Abstract
When migrating mesenchymal cells collide, they exhibit a ‘contact inhibition of locomotion’ response that results in reversal of their front–rear polarity by extension of a new leading edge, which enables their migration away from the opposing contacted cell. The critical cytoskeletal rearrangements underpinning these mutual repulsion events are currently unknown. We found that during fibroblast cell–cell collisions, microtubules at the region of contact increase their frequency of catastrophe, their rates of shrinkage and growth, and concomitantly, a new microtubule array is established at a new leading edge. We show that Rho and ROCK activity is necessary for this repulsion response, and we observed increased microtubule stabilisation as a consequence of ROCK inhibition. Importantly, partial destabilisation of microtubules, by co-treatment with a low dose of nocodazole, restored microtubule dynamics to that of untreated cells and rescued contact inhibition of locomotion in ROCK-inhibited cells. Although there was an increase in microtubule growth or shrinkage rates in Y27632 cell–cell collisions, these failed to reach the same level of dynamicity compared with untreated collisions. Our data suggest that microtubule dynamics at contact sites must increase beyond a threshold for a cell to switch its front–rear polarity, and that microtubule stabilisation can lead to a failure of contact inhibition of locomotion.
Key words: Contact inhibition of locomotion, Microtubules, Polarity switching
Introduction
Any migratory episode requires activation signals to initiate migration followed by appropriate ‘stop’ signals when a cell has reached its destination. ‘Contact inhibition of locomotion’, a term coined over 50 years ago by Abercrombie and Heaysman (Abercrombie and Heaysman, 1954), is defined as ‘the stopping of the continual locomotion of a cell in the same direction after collision with another cell’ (Abercrombie, 1970). It is a pivotal component of all normal cell migrations in vitro and in vivo (Carmona-Fontaine et al., 2008; Mayor and Carmona-Fontaine, 2010) and is considered a regulatory mechanism ensuring that cells do not overstep their permitted boundaries. Significantly, many malignant cells appear to be released from normal contact inhibition restraints, which might contribute to their invasive behaviour (Abercrombie, 1979; Astin et al., 2010; Heaysman, 1978; Paddock and Dunn, 1986). The original studies of contact inhibition detailed morphologically distinct and consecutive events commencing with adhesion, followed by lamella paralysis and then retraction and reversal of front–rear polarity occurring after two chick embryo fibroblasts collide. However, the details of the molecular mechanisms involved in this process remain unclear.
Directed cell migration requires that a cell establishes and maintains a polarised morphology. A predominantly ‘front’, forward protruding portion and a predominantly ‘back’, retracting rear are defined by distinct spatially restricted signalling events (Ridley et al., 2003). Localised actin polymerisation and contractility generate directed motility and these actin regulatory episodes are signalled through members of the Rho family of small GTPases and their downstream effectors (Etienne-Manneville and Hall, 2002; Raftopoulou and Hall, 2004). However, in many cell types, it is the microtubules and their polymerisation dynamics that provide the necessary framework for polarisation and thus guide the direction of cell motility (Watanabe et al., 2005). There is increasing evidence for crosstalk between these two cytoskeletal elements through common signalling regulators as cells polarise and migrate (Etienne-Manneville, 2004; Li and Gundersen, 2008). However, the precise cytoskeletal rearrangements that permit a dramatic switch in front–rear polarity in response to cell–cell collision, and how these are regulated, are still unknown.
A recent study demonstrated that contact inhibition of locomotion is regulated by ROCK (also known as Rho-associated kinase) activity in contacting neural crest cells (Carmona-Fontaine et al., 2008). Because Rho signalling regulates both actin (membrane retraction) and microtubule (cell polarity)-driven processes (Heasman and Ridley, 2008; Wittmann and Waterman-Storer, 2001), we investigated which of these downstream components might be critical during normal repulsive collisions.
Results
Contact inhibition of locomotion between CEFs involves a contact-induced polarity switch
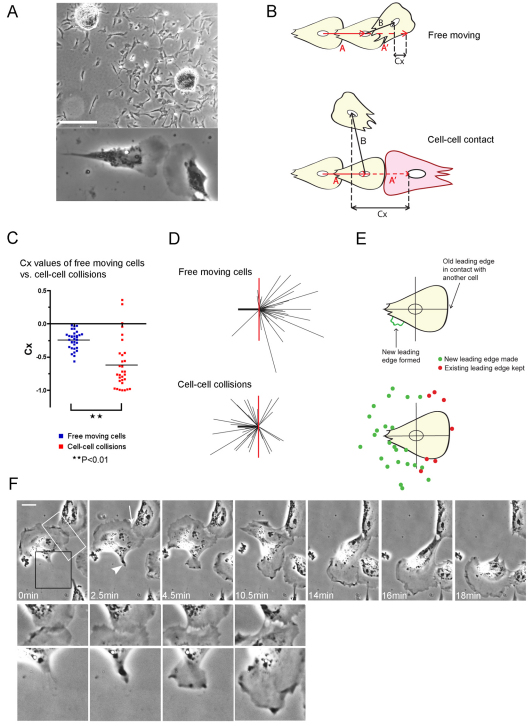
Chick embryonic heart fibroblasts (CEFs) are highly motile cells and were used by Abercrombie and Heaysman in their initial experiments characterising the phenomenon of contact inhibition of locomotion (Abercrombie and Heaysman, 1954). We have cultured chick heart explants at a sufficient distance apart (0.5–1 mm) so that their cellular outgrowths meet approximately 24 hours after plating, and using time-lapse microscopy, we selected leading-edge lamellae collisions for analysis of the contact inhibition process (Fig. 1A).
Fig. 1.
Leading-edge lamellae collisions between CEFs induce a switch in front–rear polarity. (A) Outgrowths of chick embryo heart fibroblasts from explants 24 hours after plating (top) and a typical leading-edge lamellae head-on-head collision (bottom). Scale bar: 250 μm. (B) The displacement trajectories 15 minutes before (vector A) and 15 minutes after (vector B) collisions, and the trajectories of free-moving cells over the 30 minute period. (C) Contact acceleration indices (Cx values) of free-moving cells (n=32) and colliding cells (n=31) in 2% serum; **P<0.01. (D) Velocity vectors for free-moving cells (n=32) and cell–cell collisions (n=31). The heavy black line represents the scaled displacement of all cells before contact and the thin black lines are the scaled displacements of each cell after contact. The red line is a reference line to mark the angle of 90° in relation to the displacement before contact (heavy black line).(E) Tracking the formation of new leading edges upon cell–cell collision (n=31). Green spots denote from where a new leading edge lamella extended after collision; the red spots indicate the position of the existing leading edge that was maintained. (F) Time-lapse stills of a CEF collision (supplementary material Movie 1). The arrowhead indicates a new leading edge forming; white arrow indicates retraction of the lamella. Higher magnifications of the region of contact (white box) and the formation of the new leading edge (black box) are shown underneath their corresponding images. Scale bar: 16 μm.
Quantification of contact inhibition of locomotion was largely performed as previously described (Paddock and Dunn, 1986). Vector analysis of collisions indicates how the motion of a cell changes as a result of a collision, and this is compared with the spontaneous changes of motion in freely moving cells (Fig. 1B). This measurement describes a ‘mean longitudinal component of acceleration due to collision’, known as the ‘contact acceleration index’ (Cx). A more negative Cx value implies a greater contact inhibition response. We saw a large and statistically significant difference between the Cx values of a population of free-moving CEFs and colliding CEFs, which shows that these cells exhibit contact inhibition of locomotion behaviour (Fig. 1C). The velocity vectors reveal that CEFs most frequently recoil backwards as a consequence of contact-mediated repulsion (Fig. 1D). A small number of CEFs appeared to show a reduced contact inhibition response and on closer inspection of time-lapse movies, we found that these tended to be cells that had recently collided with a different cell shortly before the measurement was taken. This suggests that there is perhaps a signalling insensitivity or dampening effect when cells repeatedly collide with their neighbours.
The general dramatic contact-induced switch in polarity was of interest to us in particular, and from time-lapse movies, we tracked the formation of new leading edges after collision. We found that 77% of colliding CEFs did indeed disassemble their old leading edge and made a new leading edge remote from the one that led to cell contact (Fig. 1E). This suggests that the collapse and retraction of lamellae at sites of contact is an important step in contact inhibition of locomotion.
The temporal relationship between retraction of the confronting original lamellae and the formation of a new leading edge was examined. Upon initial contact, active ruffling in the region of contact ceases and the cell stops moving in the direction that led to the collision (Fig. 1F, 0 minutes; supplementary material Movie 1). Within a few minutes, retraction of the leading edge occurred and concomitantly was accompanied by the establishment of a new leading edge elsewhere at the cell margin (Fig. 1F, 2.5 minutes, arrowhead). Higher magnification revealed strands of stretched cytoplasm linking the two cells as they continued to withdraw from one another (Fig. 1F, 4.5–10.5 minutes), until migration is reinitiated in the direction of the new leading edge (Fig. 1F, 14–18 minutes). Normal migration then resumed until the cells collided again with other cells. Clearly, the processes whereby a cell reorientates its polarity upon collision with another cell involves complex remodelling of various cytoskeletal players, and we wished to understand the signalling that underpins these changes.
Rho- and ROCK-inhibited CEFs do not switch polarity following collision with another cell
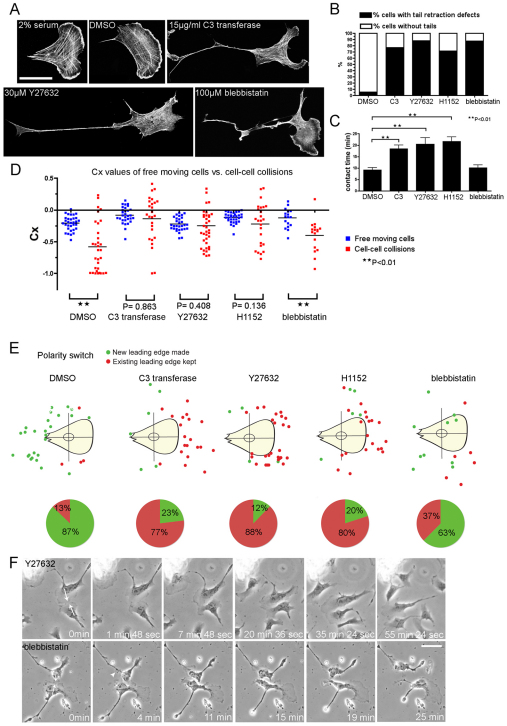
Recently, it has been shown that contact inhibition of locomotion in neural crest cells is in part regulated by RhoA and ROCK (Rho/ROCK) signalling at cell–cell contacts (Carmona-Fontaine et al., 2008). We used various inhibitors to determine precisely which aspects of contact inhibition are Rho/ROCK dependent in CEFs. Rho activity was blocked using C3 transferase (15 μg/ml), ROCK signalling was inhibited by treatment with either Y27632 (30 μM) or H1152 (10 μM) and we also ablated myosin II activity using blebbistatin (100 μM). As expected, blocking Rho/ROCK signalling or preventing myosin-II-mediated contraction, both reduced actin stress fibres and led to the formation of long immobile tails with impaired retraction (Fig. 2A,B), but did not interfere with the ability of cells to migrate; nor did it affect cell speed (data not shown). However, when analysing contact inhibition of locomotion following these blocking regimes, we noticed dramatic differences. There was a clear difference in the contact time of the leading edge lamellae between either Rho- or ROCK-inhibited CEFs and control or blebbistatin-treated CEFs (Fig. 2C). Furthermore, the contact acceleration indices (Cx values) of C3, Y27632 and H1152 collisions showed no significant difference from that of their equivalent free-moving cells (Fig. 2D), indicating that Rho/ROCK-inhibited CEFs have completely lost contact inhibition of locomotion responses. Rho/ROCK-inhibited cells appeared to slide past one another instead of exhibiting dramatic contact repulsion that commonly results in a reversal of polarity in control cells (Fig. 2E,F; supplementary material Movie 2). By contrast, the blebbistatin-treated cells still exhibited almost normal contact inhibition properties (Fig. 2D,F; supplementary material Movie 3). However, they did show a reduced ability to switch polarity upon cell–cell contact, with only 63% of blebbistatin-treated cells creating new leading edges upon collision compared with 87% of the control cells (Fig. 2E). Our data suggest that blocking Rho/ROCK signalling regulates the contact inhibition response, but not predominantly by the expected pathway leading to myosin contractility, which would be sensitive to blebbistatin.
Fig. 2.
Rho- and ROCK-inhibited CEFs have lost contact inhibition of locomotion responses and fail to make new leading edges upon contact. (A) Control CEFs (2% serum), vehicle-treated (DMSO) CEFs and CEFs treated with 15 μg/ml cell-permeable C3 transferase, 30 μM Y27632 and 100 μM blebbistatin stained for F-actin. Scale bar: 40 μm. (B) Percentage of cells with tail-retraction defects (n>180 for each treatment; 10 μM H1152). (C) Contact time of leading edge lamellae during cell–cell collisions of the indicated treatments (DMSO, n=32; C3 transferase, n=26; Y27632, n=35; H1152, n=25; blebbistatin, n=16). Error bars indicate s.e.m.; **P<0.01. (D) Contact acceleration indices (Cx values) of free-moving cells versus colliding cells treated with DMSO (n=32 free, n=32 colliding), C3 transferase (n=30 free, n=26 colliding), Y27632 (n=30 free, n=38 colliding), H1152 (n=32 free, n=25 colliding) and blebbistatin (n=17 free, n=17 colliding); **P<0.01. (E) Top panel shows tracking the formation of new leading edges upon cell–cell collision; green spots denote new leading edges made and red spots denote existing leading edges kept. Pie charts representing the percentage of new leading edges and existing leading edges kept in the corresponding treatments are shown below. (F) Collision between two Y27632-treated CEFs (top panel; supplementary material Movie 2) and two blebbistatin-treated cells colliding (bottom panel; supplementary material Movie 3). White arrows indicate direction of migration that led to the collision; white arrowhead indicates a new leading edge made. Scale bar: 30 μm.
A feature of Rho/ROCK-inhibited CEFs was their persistent forward migration and maintenance of polarity following collision with another cell (Fig. 2E,F). It is well established that Rho/ROCK signalling regulates polarised cell migration (Ridley et al., 2003) and that an intact microtubule cytoskeleton is essential for the maintenance of polarity in many cell types, including fibroblasts (Siegrist and Doe, 2007). Furthermore, because microtubule stabilisation can convert a cell to a strongly polarised form with increased migratory persistence (Drabek et al., 2006; Small et al., 2002), we asked whether impaired contact inhibition of locomotion in Rho/ROCK-inhibited CEFs might be caused by aberrant stabilisation of the microtubule cytoskeleton.
ROCK-inhibited CEFs display a greater proportion of stable microtubules
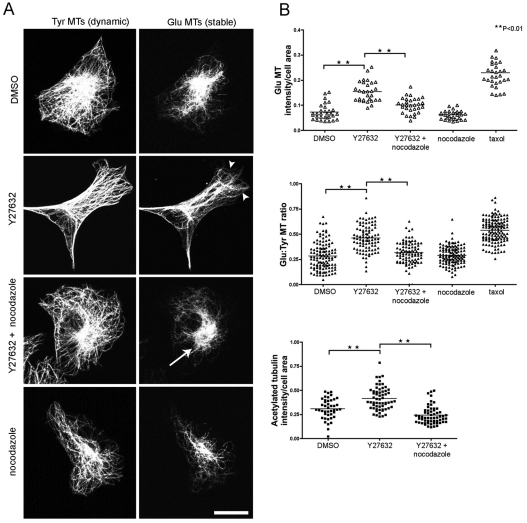
We used immunofluorescence staining to determine whether the distribution and orientation of stable detyrosinated (Glu) microtubules (MTs) was altered following ROCK inhibition. Y27632-treated CEFs or those treated with the microtubule-stabilising drug taxol (5 nM) have a greater proportion of stable Glu and acetylated microtubules (Fig. 3A,B). Interestingly, addition of a low dose of the microtubule-depolymerising drug nocodazole (25 nM) disrupted the Y27632-stabilised microtubule array so that it became more localised to the perinuclear region (Fig. 3A), and also rescued the levels of Glu and acetylated microtubules to that of control CEFs (Fig. 3B). These experiments demonstrate that reduced ROCK activity leads to an increase in the proportion of stable microtubules that can be reversed by treatment with a low concentration of nocodazole, and suggests that the ROCK pathway might indeed regulate contact inhibition of locomotion by regulation of microtubule dynamics.
Fig. 3.
ROCK inhibition leads to an increase in stable microtubules. (A) CEFs were fixed and double stained for Tyr tubulin (left) and Glu tubulin (right) following the indicated drug treatments. Arrowheads indicate stable Glu microtubules (MTs) in Y27362-treated CEFs. Arrow indicates the stable Glu MT array becomes more localised to the perinuclear region after addition of a low concentration of nocodazole (25 nM) to Y27632 treated CEFs. Scale bar: 20 μm. (B) The pixel intensity of individual cells with fluorescently labelled Glu and Tyr MTs was measured. The proportion of Glu MTs present in treated cells was determined by dividing the pixel volume by cell area (top graph; n=30 for each condition). Pixel intensity ratio of Glu:Tyr MTs (middle graph; n>92 for all treatments). Cells stained for acetylated tubulin showed that Y27632-treated CEFs have more acetylated MTs than controls and this is reduced with the addition of nocodazole to Y27632-treated cells (bottom graph; n≥43 for all treatments; **P<0.01).
Nocodazole rescues the contact inhibition defects observed in ROCK-inhibited CEFs
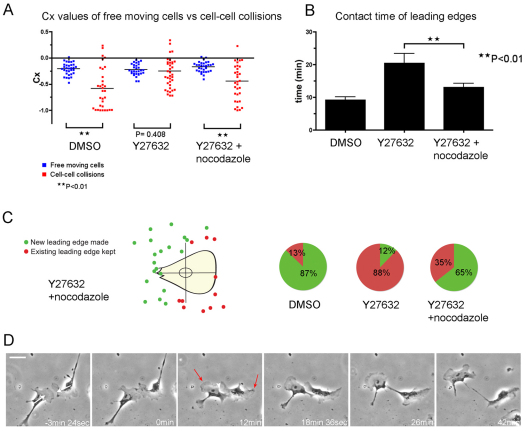
We observed that the loss of contact inhibition of locomotion seen in ROCK-inhibited CEFs was rescued when Y27632 and nocodazole were added in combination (Fig. 4A, P<0.01). The majority of cells still showed distinct tail retraction defects (62.1%, n=180 cells); however, the time that leading edges remained in contact during cell–cell collision was significantly reduced, almost to control levels (Fig. 4B). We also observed a far greater number of cells that switched front–rear polarity upon contact, again suggesting that the capacity to exhibit a normal contact inhibition response had been restored (Fig. 4C,D; supplementary material Movie 4). Critically, this experiment suggested that microtubule stabilisation might be responsible for the impaired contact inhibition of locomotion response. These data parallel our previous observation that nocodazole disrupted polarity of macrophage migration towards wounds in zebrafish larvae can be rescued by Y27632 (Redd et al., 2006), and are also consistent with a recent study showing that inhibition of ROCK restores the migration and polarity of T cells treated with microtubule-depolymerising drugs (Takesono et al., 2010). These results suggest a strong link between ROCK signalling and microtubule dynamics.
Fig. 4.
Destabilisation of microtubules with nocodazole rescues the loss of contact inhibition of locomotion of ROCK-inhibited cells. (A) Contact acceleration indices (Cx values) of free-moving cells versus colliding cells: DMSO (n=32 free moving cells, n=32 cell–cell collisions), Y27632 (n=30 free moving cells, n=38 cell–cell collisions), Y27632 + nocodazole (n=30 free-moving cells; n=32 cell–cell collisions); **P<0.01. (B) Contact time of leading edge lamellae during cell–cell collisions (DMSO, n=32; Y27632, n=35; Y27632 + nocodazole, n=32); **P<0.01. (C) Tracking the formation of new leading edges upon cell–cell collision showed that the ability to switch front–rear polarity is restored (green spots denoting new leading edges made, n=20; red spots denoting existing leading edges kept, n=11). Pie charts compare the percentage of leading edges kept in DMSO, Y27632 (from Fig. 2E) and Y27632 + nocodazole-treated cells. (D) Collision between Y27632 + nocodazole-treated CEFs (supplementary material Movie 4). White arrows indicate the direction of migration that led to the collision; white arrowhead indicates cell–cell retraction; red arrows indicate new leading edges that become prominent. Scale bar: 32 μm.
ROCK inhibition causes a decrease in microtubule dynamicity
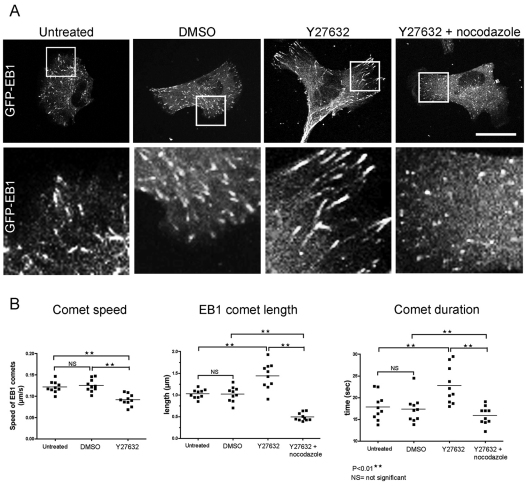
To investigate the effects of ROCK inhibition on the growth dynamics of microtubules within the leading edge of individual migrating cells, we used live imaging of CEFs expressing fluorescent end-binding protein 1 (EB1) in the absence and presence of Y27632 (Fig. 5A). Microtubule-associated proteins (MAPs) that bind specifically to the growing plus-ends of microtubules are known as plus-end tracking proteins (+TIPs), and they are involved with coordinating many aspects of cell behaviour (Akhmanova and Steinmetz, 2008). High-resolution imaging of the +TIP EB1 has shown these selectively accumulate at, and ‘track’ along the extending microtubule plus-ends (Vaughan, 2005). We found that the speed of EB1-decorated comets in Y27632-treated CEFs was significantly reduced by comparison with untreated EB1 comets (Fig. 5B; supplementary material Movies 5 and 6). Furthermore, these comets persisted for up to 30% longer (Fig. 5B) and were almost 1.5-times the length of the comets in the control cells (Fig. 5A,B). Interestingly, our observations contrast with other studies, which have shown that EB1 comet length is proportional to the rate of microtubule polymerisation (Bieling et al., 2007). A possible explanation could be that ROCK inhibition affects the activity of EB1 itself, or the numerous proteins that EB1 recruits, such as CLIP-170, CLASPs and p150Glued, which form larger complexes at microtubule plus-ends (Ligon et al., 2006; Vaughan, 2005). Phosphorylation of MAPs is a means of regulation, and in most cases leads to MAP dissociation from microtubules, thereby reducing their stabilisation capacities (Akhmanova and Steinmetz, 2008). It is possible that MAPs are a target of phosphorylation by ROCK in CEFs, although so far, ROCK has only been reported to interact with and phosphorylate neuronal MAPs Tau, MAP2 (Amano et al., 2003) and CRMP-2 (Arimura et al., 2005).
Fig. 5.
Growth dynamics of microtubules in ROCK-inhibited cells is altered. (A) Untreated, DMSO, Y27632, and Y27362 + nocodazole-treated CEFs expressing GFP–EB1 (supplementary material Movies 5–7). Higher magnifications of EB1 comets present at the leading lamellae (white boxes) are shown below. Scale bar: 16 μm. (B) Comet speed of untreated CEFs and CEFs treated with DMSO and Y27632 (left); comet length (middle) and comet duration (right) of untreated CEFs and CEFs treated with DMSO, Y27632 and Y27632 + nocodazole. 30 individual comets per frame (from eight different time-points) of ten individual cells were used in each case; **P<0.01.
Because there was a significant reduction in stable Glu and acetylated microtubules in Y27632- and nocodazole-treated CEFs (Fig. 3B), we looked to see whether microtubule dynamics had also been rescued by this low concentration of nocodazole. We found that EB1 comets in these CEFs were noticeably shorter in length than in either Y27632 or control CEFs (Fig. 5A,B; supplementary material Movie 7), and that their duration was similar to that of controls, which suggested increased rates of microtubule catastrophe (Fig. 5B) compared with cells treated with Y27632 alone.
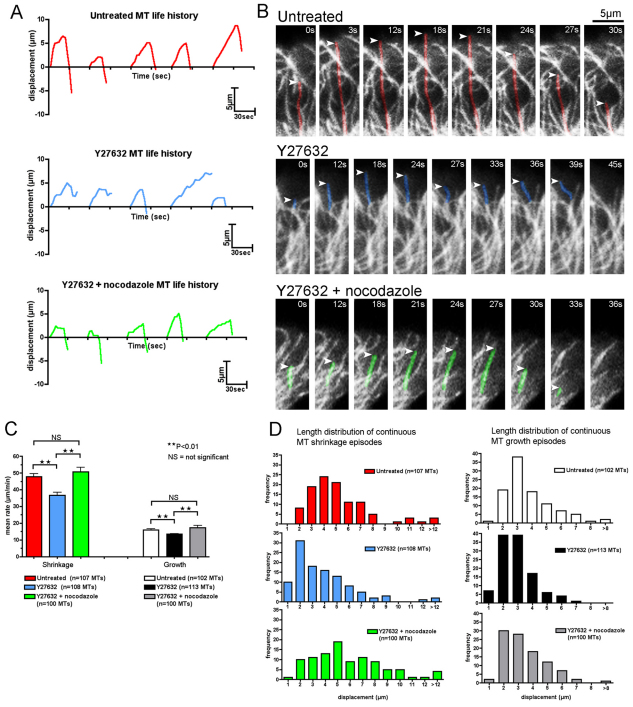
We further investigated microtubule dynamics by expressing YFP–tubulin in CEFs. In this way, we were able to measure the rate of microtubule shrinkage and growth, as well as the rates of microtubule catastrophe (Fig. 6A,B). We found that the rate of microtubule shrinkage and growth in Y27632-treated CEFs was significantly reduced in comparison with untreated CEFs (Fig. 6B,C; supplementary material Movie 8). Furthermore, the mean displacement of microtubules during either collapse or growth was smaller in Y27632 cells (Fig. 6D). In addition, the frequency of microtubule catastrophe was also significantly reduced in ROCK-inhibited cells (from 1.8±0.18 minute−1 in control to 1.06±0.10 minute−1 in Y27632; mean ± s.d.; P<0.01). From these data, we conclude that ROCK inhibition causes an increase in microtubule stability, which is consistent with our previous data showing an increase in the proportion of stable microtubules and an increase in EB1 comet length. Importantly, the addition of a low concentration of nocadozole, which does not cause an extensive loss of the microtubule network (Fig. 6A,B), was able to restore microtubule dynamics almost to control levels (Fig. 6C,D; supplementary material Movie 8).
Fig. 6.
Reduced microtubule shrinkage and growth rates in ROCK-inhibited CEFs are rescued with the addition of nocodazole. MT dynamics at the leading edge lamella in non-contacting single YFP–tubulin-expressing cells. (A) Life history plots (length vs time) of five different MTs from a single untreated (red), Y27632 (blue) and Y27632 + nocodazole (green) cell. Zero length represents the initial position of the MT filament from where the growth or shrinkage episode was tracked. For clarity, plots were arbitrarily staggered along the time axis and scales are indicated at the bottom right-hand corner of the graphs. (B) High-magnification time-lapse stills of the life history of a single MT from an untreated CEF (top panels, coloured red), Y27632 CEF (middle, coloured blue) and Y27632 + nocodazole (bottom, coloured green) (supplementary material Movie 8). Scale bar: 5 μm. (C) Mean rates of MT shrinkage and growth at the leading edge of non-contacting single untreated, Y27632, and Y27632 + nocodazole cells (from ten different cells n≥100 MTs each for shrinking and growing filaments for all treatments; **P<0.01; NS, not significant). (D) Graphs comparing the length distribution of continuous MT shrinkage and growth episodes at the leading edge of non-contacting single untreated, Y27632 and Y27632 + nocodazole cells (from ten different cells n≥100 MTs each). Mean displacement of continuous MT shrinkage episodes for untreated cells=4.75 μm, Y27632 cells=3.23 μm and Y27632 + nocodazole cells=5.47 μm. Mean displacement of continuous MT growth episodes for untreated=3.38 μm, Y27632=2.42 μm, and Y27632 + nocodazole=2.96 μm. Untreated vs Y27632 cells, P<0.01 for both shrinkage and growth. Y27632 cells vs Y27632 + nocodazole cells, P<0.01 for both shrinkage and growth.
Dynamic microtubules are necessary for switching polarity during contact inhibition of locomotion
Because inhibition of the Rho–ROCK pathway causes microtubule stabilisation and a corresponding failure of the polarity switch following collision with another cell, we wanted to investigate how dynamic microtubules behave during contact inhibition of locomotion. CEFs expressing YFP–tubulin were imaged as they underwent cell–cell collisions and we found that the microtubule network became reorganised as the cell repolarised, with microtubules being progressively lost at the contacting edge (Fig. 7A; supplementary material Movie 9).
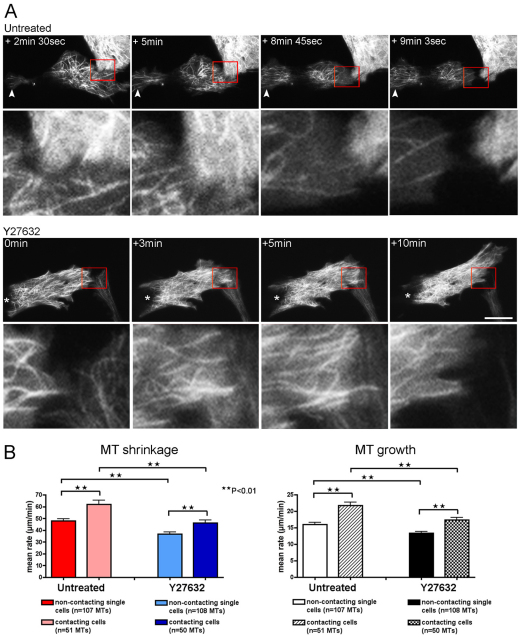
Fig. 7.
Microtubule dynamics changes at the leading edge of cells during cell–cell collision. (A) Top panels show a YFP–tubulin-expressing untreated CEF colliding with the lamella of the cell above and rapidly switching polarity (supplementary material Movie 9). Higher magnifications of the region of cell–cell contact (red box) are shown below their corresponding images. MT filaments flood into the tail end of the cell (white arrowhead) and away from the site of cell–cell contact to form a new leading edge, and subsequently, the cell is able renew migration in the opposite direction. Bottom panels show a YFP–tubulin-expressing Y27632-treated CEF colliding with the lamella of the cell below right (supplementary material Movie 10). The cell does not recoil away, and MTs are not lost from the region of cell–cell contact. Higher magnifications of the region of cell–cell contact (red box) are shown below their corresponding images. Asterisk denotes the tail end of the cell that is maintained throughout the collision episode. Scale bar: 18 μm. (B) Mean rates of MT shrinkage and growth at the region of cell–cell contact in untreated and Y27632-treated CEFs (from four different untreated CEFs, n=51 shrinking and n=53 growing filaments; three different Y27632 CEFs, n=50 shrinking and n=51 growing filaments; **P<0.01).
Comparison of microtubule dynamics in the region of cell–cell contact with that of the leading edge of isolated untreated cells, showed that rates of microtubule shrinkage and growth both increased during collision (by 29.7% and 35.6% respectively), and there was also a corresponding rise in the mean frequency of microtubule catastrophe (from 1.80±0.18 minute−1 in single cells to 2.26±0.31 minute−1 in colliding cells; P<0.01), suggesting an increase in microtubule dynamics at the leading edge of colliding cells (Fig. 7B). Interestingly, the microtubules at the leading edges of Y27632 treated CEFs also showed an increased rate of shrinkage and growth (by 26.2% and 29.9%, respectively) during collision when compared with isolated cells; but importantly, both microtubule shrinkage and growth rates remained significantly less than those in control cells during collision (Fig. 7B). However, unlike control cells, the mean frequency of microtubule catastrophe did not significantly change during collision (from 1.06±0.10 minute−1 in single Y27632 cells to 1.22±0.17 minute−1 in Y27632 cells during collision; P>0.05), (supplementary material Movie 10). The data suggest that microtubule dynamics at the site of contact needs to increase beyond a certain threshold for a cell to successfully reverse its front–rear polarity during contact inhibition of locomotion. Failure to reach this critical level of dynamicity, as in the case of Y27632-treated cells, which have hyper-stable microtubules, might result in cells being unable to re-orientate following collision with another cell. Although we have experimentally challenged the system by modulating ROCK activity, we speculate that the standard collision-induced increase in microtubule dynamics might not be mediated by ROCK signalling, because an increase in both growth and shrinkage rates is still observed in Y27632-treated cells during collision.
Discussion
When two fibroblasts collide they undergo contact inhibition of locomotion. This involves a number of distinct steps including: lamella paralysis, cell retraction and reversal of front–rear polarity. It is this polarity switching that appears to be most important in the contact inhibition of locomotion response because it permits a cell to reverse its direction of migration and move away into cell-free space. In this study, we have shown that blocking Rho–ROCK signalling, which leads to an increase in microtubule stability and reduced actomyosin contractility, inhibits the capacity of cells to switch polarity during contact inhibition of locomotion. By contrast, blocking actomyosin contraction alone, with blebbistatin, only weakly interfered with the cell's ability to create a new leading edge following contact. This contrasts with other studies showing that axonal retraction events triggered by repulsive guidance molecules, such as the ephrins, are entirely dependent upon myosin II contractility (Harbott and Nobes, 2005; Wahl et al., 2000). Because contact inhibition of locomotion was not completely dependent on actomyosin contraction, we investigated what role the microtubule network might have during this process.
Microtubules are important in generating and maintaining cell polarity in many cell types, and their rapid turnover allows for remodelling of the cytoskeleton in response to environmental cues. Previously, it has been shown that stable detyrosinated microtubules (Glu microtubules) are selectively orientated towards the leading edge of cells at an in vitro scratch-wound margin as cells polarise and begin to move forward to fill the gap. Importantly, cell contact triggers a rapid depletion of these stable Glu microtubules (Gundersen and Bulinski, 1988; Nagasaki et al., 1992), and we show here that collision between isolated cells leads to an increase in microtubule dynamics in the region of contact. Numerous studies demonstrate that Rho signalling regulates microtubules through the Rho effector mDia1 to generate stabilized microtubules (Palazzo et al., 2001; Yamana et al., 2006) and conversely, there is some evidence that microtubules can modulate Rho activity (Chang et al., 2008; Krendel et al., 2002; Takesono et al., 2010). We show that reorganisation of the microtubule network is necessary to mediate successful contact-dependent repulsion. During collision, the microtubules present at the contact site increase their frequency of catastrophe and increase their rates of shrinkage and growth. This increase in dynamic microtubule behaviour might favour the disassembly of the microtubule network at the site of cell–cell contact. We also observed a concomitant assembly of a new microtubule array away from the contact site (supplementary material Movie 9), which eventually results in the cell switching polarity with a new leading edge being formed and the cell migrating away from the point of collision.
We observed microtubule stabilisation in Y27632-treated cells by staining for markers of stable microtubules and also by live imaging the microtubule network. We found there to be an increase in the proportion of both Glu (detyrosinated) and acetylated microtubules, as well as a decrease in the growth and shrinkage rates of microtubules in cells expressing either GFP–EB1 or YFP–tubulin in Y27632-treated cells compared with untreated cells. In support of this data, microtubule stabilisation as a result of ROCK inhibition has also been observed in T cells (Takesono et al., 2010).
Importantly, the addition of the microtubule-destabilising agent nocodazole at a sufficiently low concentration that did not completely disrupt the microtubule network was able to restore microtubule dynamics to levels observed in untreated cells, and this correlated with a rescue of the polarity-switching defect. From these results, we hypothesise that reorganisation of the microtubule network is essential for polarity switching during contact inhibition of locomotion. Our data is supported by a recent study showing that contact repulsion between Drosophila macrophages requires a correctly polarised microtubule network (Stramer et al., 2010).
In this study, we have shown that microtubule dynamic behaviour is modulated by cell–cell contact. Comparison of microtubule growth and shrinkage at the leading edge of contacting untreated cells, with that of contacting ROCK-inhibited cells indicate that a certain threshold of increased microtubule dynamics is required for cells to repolarise upon cell–cell contact. In a similar fashion to untreated cells, microtubule growth and shrinkage in Y27632-treated cells are both enhanced compared with equivalent non-contacting migrating cells; however, they do not reach the highly dynamic levels observed in untreated colliding cells. We hypothesise that if microtubules at the region of cell–cell contact fail to reach this threshold, then cells lose the ability to repolarise and, as a consequence, exhibit defective contact inhibition of locomotion.
How might ROCK inhibition cause microtubule stability? Recently, ROCK has been shown to phosphorylate PAR-3, which disrupts the PAR complex (aPKC–PAR-6–PAR-3) required for front–rear polarisation of migrating cells (Nakayama et al., 2008). The maintenance of an active PAR complex in ROCK-inhibited cells might stabilise microtubules during contact inhibition of locomotion by promoting the association of adenomatous polyposis coli (APC) with the plus-ends of microtubules (Etienne-Manneville and Hall, 2003; Zumbrunn et al., 2001). Other well-characterised modulators of microtubule dynamics are the microtubule-associated proteins (MAPs). Phosphorylation of MAPs interferes with their microtubule-stabilising capacity and has an important role in controlling the dynamics of the leading edge in migrating cells and in neuronal growth cones (Akhmanova and Steinmetz, 2008). In neurons, ROCK phosphorylation of Tau and MAP2 promotes their dissociation from microtubules, thereby enhancing microtubule destabilisation (Amano et al., 2003).
Whether Rho–ROCK signalling can locally regulate microtubule-stabilising factors in the leading edge of colliding cells will be fascinating to investigate. Activation of Rho has been observed at sites of cell–cell contact during contact inhibition of locomotion (Carmona-Fontaine et al., 2008), so it is possible that ROCK activation could be triggered by cell–cell contact. Active ROCK might induce depletion of stable microtubules at the leading edge, and subsequently microtubules might stabilise at a new location, to facilitate a new leading edge. Another possibility is that ROCK activity might enable dynamic remodelling, or maintain a dynamic population of microtubules needed for repolarisation upon cell–cell contact (Fig. 8). However, it is also possible that ROCK activation is not specifically required for contact inhibition of locomotion. Because ROCK-inhibited cells still show enhanced microtubule growth and shrinkage upon collision compared with free-migrating cells under a ROCK-blocking regime, we suspect that other signalling pathways might also influence microtubule dynamics during contact inhibition of locomotion. Also, our conclusions on the function of ROCK are based on the use of pharmacological inhibitors, and therefore, it is possible that these inhibitors have additional ROCK-independent functions. We predict that any microtubule-stabilisation regime will result in defective polarity switching. Quite simply, having more-persistent stable microtubules makes it harder for a migrating cell to rapidly repolarise and make a new leading edge to drive migration in an alternative direction. Following from this study, it would be interesting also to see whether malignant cells, which are known to escape the restraints of contact inhibition of locomotion (Astin et al., 2010), have a greater proportion of stable microtubules and whether this contributes to their invasive capacity.
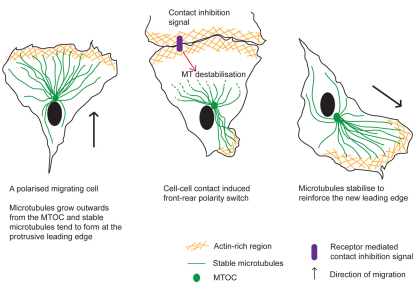
Fig. 8.
Regulation of contact inhibition of locomotion by microtubule remodelling. A normal polarised migrating cell has growing stable microtubules protruding into the leading edge lamella. Upon contact with another cell, microtubules close to the contact site become less stable and colliding cells switch their front–rear polarity. Microtubules might stabilise to another location in the cell body to reinforce the new leading edge, and the cell renews migration in the opposite direction.
Materials and Methods
Cell-confrontation assay
Chick embryo (E7–E8) heart explants were prepared as previously described (Abercrombie and Heaysman, 1954). Explants were placed 0.5–1 mm apart on coverslips coated with poly-L-lysine and then ECM gel (Sigma), in CEF medium (DMEM, sodium pyruvate, pyroxidine, 1 g/l glucose, 10% fetal bovine serum (FBS), 10% chick serum, 1% penicillin-streptomycin) and left overnight to adhere.
Inhibitors
CEFs were pre-incubated with 15 μg/ml cell-permeable C3 transferase (Cytoskeleton) overnight in CO2-independent medium, 2% serum; or with 30 μM Y27632 (Calbiochem) or 10 μM H1152 (Calbiochem) for 2 hours; or with 100 μM blebbistatin (Tocris) for 1 hour in CEF medium, 2% serum or CO2-independent medium, 2% serum (Invitrogen) and time-lapsed for up to 3 hours. 25 nM nocodazole (Sigma) was added alone or in combination with 30 μM Y27632 for 2 hours and cells were subsequently time-lapsed for up to 3 hours. Cells were incubated with 5 nM taxol (Sigma) for up to 5 hours before fixation. Control cells were treated with DMSO.
Analysis of contact inhibition of locomotion
Quantification of contact inhibition of locomotion was carried out as described previously (Paddock and Dunn, 1986). Briefly, the displacement 15 minutes before a cell contacts another cell is measured (vector A on Fig. 1B); the displacement 15 minutes later is recorded as vector B. The component Cx of vector B–A represents the difference between how far the cell has progressed in the direction of A′ and how far it would have gone had there been no collision. The statistical significance between the mean Cx values of free-moving cells and the Cx values of cell–cell collisions, determined using a Mann–Whitney test (**P<0.01), is used to assess the contact inhibition response. The centre of the nucleus was used as a marker to track vectors A and B. For the most part, lamella–lamella collisions were analysed. When collisions were ‘unequal’, we analysed only the head-on cell and only if this cell collided with the front part of the opposing cell. We excluded collisions where a third cell was involved. Cx measurements were scaled to ignore differences in speed between the two cells.
Average cell speed was determined by tracking the position of the nucleus in the first and last images divided by the time. Total contact time was determined by measuring the time taken for the leading edge lamellae (that originally led to the collision between two cells) to pull apart.
Tracking the formation of new leading edges upon cell–cell collision
The cell was split into four segments through the nucleus and a green spot marked where a new leading edge lamella was made on the cell body. A red spot marked when an existing leading edge was maintained and the rest of the cell body continued to move in that direction.
Immunocytochemistry
For visualisation of F-actin, cells were fixed in 4% paraformaldehyde for 10 minutes, rinsed with PBS, permeabilised in 0.2% Triton X-100 for 5 minutes, and then quenched with NaBH4 for 7 minutes at room temperature. Cells were stained with TRITC phalloidin (Sigma). Fixed cells were scored positive for tail retraction defects when the tail length of cells was longer than the cell body. For detection of Glu, acetylated and Tyr MTs, cells were fixed in cold (−20°C) methanol for 5 minutes, rehydrated in PBS and incubated with either a polyclonal rabbit anti-Glu tubulin antibody (1:500 dilution; Chemicon) or a monoclonal mouse anti-acetylated α-tubulin antibody (1:1000 dilution; Sigma) and a rat monoclonal antibody YL1/2 against Tyr tubulin (1:250 dilution; AbD Serotec). Cells were imaged using a Leica SP5-AOBS confocal microscope with a 40×, 1.25 NA objective.
Data analysis of stable and dynamic microtubules
A Volocity (Improvision) program was designed to measure the pixel volume of individual cells with fluorescently labelled Glu and Tyr MTs, from which the ratio of Glu:Tyr MTs was calculated. To determine the magnitude of stable Glu MTs or acetylated MTs present, pixel volume was divided by cell area. A Mann–Whitney test was used to assess statistical significance (**P<0.01) between two data sets.
Preparation of GFP–EB1 and YFP–tubulin adenovirus
The AdEasy system (Stratagene) was used to generate recombinant GFP–EB1 adenovirus. The p-Shuttle-CMV-GFP-EB1 vector was a generous gift from Jim Bamburg (Colorado State University, Fort Collins, CO) and YFP–tubulin adenovirus a kind gift from Louise Cramer (UCL, London, UK). Production and expansion of GFP–EB1 and YFP–tubulin viruses were done following the manufacturer's instructions. Virus titre was determined by an end-point cytopathic effect (CPE) assay. Explants in suspension were infected for 24 hours with GFP–EB1 or YFP–tubulin adenovirus at doses of 107 and 106 p.f.u./ml, respectively, before plating.
Live cell imaging
Individual collisions were analysed by phase-contrast time-lapse microscopy, with the 20× or 40× objective for up to 4 hours (37°C, 5% CO2). For multi-acquisition phase-contrast time-lapse microscopy, images were taken at 30 second intervals for 3 hours at 37°C, with a 10× 0.3 NA objective. Prior to imaging, the CEF medium was changed to CO2-independent medium. Time-lapse imaging of EB1 comets was performed at 1 frame/3 seconds at 37°C; 63× 1.3 NA objective; using a Perkin Elmer UltraVIEW ERS 6FE spinning-disk confocal with a Hamamatsu C9100-50 EM-CCD camera. Time-lapse imaging of YFP–tubulin-expressing cells was performed at 1 frame/3 seconds at a penetration depth of 150 nm; 37°C; 63× 1.47 NA objective; using a Leica AM TIRF multi-colour system attached to a Leica DMI 6000 inverted epifluorescence microscope with a Hamamatsu C9100-13 back-thinned EM-CCD camera. All movies were compiled and cell tracking was done using Volocity software (Improvision).
Data analysis of EB1 comets
The longevity of 30 individual comets per cell was recorded and the average duration time calculated from ten different cells. The lengths per frame of 30 comets (from eight different time points) of ten individual cells were measured to determine the average comet length. To calculate comet speed, 5 frames (15 seconds) from a movie were merged using ImageJ (NIH), and the displacement of the comets measured. EB1 comets only at the leading edge of cells were analysed using Volocity software. Error bars represent ± s.e.m. and a Mann–Whitney test was used to assess statistical significance (**P<0.01) between two data sets.
Data analysis of microtubule filaments
MT dynamics parameters were measured manually using Volocity software (Improvision). Instantaneous rates of shrinkage and growth (μm/minute) were measured as the displacement of the shrinking or growing filament end divided by the time between successive images in a time-lapse series. Only MTs within a 12 μm region from the leading edge were analysed; ~100 MTs (each for growth and shrinkage) from 8–10 non-contacting individual cells were analysed; ~50 MTs (each for growth and shrinkage) from the leading edge of 3–4 contacting cells were analysed. The frequency of catastrophe was calculated by dividing the total number of observed MT catastrophes by the total time of elongations for all MTs observed (in growth phase until they collapse). s.d. was calculated by dividing the frequency of catastrophe by the square root of the total number of events observed (Walker et al., 1988). The life histories of MTs from non-contacting single cells were plotted as length (μm) vs time (seconds). To highlight MTs of interest in Fig. 6, the colour balance was adjusted using Adobe Photoshop. Error bars represent ± s.e.m. and a Mann–Whitney test was used to assess statistical significance (**P<0.01; *P<0.05) between two data sets.
Supplementary Material
Acknowledgments
We thank Jim Bamburg for helpful comments on the manuscript and for research support for L.T., Louise Cramer for her advice on adenoviruses and chick embryo dissection and David Stephens for help with data analysis of EB1 comets and microtubules. We are grateful to John Leach, Landsdown Poultry Farm, Bath, for supplying fertilized chicken eggs. This study was financed by a Medical Research Council (MRC) studentship to S.K., by a Jordanian Graduate Fellowship (L.T.) and by Cancer Research UK (C.D.N.). Deposited in PMC for release after 6 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/124/15/2642/DC1
References
- Abercrombie M. (1970). Contact inhibition in tissue culture. In Vitro 6, 128-142 [DOI] [PubMed] [Google Scholar]
- Abercrombie M. (1979). Contact inhibition and malignancy. Nature 281, 259-262 [DOI] [PubMed] [Google Scholar]
- Abercrombie M., Heaysman J. E. M. (1954). Observations on the social behaviour of cells in tissue culture. 2. Monolayering of fibroblasts. Exp. Cell Res. 6, 293-306 [DOI] [PubMed] [Google Scholar]
- Akhmanova A., Steinmetz M. O. (2008). Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat. Rev. Mol. Cell Biol. 9, 309-322 [DOI] [PubMed] [Google Scholar]
- Amano M., Kaneko T., Maeda A., Nakayama M., Ito M., Yamauchi T., Goto H., Fukata Y., Oshiro N., Shinohara A., et al. (2003). Identification of Tau and MAP2 as novel substrates of Rho-kinase and myosin phosphatase. J. Neurochem. 87, 780-790 [DOI] [PubMed] [Google Scholar]
- Arimura N., Menager C., Kawano Y., Yoshimura T., Kawabata S., Hattori A., Fukata Y., Amano M., Goshima Y., Inagaki M., et al. (2005). Phosphorylation by Rho kinase regulates CRMP-2 activity in growth cones. Mol. Cell. Biol. 25, 9973-9984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astin J. W., Batson J., Kadir S., Charlet J., Persad R. A., Gillatt D., Oxley J. D., Nobes C. D. (2010). Competition amongst Eph receptors regulates contact inhibition of locomotion and invasiveness in prostate cancer cells. Nat. Cell Biol. 12, 1194-1204 [DOI] [PubMed] [Google Scholar]
- Bieling P., Laan L., Schek H., Munteanu E. L., Sandblad L., Dogterom M., Brunner D., Surrey T. (2007). Reconstitution of a microtubule plus-end tracking system in vitro. Nature 450, 1100-1105 [DOI] [PubMed] [Google Scholar]
- Carmona-Fontaine C., Matthews H. K., Kuriyama S., Moreno M., Dunn G. A., Parsons M., Stern C. D., Mayor R. (2008). Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature 456, 957-961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. C., Nalbant P., Birkenfeld J., Chang Z. F., Bokoch G. M. (2008). GEF-H1 couples nocodazole-induced microtubule disassembly to cell contractility via RhoA. Mol. Biol. Cell 19, 2147-2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabek K., van Ham M., Stepanova T., Draegestein K., van Horssen R., Sayas C. L., Akhmanova A., Ten Hagen T., Smits R., Fodde R., et al. (2006). Role of CLASP2 in microtubule stabilization and the regulation of persistent motility. Curr. Biol. 16, 2259-2264 [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S. (2004). Actin and microtubules in cell motility: which one is in control? Traffic 5, 470-477 [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S., Hall A. (2002). Rho GTPases in cell biology. Nature 420, 629-635 [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S., Hall A. (2003). Cdc42 regulates GSK-3 beta and adenomatous polyposis coli to control cell polarity. Nature 421, 753-756 [DOI] [PubMed] [Google Scholar]
- Gundersen G. G., Bulinski J. C. (1988). Selective stabilization of microtubules oriented toward the direction of cell migration. Proc. Natl. Acad. Sci. USA 85, 5946-5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbott L. K., Nobes C. D. (2005). A key role for Abl family kinases in EphA receptor-mediated growth cone collapse. Mol. Cell. Neurosci. 30, 1-11 [DOI] [PubMed] [Google Scholar]
- Heasman S. J., Ridley A. J. (2008). Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 9, 690-701 [DOI] [PubMed] [Google Scholar]
- Heaysman J. E. (1978). Contact inhibition of locomotion: a reappraisal. Int. Rev. Cytol. 55, 49-66 [DOI] [PubMed] [Google Scholar]
- Krendel M., Zenke F. T., Bokoch G. M. (2002). Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat. Cell Biol. 4, 294-301 [DOI] [PubMed] [Google Scholar]
- Li R., Gundersen G. G. (2008). Beyond polymer polarity: how the cytoskeleton builds a polarized cell. Nat. Rev. Mol. Cell Biol. 9, 860-873 [DOI] [PubMed] [Google Scholar]
- Ligon L. A., Shelly S. S., Tokito M. K., Holzbaur E. L. F. (2006). Microtubule binding proteins CLIP-170, EB1, and p150(Glued) form distinct plus-end complexes. FEBS Lett. 580, 1327-1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor R., Carmona-Fontaine C. (2010). Keeping in touch with contact inhibition of locomotion. Trends Cell Biol. 20, 319-328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaki T., Chapin C. J., Gundersen G. G. (1992). Distribution of detyrosinated microtubules in motile NRK fibroblasts is rapidly altered upon cell-cell contact: implications for contact inhibition of locomotion. Cell Motil. Cytoskeleton 23, 45-60 [DOI] [PubMed] [Google Scholar]
- Nakayama M., Goto T. M., Sugimoto M., Nishimura T., Shinagawa T., Ohno S., Amano M., Kaibuchi K. (2008). Rho-kinase phosphorylates PAR-3 and disrupts PAR complex formation. Dev. Cell 14, 205-215 [DOI] [PubMed] [Google Scholar]
- Paddock S. W., Dunn G. A. (1986). Analyzing collisions between fibroblasts and fibrosarcoma cells: fibrosarcoma cells show an active invasionary response. J. Cell Sci. 81, 163-187 [DOI] [PubMed] [Google Scholar]
- Palazzo A. F., Cook T. A., Alberts A. S., Gundersen G. G. (2001). mDia mediates Rho-regulated formation and orientation of stable microtubules. Nat. Cell Biol. 3, 723-729 [DOI] [PubMed] [Google Scholar]
- Raftopoulou M., Hall A. (2004). Cell migration: Rho GTPases lead the way. Dev. Biol. 265, 23-32 [DOI] [PubMed] [Google Scholar]
- Redd M. J., Kelly G., Dunn G., Way M., Martin P. (2006). Imaging macrophage chemotaxis in vivo: studies of microtubule function in zebrafish wound inflammation. Cell Motil. Cytoskeleton 63, 415-422 [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. (2003). Cell migration: integrating signals from front to back. Science 302, 1704-1709 [DOI] [PubMed] [Google Scholar]
- Siegrist S. E., Doe C. Q. (2007). Microtubule-induced cortical cell polarity. Genes Dev. 21, 483-496 [DOI] [PubMed] [Google Scholar]
- Small J. V., Geiger B., Kaverina I., Bershadsky A. (2002). How do microtubules guide migrating cells? Nat. Rev. Mol. Cell Biol. 3, 957-964 [DOI] [PubMed] [Google Scholar]
- Stramer B., Moreira S., Millard T., Evans I., Huang C.-Y., Sabet O., Milner M., Dunn G., Martin P., Wood W. (2010). Clasp-mediated microtubule bundling regulates persistent motility and contact repulsion in Drosophila macrophages in vivo. J. Cell Biol. 189, 681-689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesono A., Heasman S. J., Wojciak-Stothard B., Garg R., Ridley A. J. (2010). Microtubules regulate migratory polarity through Rho/ROCK signaling in T cells. PLoS ONE 5, e8774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan K. T. (2005). TIP maker and TIP marker; EB1 as a master controller of microtubule plus ends. J. Cell Biol. 171, 197-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S., Barth H., Ciossek T., Aktories K., Mueller B. K. (2000). Ephrin-A5 induces collapse of growth cones by activating Rho and Rho kinase. J. Cell Biol. 149, 263-270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. A., O'Brien E. T., Pryer N. K., Soboeiro M. F., Voter W. A., Erickson H. P., Salmon E. D. (1988). Dynamic instability of individual microtubules analyzed by video light microscopy: rate constants and transition frequencies. J. Cell Biol. 107, 1437-1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Noritake J., Kaibuchi K. (2005). Regulation of microtubules in cell migration. Trends Cell Biol. 15, 76-83 [DOI] [PubMed] [Google Scholar]
- Wittmann T., Waterman-Storer C. M. (2001). Cell motility: can Rho GTPases and microtubules point the way? J. Cell Sci. 114, 3795-3803 [DOI] [PubMed] [Google Scholar]
- Yamana N., Arakawa Y., Nishino T., Kurokawa K., Tanji M., Itoh R. E., Monypenny J., Ishizaki T., Bito H., Nozaki K., et al. (2006). The Rho-mDia1 pathway regulates cell polarity and focal adhesion turnover in migrating cells through mobilizing Apc and c-Src. Mol. Cell. Biol. 26, 6844-6858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumbrunn J., Kinoshita K., Hyman A. A., Nathke I. S. (2001). Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3 beta phosphorylation. Curr. Biol. 11, 44-49 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.