Abstract
Filamins are an important family of actin-binding and crosslinking proteins that mediate remodeling of the actin cytoskeleton and maintain extracellular matrix connections by anchoring transmembrane proteins to actin filaments and linking them to intracellular signaling cascades. We recently found that filamins are targeted for proteasomal degradation by the E3 ubiquitin ligase specificity subunit ASBα and that acute degradation of filamins through this ubiquitin–proteasome pathway correlates with cell differentiation. Specifically, in myeloid leukemia cells retinoic-acid-induced expression of ASB2α triggers filamin degradation and recapitulates early events crucial for cell differentiation. ASB2α is thought to link substrates to the ubiquitin transferase machinery; however, the mechanism by which ASB2α interacts with filamin to induce degradation remained unknown. Here, we use cell-based and biochemical assays to show that the subcellular localization of ASB2α to actin-rich structures is dependent on filamin and that the actin-binding domain (ABD) of filamin mediates the interaction with ASB2α. Furthermore, we show that the ABD is necessary and sufficient for ASB2α-mediated filamin degradation. We propose that ASB2α exerts its effect by binding the ABD and mediating its polyubiquitylation, so targeting filamins for degradation. These studies provide the molecular basis for ASB2α-mediated filamin degradation and unravel an important mechanism by which filamin levels can be acutely regulated.
Key words: Filamin, ASB2, Calponin homology domain, Ubiquitylation
Introduction
Filamins (FLNs) are essential, evolutionarily conserved, actin-binding proteins that crosslink and bundle actin filaments and link transmembrane receptors to intracellular cytoskeletal and signaling networks. Humans have three highly homologous FLN isoforms (FLNa, FLNb and FLNc). FLNa and FLNb show ubiquitous cellular and tissue expression patterns, whereas FLNc is thought to be largely restricted to skeletal and cardiac muscles (Thompson et al., 2000; van der Flier and Sonnenberg, 2001). FLN expression is essential to mammalian development (Zhou et al., 2007), and mutations in the human FLN genes result in diverse congenital anomalies including defects in the brain, bone, cardiovascular system and many other organs (Krakow et al., 2004; Robertson et al., 2003; Sheen et al., 2001). Although the mechanisms underlying the genetic mutations that disrupt development are unknown, the wide range of diseases suggests involvement of FLNs in a diverse variety of interactions.
FLNs are cytoplasmic homodimers composed of two ~250 kDa subunits. Each FLN subunit consists of an N-terminal actin-binding domain (ABD), composed of two calponin homology (CH) domains, followed by 24 tandem immunoglobulin-like domains (IgFLN1–IgFLN24) of ~96 amino acids interrupted by two hinge regions (Gorlin et al., 1990; Pudas et al., 2005; van der Flier and Sonnenberg, 2001). Dimerization of FLN subunits through their C-terminal IgFLN domain allows formation of a 160-nm V-shaped flexible structure that tethers actin filaments (Gorlin et al., 1990; Hartwig et al., 1980). In addition to F-actin, FLNs bind to more than 70 diverse proteins, including transmembrane receptors, signaling and adaptor proteins, and act as scaffolds for a wide range of signaling complexes (Feng and Walsh, 2004; Stossel et al., 2001; Zhou et al., 2010; Zhou et al., 2007). Through these interactions, mainly mediated by IgFLN16–IgFLN24, FLNs link matrix and cytoskeletal signaling pathways and regulate re-organization of the actin cytoskeleton, cell shape, cell adhesion and migration (Stossel et al., 2001; Zhou et al., 2010).
The importance of FLNs in a range of cellular processes and the numerous FLN-binding proteins suggests that FLN activity and interactions will be tightly regulated. A number of regulatory mechanisms have been identified, including dynamic phosphorylation and dephosphorylation of FLN or FLN-binding proteins (Jay et al., 2000; Kiema et al., 2006; Vadlamudi et al., 2002; Woo et al., 2004), intramolecular autoinhibition (Lad et al., 2007), mechanical force (Chen et al., 2009; Glogauer et al., 1998; Pentikainen and Ylanne, 2009) and competition between FLN-binding partners (Ithychanda et al., 2009; Lad et al., 2008). In addition, FLNs can be regulated by calpain and caspase proteolysis and, in turn, affect upon many processes, including apoptosis and motility (Browne et al., 2000; O'Connell et al., 2009; Umeda et al., 2001). Finally, we recently found that FLN levels can be acutely controlled by the ubiquitin–proteasome pathway, and that transient loss of FLNs is important during cell differentiation (Bello et al., 2009; Heuze et al., 2008). This is achieved through the action of ASB2 [for ‘ankyrin repeat containing protein with a suppressor of cytokine signaling (SOCS) box 2’] proteins, the specificity subunits of E3 ubiquitin ligase complexes (Bello et al., 2009; Heuze et al., 2008).
ASB2, initially identified as a retinoic-acid-response gene in myeloid leukemia cells (Guibal et al., 2002), encodes two isoforms, ASB2α and ASB2β, which are expressed in hematopoietic and muscle cells, respectively (Bello et al., 2009). ASB2α contains an N-terminal region followed by 15 predicted ankyrin repeats and a C-terminal SOCS box. The SOCS box mediates interaction with a cullin family member (cullin 5) and RING finger proteins (Rbx1 or Rbx2) by interacting with elongin BC to form an E3 ubiquitin ligase complex (Heuze et al., 2005; Kohroki et al., 2005). It has been proposed that ASB2α mediates polyubiquitylation and proteasomal degradation of bound proteins and regulates myeloid cell proliferation and differentiation by targeting regulators of hematopoiesis for degradation (Heuze et al., 2005). Other members of the ASB family have also been proposed to exert their effects by targeting specific regulatory proteins for degradation (Chung et al., 2005; Debrincat et al., 2007; Wilcox et al., 2004) and have been implicated in many biological processes (Boengler et al., 2003; Diks et al., 2006; Kile et al., 2001; Kohroki et al., 2001; McDaneld et al., 2004; McDaneld et al., 2006).
We recently identified FLNa, FLNb and FLNc as ASB2α substrates and showed that ASB2α induces proteasome-mediated degradation of FLNs, whereas an inactive ASB2α mutant lacking the SOCS box, rendering it unable to assemble an E3 ubiquitin ligase complex, does not induce degradation of FLNs (Baldassarre et al., 2009; Heuze et al., 2008). We further showed that ASB2α-mediated degradation of FLNs impacts upon cell spreading and migration and might play a role in cell differentiation (Baldassarre et al., 2009; Heuze et al., 2008). In differentiating myeloid leukemia cells, ASB2α expression correlates with FLN downregulation, and knockdown of endogenous ASB2α delays FLN degradation and differentiation (Heuze et al., 2008), implicating ASB2α-induced FLN degradation in hematopoietic differentiation.
ASB2α represents the first example of FLN regulation through the proteasomal degradation pathway; however, the mechanism by which ASB2α targets FLNs for degradation remained unknown. Here, we use cell-based and biochemical assays to map and characterize the FLN–ASB2α interaction. We find that the subcellular localization of ASB2α is dependent on FLNs, and we map the ABD of FLNa as an ASB2α-binding site. In addition, we show that the ABD is not only necessary but sufficient for ASB2α-mediated proteasomal degradation. Furthermore, we show that ASB2α specifically targets the ABD of all the three FLN isoforms for degradation. These findings provide insight into the molecular basis for ASB2α-mediated FLN degradation and characterize an important mechanism by which FLN levels can be regulated through the targeting of the ABD for proteasomal degradation.
Results
The subcellular localization of ASB2α to actin-rich structures is dependent on FLN
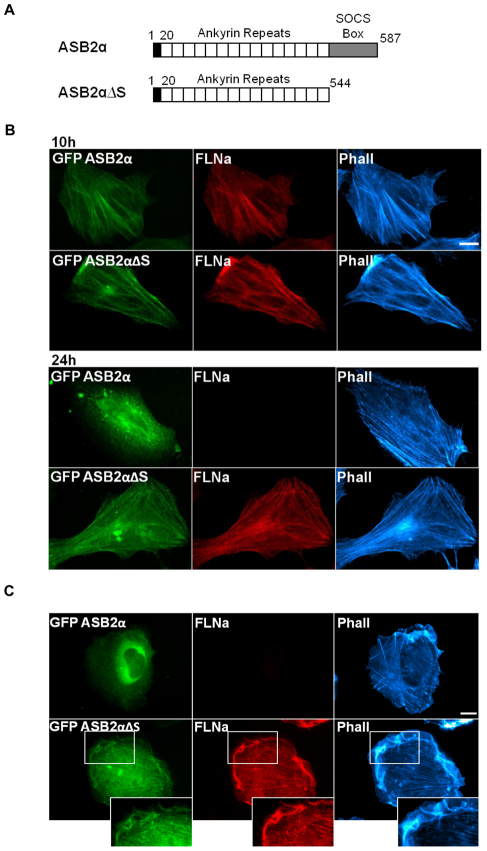
We previously showed that ASB2α serves as the substrate recognition subunit of the E3 ubiquitin ligase complex that mediates polyubiquitylation and proteasomal-mediated degradation of FLNs (Baldassarre et al., 2009; Bello et al., 2009; Burande et al., 2009; Heuze et al., 2008). In order for this process to occur, FLNs and ASB2α must, at least transiently, interact. Consistent with this hypothesis, when expressed in HeLa cells, ASB2α initially colocalizes with FLNa and F-actin at stress fibers (Fig. 1B). However, at later time points, although stress fibers remain, ASB2α is diffuse throughout the cytoplasm (Fig. 1B) and FLNa staining is undetectable in the ASB2α-expressing cells (Fig. 1B). As previously reported (Heuze et al., 2008), expression of an inactive ASB2α mutant (ASB2αΔS) lacking the SOCS box (Fig. 1A), rendering it unable to assemble into an E3 ubiquitin ligase complex, does not induce FLNa degradation (Fig. 1B). This inactive mutant continues to accumulate on stress fibers even at later time points (Fig. 1B).
Fig. 1.
ASB2α exhibits cytoplasmic localization upon FLN degradation. (A) Schematic representation of wild-type ASB2α and the inactive mutant form, ASB2αΔS, lacking the SOCS box. The N-terminus is shaded in black, ankyrin repeats are in white and SOCS box is in gray. (B) HeLa cells were imaged 10 hours and 24 hours after transfection with GFP–ASB2α or GFP–ASB2αΔS. Cells were fixed and stained for FLNa and phalloidin (Phall). (C) HT1080 cells were imaged 24 hours after transfection with GFP–ASB2α or GFP–ASB2αΔS. Cells were fixed and stained as in B. Scale bars: 10 μm.
In HeLa cells, FLNs largely localize to stress fibers (Fig. 1B), and ASB2α regulates FLN abundance at these sites. To determine whether ASB2α regulates FLN abundance in the cell cortex, ASB2α was expressed in HT1080 cells, where FLNs primarily localize to the cell cortex. As in the HeLa cells, 24 hours after transfection, ASB2α is diffuse throughout the cytoplasm and FLNa staining is undetectable in the ASB2α-expressing HT1080 cells (Fig. 1C). However, the inactive ASB2αΔS mutant colocalizes with FLNa to the cell cortex and stress fibers (Fig. 1C). These findings are consistent with the hypothesis that subcellular localization of ASB2α to actin-rich structures is due to association with FLNs because after FLN degradation ASB2α is diffuse throughout the cytoplasm.
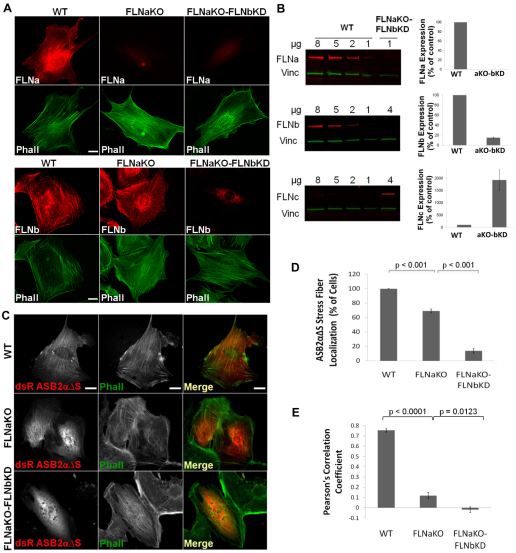
To validate that ASB2α subcellular localization is FLN dependent, we generated FLN-deficient cells. Fibroblasts from wild-type (WT) or FLNa-deficient mouse embryos (Hart et al., 2006) were immortalized using the 3T3 protocol (Todaro and Green, 1963). Although FLNa is the most abundant FLN isoform, we recently showed that FLNb can compensate for the absence of FLNa, at least in terms of the cell spreading and motility effects of FLNs (Baldassarre et al., 2009; Heuze et al., 2008). Therefore, FLNa-deficient FLNb-knockdown (FLNaKO-bKD) fibroblasts were generated by stably transfecting FLNa-deficient cells with short hairpin RNA (shRNA) against FLNb. As shown in Fig. 2A,B, FLNa is not expressed in either of the FLNa deficient lines and FLNb expression is substantially reduced in the FLNaKO-bKD lines. Consistent with our previous studies (Baldassarre et al., 2009), FLNa and FLNb removal did not dramatically affect the actin cytoskeleton, and stress fibers were still present and intact in the FLNaKO and FLNaKO-bKD cells, as shown by the phalloidin staining in Fig. 2A,C.
Fig. 2.
The subcellular localization of ASB2α to stress fibers in fibroblasts is dependent on FLN. (A)WT, FLNaKO and FLNaKO-bKD fibroblasts plated on fibronectin-coated coverslips were fixed and stained for FLNa and phalloidin (Phall) (top panels) or FLNb and phalloidin (Phall) (bottom panels). (B) Western blot of FLNa, FLNb and FLNc expression in FLNaKO-bKD fibroblasts compared with a curve from wild-type (WT) cells. Vinculin was used as a loading control. Results are means ± s.e.m. for three independent experiments. (C)WT, FLNaKO and FLNaKO-bKD fibroblasts plated on fibronectin-coated coverslips were transfected with dsRed–ASB2αΔS. At 24 hours after transfection cells were fixed and stained for phalloidin (Phall). (D) Quantification of dsRed–ASB2αΔS localization to stress fibers in WT, FLNaKO and FLNaKO-bKD cells. Results are means ± s.e.m. for eight independent experiments. P-values were calculated using the Mann–Whitney test. (E) Pearson's correlation coefficient of dsRed–ASB2αΔS colocalization with F-actin in WT, FLNaKO and FLNaKO-FLNbKD cells. Results are mean ± s.e.m. for five random profiles from at least five cells. P-values were calculated using the Mann–Whitney test. Scale bars: 20 μm.
To examine the effect of FLNab-deficiency (i.e. deficiency in both FLNa and FLNb) on ASB2α localization, WT, FLNaKO and FLNaKO-bKD fibroblasts were transfected with dsRed–ASB2αΔS and stained for phalloidin 24 hours later (Fig. 2C). Given that the inactive mutant ASB2αΔS does not induce degradation of FLNs and accumulates primarily on stress fibers in fibroblasts, this protein was used in the localization assays to assess ASB2α targeting to FLNs. Localization of dsRed–ASB2αΔS in each cell line was quantified by scoring the number of ASB2αΔS-expressing cells that showed ASB2αΔS targeting to at least one stress fiber in the red channel (Fig. 2D). ASB2αΔS normally localized to stress fibers in 100% of the WT cells. In the FLNaKO cells, only 70% of cells exhibited any ASB2αΔS localization to stress fibers, and that localization was much less prominent than in the WT cells (Fig. 2C,D). Interestingly, <15% of the FLNaKO-bKD fibroblasts exhibited ASB2αΔS stress fiber localization, and the few cells showing ASB2αΔS targeting to stress fibers exhibited very weak localization, whereas in the majority of the FLNaKO-bKD cells ASB2αΔS was diffuse throughout the cytoplasm (Fig. 2C,D). To better quantify this phenomenon, we measured the colocalization correlation coefficient between ASB2αΔS and F-actin staining (see Materials and Methods for details). The Pearson's coefficient for WT cells was 0.75±0.07, for FLNaKO cells was 0.12±0.17 and for FLNaKO-bKD cells was −0.018±0.12 (Fig. 2E). The colocalization coefficient is consistent with our colocalization scoring and indicates strong correlation of ASB2αΔS with F-actin in WT cells and significantly (P<0.0001) reduced correlation in the FLNaKO and FLNaKO-bKD cells. Note that despite the presence of stress fibers, ASB2αΔS is largely cytoplasmic in the FLNaKO and FLNaKO-bKD cells (Fig. 2C).
The more pronounced reduction of ASB2αΔS localization to stress fibers in the FLNaKO-bKD cells compared with that in the FLNaKO cells suggests that endogenous FLNb is compensating for lack of FLNa. We previously observed that knockdown of FLNa and FLNb in HT1080 human fibrosarcoma cells resulted in upregulation of FLNc (Baldassarre et al., 2009). This compensatory adaptation suggests that a threshold level of FLNs is essential for cell viability. Consistent with this, FLNc expression, although barely detectable in WT fibroblasts, is substantially upregulated in the FLNaKO-bKD cells (Fig. 2A). In the absence of a pan anti-FLN antibody capable of detecting all three isoforms we cannot accurately quantify total FLN levels but, consistent with our data in FLNabKD HT1080 cells (Baldassarre et al., 2009), we believe that total FLN levels are substantially reduced in the FLNaKO-bKD cells, which lack the two major FLN isoforms. This is supported by the near complete lack of ASB2α targeting to stress fibers in the FLNaKO-bKD cells, suggesting that, despite increased FLNc levels, the overall FLN levels remain too low to target ASB2αΔS. Overall, these results are consistent with our observations in HeLa and HT1080 cells and demonstrate that ASB2α localization to F-actin-rich structures is indeed FLN dependent.
The ABD of FLNa is necessary and sufficient for ASB2α-mediated degradation
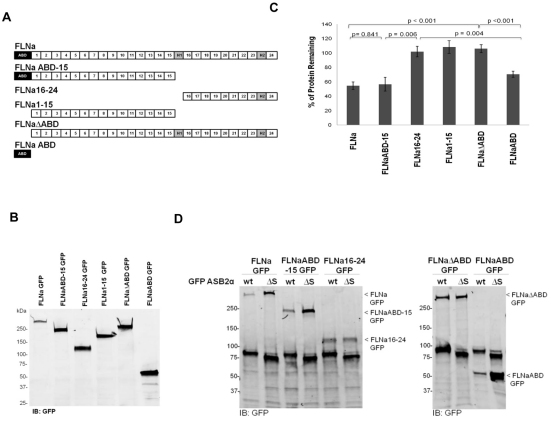
E3 ubiquitin ligase complexes are thought to work by linking substrates to the ubiquitin transferase machinery. Indeed, ASB3 and ASB9 binding to tumor necrosis factor receptor II and creatine kinase B, respectively, results in polyubiquitylation and subsequent degradation of their substrate (Chung et al., 2005; Debrincat et al., 2007). The above results demonstrate that ASB2α targeting to actin-rich structures is dependent on FLNa and FLNb and suggest that ASB2α triggers FLN polyubiquitylation and degradation through an association with FLNs. Thus, the minimal fragment of FLN that can be targeted for degradation by ASB2α is likely to contain the ASB2α-binding site. We therefore generated a series of GFP-tagged FLNa fragments (Fig. 3A); transfection of CHO cells with these constructs resulted in expression of GFP-tagged proteins of the expected sizes as assessed by western blotting (Fig. 3B). We then tested the ability of the FLNa fragments to be targeted for degradation by ASB2α using a FACS-based assay. To do so, each GFP-tagged FLNa construct was transfected into CHO cells, and the cells were divided between two plates. After 24 hours, each plate was re-transfected with either dsRed–ASB2α or dsRed–ASB2αΔS. This serial transfection allowed us to verify expression of the GFP-tagged FLNa constructs before transfection of dsRed–ASB2α or dsRed–ASB2αΔS. At 48 hours after the second transfection, the GFP intensity of dsRed-ASB2α expressing cells was measured and compared with that in the cells expressing the inactive mutant dsRed–ASB2αΔS. ASB2αΔS serves as an internal control for non-specific effects on FLN fragment expression and allows us to compare different experiments and different FLN constructs, independent of differential expression levels or transfection efficiency. Thus, the percentage of protein remaining is reported as a ratio of the GFP intensity in dsRed-ASB2α-expressing cells compared with that in dsRed-ASB2αΔS-expressing cells.
Fig. 3.
The ABD of FLNa is necessary and sufficient for ASB2α-mediated degradation. (A) Schematic diagram of the various FLNa–GFP constructs used in the FACS assay. (B) CHO cells transfected with the various FLNa–GFP constructs were lysed and analyzed by western blotting using anti-GFP antibodies. (C) CHO cells transiently expressing FLNa–GFP, FLNaABD-15–GFP, FLNa16-24–GFP, FLNa1-15–GFP, FLNaΔABD–GFP and FLNaABD–GFP were transfected with either dsRed–ASB2α or dsRed–ASB2αΔS. At 48 hours after transfection, cells were detached, washed with PBS and subjected to a FACS-based assay to measure the GFP intensity of dsRed-expressing cells. The bar chart depicts the mean percentage of GFP-tagged protein remaining (± s.e.m.) in dsRed–ASB2α-expressing cells normalized to levels in dsRed–ASB2αΔS-expressing cells (see Materials and Methods for details). Data are from at least five independent experiments. P-values were calculated using the Mann–Whitney test. (D) CHO cells were co-transfected with GFP–ASB2α (wt, wild-type) or GFP–ASB2αΔS and FLNa–GFP, FLNaABD-15–GFP, FLNa16-24–GFP, FLNaΔABD–GFP or FLNaABD–GFP expression vectors, as indicated. At 48 hours after transaction, 15 μg aliquots of cell lysates were immunoblotted using anti-GFP antibody.
As shown in Fig. 3C, FLNa–GFP and FLNaABD-15–GFP levels were substantially decreased in ASB2α-expressing cells compared with those in ASB2αΔS-expressing cells. By contrast, the expression of FLNa16-24–GFP was unaffected (Fig. 3C). These data narrowed down the region of FLNa that is targeted for degradation by ASB2α to the region from the ABD to Ig domain 15 of FLNa. We next tested ASB2α-mediated degradation of FLNa1-15, a variant of FLNaABD-15 lacking the ABD (Fig. 3A). Similar to FLNa16-24–GFP, FLNa1-15–GFP was not degraded (Fig. 3C), suggesting that the ABD is required for targeted degradation. Consistent with this, FLNaΔABD-GFP, containing Ig domains 1–24 but lacking the ABD, was also resistant to ASB2α-mediated degradation. Furthermore, FLNaABD–GFP was degraded following ASB2α expression (Fig. 3C), indicating that the ABD is not only necessary but also sufficient for ASB2α-mediated degradation. To confirm these results using a biochemical assay, CHO cells were co-transfected with the various FLNa–GFP constructs and GFP-ASB2α or GFP-ASB2αΔS. At 48 hours after transfection, western blotting showed that levels of FLNa constructs containing the ABD were reduced in presence of ASB2α but not the inactive ASB2αΔS mutant (Fig. 3D). The results of the biochemical assay are consistent with the FACS assay and confirm that the ABD is not only necessary but also sufficient for ASB2α-mediated degradation.
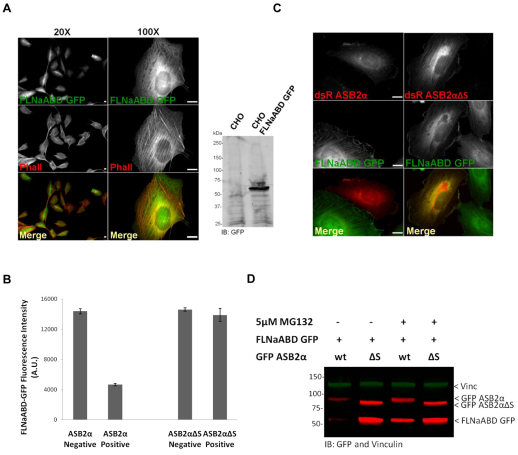
To further validate that FLNaABD is sufficient for ASB2α-mediated degradation, a monoclonal CHO cell line stably expressing FLNaABD–GFP was generated (Fig. 4A) and transfected with dsRed–ASB2α or dsRed–ASB2αΔS. The 40% transfection efficiency of dsRed–ASB2α and dsRed–ASB2αΔS allowed us to gate on dsRed-positive or -negative cells and measure the FLNaABD–GFP intensity of both populations within the same sample. As shown in Fig. 4B, FLNaABD–GFP levels were substantially decreased in ASB2α-expressing cells compared with those in ASB2α non-expressing cells. However, ASB2αΔS expression did not affect FLNaABD–GFP levels (Fig. 4B). Consistently, as shown in Fig. 4C, FLNaABD–GFP levels were greatly reduced in ASB2α-expressing cells, whereas ASB2αΔS did not induce degradation of FLNaABD–GFP. Notably, both ASB2αΔS and FLNaABD accumulate on stress fibers but ASB2α is diffusely distributed in cells where FLNaABD had been degraded (Fig. 4A,C).
Fig. 4.
The ABD of FLNa is targeted to proteasomal degradation by ASB2α. (A) A monoclonal CHO cell line stably expressing FLNaABD–GFP (CHO FLNaABD–GFP) was stained for phalloidin (Phall) and analyzed by western blotting using anti-GFP antibodies. (B) CHO FLNaABD-GFP cells were transfected with either dsRed–ASB2α or dsRed–ASB2αΔS. At 48 hours after transfection, cells were detached, washed with PBS and subjected to a FACS-based assay to measure the GFP intensity of dsRed-expressing and non-expressing cells. The bar chart depicts the mean GFP intensity of dsRed-expressing (positive) and non-expressing (negative) cells (± s.e.m.) for three independent experiments. (C) CHO FLNaABD–GFP cells were transfected with dsRed–ASB2α or dsRed–ASB2αΔS. Cells were imaged 48 hours after transfection. (D) CHO cells were co-transfected with FLNaABD–GFP and GFP–ASB2α or GFP–ASB2αΔS. At 30 hours after transfection, cells were untreated or treated with 5 μM MG132 for 18 hours. At 48 hours after transfection, cells were lysed and immunoblotted using anti-GFP antibodies. Vinculin was used as a loading control. Scale bars: 10 μm.
We previously showed that ASB2α E3 ubiquitin ligase activity mediates polyubiquitylation and proteasomal degradation of FLNa (Heuze et al., 2008). To determine whether ASB2α-induced degradation of FLNaABD–GFP is mediated through the proteasome, CHO cells co-transfected with FLNaABD–GFP and GFP–ASB2α or GFP–ASB2αΔS were untreated or treated with the proteasome inhibitor MG132. Consistent with our above results, in untreated cells the level of FLNaABD–GFP was reduced in presence of ASB2α but not ASB2αΔS. However, MG132 inhibited ASB2α-induced FLNaABD–GFP degradation (Fig. 4D). Taken together, these results indicate that the ABD of FLNa is a substrate of the ASB2α E3 ubiquitin ligase complex and that ASB2α-induced degradation of FLNaABD is proteasome dependent.
The ABD of FLNa is sufficient for ASB2α binding and targeting to stress fibers in FLNab-deficient fibroblasts
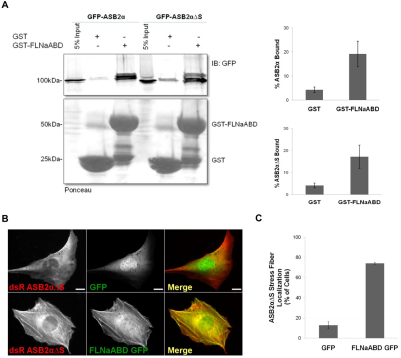
The above results, demonstrating that FLNaABD is the minimal domain of FLNa necessary and sufficient for ASB2α-mediated degradation, suggest that this region contains the ASB2α-binding site. To test whether FLNaABD is sufficient for ASB2α binding, pull-down assays were performed. GST and GST–FLNaABD were expressed in Escherichia coli and purified on glutathione affinity resin. Immobilized GST or GST–FLNaABD was then used in pull-down assays to assess ASB2α binding. Both GFP–ASB2α and GFP–ASB2αΔS bound to GST–FLNaABD, whereas only background binding was seen to the GST beads (Fig. 5A) demonstrating that the ABD of FLNa is sufficient for ASB2α binding.
Fig. 5.
The ABD of FLNa is sufficient for ASB2α binding and targeting to stress fibers in the FLNab-deficient fibroblasts. (A) CHO cells transfected with GFP–ASB2α or ASB2αΔS were lysed, and lysates were incubated with GST or GST–FLNaABD coated onto glutathione–Sepharose beads. After incubation, beads were washed and resuspended in sample buffer. Bound proteins were fractionated by SDS-PAGE and analyzed by western blotting using anti-GFP antibodies. Results are means ± s.e.m. for eight independent experiments for ASB2α and four independent experiments for ASB2αΔS. (B) FLNaKO-bKD fibroblasts were co-transfected with dsRed–ASB2αΔS and GFP or FLNaABD–GFP. At 24 hours after transfection cells were fixed and imaged. Scale bars: 20 μm. (C) Quantification of dsRed–ASB2αΔS localization to stress fibers in FLNaKO-bKD fibroblasts expressing GFP or FLNaABD–GFP. Results are means ± s.e.m. for five independent experiments.
We have shown that ASB2αΔS fails to target to stress fibers in the FLNaKO-bKD cells (Fig. 2C,D), that FLNaABD is effectively targeted for degradation by ASB2α in a proteasome-dependent manner (Fig. 3C,D and Fig. 4B,C,D) and that FLNaABD binds ASB2α and ASB2αΔS (Fig. 5A). As FLNaABD efficiently targets to stress fibers (Fig. 4A,C) we next tested whether FLNaABD is sufficient to restore targeting of ASB2αΔS to stress fibers in the FLNab-deficient fibroblasts. Expression of GFP–FLNaABD, but not GFP, rescued the stress fiber localization of ASB2αΔS in FLNaKO-bKD fibroblasts (Fig. 5B,C), suggesting that FLNaABD is sufficient to target ASB2α to stress fibers. Taken together, our results indicate that the ABD is the minimal FLNa domain sufficient for ASB2α binding and targeting.
ASB2α targets the ABD of FLNb and FLNc for degradation
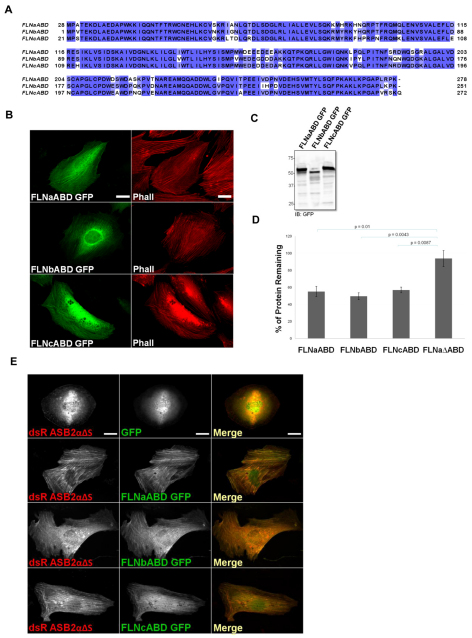
We previously showed that ASB2α targets FLNa, FLNb and FLNc for proteasomal degradation (Baldassarre et al., 2009; Bello et al., 2009; Burande et al., 2009; Heuze et al., 2008) and have now mapped the ABD of FLNa as the minimal fragment sufficient for ASB2α-mediated degradation. The structure of FLNaABD is highly similar to the structure of FLNbABD [root mean square deviation (r.m.s.d.) of 0.425 Å; 1 Å=0.1 nm] with 89% sequence identity (Ruskamo and Ylanne, 2009), whereas FLNaABD and FLNcABD show 82% sequence identify. Considering the high sequence identity among the ABDs of the three FLN isoforms (Fig. 6A), we asked whether ASB2α could also target the ABD of FLNb and FLNc for degradation. We generated FLNbABD–GFP and FLNcABD–GFP constructs (Fig. 6B,C) and showed that, like FLNaABD–GFP, they target to stress fibers (Fig. 6B). Furthermore, similar to FLNaABD–GFP, FLNbABD–GFP and FLNcABD–GFP are efficiently degraded in the presence of ASB2α, as assessed by FACS analysis (Fig. 6D).
Fig. 6.
The ABD of FLNb and FLNc are targeted for degradation by ASB2α and rescue ASB2α localization in the FLNab-deficient fibroblasts. (A) Sequence alignment of human FLN ABDs. The sequences are aligned with CLUSTALW2 and colored by sequence identity. (B) Fibroblast cells transfected with FLNaABD–GFP, FLNbABD–GFP and FLNcABD–GFP were stained for phallodin (Phall). (C) CHO cells transfected with FLNaABD–GFP, FLNbABD–GFP and FLNcABD-GFP were lysed and immunoblotted using anti-GFP antibodies. (D) CHO cells transiently expressing FLNaABD–GFP, FLNbABD–GFP, FLNcABD–GFP and FLNaΔABD–GFP were transfected with either dsRed–ASB2α or dsRed–ASB2αΔS. At 48 hours after transfection, cells were detached, washed with PBS and subjected to a FACS-based assay to measure the GFP intensity of dsRed-expressing cells. The bar chart depicts the mean percentage of GFP-tagged protein remaining (± s.e.m.) in dsRed–ASB2α-expressing cells normalized to levels in dsRed–ASB2αΔS-expressing cells (see Materials and Methods for details). Data are from at least five independent experiments. P-values were calculated using the Mann–Whitney test. (E) FLNaKO-bKD fibroblasts were co-transfected with dsRed–ASB2αΔS and GFP, FLNaABD–GFP, FLNbABD–GFP or FLNcABD–GFP. At 24 hours after transfection cells were fixed and imaged. Scale bars: 20 μm.
The ABD of FLNb and FLNc rescue ASB2α targeting to stress fibers in FLNab-deficient fibroblasts
The above results, demonstrating that the ABD of FLNa, FLNb and FLNc are efficiently targeted for degradation, establish the specificity of ASB2α for the ABD of three FLN isoforms. We next asked whether FLNbABD or FLNcABD could restore F-actin targeting of ASB2αΔS in FLNab-deficient fibroblasts. Similar to FLNaABD–GFP, expression of FLNbABD–GFP and FLNcABD–GFP, but not GFP, rescued the stress fiber localization of ASB2αΔS in FLNab-deficient fibroblasts (Fig. 6E), suggesting that FLNbABD and FLNcABD are sufficient to target ASB2α to stress fibers and are capable of binding ASB2α. The ability of FLNcABD to recruit ASB2α to stress fibers suggests that the levels of FLNc in the FLNab-deficient cells remain below the threshold and that rescue of targeting requires higher FLNc expression levels.
ASB2α specifically targets the ABD of FLNa and not α-actinin for degradation
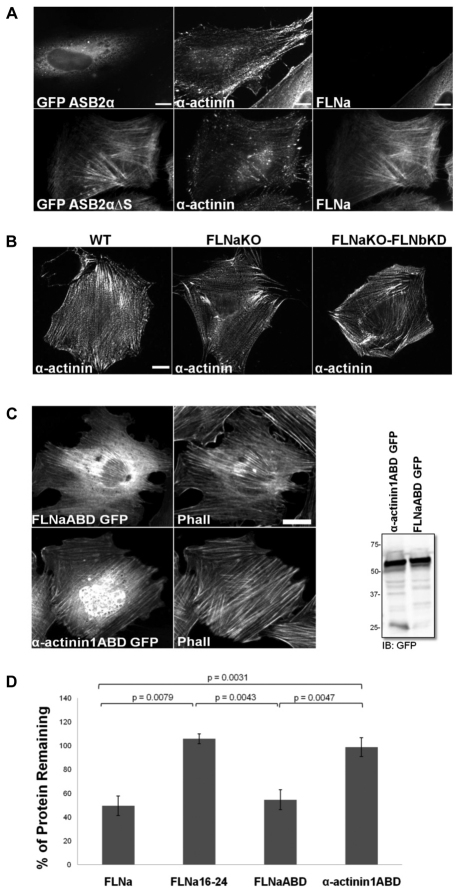
The ABDs of FLNs are composed of two calponin homology (CH) domains, and structurally related domains are found in several F-actin binding, bundling and crosslinking proteins, such as α-actinins, spectrins, dystrophin, utrophin and plectins (Gimona et al., 2002). α-Actinin forms an anti-parallel dimer with an ABD followed by four spectrin repeats and a calmodulin-like domain (Otey and Carpen, 2004). The α-actinin1ABD has 36% sequence identity to FLNaABD and is structurally closely related to FLNaABD (r.m.s.d. of 1.4 Å) (Ruskamo and Ylanne, 2009), and, like FLNs, α-actinin localizes to stress fibers (Lorenzi and Gimona, 2008). We therefore asked whether ASB2α is specific for FLNs or whether it could also induce degradation of α-actinin, and whether α-actinin is sufficient to target ASB2α to stress fibers.
To determine the specificity of ASB2α-mediated degradation, HeLa cells were transfected with GFP–ASB2α or GFP–ASB2αΔS and 48 hours after transfection cells were stained for α-actinin and FLNa. In GFP–ASB2α-expressing cells FLNa was undetectable but α-actinin staining remained, demonstrating the selectivity of ASB2α for FLNs (Fig. 7A). Furthermore, despite the presence of α-actinin on stress fibers, GFP–ASB2α was diffuse throughout the cytoplasm indicating that α-actinin is not sufficient to recruit ASB2α to stress fibers (Fig. 7A). This latter point was confirmed in FLNaKO-bKD fibroblasts, as α-actinin staining on stress fibers was comparable in WT, FLNaKO and FLNaKO-bKD cells (Fig. 7B) but ASB2α targeting to stress fibers was impaired in the FLNaKO-bKD cells (Fig. 2C). These results suggest that ASB2α localization is independent of α-actinin and further validates the requirement of FLNa and FLNb for targeting to stress fibers.
Fig. 7.
ASB2α specifically targets the ABD of FLNa but not α-actinin1 for degradation. (A) HeLa cells plated on fibronectin-coated coverslips were transfected with GFP–ASB2α or GFP–ASB2αΔS. At 48 hours after transfection, cells were treated with 0.05% Triton X-100 before fixation, and stained for α-actinin and FLNa. (B) WT, FLNaKO and FLNaKO-bKD fibroblasts plated on FN-coated coverslips were treated with 0.05% Triton X-100 before fixation and stained for α-actinin. (C) CHO cells transfected with FLNaABD–GFP or α-actinin1ABD–GFP were stained for phalloidin (Phall) and immunoblotted using anti-GFP antibodies. (D) CHO cells transiently expressing FLNa–GFP, FLNa16-24–GFP, FLNaABD-GFP and GFP-α-actinin1ABD were transfected with either dsRed–ASB2α or dsRed–ASB2αΔS. 48 hours after transfection, cells were detached, washed in PBS and subjected to a FACS based assay to measure the GFP intensity of dsRed-expressing cells. Bar chart depicts mean percentage of GFP-tagged protein remaining ± s.e.m. in dsRed-ASB2α expressing cells normalized to levels in dsRed-ASB2αΔS expressing cells (see Materials and Methods for details). Data are from at least five independent experiments. P-values were calculated using the Mann–Whitney test. Scale bars: 10 μm (A); 20 μm (B); 10 μm (C).
As a final test of the specificity of ASB2α, we used the FACS-based assay to assess ASB2α-mediated degradation of α-actinin1ABD. Similar to FLNaABD–GFP, α-actinin1ABD–GFP is sufficient to localize to stress fibers (Fig. 7C) (Lorenzi and Gimona, 2008). As shown above, both full-length FLNa and FLNaABD were degraded (Fig. 7D). By contrast, similar to FLNa16-24, α-actinin1ABD was not degraded (Fig. 7D). In summary, despite their similar structure and their related sequence, ASB2α specifically targets FLNaABD and not α-actinin1ABD for degradation, and the localization of ASB2α to stress fibers in fibroblasts is dependent on FLNs despite the presence of α-actinin and F-actin.
Discussion
ASB2α-mediated proteasomal degradation is a new mechanism by which FLN levels can be acutely regulated and has the potential to affect many biological processes but specifically hematopoiesis. In acute myeloid leukemia, cells are arrested at an immature step of differentiation leading to an accumulation of granulocyte and monocyte precursors in the bone marrow and blood (Heuze et al., 2008). All-trans retinoic acid serves as an effective therapy for inducing differentiation of acute promyelocytic leukemia. ASB2α is expressed upon retinoic acid treatment and proposed to regulate myeloid cell proliferation and differentiation by targeting regulators of hematopoiesis for degradation (Guibal et al., 2002; Heuze et al., 2005; Heuze et al., 2008). We identified FLNs as ASB2α substrates and showed that ASB2α triggers degradation of these proteins in various cell types, including differentiating myeloid leukemia cells (Baldassarre et al., 2009; Heuze et al., 2008). Retinoic-acid-induced expression of ASB2α correlates with FLN downregulation in myeloid leukemia cells induced to differentiate. Although, retinoic acid is the only pathway known to trigger ASB2α-mediated FLN degradation, other pathways and stimuli might be linked to degradation of FLNs. Knockdown of endogenous ASB2α delays FLN degradation and differentiation (Heuze et al., 2008), suggesting that FLNs can play a role in hematopoietic cell differentiation.
Despite the ample evidence that ASB2α triggers FLN degradation, the mechanism by which ASB2α interacts with FLN to induce degradation remained unknown. Here, we used a systematic approach to characterize the FLN–ASB2α interaction and map the ASB2α-binding site within FLNa. Our initial observations suggested that the subcellular localization of ASB2α to actin-rich structures is due to association with FLNs. ASB2α initially colocalizes with FLNs to stress fibers in HeLa cells and fibroblasts, or to the cell cortex in HT1080 cells. However, after FLN degradation ASB2α is diffuse throughout the cytoplasm despite the presence of actin-rich structures. Thus, regardless of FLN localization, transiently expressed ASB2α colocalizes with FLNs and triggers FLN loss from stress fibers and the cell cortex. Furthermore, ASB2α effectively triggers loss of FLNs in promyelocytic NB4 and myeloblastic PLB985 cells (Heuze et al., 2008), which do not form evident stress fibers. Following loss of FLNs, ASB2α localization is cytoplasmic, consistent with an interaction between ASB2α and FLNs.
To assess the dependency of the subcellular localization of ASB2 on FLNs more directly, FLNab-deficient fibroblast cells were used as a model system in our studies. In WT fibroblast cells, FLNs and ASB2α primarily localize to stress fibers, whereas in the FLNab-deficient cells ASB2α exhibits cytoplasmic localization, suggesting that the ASB2α subcellular localization is indeed dependent on FLNs. Furthermore, expression of FLNaABD rescues the ASB2α subcellular localization to stress fibers in FLNab-deficient fibroblasts. This result is consistent with our binding assay indicating that the ABD is capable of binding ASB2α. The ability of FLNaABD to target ASB2α to F-actin strongly suggests that ASB2α binding does not affect actin binding to the ABD.
ASB2α is expressed in hematopoietic cells (Bello et al., 2009) and its primary function appears to be in the differentiation of blood cells rather than in differentiation of adherent fibroblast cells, as used in our study. The cells used in our study provided a facile means to investigate ASB2α function and its mode of interaction with FLNs. Here, we show that exogenous expression of ASB2α induces FLN degradation in adherent cell types, as it does in hematopoietic cells, validating that conserved mechanisms are involved. Whether there is endogenous expression of ASB2α, and whether ASB2α can regulate cell differentiation, in such cell types remains to be determined. Additionally, the dependency of ASB2α-induced FLN degradation and differentiation on F-actin are subjects of ongoing investigation.
We previously showed that some non-muscle cells express low but detectable levels of FLNc and that FLNc is upregulated after FLNa and FLNb knockdown (Baldassarre et al., 2009). Consistent with this observation, FLNc expression was barely detectable in WT fibroblasts but was upregulated in FLNab-deficient cells. The significant upregulation of FLNc might explain the lack of effect on the actin cytoskeleton. However, FLNc upregulation was insufficient to rescue ASB2α targeting, whereas overexpression of FLNcABD rescued ASB2α localization, suggesting that FLNc levels remained too low to support stress fiber targeting. Unfortunately our anti-FLNc antibody does not work for immunofluorescence (data not shown) so we cannot determine the localization of the upregulated FLNc in the FLNab-deficient cells but, like FLNcABD–GFP, overexpressed FLNc-GFP localizes to stress fibers (data not shown) suggesting that FLNc is capable of stress fiber targeting.
Using a FACS-based assay we identified the ABD of FLNa as the minimal fragment necessary for ASB2α-mediated degradation and showed that the isolated ABD is efficiently targeted for degradation by ASB2α. The FACS assay allowed us to gate specifically on dsRed–ASB2α- or dsRed–ASB2αΔS-expressing cells and measure GFP intensity of thousands of ‘red’ cells. Thus, this assay is extremely sensitive and quantitative, and provides an accurate measure for degradation. We note that the level of FLN degradation achieved in this assay was not complete and attribute this to the high expression of the GFP-tagged FLN constructs. To validate the results from the serial transfection, the FACS assay was repeated using a clonal CHO cell line stably expressing FLNaABD–GFP. Indeed, using this line, the expression of FLNaABD–GFP was significantly reduced in the ASB2α-expressing cells, further validating the ABD of FLNa as a substrate of ASB2α.
We previously showed that ASB2α E3 ubiquitin ligase activity mediates proteasomal degradation of FLNa (Heuze et al., 2008) and now show that ASB2α-induced degradation of FLNaABD is also proteasome dependent. Furthermore, we demonstrate the specificity of ASB2α for the ABD of all the three members of the FLN family and show that the ABD of FLNb and FLNc are also efficiently targeted for degradation by ASB2α. In addition, we show that expression of FLNbABD and FLNcABD rescue stress fiber targeting of ASB2α in FLNab-deficient cells. Interestingly, we show that, despite their similar structure and related sequence, ASB2α specifically targets FLNaABD and not α-actinin1ABD for degradation. Taken together, these results demonstrate the specificity of ASB2α for the ABD of the three FLN isoforms and suggest that FLNbABD and FLNcABD are also ASB2α substrates and capable of interacting with ASB2α. Thus, we propose that ASB2α, upon binding FLN, mediates polyubiquitylation of the lysine residue(s) within the ABD and targets FLN for proteasomal degradation. Identification of lysine residue(s) within the ABD that might be targeted for polyubiquitylation by the ASB2α E3 ubiquitin ligase complex is subject of ongoing investigation.
FLNs play an important role in the differentiation of various cell types (Bello et al., 2009; Heuze et al., 2008; Lu et al., 2007; van der Flier et al., 2002; Zheng et al., 2007). Deciphering the exact mechanism by which ASB2α-mediated FLN degradation impacts upon cell differentiation is a major challenge. Knockdown of FLNa and FLNb inhibits cells spreading (Baldassarre et al., 2009; Heuze et al., 2008), and this effect is recapitulated in ASB2α-expressing cells, which display decreased area compared with that of cells expressing inactive mutants of ASB2α (Heuze et al., 2008). Cytoskeletal reorganization, cell spreading and changes in cell shape are integral aspects of differentiation (McBeath et al., 2004). Cell spreading and changes in cell shape regulate differentiation by altering focal adhesion assembly, RhoA downstream signaling pathways, and expression and signaling of integrins and cytoskeletal proteins (McBeath et al., 2004). Thus, we speculate that ASB2α can control hematopoietic differentiation by modulating cell spreading and actin remodeling through targeting of FLNs for degradation.
In addition to F-actin, FLNs interact with transmembrane receptors and many signaling and adaptor proteins, and act as scaffold for a wide range of signaling pathways. They regulate integrin signaling and the activity of small GTPases of the Rho family, as well as factors upstream and downstream of GTPases (Zhou et al., 2010). Thus, ASB2α-mediated FLN degradation can impact upon many pathways involving FLNs, specifically those related to changes in cell shape and spreading. In our studies, we demonstrate the specificity of ASB2α for the ABD of the three members of the FLN family and show that the ABD is necessary for ASB2α-mediated degradation and binding. This latter finding is of particular importance and will facilitate future mutational and structural studies. Importantly, a detailed understanding of the nature of the ASB2α–FLN interaction will facilitate future studies on ASB2α and allow additional mechanistic studies into the significance of ASB2α-mediated FLN degradation in cell function and differentiation.
Materials and Methods
Reagents and DNA constructs
Monoclonal anti-vinculin (Sigma), polyclonal anti-FLNc (Kinasource), polyclonal anti-GFP (Rockland), monoclonal anti-α-actinin (Sigma), secondary Alexa-Fluor-568-conjugated anti-rabbit-IgG (Invitrogen), Alexa-Fluor-800-conjugated anti-rabbit-IgG (Invitrogen), Alexa-Fluor-647-conjugated anti-rabbit-IgG (Invitrogen), Alexa-Fluor-568-conjugated anti-mouse-IgG (Invitrogen), Alexa-Fluor-680-conjugated anti-goat-IgG (Invitrogen) antibodies, and phalloidin–Alexa-Fluor-568, phalloidin–Alexa-Fluor-350 and phalloidin–Alexa-Fluor-647 (Invitrogen), were purchased. The anti-FLNa and anti-FLNb antisera were raised against domains 19–21 of the respective proteins (Baldassarre et al., 2009; Heuze et al., 2008; Kiema et al., 2006). Fibronectin (FN) solution (1 mg/ml) was from Sigma. Full-length FLNa–GFP, FLNaABD-15 (amino acids 1–1761) and FLNa16-24 (amino acids 1762–2647) conjugated to GFP have been described previously (Lad et al., 2007; Lad et al., 2008). FLNaABD (amino acids 1–275), FLNa1-15 (amino acids 276–1761), FLNbABD (amino acids 1–251) and FLNcABD (amino acids 1–272), α-actinin1ABD (amino acids 1–253) were generated using PCRs and subcloned into GFP pCDNA3, a modified version of pCDNA3 expression vector (Invitrogen). FLNaΔABD (amino acids 276–2647) conjugated to GFP was generated by subcloning FLNaΔABD (kindly provided by Thomas P. Stossel, Harvard Medical School, Boston, MA) (Nakamura et al., 2007) into GFP pCDNA3. GST-FLNaABD was a gift from Jari Ylänne (University of Jyväskylä, Finland) (Ruskamo and Ylanne, 2009). GFP–ASB2α, GFP–ASB2αΔS and dsRed–ASB2α expression constructs were as described previously (Heuze et al., 2008). DsRed–ASB2αΔS was generated by subcloning ASB2αΔS (amino acids 1–544) into the pDsRed-monomerC1 expression vector (Clontech). FLNb shRNA in the pGIPZ vector was purchased from OpenBiosystems and MG132 was kindly provided by Mark Hochstrasser (Yale University, New Haven, CT).
Cell lines, culture conditions and transfection
HT1080 and HeLa cells were cultured in Dulbecco's modified essential medium (DMEM) (Invitrogen) containing 9% fetal bovine serum (FBS) (Atlanta Biological), sodium pyruvate (Invitrogen) and penicillin-streptomycin (Invitrogen). Immortalized fibroblasts from mouse embryos were cultured in DMEM containing 9% fetal clone III (Hyclone), sodium pyruvate and penicillin-streptomycin. CHO cells were cultured in DMEM containing 9% FBS, sodium pyruvate, non-essential amino acids (Invitrogen) and penicillin-streptomycin and were incubated at 37°C in a humidified atmosphere containing 5% CO2. For transfection, cells were either plated at 50% confluence and transfected 24 hours after plating or transfected in suspension using Lipofectamine 2000 (Invitrogen). For proteasome inhibition, cells were treated with 5 μM MG132 for 18 hours.
Generation of FLNa-deficient FLNb-knockdown fibroblast cell lines
Fibroblasts from FLNa-deficient mouse embryos (Hart et al., 2006) were immortalized using the 3T3 protocol (Todaro and Green, 1963) by continual passage of 3×105 cells every 3 days on 50-mm dishes for 15–20 passages. Stable FLNa-deficient FLNb-knockdown (FLNaKO-bKD) lines were established by transfecting immortalized FLNa deficient fibroblasts with pGIPZ vector expressing FLNb shRNA. Polyclonal populations were selected using 4 μg/ml puromycin (Sigma). FLN expression was quantified by western blotting using anti-FLNa, anti-FLNb and anti-FLNc antibodies. Anti-vinculin antibody was used as a loading control.
Quantification of colocalization
A special macro on the open source software ImageJ was developed and applied to images of dsRed–ASB2αΔS-expressing cells stained for F-actin (phalloidin conjugated to Alexa-Fluor-350) as follows. ASB2αΔS and the F-actin stain were normalized to account for intensity variation. Then a line was drawn across the cell, and fluorescence profiles along that line were collected for both ASB2αΔS and F-actin. The profiles were then compared and Pearson's correlation coefficient was obtained using the JACoP plugIn (http://fsbweb.nih.gov/ij/plugins/track/jacop.html).
Generation of CHO cell line expressing FLNaABD–GFP
A CHO cell line stably expressing FLNaABD–GFP was established by transfecting CHO wild-type cells with FLNaABD–GFP pCDNA3 vector. Polyclonal populations were selected using 2 mg/ml Geneticin. Single clones were obtained by limiting dilution of the polyclonal population and screened by immunofluorescence and western blotting.
Immunofluorescence
Cells plated on fibronectin-coated (5 μg/ml) coverslips were fixed in 4% paraformaldehyde in PBS (pH 7.4) for 15 minutes and permeabilized for 30 minutes with PBS containing 0.2% BSA, 50 mM NH4Cl and 0.3% Triton X-100. After three washes with PBS, the coverslips were incubated with primary antibody or fluorophore-conjugated phalloidin for 1 hour at room temperature, washed in PBS and incubated with secondary antibody for 1 hour at room temperature. Coverslips were mounted using the ProLongGold anti-fade mounting agent (Invitrogen). Images were acquired using a Nikon TE2000, with a 20×, 40× or 100× objective and IPLab (version 3.5.2; Scanlytics, Fairfax, VA) software, and were analyzed using ImageJ. For α-actinin staining cells were permeabilized with 0.05% Triton X-100 for 30 seconds before fixation.
Immunoblotting
Cells were lysed in RIPA buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate and 0.1% SDS) containing protease inhibitor cocktail tablets (Roche). Proteins were resolved by SDS-PAGE, transferred onto nitrocellulose membrane (Bio-Rad) and blocked for 1 hour with 2% BSA in TBS-T (0.1 M Tris-HCl pH 7.4, 135 mM NaCl and 0.05% Tween-20). The membranes were incubated with primary antibodies overnight at 4°C, washed in TBS-T and incubated with fluorescent secondary antibodies. The signal was detected using the Odyssey infrared imaging system (LI-COR Biotechnology). Band intensities were quantified using ImageJ.
FACS assays
For the serial transfection, CHO cells were transfected with FLNa–GFP, FLNaABD-15–GFP, FLNa16-24–GFP, FLNa1-15–GFP, FLNaΔABD–GFP, FLNaABD–GFP, FLNbABD–GFP, FLNcABD–GFP and α-actinin1ABD–GFP. At 24 hours after transfection cells were re-transfected with either dsRed–ASB2α or dsRed–ASB2αΔS. At 48 hours after the second transfection, cells were detached, washed in PBS and the GFP fluorescence intensity of dsRed-expressing cells was quantified using a FACSCalibur or LSRII instrument (BD Biosciences). FACS data analysis was carried out using FlowJo FACS analysis software. The percentage of protein remaining was defined as Fα/FαΔS × 100, where Fα is the GFP geometric mean fluorescence intensity (MFI) of dsRed–ASB2α-expressing cells and FαΔS is the GFP geometric mean fluorescence intensity of dsRed–ASB2αΔS-expressing cells.
The CHO cell line stably expressing FLNaABD–GFP was transfected with either dsRed–ASB2α or dsRed–ASB2αΔS. At 48 hours after transfection cells were detached, washed in PBS and the GFP fluorescence intensity of dsRed-expressing and non-expressing cells was quantified using the FACSCalibur or LSRII instrument (BD Biosciences). FACS data analysis was carried out using FlowJo FACS analysis software and the GFP geometric mean fluorescence intensity of dsRed-positive and -negative cells was reported.
Binding assays
GST fusion proteins were produced in E. coli BL21 Gold (Stratagene) and purified on glutathione–Sepharose 4 Fast Flow medium (GE Healthcare) according to the manufacturer's instructions. CHO cells were transiently transfected with GFP-ASB2α or ASB2αΔS, harvested 24 hours later and lysed. Cell lysates were incubated overnight with GST, or GST–FLNaABD bound to glutathione–Sepharose beads, washed and resuspended in SDS sample buffer. Bound proteins were fractionated by SDS-PAGE and analyzed by western blotting using anti-GFP antibodies. Band intensities were quantified using ImageJ and the percentage of bound protein was defined as B/Btotal × 100 where B is the band intensity of GFP–ASB2α or GFP–ASB2αΔS from the pull-down and Btotal is the band intensity of the total input.
Acknowledgments
We thank Jari Ylänne (University of Jyväskylä, Finland) and Thomas P. Stossel (Harvard Medical School, Boston, MA) for kindly providing the GST–FLNaABD and FLNaΔABD constructs, respectively. FLNa-deficient mouse embryonic fibroblasts, originally generated with support from GlaxoSmithKline, were provided by Sally H. Cross (MRC Human Genetics Unit, Edinburgh, UK). This work was supported by a grant from the National Institutes of Health (RO1 GM-068600) to D.A.C., an award from the American Heart Association to Z.R., by the Centre National de la Recherche Scientifique and Université Paul Sabatier and by grants to P.G.L. from the Comité Leucémie de la Fondation de France and from the Association pour la Recherche sur le Cancer. I.L. was supported by fellowships from the Lady TATA Foundation and the Comité Leucémie de la Fondation de France. Deposited in PMC for release after 12 months.
References
- Baldassarre M., Razinia Z., Burande C. F., Lamsoul I., Lutz P. G., Calderwood D. A. (2009). Filamins regulate cell spreading and initiation of cell migration. PLoS ONE 4, e7830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello N. F., Lamsoul I., Heuze M. L., Metais A., Moreaux G., Calderwood D. A., Duprez D., Moog-Lutz C., Lutz P. G. (2009). The E3 ubiquitin ligase specificity subunit ASB2beta is a novel regulator of muscle differentiation that targets filamin B to proteasomal degradation. Cell Death Differ. 16, 921-932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boengler K., Pipp F., Fernandez B., Richter A., Schaper W., Deindl E. (2003). The ankyrin repeat containing SOCS box protein 5, a novel protein associated with arteriogenesis. Biochem. Biophys. Res. Commun. 302, 17-22 [DOI] [PubMed] [Google Scholar]
- Browne K. A., Johnstone R. W., Jans D. A., Trapani J. A. (2000). Filamin (280-kDa actin-binding protein) is a caspase substrate and is also cleaved directly by the cytotoxic T lymphocyte protease granzyme B during apoptosis. J. Biol. Chem. 275, 39262-39266 [DOI] [PubMed] [Google Scholar]
- Burande C. F., Heuze M. L., Lamsoul I., Monsarrat B., Uttenweiler-Joseph S., Lutz P. G. (2009). A label-free quantitative proteomics strategy to identify E3 ubiquitin ligase substrates targeted to proteasome degradation. Mol. Cell. Proteomics 8, 1719-1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. S., Kolahi K. S., Mofrad M. R. (2009). Phosphorylation facilitates the integrin binding of filamin under force. Biophys. J. 97, 3095-3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung A. S., Guan Y. J., Yuan Z. L., Albina J. E., Chin Y. E. (2005). Ankyrin repeat and SOCS box 3 (ASB3) mediates ubiquitination and degradation of tumor necrosis factor receptor II. Mol. Cell. Biol. 25, 4716-4726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debrincat M. A., Zhang J. G., Willson T. A., Silke J., Connolly L. M., Simpson R. J., Alexander W. S., Nicola N. A., Kile B. T., Hilton D. J. (2007). Ankyrin repeat and suppressors of cytokine signaling box protein asb-9 targets creatine kinase B for degradation. J. Biol. Chem. 282, 4728-4737 [DOI] [PubMed] [Google Scholar]
- Diks S. H., Bink R. J., van de Water S., Joore J., van Rooijen C., Verbeek F. J., den Hertog J., Peppelenbosch M. P., Zivkovic D. (2006). The novel gene asb11: a regulator of the size of the neural progenitor compartment. J. Cell Biol. 174, 581-592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Walsh C. A. (2004). The many faces of filamin: a versatile molecular scaffold for cell motility and signalling. Nat. Cell Biol. 6, 1034-1038 [DOI] [PubMed] [Google Scholar]
- Gimona M., Djinovic-Carugo K., Kranewitter W. J., Winder S. J. (2002). Functional plasticity of CH domains. FEBS Lett. 513, 98-106 [DOI] [PubMed] [Google Scholar]
- Glogauer M., Arora P., Chou D., Janmey P. A., Downey G. P., McCulloch C. A. (1998). The role of actin-binding protein 280 in integrin-dependent mechanoprotection. J. Biol. Chem. 273, 1689-1698 [DOI] [PubMed] [Google Scholar]
- Gorlin J. B., Yamin R., Egan S., Stewart M., Stossel T. P., Kwiatkowski D. J., Hartwig J. H. (1990). Human endothelial actin-binding protein (ABP-280, nonmuscle filamin): a molecular leaf spring. J. Cell Biol. 111, 1089-1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guibal F. C., Moog-Lutz C., Smolewski P., Di Gioia Y., Darzynkiewicz Z., Lutz P. G., Cayre Y. E. (2002). ASB-2 inhibits growth and promotes commitment in myeloid leukemia cells. J. Biol. Chem. 277, 218-224 [DOI] [PubMed] [Google Scholar]
- Hart A. W., Morgan J. E., Schneider J., West K., McKie L., Bhattacharya S., Jackson I. J., Cross S. H. (2006). Cardiac malformations and midline skeletal defects in mice lacking filamin A. Hum. Mol. Genet. 15, 2457-2467 [DOI] [PubMed] [Google Scholar]
- Hartwig J. H., Tyler J., Stossel T. P. (1980). Actin-binding protein promotes the bipolar and perpendicular branching of actin filaments. J. Cell Biol. 87, 841-848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuze M. L., Guibal F. C., Banks C. A., Conaway J. W., Conaway R. C., Cayre Y. E., Benecke A., Lutz P. G. (2005). ASB2 is an Elongin BC-interacting protein that can assemble with Cullin 5 and Rbx1 to reconstitute an E3 ubiquitin ligase complex. J. Biol. Chem. 280, 5468-5474 [DOI] [PubMed] [Google Scholar]
- Heuze M. L., Lamsoul I., Baldassarre M., Lad Y., Leveque S., Razinia Z., Moog-Lutz C., Calderwood D. A., Lutz P. G. (2008). ASB2 targets filamins A and B to proteasomal degradation. Blood 112, 5130-5140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ithychanda S. S., Das M., Ma Y. Q., Ding K., Wang X., Gupta S., Wu C., Plow E. F., Qin J. (2009). Migfilin, a molecular switch in regulation of integrin activation. J. Biol. Chem. 284, 4713-4722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay D., Garcia E. J., Lara J. E., Medina M. A., de la Luz Ibarra M. (2000). Determination of a cAMP-dependent protein kinase phosphorylation site in the C-terminal region of human endothelial actin-binding protein. Arch. Biochem. Biophys. 377, 80-84 [DOI] [PubMed] [Google Scholar]
- Kiema T., Lad Y., Jiang P., Oxley C. L., Baldassarre M., Wegener K. L., Campbell I. D., Ylanne J., Calderwood D. A. (2006). The molecular basis of filamin binding to integrins and competition with talin. Mol. Cell 21, 337-347 [DOI] [PubMed] [Google Scholar]
- Kile B. T., Metcalf D., Mifsud S., DiRago L., Nicola N. A., Hilton D. J., Alexander W. S. (2001). Functional analysis of Asb-1 using genetic modification in mice. Mol. Cell. Biol. 21, 6189-6197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohroki J., Fujita S., Itoh N., Yamada Y., Imai H., Yumoto N., Nakanishi T., Tanaka K. (2001). ATRA-regulated Asb-2 gene induced in differentiation of HL-60 leukemia cells. FEBS Lett. 505, 223-228 [DOI] [PubMed] [Google Scholar]
- Kohroki J., Nishiyama T., Nakamura T., Masuho Y. (2005). ASB proteins interact with Cullin5 and Rbx2 to form E3 ubiquitin ligase complexes. FEBS Lett. 579, 6796-6802 [DOI] [PubMed] [Google Scholar]
- Krakow D., Robertson S. P., King L. M., Morgan T., Sebald E. T., Bertolotto C., Wachsmann-Hogiu S., Acuna D., Shapiro S. S., Takafuta T., et al. (2004). Mutations in the gene encoding filamin B disrupt vertebral segmentation, joint formation and skeletogenesis. Nat. Genet. 36, 405-410 [DOI] [PubMed] [Google Scholar]
- Lad Y., Kiema T., Jiang P., Pentikainen O. T., Coles C. H., Campbell I. D., Calderwood D. A., Ylanne J. (2007). Structure of three tandem filamin domains reveals auto-inhibition of ligand binding. EMBO J. 26, 3993-4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad Y., Jiang P., Ruskamo S., Harburger D. S., Ylanne J., Campbell I. D., Calderwood D. A. (2008). Structural basis of the migfilin-filamin interaction and competition with integrin beta tails. J. Biol. Chem. 283, 35154-35163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi M., Gimona M. (2008). Synthetic actin-binding domains reveal compositional constraints for function. Int. J. Biochem. Cell Biol. 40, 1806-1816 [DOI] [PubMed] [Google Scholar]
- Lu J., Lian G., Lenkinski R., De Grand A., Vaid R. R., Bryce T., Stasenko M., Boskey A., Walsh C., Sheen V. (2007). Filamin B mutations cause chondrocyte defects in skeletal development. Hum. Mol. Genet. 16, 1661-1675 [DOI] [PubMed] [Google Scholar]
- McBeath R., Pirone D. M., Nelson C. M., Bhadriraju K., Chen C. S. (2004). Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483-495 [DOI] [PubMed] [Google Scholar]
- McDaneld T. G., Hancock D. L., Moody D. E. (2004). Altered mRNA abundance of ASB15 and four other genes in skeletal muscle following administration of beta-adrenergic receptor agonists. Physiol. Genomics 16, 275-283 [DOI] [PubMed] [Google Scholar]
- McDaneld T. G., Hannon K., Moody D. E. (2006). Ankyrin repeat and SOCS box protein 15 regulates protein synthesis in skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R1672-R1682 [DOI] [PubMed] [Google Scholar]
- Nakamura F., Osborn T. M., Hartemink C. A., Hartwig J. H., Stossel T. P. (2007). Structural basis of filamin A functions. J. Cell Biol. 179, 1011-1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell M. P., Fiori J. L., Baugher K. M., Indig F. E., French A. D., Camilli T. C., Frank B. P., Earley R., Hoek K. S., Hasskamp J. H., et al. (2009). Wnt5A activates the calpain-mediated cleavage of filamin A. J. Invest. Dermatol. 129, 1782-1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otey C. A., Carpen O. (2004). Alpha-actinin revisited: a fresh look at an old player. Cell Motil. Cytoskeleton 58, 104-111 [DOI] [PubMed] [Google Scholar]
- Pentikainen U., Ylanne J. (2009). The regulation mechanism for the auto-inhibition of binding of human filamin A to integrin. J. Mol. Biol. 393, 644-657 [DOI] [PubMed] [Google Scholar]
- Pudas R., Kiema T. R., Butler P. J., Stewart M., Ylanne J. (2005). Structural basis for vertebrate filamin dimerization. Structure 13, 111-119 [DOI] [PubMed] [Google Scholar]
- Robertson S. P., Twigg S. R., Sutherland-Smith A. J., Biancalana V., Gorlin R. J., Horn D., Kenwrick S. J., Kim C. A., Morava E., Newbury-Ecob R., et al. (2003). Localized mutations in the gene encoding the cytoskeletal protein filamin A cause diverse malformations in humans. Nat. Genet. 33, 487-491 [DOI] [PubMed] [Google Scholar]
- Ruskamo S., Ylanne J. (2009). Structure of the human filamin A actin-binding domain. Acta Crystallogr. D Biol. Crystallogr. 65, 1217-1221 [DOI] [PubMed] [Google Scholar]
- Sheen V. L., Dixon P. H., Fox J. W., Hong S. E., Kinton L., Sisodiya S. M., Duncan J. S., Dubeau F., Scheffer I. E., Schachter S. C., et al. (2001). Mutations in the X-linked filamin 1 gene cause periventricular nodular heterotopia in males as well as in females. Hum. Mol. Genet. 10, 1775-1783 [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Condeelis J., Cooley L., Hartwig J. H., Noegel A., Schleicher M., Shapiro S. S. (2001). Filamins as integrators of cell mechanics and signalling. Nat. Rev. Mol. Cell Biol. 2, 138-145 [DOI] [PubMed] [Google Scholar]
- Thompson T. G., Chan Y. M., Hack A. A., Brosius M., Rajala M., Lidov H. G., McNally E. M., Watkins S., Kunkel L. M. (2000). Filamin 2 (FLN2): a muscle-specific sarcoglycan interacting protein. J. Cell Biol. 148, 115-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Green H. (1963). Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 17, 299-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda T., Kouchi Z., Kawahara H., Tomioka S., Sasagawa N., Maeda T., Sorimachi H., Ishiura S., Suzuki K. (2001). Limited proteolysis of filamin is catalyzed by caspase-3 in U937 and Jurkat cells. J. Biochem. 130, 535-542 [DOI] [PubMed] [Google Scholar]
- Vadlamudi R. K., Li F., Adam L., Nguyen D., Ohta Y., Stossel T. P., Kumar R. (2002). Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nat. Cell Biol. 4, 681-690 [DOI] [PubMed] [Google Scholar]
- van der Flier A., Sonnenberg A. (2001). Structural and functional aspects of filamins. Biochim. Biophys. Acta 1538, 99-117 [DOI] [PubMed] [Google Scholar]
- van der Flier A., Kuikman I., Kramer D., Geerts D., Kreft M., Takafuta T., Shapiro S. S., Sonnenberg A. (2002). Different splice variants of filamin-B affect myogenesis, subcellular distribution, and determine binding to integrin [beta] subunits. J. Cell Biol. 156, 361-376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox A., Katsanakis K. D., Bheda F., Pillay T. S. (2004). Asb6, an adipocyte-specific ankyrin and SOCS box protein, interacts with APS to enable recruitment of elongins B and C to the insulin receptor signaling complex. J. Biol. Chem. 279, 38881-38888 [DOI] [PubMed] [Google Scholar]
- Woo M. S., Ohta Y., Rabinovitz I., Stossel T. P., Blenis J. (2004). Ribosomal S6 kinase (RSK) regulates phosphorylation of filamin A on an important regulatory site. Mol. Cell. Biol. 24, 3025-3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Baek H. J., Karsenty G., Justice M. J. (2007). Filamin B represses chondrocyte hypertrophy in a Runx2/Smad3-dependent manner. J. Cell Biol. 178, 121-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A. X., Hartwig J. H., Akyurek L. M. (2010). Filamins in cell signaling, transcription and organ development. Trends Cell Biol. 20, 113-123 [DOI] [PubMed] [Google Scholar]
- Zhou X., Boren J., Akyurek L. M. (2007). Filamins in cardiovascular development. Trends Cardiovasc. Med. 17, 222-229 [DOI] [PubMed] [Google Scholar]