Cocaine than GTs
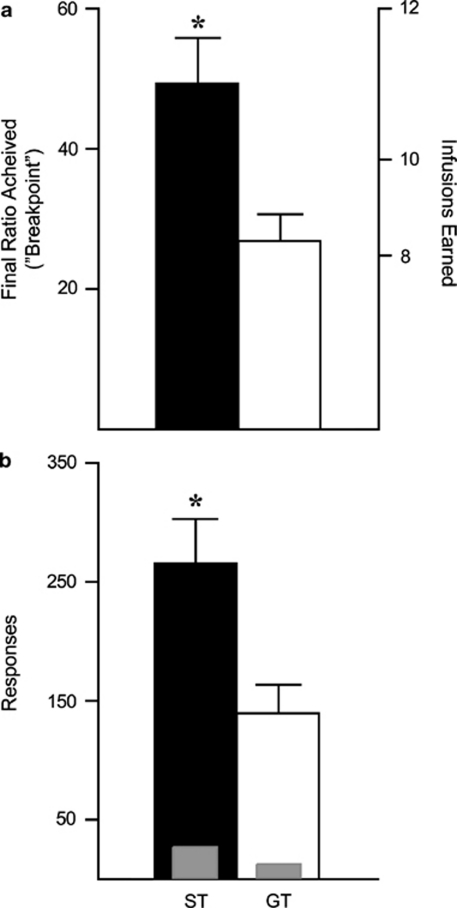
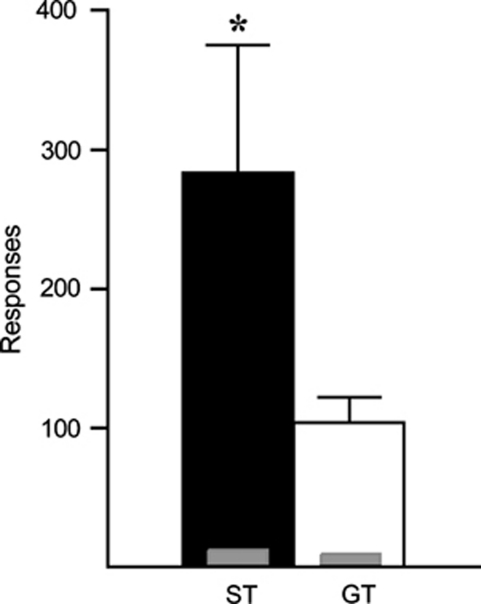
By the end of self-administration training on a FR 1 schedule STs and GTs showed identical, stable behavior (Figure 3c). All rats were then transferred to a PR schedule for two consecutive sessions. We found no effect of PR session in either group, so the data from the two PR sessions were averaged together. STs made more active responses and attained higher final ratios (‘breakpoints') than GTs (effect of group, F(1,50)=9.632, p=0.003), reaching a final ratio nearly twice that of GTs (Figure 4a). Accordingly, STs received more cocaine injections (F(1,50)=11.531, p=0.001), emitted more responses (F(1,50)=8.534, p=0.005; Figure 4b), and took longer to reach breakpoint (F(1,50)=6.916, p=0.015) than GTs. It should be emphasized that although STs worked nearly twice as much as GTs during PR testing, this amounted to, on average, only three more cocaine infusions relative to GTs (Figure 4a). Given the large number of infusions all rats received across training, this is a negligible difference in drug exposure that unlikely had any carryover effects on subsequent self-administration behavior (see below).
Figure 4.
Self-administration behavior during progressive ratio (PR) testing for sign trackers and goal trackers. (a) The mean+SEM final PR ratio achieved (‘breakpoint') and total infusions earned. (b) The mean+SEM number of active (thick bars) nose-poke responses made during PR testing. The inset gray bars represent the number of inactive nose-poke responses. *, indicates a significant group difference, p<0.05.
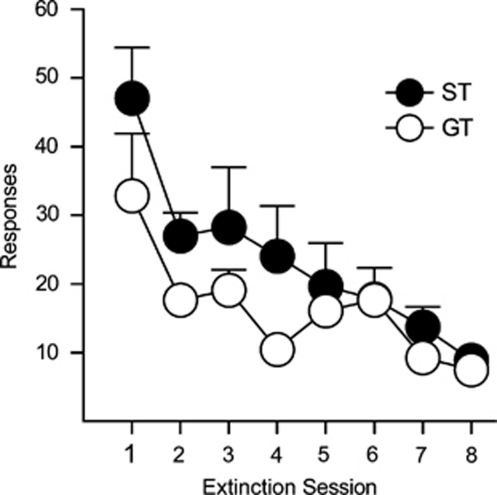
Following PR test sessions, rats were returned to an FR1 schedule at IC 80 to re-stabilize behavior. During these sessions there were no group differences in the rate of self-administration (F(1,25.02)=0.001, p=0.971), or number of responses (F(1,25.03)=0.209, p=0.652) (data not shown). After the resumption of baseline levels of self-administration, rats underwent eight sessions of extinction training, during which nose pokes no longer produced cocaine. During this period STs and GTs decreased responding (effect of session, F(7,43.65)=9.352, p<0.0001), and they did so at a similar rate (no group × session interaction, F(1,43.65)=1.612, p=0.157; Figure 5).
Figure 5.
Extinction of responding for cocaine in sign trackers (n=12) and goal trackers (n=15). The mean+SEM number of active nose-poke responses is shown.
Cocaine-Induced Reinstatement in STs and GTs
Following extinction training all rats were tested for cocaine-induced reinstatement of drug-seeking behavior. Rats were given a priming injection of cocaine (15 mg/kg, i.p.) immediately before being placed into the test chambers for a 60-min session under extinction conditions. STs showed more robust reinstatement of cocaine-seeking than GTs (F(1,50)=4.657, p=0.036; Figure 6). Although both groups discriminated between active and inactive nose pokes (effect of nose poke port, F(1,50)=19.856, p<0.0001), STs reinstated to a greater degree than GTs (significant group × nose-poke interaction, F(1,50)=4.526, p=0.038; Figure 6). We next assessed the relation between performance during PR testing and the degree of reinstatement following cocaine priming. For STs, performance during PR sessions was significantly correlated with performance during reinstatement (Spearman's r=0.504, p=0.048), but for GTs the correlation was not significant (Spearman's r=0.007, p=0.490). Thus, ST rats that made the most responses under a PR schedule tended to reinstate the most following a cocaine prime.
Figure 6.
Cocaine-primed (15 mg/kg i.p. injection) reinstatement of drug-seeking behavior for sign trackers (n=12) and goal trackers (n=15) during a 60-min test session, in which an active response had no consequences. The mean+SEM number of active nose-poke responses (thick bars) are shown. The inset gray bars represent the number of inactive nose-poke responses. *, indicates a significant group difference, p<0.05.
DISCUSSION
We asked whether variation in the propensity to attribute incentive salience to a localizable food cue predicts variation in the ability of cocaine to spur cocaine-seeking behavior. We found that it does. At the doses tested, STs worked harder to receive cocaine injections (ie, had higher ‘breakpoints' on a PR schedule), and showed more robust cocaine-induced reinstatement, than GTs. These findings, together with our previous report (Saunders and Robinson, 2010), suggest that both interoceptive and exteroceptive drug cues acquire more potent incentive motivational properties, and thus the ability to spur drug-seeking behavior, in STs than GTs.
The ability of different types of drug cues to motivate behavior is especially important in the context of addiction, as drug-associated stimuli of many kinds can initiate a cascade of behaviors leading to relapse (de Wit, 1996; Milton and Everitt, 2010; Shaham et al, 2003). Cues gain this control over behavior if they come to act as incentive stimuli, which have three fundamental properties: (1) they attract and elicit approach; (2) they acquire conditioned reinforcing properties and become ‘wanted,' in the sense that animals will work to obtain them; and (3) they energize ongoing instrumental actions (Berridge, 2001; Cardinal et al, 2002; Milton and Everitt, 2010). There is, however, considerable individual variation in the propensity to attribute incentive salience to reward cues. A food cue acquires the properties of an incentive stimulus: it attracts (Flagel et al, 2007), it serves as an effective conditioned reinforcer (Robinson and Flagel, 2009), and it spurs food-seeking behavior (Yager and Robinson, 2010), to a greater extent in STs than GTs. This variation in incentive salience attribution extends to drug cues as well, as a discrete cocaine-associated cue motivates self-administration and instigates more robust reinstatement in STs than GTs (Saunders and Robinson, 2010). Given the established differences between ST and GT rats, our current results suggest that cocaine itself also motivates drug-seeking behavior to a greater degree in rats with a propensity to attribute incentive salience to reward-associated cues (ie, STs).
There is more than one potential psychological process that can account for the variation in drug-seeking behavior exhibited by ST and GT rats in the current experiment. First, the subjective experience associated with cocaine, the constellation of interoceptive cues produced by cocaine, may be more pleasurable and/or attributed with greater incentive salience in STs than GTs. Thus, STs may come to ‘want' the experience of cocaine more than GTs (Robinson and Berridge, 2000). This interpretation is consistent with a recent finding that under some conditions STs acquire cocaine self-administration behavior more readily than GTs (Beckmann et al, 2011). With the procedure used here, there was no difference between STs and GTs in the acquisition of cocaine self-administration (see also Saunders and Robinson, 2010), but our procedure was explicitly designed to limit any potential group difference in acquisition by limiting self-administration sessions by the number of cocaine infusions, not time. Indeed, when allowed to self-administer cocaine unrestrained during PR sessions STs sought out more drug than GTs. Thus, the interoceptive cues produced by cocaine seem to motivate greater drug-seeking behavior in STs than GTs.
One limitation of this study is that only one dose of cocaine was used. Extensive dose-effect studies would be required to determine if cocaine is more potent in STs (ie, their dose-response function is shifted to the left) compared with GTs, or if cocaine has a larger maximum effect in STs (ie, their dose-response function is shifted up). However, the opposite—that cocaine is more potent and/or efficacious in GTs—is very unlikely. In the context of our results, if this were the case, GTs would have to show more robust reinstatement compared with STs to a cocaine prime dose below 15 mg/kg, and then the opposite effect we observed at 15 mg/kg. There is no precedent in the reinstatement literature for this type of dose-effect relationship and, in general, cocaine-primed reinstatement is weak at doses below 15 mg/kg and maximal at doses of 15–20 mg/kg. Therefore, we think the data are consistent with the conclusion that cocaine is more potent and/or has greater maximum effect, in STs relative to GTs.
Variation in the motivational effects of cocaine described here in rats has parallels in humans. The ability of a drug prime to instigate relapse is well established, but there are also marked individual differences in both the subjective effects of drugs (de Wit et al, 1986; de Wit et al, 1987) and the drug-priming effect (de Wit et al, 1987; Kirk and de Wit, 2000). Specifically, individuals reporting the greatest subjective drug effects have the highest motivation to obtain drug. This is supported by clinical reports indicating that not all drug users relapse after drug-prime exposure (Lloyd and Salzberg, 1975). Our results suggest, therefore, that some of the variation in drug-induced relapse potential may be due to differences in the tendency of an individual to attribute incentive value to interoceptive drug cues.
A second, although not mutually exclusive interpretation of our results, is that cocaine enhances the motivational properties of external cues present during drug exposure to a greater degree in STs than GTs. Drugs can influence responding due to their direct subjective effects, but also by amplifying the motivational value of other stimuli. These effects are behaviorally dissociable but generally have an impact in a synergistic manner (Caggiula et al, 2009; Palmatier et al, 2006). Stein (1964) and Hill (1970) originally conceptualized this enhancement effect that was later confirmed in experiments by Robbins (1975, 1976, 1984) and others (Beninger et al, 1981; Caggiula et al, 2009; Chaudhri et al, 2006; Palmatier et al, 2006; Phillips and Fibiger, 1990), who demonstrated the ability of drugs to enhance the conditioned reinforcing properties of environmental stimuli. It is notable that there is considerable individual variation in the strength of this effect (Hill, 1970; Phillips and Fibiger, 1990; Robbins, 1975). Thus, the differences between STs and GTs in PR performance and cocaine-primed reinstatement may be due to differences in cocaine's ability to enhance the motivational value of non-contingent external cues independent of, or in addition to, cocaine's interoceptive effects. Perhaps STs responded more than GTs during PR testing and following cocaine priming in part because contextual stimuli continuously present during experience with cocaine acquired enhanced incentive salience—they became more motivating—and thus spurred greater drug-seeking in STs than GTs (Berridge, 2001; Robinson and Berridge, 1993). Although we took care to eliminate discrete drug-paired cues (CSs), there were stimuli—the context of the experimental chamber, the nose-poke port—that remained present while the rats experienced cocaine. It is possible that the action of approaching and emitting an active nose poke became more likely in STs because cocaine potentiated the incentive value of these stimuli.
It is not known what neurobiological differences between STs and GTs account for their varying propensity to attribute incentive salience to food and drug cues. Of course, there is a wealth of evidence implicating ascending mesotelencephalic dopamine (DA) systems in the assignment of incentive motivational properties to rewards and associated stimuli (Berridge, 2007; Berridge and Robinson, 1998; Cardinal et al, 2002; Di Chiara, 1998; Robbins et al, 1989). For example, blockade of DA receptors attenuates cocaine self-administration and reinstatement behavior (Pilla et al, 1999; Woolverton and Virus, 1989). Additionally, potentiation of DA activity in the nucleus accumbens (NAc) via local amphetamine injection increases the conditioned reinforcing properties of reward cues, whereas DA blockade in NAc attenuates it (Taylor and Robbins, 1984, 1986). Unfortunately, there has been little research on potential neurobiological differences in STs and GTs, but recent evidence suggests that differences in DA systems do exist. For example, the acquisition of a sign-tracking CR is DA-dependent, but learning a goal-tracking CR is not (Flagel et al, 2011). Furthermore, during Pavlovian training there is a transition of the phasic DA signal from a food US to a lever CS in STs, but this transfer does not occur in GTs (Flagel et al, 2011). These studies implicate differences in DA signaling in the behavioral differences seen with food-associated cues, but further research is needed to determine whether DA system differences account for variation in the motivational properties of cocaine we report here.
In conclusion, we report that it is possible to predict, before any drug experience, which rats later will be more motivated to work for cocaine and to seek cocaine following a priming injection. Interestingly, Mahler and de Wit (2010) recently reported what may be a related phenomenon—smokers who show high craving to food cues when food deprived also show the highest craving to smoking cues. The results reported here extend our previous studies with STs and GTs and suggest that for some individuals not only do exteroceptive drug-associated cues acquire greater motivational control over behavior, but interoceptive drug cues do as well. Individuals for whom drug cues exert an effect as potent incentive stimuli will have more difficulty resisting them than individuals for whom cues merely have an impact as predictors of reward. Thus, a complex set of external and internal cues may continually goad some individuals to action (Robinson and Berridge, 1993; Stewart et al, 1984), and therefore these individuals may be most susceptible to addiction. It will be important to further understand the psychological and neurobiological mechanisms that underlie variation in propensity to attribute incentive salience to reward cues because this might not only be relevant to the propensity for addiction, but for other impulse control disorders as well.
Acknowledgments
This research was supported by a grant from the National Institute on Drug Abuse to TER (R37 DA04294). We thank Jacqueline Antonishek and Viktoria Krajnc for technical assistance and Vedran Lovic and Paul Meyer for helpful comments on an earlier version of this article.
The authors declare no conflict of interest.
References
- Arroyo M, Markou A, Robbins TW, Everitt BJ. Acquisition, maintenance and reinstatement of intravenous cocaine self-administration under a second-order schedule of reinforcement in rats: effects of conditioned cues and continuous access to cocaine. Psychopharmacology. 1998;140:331–344. doi: 10.1007/s002130050774. [DOI] [PubMed] [Google Scholar]
- Beckmann JS, Marusich JA, Gipson CD, Bardo MT. Novelty seeking, incentive salience and acquisition of cocaine self-administration in the rat. Behav Brain Res. 2011;216:159–165. doi: 10.1016/j.bbr.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninger RJ, Hanson DR, Phillips AG. The acquisition of responding with conditioned reinforcement: effects of cocaine, (+)-amphetamine and pipradrol. Br J Pharmacol. 1981;74:149–154. doi: 10.1111/j.1476-5381.1981.tb09967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC.2001Reward learning: Reinforcement, incentives, and expectations Psychology of Learning and Motivation: Advances in Research and Theoryvol 40. Academic Press Inc.: San Diego; 223–278. [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience. Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bindra D. How adaptive behavior is produced: a perceptual-motivation alternative to response reinforcement. Behav Brain Sci. 1978;1:41–91. [Google Scholar]
- Boakes RA.1977Performance on learning to associate a stimulus with positive reinforcementIn: Davis H, Hurwitz H (eds).Operant-Pavlovian Interactions Earlbaum: Hillsdale, NJ; 67–97. [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, et al. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Acquisition of i.v. amphetamine and cocaine self-administration in rats as a function of dose. Psychopharmacology (Berl) 1997;129:206–214. doi: 10.1007/s002130050182. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Maren S, Robinson TE. The role of contextual versus discrete drug-associated cues in promoting the induction of psychomotor sensitization to intravenous amphetamine. Behav Brain Res. 2000;116:1–22. doi: 10.1016/s0166-4328(00)00243-6. [DOI] [PubMed] [Google Scholar]
- de Wit H. Priming effects with drugs and other reinforcers. Exp Clin Psychopharmacol. 1996;4:5–10. [Google Scholar]
- de Wit H, Chutuape MA. Increased ethanol choice in social drinkers following ethanol preload. Behav Pharmacol. 1993;4:29–36. [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- de Wit H, Uhlenhuth EH, Johanson CE. Individual-differences in the reinforcing and subjective effects of amphetamine and diazepam. Drug Alcohol Depend. 1986;16:341–360. doi: 10.1016/0376-8716(86)90068-2. [DOI] [PubMed] [Google Scholar]
- de Wit H, Uhlenhuth EH, Pierri J, Johanson CE. Individual differences in behavioral and subjective responses to alcohol. Alcohol Clin Exp Res. 1987;11:52–59. doi: 10.1111/j.1530-0277.1987.tb01263.x. [DOI] [PubMed] [Google Scholar]
- DeJong W. Relapse prevention: an emerging technology for promoting long-term drug abstinence. Int J Addict. 1994;29:681–705. doi: 10.3109/10826089409047904. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. J Psychopharmacol. 1998;12:54–67. doi: 10.1177/026988119801200108. [DOI] [PubMed] [Google Scholar]
- Duka T, Townshend JM. The priming effect of alcohol pre-load on attentional bias to alcohol-related stimuli. Psychopharmacology (Berl) 2004;176:353–361. doi: 10.1007/s00213-004-1906-7. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O'Brien CP. Conditioned-responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology. 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: implications for addiction. Neuropharmacology. 2009;56:139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology. 2007;191:599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Hearst E, Jenkins H. Sign-tracking: The stimulus-reinforcer relation and directed action. Monograph of the Psychonomic Society: Austin; 1974. [Google Scholar]
- Hill RT.1970Facilitation of conditioned reinforcement as a mechanism of psychomotor stimulationIn: Costa E, Garattini S (eds).Amphetamine and related compounds Raven: New York; 781–795. [Google Scholar]
- Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology. 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Kirk JM, de Wit H. Individual differences in the priming effect of ethanol in social drinkers. J Stud Alcohol. 2000;61:64–71. doi: 10.15288/jsa.2000.61.64. [DOI] [PubMed] [Google Scholar]
- Lloyd RW, Jr, Salzberg HC. Controlled social drinking: an alternative to abstinence as a treatment goal for some alcohol abusers. Psychol Bull. 1975;82:815–842. [PubMed] [Google Scholar]
- Mahler SV, de Wit H. Cue-reactors: individual differences in cue-induced craving after food or smoking abstinence. PLoS One. 2010;5:e15475. doi: 10.1371/journal.pone.0015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Everitt BJ. The psychological and neurochemical mechanisms of drug memory reconsolidation: implications for the treatment of addiction. Eur J Neurosci. 2010;31:2308–2319. doi: 10.1111/j.1460-9568.2010.07249.x. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, McLellan AT, Ehrman RN.1992Classical Conditioning in Drug-Dependent Humansa Ann NY Acad Sci 654(The Neurobiology of Drug and Alcohol Addiction)400–415. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, et al. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl) 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Fibiger HC. Role of reward and enhancement of conditioned reward in persistence of responding for cocaine. Behav Pharmacol. 1990;1:269–282. doi: 10.1097/00008877-199000140-00002. [DOI] [PubMed] [Google Scholar]
- Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, et al. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian conditioning - It′s not what you think it is. American Psychologist. 1988;43:151–160. doi: 10.1037//0003-066x.43.3.151. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The potentiation of conditioned reinforcement by psychomotor stimulant drugs. A test of Hill′s hypothesis. Psychopharmacology. 1975;45:103–114. [Google Scholar]
- Robbins TW. Relationship between reward-enhancing and stereotypical effects of psychomotor stimulant drugs. Nature. 1976;264:57–59. doi: 10.1038/264057a0. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Cador M, Taylor JR, Everitt BJ. Limbic-striatal interactions in reward-related processes. Neurosci Biobehav Rev. 1989;13:155–162. doi: 10.1016/s0149-7634(89)80025-9. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Richardson NR.1992Self-administration of psychomotor stimulants using progressive ratio schedules of reinforcementIn: Boulton A, Baker G, Wu PH (eds).Neuromethodsvol 24. Humana: Totowa; 223–269. [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving—an incentive sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95:S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiat. 2009;65:869–873. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biol Psychiat. 2010;67:730–736. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Influence of a conditioned light stimulus on cocaine self-administration in rats. Psychopharmacology (Berl) 2001;154:390–396. doi: 10.1007/s002130000608. [DOI] [PubMed] [Google Scholar]
- Schoenmakers T, Wiers RW, Field M. Effects of a low dose of alcohol on cognitive biases and craving in heavy drinkers. Psychopharmacology (Berl) 2008;197:169–178. doi: 10.1007/s00213-007-1023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Stein L.1964Amphetamine and neural reward mechanismsIn: Steinberg H, de Reuck AVS, Knight J (eds).Animal behaviour and drug action Churchill: London; 91–118. [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- Stretch R, Gerber GJ, Wood SM. Factors affecting behavior maintained by response-contingent intravenous infusions of amphetamine in squirrel monkeys. Can J Physiol Pharmacol. 1971;49:581–589. doi: 10.1139/y71-075. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Robbins TW. Enhanced behavioural control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology (Berl) 1984;84:405–412. doi: 10.1007/BF00555222. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Robbins TW. 6-Hydroxydopamine lesions of the nucleus accumbens, but not of the caudate nucleus, attenuate enhanced responding with reward-related stimuli produced by intra-accumbens d-amphetamine. Psychopharmacology (Berl) 1986;90:390–397. doi: 10.1007/BF00179197. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Acerbo MJ, Jones SA, Robinson TE. The attribution of incentive salience to a stimulus that signals an intravenous injection of cocaine. Behav Brain Res. 2006;169:320–324. doi: 10.1016/j.bbr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Verbeke G. Linear mixed models for longitudinal data. Springer: New York; 2009. [Google Scholar]
- Wiers RW, Rinck M, Dictus M, van den Wildenberg E. Relatively strong automatic appetitive action-tendencies in male carriers of the OPRM1 G-allele. Genes Brain Behav. 2009;8:101–106. doi: 10.1111/j.1601-183X.2008.00454.x. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Virus RM. The effects of a D1 and a D2 dopamine antagonist on behavior maintained by cocaine or food. Pharmacol Biochem Behav. 1989;32:691–697. doi: 10.1016/0091-3057(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Yager LM, Robinson TE. Cue-induced reinstatement of food seeking in rats that differ in their propensity to attribute incentive salience to food cues. Behav Brain Res. 2010;214:30–34. doi: 10.1016/j.bbr.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zener K. The significance of behavior accompanying conditioned salivary secretion for theories of the conditioned response. Am J Psychol. 1937;50:384–403. [Google Scholar]