Abstract
BACKGROUND
The study of breast cancer in women with African ancestry offers the promise of identifying markers for risk assessment and treatment of triple-negative disease.
METHODS
African American and white American women with invasive cancer diagnosed at the Henry Ford Health System comprised the primary study population, and Ghanaian patients diagnosed and/or treated at the Komfo Anokye Teaching Hospital in Kumasi, Ghana constituted the comparison group. Formalin-fixed, paraffin-embedded specimens were transported to the University of Michigan for histopathology confirmation, and assessment of estrogen and progesterone receptors and HER-2/neu expression.
RESULTS
The study population included 1008 white Americans, 581 African Americans, and 75 Ghanaians. Mean age at diagnosis was 48.0 years for Ghanaian, 60.8 years for African American, and 62.4 for white American cases (P =.002). Proportions of Ghanaian, African American, and white American cases with estrogen receptor-negative tumors were 76%, 36%, and 22%, respectively (P < .001), and proportions with triple-negative disease were 82%, 26%, and 16%, respectively (P < .001). All Ghanaian cases were palpable, locally advanced cancers; 57 (76%) were grade 3. A total of 147 American women were diagnosed as stage III or IV; of these, 67.5% (n =46) of African Americans and 44.6% (n = 29) of white Americans were grade 3. Among palpable, grade 3 cancers, Ghanaians had the highest prevalence of triple-negative tumors (82.2%), followed by African Americans (32.8%) and white Americans (10.2%).
CONCLUSIONS
Our study demonstrates progressively increasing frequency of estrogen receptor-negative and triple-negative tumors among breast cancer patients with white American, African American, and Ghanaian/African backgrounds. This pattern indicates a need for additional investigations correlating the extent of African ancestry and high-risk breast cancer subtypes.
Keywords: breast cancer, triple negative, race, African American, white American, African ancestry
Breast cancer, with an annual estimate of >180,000 new cases is the most common malignancy among women in the United States.1,2 Because of the extensive effort and dedication of the research and medical community and different cancer advocacy organizations, significant milestones have been achieved in reducing the disease-specific mortality across different socioeconomic strata and racial/ethnic lines.3–6 Healthy People 2010, a national health promotion and disease prevention initiative, has as 1 of its 2 main goals to eliminate health disparities across different segments of population.7 The notion of health disparities implies that the disease-specific risk for different subpopulations should be identified and resources should be allocated accordingly.
African American women have a lower lifetime incidence of breast cancer compared with white American women, yet they have higher breast cancer mortality rates.1 African American women are also more likely to be diagnosed with breast cancer at younger ages, and with high-grade tumors that are negative for expression of the estrogen receptor (ER), progesterone receptor (PR), and the HER-2/neu marker.8–10 Because these features are more common in BRCA-1 mutation-associated breast cancer, it has been postulated that African ancestry might be associated with hereditary predisposition for high-risk breast cancer of a specific subtype.11 The study of breast cancer in women with African ancestry, therefore, has the potential for leading to the identification of biomarkers that might be useful for the risk assessment and treatment of triple-negative breast cancer. The University of Michigan has established international breast cancer research collaboration with the Komfo Anokye Teaching Hospital in Kumasi, Ghana, and the Henry Ford Health System in Detroit, Michigan, with the goal of studying the genetics of breast cancer in African, African American, and white American women. We report herein our initial findings with respect to comparing patterns of disease and selected clinicopathologic features.
MATERIALS AND METHODS
The conduct of this study was approved by the institutional review boards affiliated with the University of Michigan in Ann Arbor, Michigan; the Henry Ford Health System in Detroit, Michigan; and the Komfo Anokye Teaching Hospital in Kumasi, Ghana.
Study Population
African American and white American women with invasive breast cancer diagnosed through the Henry Ford Health System between January 1, 2001 and December 31, 2007 comprised the primary study population. Details regarding the Henry Ford Health System tumor registry and data acquisition process have been reported previously.12,13 Briefly, information on individual patient demographics (self-reported race, date of birth) and breast cancer clinicopathologic features (date of diagnosis, stage, grade, expression of molecular markers estrogen receptor, progesterone receptor, and HER-2/neu) were downloaded from the Pathology Information System (Mysis-CoPath).
African women with invasive breast cancer diagnosed and/or treated at the Komfo Anokye Teaching Hospital in Kumasi, Ghana between January 1, 2007 and December 31, 2008 comprised the comparison group. Patient demographics and selected clinicopathologic features (age, tumor size) were abstracted from the Komfo Anokye Teaching Hospital hardcopy medical records and pathology reports. Information regarding the extended medical history such as menopausal status was rarely available, because hospital-based financial limitations preclude the ability to maintain a tumor registry or centralized medical records system. Furthermore, image-guided wire localization biopsy procedures are not available at Komfo Anokye Teaching Hospital, and screening mammography is limited. All of the Ghanaian breast cancer patients included in this study therefore presented with clinically evident disease. All breast cancers from Komfo Anokye Teaching Hospital included in the present analysis had formalin-fixed, paraffin-embedded specimens transported to the University of Michigan Department of Pathology, where they were recut, stained by hematoxylin and eosin for histopathology confirmation, and then stained by immunohistochemistry for ER, PR, and HER-2/neu expression.
Assessment of ER and PR
Hormone receptor proteins in the nucleus of cells were detected with specific monoclonal antibodies using a labeled streptavidin-biotin immunoperoxidase method. The immunocytochemical assay was performed on depar-affinized formalin-fixed tissue sections of the specimens. Monoclonal mouse antibodies to human ER (Dako [Glostrup, Denmark] clone ID5) and to human PR (Dako clone PgR636) were used with a Dako automated immunostainer following the manufacturer’s protocol. Slides were then reviewed using light microscopy, and the percentage of cells with nuclear immunoreactivity was semiquantitatively assessed. Slides were graded as positive, negative, or focal positive, indicating a lower level of receptor protein.14,15 For the purpose of the present study, the status of ER and PR was dichotomized as positive or negative. Therefore, cells with focal positive status for ER and/or PR were classified as positive.
Assessment of HER-2/neu
Fluorescent in situ hybridization (FISH) and immunohistochemistry (IHC) were used to assess amplification of the HER2 gene and overexpression of its protein, p185, respectively. IHC was performed on formalin-fixed, paraffin-embedded tissue sections using the HerceptTest (Dako) according to the manufacturer’s instructions. The Dako HerceptTest is a US Food and Drug Administration-approved clinical test that qualitatively identifies by light microscopy p185 HER2 overexpression in breast cancer cells. The relative expression of HER2 was scored as either 1+ or weakly positive (staining in <10% of tumor cells), 2+ or weak to moderate positive (complete membrane staining in >10%), or 3+ or strongly positive (strong complete membrane staining in >10%). Specimens that were scored as 2+ were further evaluated by the FISH technique. The method was performed on formalin-fixed, paraffin-embedded tissue sections using a DNA probe cocktail specific for the HER-2/neu gene locus (17q11.2-q12) and an internal control probe for chromosome 17 (CEP17-D17Z1) (Vysis, Downers Grove, Ill). After hybridization cells were scored, the ratio of HER-2/neu to CEP17 was calculated. A ratio of >2 was considered as positive for amplification. For the purpose of the present study, HER-2/neu status was dichotomized as either positive or negative. A specimen scored as 0 was classified as HER-2/neu negative; a specimen was considered positive if it received an IHC score of 3+. For specimens with an IHC score of either 1+ or 2+, an amplification ratio of >2 was classified as positive for HER-2/neu, a ratio ≤2 was considered negative.
Definition of Subtypes of Breast Carcinoma
Presently, application of DNA microarray technology is not readily available in many clinical settings. IHC and/or FISH provide valid, reliable, and cost-effective methods for evaluation of prognostic biomarkers.16 Reproducible correlation of IHC with microarray technique supports the validity of IHC for the purpose of subtyping of breast carcinoma.17 In addition, hormone receptors and HER-2/neu status are the primary biomarkers for subtyping.18,19 For the purpose of the present study, we adopted an IHC classification that categorizes breast carcinoma according to the expression status of ER, PR, and HER-2/neu.
Statistical Methods
Prevalence distribution of categorical data, ER, PR, HER-2/neu, histologic grade, and histopathology of cancer among the 3 groups of women was evaluated using the Kruskal-Wallis test. Analysis of variance with Tukey post hoc test of significance was applied to compare the location of mean values for the 2 continuous variables, age at the time of diagnosis and tumor size in the greatest dimension, among the 3 groups of women. All statistical comparisons were 2-sided, and analysis were performed using SAS v. 9.1 (SAS Institute, Cary, NC).
RESULTS
A total of 1008 white American, 581 African American, and 75 Ghanaian women contributed to this study. The mean age at the time of the diagnosis for Ghanaian women was 48.0 years (±6.4 years) compared with 60.7 years (±13.7 years) for African American women and 62.4 years (±13.7 years) for white American women (P = .0019). (Table 1) All of the Ghanaian women had palpable cancers that were diagnosed by clinical breast examination followed by subsequent pathologic confirmation via freehand (ie, without image guidance) percutaneous core needle biopsy (⅓) or surgical resection (⅔). Mean primary breast tumor size for the Ghanaian, African American, and white American women was 3.20 cm, 2.3 cm, and 1.95 cm, respectively (P < .001). Approximately 75% (n = 56) of the Ghanaian cases were grade 3 lesions. Data for histologic grade were available for a total of 828 white American and 450 African American women. Of these women, 44.9% (n = 202) of African American and 29.3% (n = 243) of white American women were presented with grade 3 cancers (Table 1).
Table 1.
Comparisons of Clinicopathologic Features in Study Populations
| Feature | HFH WA,a n=1008 | HFH AA,b n=581 | Ghanaian,c n=75 | P |
|---|---|---|---|---|
| Mean age, y (±SD) | 62.4 (±13.7) | 60.7 (±13.7) | 48.0 (±6.4) | .002 |
| Mean tumor size, cm (range) | 1.95 (0.1–14.0) | 2.30 (0.1–15.0) | 3.20 (0.9–9.0) | <.001 |
| Histology (% with invasive ductal carcinoma) | 821/1008 (81.4) | 499/581 (86) | 50/75 (66.7) | <.0001 |
| Grade 3 (%) | 243/828d (29.3) | 202/450d (44.9) | 57/75 (76) | .007 |
| ER− (%) | 218/995d (21.9) | 208/576d (36.1) | 57/76 (76) | <.0001 |
| PR− (%) | 249/827d (30.1) | 199/443d (44.9) | 32/48d (66.7) | .0001 |
| HER-2/neu− (%) | 641/836d (76.7) | 332/442d (75.1) | 46/48d (95.8) | .0001 |
| ER−, PR−, HER2− (%) | 122/763 (16.0) | 107/405 (26.4) | 37/45 (82.2) | .0001 |
| ER+ and/or PR+, HER2− (%) | 472/763 (61.9) | 200/405 (49.4) | 6/45 (13.3) | .019 |
HFH indicates Henry Ford Health System; WA, white American; AA, African American; SD, standard deviation; ER, estrogen receptor; progesterone receptor.
WA breast cancer cases from the HFH.
AA breast cancer cases from the HFH.
Ghanaian women from Komfo Anokye Teaching Hospital in Kumasi, Ghana.
Missing data.
A total of 57 (76%) Ghanaian women were diagnosed with ER-negative cancers. Data on the status of PR and HER-2/neu biomarker were available for a total of 48 women, of whom 66.7% (n = 32) were diagnosed with PR-negative and 95.8% (n =46) with HER-2/neu–negative breast cancers (Table 1). Data on the status of ERs were available for 995 white American and 576 African American women. The prevalence of ER-negative cancer was 21.9% (n = 218) in white American and 36.1% (n = 208) in African American women. We were able to retrieve PR data for a total of 827 white American and 443 African American women. Twenty-nine percent (n = 249) of white American and 43.4% (n = 199) of African American women were diagnosed with cancer lacking the expression of PRs. Finally, data for HER-2/neu bio-marker were available for a total of 836 white American and 442 African American women. More than ¾ (76.7%; n = 641) of white American women and 75.1% (n = 332) of African American women were diagnosed with HER-2/neu–negative breast cancer (Table 1). We then classified the 3 groups of women by the joint expression of the 3 diagnostic biomarkers (ER, PR, and HER-2/neu). We observed the highest prevalence of triple-negative breast cancers in Ghanaian women (82.2%, n = 37), followed by African American (26.4%, n = 107) and white American (16.0%, n = 122) women. In contrast, the highest proportion of women diagnosed with ER+ and/or PR+, HER2+ breast cancers was observed in white American women (61.9%, n = 472), followed by African American (49.4%, n = 200) and Ghanaian (13.3%, n = 6) women. (Table 1).
To minimize the potential confounding effect of advanced stages and poorly differentiated grades, we stratified American women by their stage and grade of cancers and estimated the prevalence of hormone receptor-negative breast cancers. A total of 28 (2.8%) white American women, 46 (7.9%) African American women, and 57 (76%) Ghanaian women were diagnosed with poorly differentiated and advanced stage (III/IV) breast cancer (Table 2). Absence of expression of ERs was observed in 77.2% (n = 44) of Ghanaian women, 67.4% (n = 31) of African American women, and 50.0% (n = 14) of white American women (P = .043). Interestingly, African American women had the highest prevalence of PR-negative cancers (76.1%, n = 35), followed by Ghanaian (69.2%, n = 27) and white American (60.7%, n = 17) women (P = .297). Finally, 94.7% (n = 36) of Ghanaian women were diagnosed with HER-2/neu–negative breast cancer, compared with 63.0% (n = 29) of African American women and 46.4% (n = 13) of white American women (P < .0001) (Table 2). We then stratified women by the joint expression of ER, PR, and HER-2/neu bio-marker (Fig. 1). Eighty-three percent (n = 30) of Ghanaian women were diagnosed with triple-negative breast cancer, whereas the proportion of African American women with triple-negative breast cancer was 41.9% (n = 18) and of white American women was 15.4% (n = 4). In contrast, white American women experienced the highest proportion (38.5%, n = 10) of hormone-independent, HER-2/neu–positive breast cancer, followed by African American (30.2%, n = 13) and Ghanaian women (2.8%, n = 1).
Table 2.
Comparisons of Clinicopathologic Features in Study Populations Subsets With Advanced Stages and Poorly Differentiated Grades
| Feature | HFH WA,a n=28 | HFH AA,b n=46 | Ghanaian,c n=57 | P |
|---|---|---|---|---|
| Estrogen receptor negative (%) | 14/28 (50.0) | 31/46 (67.4) | 44/57 (77.2) | <.041 |
| Progesterone receptor negative (%) | 17/28 (60.7) | 35/46 (76.1) | 27/39d (69.2) | .374 |
| HER-2/neu negative (%) | 13/28 (46.4) | 29/46 (63.0) | 36/38d (94.7) | <.0001 |
HFH indicates Henry Ford Health System; WA, white American; AA, African American.
WA breast cancer cases from the HFH.
AA breast cancer cases from the HFH.
Ghanaian women from Komfo Anokye Teaching Hospital in Kumasi, Ghana.
Missing data.
Figure 1.
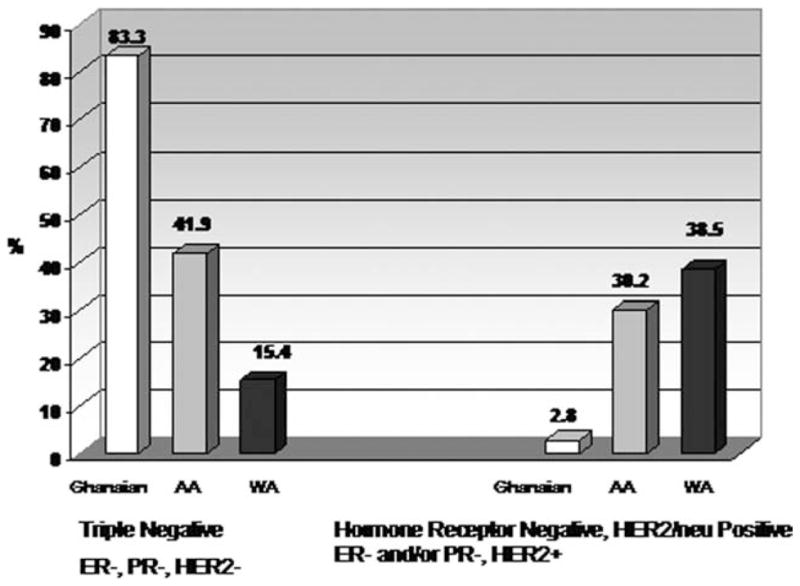
Prevalence is shown of selected subtypes among white American, African American, and Ghanaian women diagnosed with palpable cancers and poorly differentiated histologic grade. Frequencies shown are for hormone receptor-negative, HER-2/neu-positive, and triple-negative breast cancer subtypes. Data for frequencies of estrogen receptor (ER)-positive/progesterone receptor (PR)-positive/HER2-positive and ER-positive/PR-positive/HER2 negative tumors are not shown. WA indicates white American; AA, African American.
We also stratified African American and white American women by their menopausal status and compared frequencies of triple-negative breast cancers. Among premenopausal African American and white American women, the prevalence of triple-negative breast cancer was 32.3% and 25.2%, respectively. These proportions were significantly lower than the 82% triple-negative rate observed among the Ghanaian cases, the majority of whom were younger than the commonly used menopausal surrogate cutpoint of 50 years. Non–triple-negative tumors were rare among Ghanaian cases, regardless of age.
DISCUSSION
Lifetime breast cancer incidence rates are lower for African American women, yet mortality rates are paradoxically higher. The mortality differences mostly can be explained by the more advanced stages at the initial clinical presentation of the disease that is observed for African American breast cancer patients, and this in turn, although multifactorial, is likely driven by delays in diagnosis and treatment that result from the poverty rates and healthcare access barriers that are more prevalent in the African American patient population.
Several of the other features that describe the breast cancer burden of the African American community are more enigmatic and not readily explained by socioeconomic factors. For example, African American women are more likely to develop breast cancer at younger ages. In addition, at any age of diagnosis they are more likely to have aneuploidy and tumors that are negative for ER, PR, and the HER-2/neu marker. In Table 3, we have provided summary data from selected studies that have compared the frequency of triple-negative breast cancers between African American and white American women.8,13,20–23 These molecular marker patterns suggest that outcome disparities are likely to increase over the next few years, because the most significant recent advances in systemic therapy for breast cancer have been made in the management of endocrine-sensitive and/or HER-2/neu–overexpressing disease. Fewer African American women will be candidates for these treatment advances. Lastly, the incidence of male breast cancer is also higher for African American compared with white American communities.24,25 All of these latter features describing African American breast cancer also serve to describe the breast cancer burden of patients with known hereditary susceptibility, such as carriers of BRCA1 mutations. It has also been well documented that selected BRCA mutations (founder mutations) are particularly common within specific populations defined by ancestral heritage (such as the Ashkenazi Jewish community).26–28 It is therefore reasonable to explore the possibility that African ancestry might also be associated with some hereditary predisposition for early onset or high-risk breast cancer. Extent of African background can be quite variable in individuals who self-identify as African American because of the past 4 centuries of genetic admixture that has occurred in the United States. However, it can generally be assumed that extent of African ancestry is likely to be stronger for individuals who self-identify as African American compared with those who self-identify as white American, but not as strong as that seen in contemporary populations residing in continental Africa.
Table 3.
Frequency of Reported Triple-Negative Breast Cancer in AA and WA Women
| Study | Dataset/Sample Size | Frequency of TN or Basal Subtype Breast Cancer | ||
|---|---|---|---|---|
| AAs | WAs | P | ||
| Carey 20068 | 97 premenopausal AA vs 164 premenopausal non-AA women from Carolina Breast Cancer Study | 39% (basalAU: Please note asterisks removed from Table 3; no corresponding footnote. subtype) | 16% (basal subtype) | <.001 |
| Morris 200720 | 2230 Thomas Jefferson University Hospital pts and 197,274 SEER pts | 20.8% (TN) | 10.4% (TN) | <.0001 |
| Stark 200813 | Henry Ford Health System, 441 AA, 822 WA | 24.4% (TN) | 14.4% (TN) | <.0001 |
| Lund 200821 | 167 AA and 23 WA cases from Grady Hospital; urban Atlanta, GA | 29.3% (TN) | 13.0% (TN) | .05 |
| Moran 200822 | 99 AA and 968 WA breast conservation pts from Yale University School of Medicine | 21% (TN) | 8% (TN) | <.0001 |
| Lund 200923 | Population-based Atlanta GA cohort of 116 AA, 360 WA cases | 46.6% (TN) | 21.8% (TN) | <.001 |
AA indicates African American; WA, white American; TN, triple negative; pts, patients; SEER, Surveillance, Epidemiology, and End Results.
This article represents the first published study that directly compares clinicopathologic features of a contemporary African breast cancer population (as represented in Ghana) with those of white American and African American breast cancer patients diagnosed within a similar timeframe. Ghana is a particularly valuable comparison population, because of its geographic location in western, sub-Saharan Africa, where many of the colonialera slave colonies were also located. It is therefore a reasonable expectation that contemporary African American and contemporary Ghanaians have some shared ancestry. Other studies of breast cancer in selected African populations have demonstrated similarly high prevalence of ER-negative and triple-negative tumors.29,30 Our study documented provocative patterns of increasing frequency for early onset/younger age at diagnosis, ER-negative/PR-negative, and triple-negative breast cancers in association with presumed increasing extent of African ancestry. These patterns suggest that further study of the breast cancer burden in African women could lead to the identification of tumor or germline markers associated with high-risk breast cancer. Hopefully, these markers will ultimately have potential utility for targeted therapy of the triple-negative phenotype, for which we are currently limited to chemotherapy as systemic treatment.
This study has several obvious limitations. First, the younger average age at diagnosis for Ghanaian breast cancer patients may well be influenced by the overall shorter longevity expectations in developing countries. Average lifespan for Ghanaians is approximately 20 years younger than the average life expectancy of 77 years for Americans. Also, it has been suggested that racial/ethnic identity is confounded by the stronger effect of poverty as an independent risk factor for ER-negative disease.31 However, data from international registries (in countries that have more homogeneous populations and therefore less opportunity for confounding between race/ethnicity and socioeconomic factors) fail to show any consistent association between poverty and frequency of ER-negative breast cancer.32–34 Furthermore, our study is notably limited by the paucity of detailed clinicopathologic information on the Ghanaian breast cancer cases. We were therefore unable to perform any stratified comparisons based on menopausal status or disease stage beyond the subset analyses of cases with palpable tumors. This is of particular concern because the argument could be made that the high frequency of ER-negative disease among African women is a direct consequence of the fact that few Ghanaian women have access to screening mammography, and so the majority of breast cancer patients present with ER-negative tumors that were rapidly growing. Population-based data from the Surveillance, Epidemiology, and End Results (SEER) program refutes this argument, at least as it pertains to African American women. The SEER program documents higher population-based incidence rates for ER-negative breast cancers in African American compared with white American women at all age categories, and regardless of whether the cancer is diagnosed as locally advanced, nonlocally advanced, or inflammatory disease.35,36 Lastly, we have made the assumption that many African American women are likely to have shared ancestry with many Ghanaian women, because of the slave trade colonies located in western, sub-Saharan Africa. However, we did not have any data on the actual geographic ancestry for the African American women included in this study. We therefore do not know the relative contributions of East African versus West African or other geographically defined communities to their lineage.
In summary, we report herein a correlation between risk of ER-negative and triple-negative breast cancer and presumed extent of African ancestry by looking at White American, African American, and African breast cancer patients. Our findings underscore the need for further research regarding the breast cancer burden of African women.
Acknowledgments
The authors gratefully acknowledge the assistance of Dr. Francis Aitpillah, Mr. Samuel Kachunga, Ms. Goulda, Mr. Emanuel Asiameh, Ms. Beatrice Antwi, and Ms. Adwoa Bemah Bonsu, all from the KATH and without whose energy, this research would not have been possible.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
This work was supported by Susan G. Komen for the Cure and the University of Michigan Global REACH program. Dr. Kleer is supported by NIH grants R01 CA107469 and R01 CA125577.
References
- 1.Horner MJ, Ries LAG, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2006. Bethesda, MD: National Cancer Institute; 2009. [Accessed on November 15, 2009]. Based on November 2008 SEER data submission, posted to the SEER web site. Available at: http://seer.cancer.gov/csr/1975_2006/ [Google Scholar]
- 2.Peto R, Boreham J, Clarke M, et al. UK and USA breast cancer deaths down 25% in year 2000 at ages 20–69 years. Lancet. 2000;355:1822. doi: 10.1016/S0140-6736(00)02277-7. [DOI] [PubMed] [Google Scholar]
- 3.Dignam JJ. Differences in breast cancer prognosis among African-American and Caucasian women. CA Cancer J Clin. 2000;50:50–64. doi: 10.3322/canjclin.50.1.50. [DOI] [PubMed] [Google Scholar]
- 4.Jones LA, Brawley O, Johnson-Thompson M, et al. Overview of the summit meeting evaluating research in African-American women. Cancer. 2003;97:207–210. doi: 10.1002/cncr.11028. [DOI] [PubMed] [Google Scholar]
- 5.Brawley OW. Disaggregating the effects of race and poverty on breast cancer outcomes. J Natl Cancer Inst. 2002;94:471–473. doi: 10.1093/jnci/94.7.471. [DOI] [PubMed] [Google Scholar]
- 6.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 7.Healthy People 2010: Understanding and improving health. Washington, DC: Department of Health and Human Services, Office of Disease Prevention and Health Promotion; 2000. [Accessed July 4, 2009]. Available at: http://www.healthypeople.gov/Default.htm. [Google Scholar]
- 8.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 9.Newman LA. Breast cancer in African-American women. Oncologist. 2005;10:1–14. doi: 10.1634/theoncologist.10-1-1. [DOI] [PubMed] [Google Scholar]
- 10.Newman LA, Martin IK. Disparities in breast cancer. Curr Probl Cancer. 2007;31:134–156. doi: 10.1016/j.currproblcancer.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Newman LA, Bunner S, Carolin K, et al. Ethnicity related differences in the survival of young breast carcinoma patients. Cancer. 2002;95:21–27. doi: 10.1002/cncr.10639. [DOI] [PubMed] [Google Scholar]
- 12.Stark AT, Claud S, Kapke A, et al. Race modifies the association between breast carcinoma pathologic prognostic indicators and the positive status for HER-2/neu. Cancer. 2005;104:2189–2196. doi: 10.1002/cncr.21463. [DOI] [PubMed] [Google Scholar]
- 13.Stark A, Kapke A, Schultz D, et al. Advanced stages and poorly differentiated grade are associated with an increased risk of HER2/neu positive breast carcinoma only in white women: findings from a prospective cohort study of African-American and white-American women. Breast Cancer Res Treat. 2008;107:405–414. doi: 10.1007/s10549-007-9560-5. [DOI] [PubMed] [Google Scholar]
- 14.Fisher ER, Anderson S, Dean S, et al. Solving the dilemma of the immunohistochemical and other methods used for scoring estrogen receptor and progesterone receptor in patients with invasive breast carcinoma. Cancer. 2005;103:164–173. doi: 10.1002/cncr.20761. [DOI] [PubMed] [Google Scholar]
- 15.Allred DC, Harvey JM, Berardo M, et al. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 16.Wang S, Saboorian MH, Frenkel E, et al. Laboratory assessment of the status of Her-2/neu protein and oncogene in breast cancer specimens: comparison of immunohistochemistry assay with fluorescence in situ hybridisation assays. J Clin Pathol. 2000;53:374–381. doi: 10.1136/jcp.53.5.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 18.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livasy CA, Karaca G, Nanda R, et al. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006;19:264–271. doi: 10.1038/modpathol.3800528. [DOI] [PubMed] [Google Scholar]
- 20.Morris GJ, Naidu S, Topham AK, et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute’s Surveillance, Epidemiology, and End Results database. Cancer. 2007;110:876–884. doi: 10.1002/cncr.22836. [DOI] [PubMed] [Google Scholar]
- 21.Lund MJ, Butler EN, Bumpers HL, et al. High prevalence of triple-negative tumors in an urban cancer center. Cancer. 2008;113:608–615. doi: 10.1002/cncr.23569. [DOI] [PubMed] [Google Scholar]
- 22.Moran MS, Yang Q, Harris LN, et al. Long-term outcomes and clinicopathologic differences of African-American versus white patients treated with breast conservation therapy for early-stage breast cancer. Cancer. 2008;113:2565–2574. doi: 10.1002/cncr.23881. [DOI] [PubMed] [Google Scholar]
- 23.Lund MJ, Trivers KF, Porter PL, et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat. 2009;113:357–370. doi: 10.1007/s10549-008-9926-3. [DOI] [PubMed] [Google Scholar]
- 24.Anderson WF, Althuis MD, Brinton LA, et al. Is male breast cancer similar or different than female breast cancer? Breast Cancer Res Treat. 2004;83:77–86. doi: 10.1023/B:BREA.0000010701.08825.2d. [DOI] [PubMed] [Google Scholar]
- 25.O’Malley C, Shema S, White E, et al. Incidence of male breast cancer in California, 1988–2000: racial/ethnic variation in 1759 men. Breast Cancer Res Treat. 2005;93:145–150. doi: 10.1007/s10549-005-4517-z. [DOI] [PubMed] [Google Scholar]
- 26.Struewing JP, Abeliovich D, Peretz T, et al. The carrier frequency of the BRCA1 185delAG mutation is approximately 1 percent in Ashkenazi Jewish individuals. Nat Genet. 1995;11:198–200. doi: 10.1038/ng1095-198. [DOI] [PubMed] [Google Scholar]
- 27.Oddoux C, Struewing JP, Clayton CM, et al. The carrier frequency of the BRCA2 6174delT mutation among Ashkenazi Jewish individuals is approximately 1% Nat Genet. 1996;14:188–190. doi: 10.1038/ng1096-188. [DOI] [PubMed] [Google Scholar]
- 28.Warner E, Foulkes W, Goodwin P, et al. Prevalence and penetrance of BRCA1 and BRCA2 gene mutations in unselected Ashkenazi Jewish women with breast cancer. J Natl Cancer Inst. 1999;91:1241–1247. doi: 10.1093/jnci/91.14.1241. [DOI] [PubMed] [Google Scholar]
- 29.Huo D, Ikpatt F, Khramtsov A, et al. Population differences in breast cancer: survey in indigenous African women reveals over-representation of triple-negative breast cancer. J Clin Oncol. 2009;27:4515–4521. doi: 10.1200/JCO.2008.19.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bird PA, Hill AG, Houssami N. Poor hormone receptor expression in East African breast cancer: evidence of a biologically different disease? Ann Surg Oncol. 2008;15:1983–1988. doi: 10.1245/s10434-008-9900-7. [DOI] [PubMed] [Google Scholar]
- 31.Gordon NH. Socioeconomic factors and breast cancer in black and white Americans. Cancer Metastasis Rev. 2003;22:55–65. doi: 10.1023/a:1022212018158. [DOI] [PubMed] [Google Scholar]
- 32.Carnon AG, Ssemwogerere A, Lamont DW, et al. Relation between socioeconomic deprivation and pathological prognostic factors in women with breast cancer. BMJ. 1994;309:1054–1057. doi: 10.1136/bmj.309.6961.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halmin M, Bellocco R, Lagerlund M, et al. Long-term inequalities in breast cancer survival—a ten year follow-up study of patients managed within a National Health Care System (Sweden) Acta Oncol. 2008;47:216–224. doi: 10.1080/02841860701769768. [DOI] [PubMed] [Google Scholar]
- 34.Bowen RL, Duffy SW, Ryan DA, et al. Early onset of breast cancer in a group of British black women. Br J Cancer. 2008;98:277–281. doi: 10.1038/sj.bjc.6604174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hance KW, Anderson WF, Devesa SS, et al. Trends in inflammatory breast carcinoma incidence and survival: the Surveillance, Epidemiology, and End Results program at the National Cancer Institute. J Natl Cancer Inst. 2005;97:966–975. doi: 10.1093/jnci/dji172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson WF, Chatterjee N, Ershler WB, et al. Estrogen receptor breast cancer phenotypes in the Surveillance, Epidemiology, and End Results database. Breast Cancer Res Treat. 2002;76:27–36. doi: 10.1023/a:1020299707510. [DOI] [PubMed] [Google Scholar]


