Abstract
Signal transducer and activator of transcription-3 (Stat3) is frequently activated in breast cancer and multiple lines of evidence suggest that Stat3 promotes tumor progression. However, the prognostic value of Stat3 in human breast cancer remains controversial and associations range from favorable to unfavorable based on four outcome studies of 62, 102, 255 and 517 patients. Cellular Stat3 protein expression was measured in three studies whereas nuclear localized, tyrosine phosphorylated Stat3 (Nuc-pYStat3) was used as the readout in only one study. We therefore retrospectively analyzed the prognostic value of Nuc-pYStat3 in a larger material of 721 breast cancer specimens. Overall, patients whose tumors were positive for Nuc-pYStat3 tended to have improved survival, but the trend did not reach statistical significance (P=0.08). When specimens were stratified by tumor grade, patients with low grade but not high grade tumors that were positive for Nuc-pYStat3 had significantly prolonged overall survival in univariate analysis (P=0.014) but not in multivariate analyses. Unexpectedly, quantitative immunofluoresence detection revealed highest levels of Nuc-pYStat3 in normal breast epithelia and gradual loss of Nuc-pYStat3 during progression from DCIS, invasive ductal carcinoma, and lymph node metastases. Levels of Nuc-pYStat3 correlated positively with levels of Nuc-pYStat5, a favorable prognostic marker, in invasive ductal carcinomas. Furthermore, Nuc-pYStat3 levels correlated strongly with protein levels of nuclear localized Stat5a (r=0.633, P<0.001) but notStat5b. Our data does not support the notion that Nuc-pYStat3 is an independent marker of prognosis in breast cancer, although future studies may reveal prognostic utility within molecularly characterized subtypes of breast cancer.
Keywords: Stat3, breast cancer, biomarker, prognosis, survival, immunohistochemistry
Introduction
The signal transducer and activator of transcription (Stat) family includes 7 gene products (Stats 1-4, 5a, 5b, and 6) that function as mediators of cytokine and growth factor signaling. When activated, cytoplasmic Stats become phosphorylated on a positionally conserved tyrosine residue, translocate to the nucleus, and bind as dimers to DNA target sequences to modulate transcription of target genes [1, 2]. Compared with normal cells and tissues, consti-tutively activated Stats have been detected in a wide range of human cancer cell lines and primary tumors, including leukemias, lymphomas, melanomas, prostate, ovarian, lung, and breast cancers [3]. Constitutive activation of Stat3, for instance, has been reported in human breast carcinoma cell lines but not in mammary epithelial cell lines established from non-malignant tissues [4, 5]. Elevated levels of activated Stat3 have been associated with increased breast cancer cell proliferation, survival and metastasis in experimental settings [6-11]. Furthermore, suppression of Stat3 expression in breast cancer cells has been shown to cause apoptosis, inhibit cell growth, and reduce invasive potential, implicating Stat3 as a promoter of breast tumor growth and progression [12-15]. Consistent with a role for activated Stat3 in breast cancer progression, elevated Stat3 activity was detected in tumors compared with matched nonneoplastic tissues, and tumor levels of activated Stat3 were lower in patients who had a complete pathologic response to neoadjuvant docetaxel and doxorubicin therapy than those of patients who had a partial pathologic response [16].
Despite the extensive data suggesting that Stat3 promotes human breast cancer progression, the prognostic value of Stat3 in breast cancer remains controversial and unresolved based on four studies of clinical outcome. An initial immunohistochemical analysis of 62 breast cancer specimens indicated no correlation between levels of nuclear localized Stat3 and patient survival [17]. Analysis of 255 node-negative breast cancer specimens stained using a phospho-Stat3 specific antibody revealed an association between elevated levels of nuclear localized, tyrosine phosphorylated Stat3 (Nuc-pYStat3) and a modestly improved overall survival at both 5- and 20- year follow-up [18]. This effect was significant in multivariate analysis (HR=2.35, 95% CI(1.01-5.46), P=0.0469) [18]. A third study on 517 human breast cancer tissues reported that total Stat3 protein expression regardless of nuclear staining did not correlate with patient survival [19]. Finally, a fourth study on 102 primary invasive breast cancers found that elevated levels of total Stat3 protein expression was significantly correlated with a decreased overall 5 year survival rate [20]. This effect was also significant in multivariate analysis (OR=2.5, 95% CI(1.1-5.6), P=0.024) [20]. Among the four studies, only Dolled-Filhart and colleagues examined levels of Nuc-pYStat3, which are arguably more reflective of transcriptional activation than levels of cellular Stat3 protein. Furthermore, this study was unique in that it was confined to node-negative patients. Based on the apparently contradictory clinical outcome data from the four initial studies, we analyzed levels of Nuc-pYStat3 in a larger breast cancer material that included patients with both node-negative and node-positive disease.
We employed the same phospho-Stat3 antibody used by Dolled-Filhart and colleagues [18] to evaluate levels of Nuc-pYStat3 in 721 breast cancer specimens by immunohistochemical staining, and correlated the data with clinical outcome. Elevated levels of Nuc-pYStat3 were significantly associated with favorable prognosis in patients with low grade tumors in univariate analysis but not in multivariate analyses. Furthermore, Nuc-pYStat3 status was not correlated with survival when the entire material was analyzed combined, or when patient tumors were stratified by nodal status. Interestingly, quantitative analyses of levels of Nuc-pYStat3 in a breast cancer progression material showed a significant correlation with levels of Nuc-pYStat5 and unexpectedly revealed frequent reduction of levels of Nuc-pYStat3 in invasive and metastatic breast cancer compared to normal breast epithelia.
Materials and methods
Breast cancer materials
Paraffin-embedded breast cancer specimens from deidentified archival tissue microarrays were used in this retrospective study. A microarray material totaling 785 primary breast cancer specimens (0.6mm cores) for which clinical follow-up data were available in the form of overall survival data, as described previously as “Material B” [21]. Immunohistochemical analysis of levels of Nuc-pYStat3 was uninformative for 64 tumor samples because of missing or unrepresentative samples in the array sections analyzed. Demographic and clinical characteristics of the breast cancer specimens are presented in Table 1. The second tissue array was constructed using cutting edge matrix assembly [22] and represents a progression material containing normal breast tissues (N=40), DCIS (N=20), invasive ductal carconimas (N=100), and lymph node metastases (N=20) as described previously [23].
Table 1.
Characteristics of breast cancer specimens
| No. patients | % | |
|---|---|---|
| Age at diagnosis (yr) | ||
| Median | 64 | |
| Range | 30-98 | |
| No. patients | 785 | |
| Tumor diameter (mm) | ||
| Median | 25 | |
| Range | 5-140 | |
| No. patients | 776 | |
| Lymph node status | ||
| 0 | 376 | 47.9 |
| 1 | 278 | 35.4 |
| 2 | 32 | 4.1 |
| Unknown | 99 | 12.6 |
| Tumor grade | ||
| 1 | 246 | 31.3 |
| 2 | 314 | 40.0 |
| 3 | 207 | 26.4 |
| Unknown | 18 | 2.3 |
| pT stage | ||
| T1 | 252 | 32.1 |
| T2 | 398 | 50.7 |
| T3 | 58 | 7.4 |
| T4 | 74 | 9.4 |
| Unknown | 3 | 0.4 |
Immunohistochemistry of Nuc-pYStat3
Phospho-Stat3 (Tyr705) antibody was purchased from Cell Signaling Technology (Beverly, MA). Sections of paraffin-embedded, formalin-fixed tissues from human breast cancers were deparaffinized in xylene for 2 × 15 min followed by rehydration in graded ethanol. Slides containing deparaffinized tissue sections were microwave treated in a pressure cooker in citrate solution (BioGenex Laboratories, San Ramon, CA). Endogenous peroxidase activity was blocked by incubating slides in 0.3% hydrogen peroxide for 10 min at room temperature, and nonspecific binding of immunoglobulin was minimized by preincubation in normal goat serum for 2 h at room temperature. The primary antibody recognizing phosphorylated tyrosine 705 of active Stat3 (Cell Signaling Technology, Beverly, MA) was diluted in 1% bovine serum albumin in phosphate-buffered saline and incubated with the samples at a final concentration of 0.6 µg/ml for 16 h. Antigen-antibody complexes were detected using biotinylated goat anti-rabbit secondary antibody (Biogenex, San Ramon, CA) followed by streptavidin-horseradish peroxidase complex, using 3,3′-diaminobenzidine as chromogen and Mayer's hematoxylin as counterstain.
Individual breast tumor samples were scored in a blinded manner for active Stat3 levels as defined by nuclear localized and tyrosine phosphorylated Stat3 on a scale from 0 to 3, where 0 was undetectable and 1 to 3 represented detectable staining at three steps of increasing staining intensity and proportion of stained tumor cells (low, intermediate, and high). This scoring method corresponded to a simplified version of a general immunohistochemical scoring method [24]. For survival analysis, specimens with detectable levels of active Stat3 (scores of 1-3) constituted positive Nuc-pY-Stat3 status whereas specimens with undetectable levels of active Stat3 (score of 0) constituted negative Nuc-pY-Stat3 status. The second array was analyzed by AQUA using the AQUA/PM2000 Imaging Platform (HistoRx) as described previously [23].
Statistical methods
Statistical analyses were conducted using SPSS software version 15.0 (SPSS Inc, Chicago, IL). Survival curves were calculated using the Kaplan-Meier method, and statistical significance was evaluated using the log-rank test. Hazard ratios (HR) for overall survival were estimated using the Cox proportional hazards regression model [25], and the assumption of proportional hazards was verified graphically. In the multi-variate analyses, the Cox regression models were adjusted for Stat3 activation status (negative vs. positive), tumor size (continuous variable in millimeters), patient age at diagnosis (continuous variable in years), affected lymph nodes (positive vs. negative), and tumor histological grade (3 vs. 1-2). Reduced models were obtained by forward selection and exclusion level of 0.10. Considered significant was P<0.05, and 95% CI for the HR was calculated. One-way ANOVA with Dunnett's T3 pairwise post-hoc test assuming unequal variances was used to test for differences in Nuc-pYStat3 levels between breast histology groups in the progression material. Pearson correlation analyses were used to test for associations between Nuc-pYStat3 and Nuc-pYStat5 or Nuc-Stat5a or Nuc-Stat5b.
Results
Of the 785 breast cancer specimens 721 (92%) were interpretable for Nuc-pYStat3 staining. Negative Nuc-pYStat3 status was observed in 350 (49%) of these tissues (score 0; Figure 1Aa), and positive Nuc-pYStat3 staining was observed in 371 (51%) of these tissues (scores 1-3; Figure 1Ab). The distribution of Nuc-pYStat3 scores is shown in Figure 1B, and was consistent with the distribution previously reported for a set of node-negative breast cancer samples using the same antibody [18]. Kaplan-Meier analysis was performed to examine the association between Nuc-pYStat3 staining and overall survival. Among all samples, patients whose tumors had positive Nuc-pYStat3 status tended to have better survival, but the difference was not statistically significant (P=0.081, Figure 2A). When specimens were stratified by tumor grade, however, patients with low grade tumors with positive Nuc-pYStat3 status had a significantly prolonged overall survival as compared to those with negative Nuc-pYStat3 status (P=0.014, Figure 2B). Among patients with high grade tumors, there was no difference in overall survival between positive or negative Stat3 activation status (P=0.711, Figure 2C). When patients were stratified by nodal status (negative versus positive), Nuc-pYStat3 status was not associated with overall survival in this material (Table 2).
Figure 1.
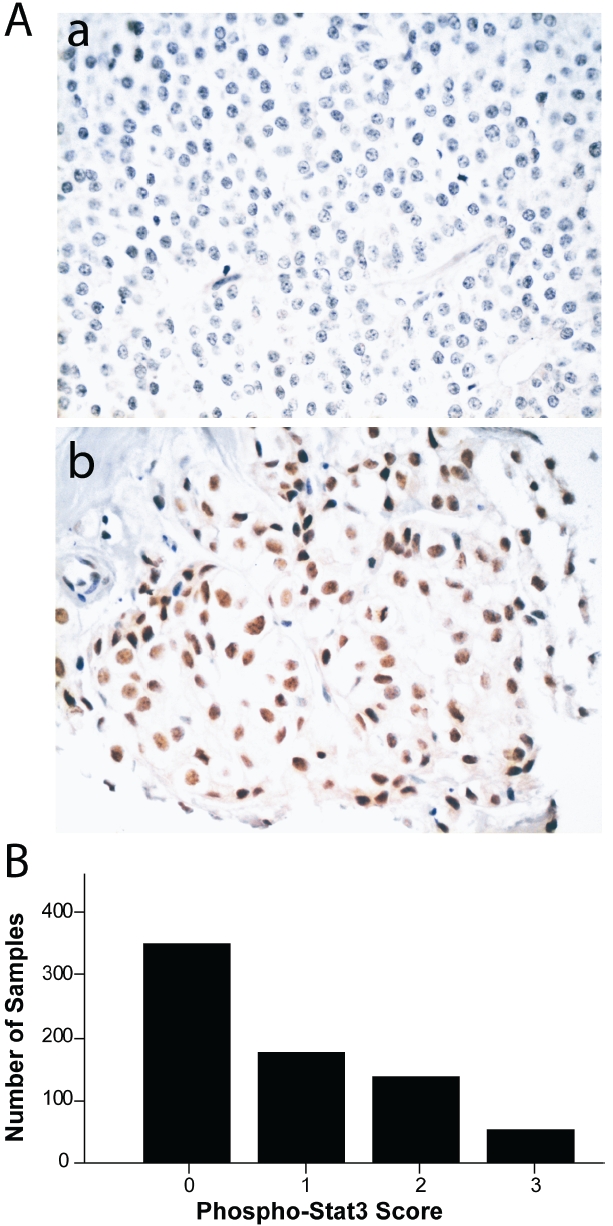
Distribution of nuclear phospho-Stat3 (Tyr705) immunohistochemical staining scores in breast cancer. A. Example of breast carcinoma with negative phospho-Stat3 staining (upper panel) and positive phospho-Stat3 staining using diaminoben-zamide chromogen (score 3; lower panel); B. Distribution of phospho-Stat3 (Tyr705) nuclear staining scores 0-3.
Figure 2.
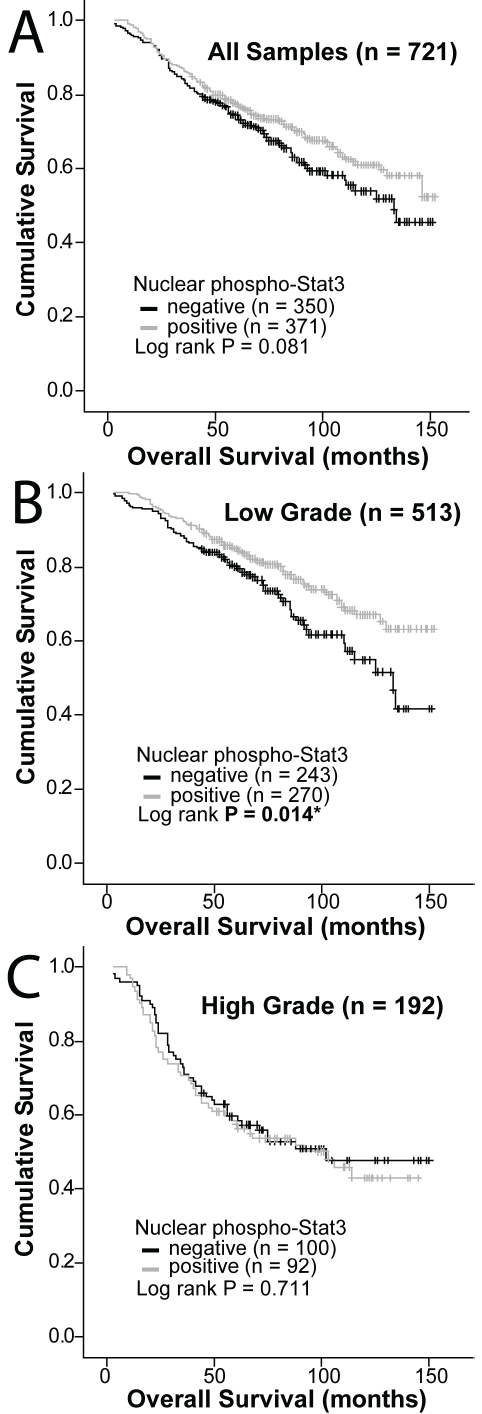
Kaplan-Meier survival curves in breast cancer according to nuclear phospho-Stat3 (Tyr705) staining status. A. Survival curves for all samples; B. Survival curves for low grade cancer (grades 1 and 2); C. Survival curves for high grade cancer (grade 3).
Table 2.
Multivariate survival analyses
| Variable | P | HR (95% CI) |
|---|---|---|
| Entire Material | ||
| Tumor grade (high) | <0.001 | 2.482 (1.841-3.347) |
| Tumor size | 0.081 | 1.007 (0.999-1.014) |
| Nodal status (positive) | <0.001 | 2.402 (1.739-3.317) |
| Age | <0.001 | 1.029 (1.017-1.041) |
| Stat3 activation status (negative) | 0.238 | 1.195 (0.889-1.608) |
| Low Grade Breast Cancer | ||
| Tumor size | 0.314 | 1.005 (0.995-1.016) |
| Nodal status (positive) | 0.001 | 2.069 (1.357-3.153) |
| Age | <0.001 | 1.044 (1.026-1.062) |
| Stat3 activation status (negative) | 0.200 | 1.304 (0.869-1.957) |
| High Grade Breast Cancer | ||
| Tumor size | 0.119 | 1.009 (0.998-1.021) |
| Nodal status (positive) | <0.001 | 2.962 (1.750-5.012) |
| Age | 0.102 | 1.014 (0.997-1.030) |
| Stat3 activation status (negative) | 0.583 | 1.130 (0.730-1.751) |
| Node Negative Breast Cancer | ||
| Tumor grade (high) | 0.014 | 2.040 (1.154-3.607) |
| Tumor size | 0.511 | 1.007 (0.986-1.028) |
| Age | <0.001 | 1.048 (1.024-1.072) |
| Stat3 activation status (negative) | 0.722 | 1.098 (0.655-1.838) |
| Node Positive Breast Cancer | ||
| Tumor grade (high) | <0.001 | 2.694 (1.877-3.867) |
| Tumor size | 0.106 | 1.007 (0.999-1.015) |
| Age | 0.004 | 1.020 (1.006-1.035) |
| Stat3 activation status (negative) | 0.167 | 1.294 (0.898-1.862) |
To assess the ability of Nuc-pYStat3 status to predict breast cancer-specific survival, multivariate analyses were performed using Cox's proportional hazards regression model. Taking into account other prognostic factors including age, nodal status, tumor grade, and tumor stage, multivariate analysis showed that Nuc-pYStat3 was not an independent marker of breast cancer prognosis among all patients (P=0.238), patients with low grade tumors (P=0.200), patients with high grade tumors (P=0.583), patients with lymph node negative disease (P=0.722) or patients with lymph node positive disease (P=0.167) (Table 2). Independent prognostic factors associated with increased risk of death in this study were 1) high tumor grade (HR=2.482, P<0.001), 2) positive lymph node status (HR=2.402, P<0.001), and 3) age (HR=1.029 per year, P<0.001).
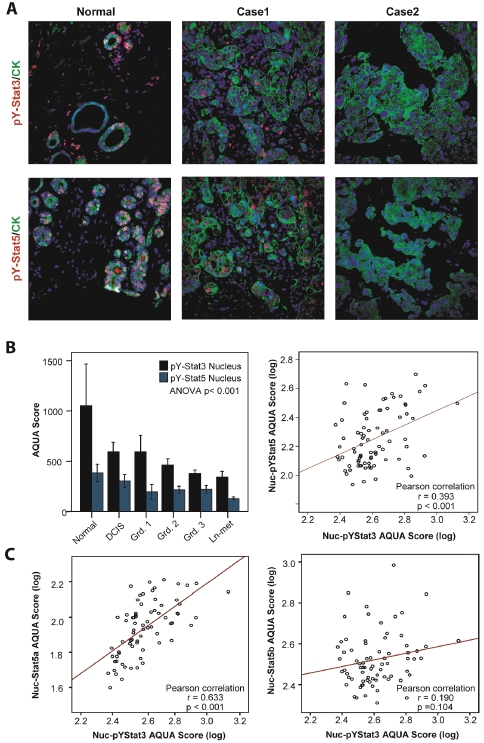
Interestingly, levels of Nuc-pYStat3 within this breast cancer material correlated with Nuc-pYStat5 (r=0.245, P<0.001). This was unexpected in light of the presumed tumor progression-promoting role of Stat3 and the fact that Nuc-pYStat5 is an established favorable prognostic marker in breast cancer [21, 26]. To validate this correlation in an independent material, we extended our studies using fluorescence -based immunohistochemistry and AQUA analysis of a progression breast cancer array, including normal tissues, ductal carcinoma in situ (DCIS), invasive ductal carcinoma (IDC), and lymph node metastases (Figure 3A). Intriguingly, levels of Nuc-pYStat3 declined as tumors progressed (ANOVA P<0.001), with significant decreases relative to normal breast epithelia observed in grade 2 (P=0.011) and grade 3 (P<0.001) invasive breast cancer as well as lymph node metastatic breast cancer (P<0.001; Figure 3B left panel). Levels of Nuc-pYStat3 also appeared to be lower in DCIS and Grade 1 invasive breast cancer but these reductions did not reach statistical significance. The reduction of Nuc-pYStat3 paralleled the reduction in Nuc-pYStat5 measured in the same progression material (Figure 3B left panel). Within invasive carcinomas, a positive correlation between Nuc-pYStat3 and Nuc-pYStat5 was confirmed (r=0.393, P<0.001; Figure 3B right panel). Intriguingly, a strong positive correlation was observed between levels of Nuc-pYStat3 and nuclear Stat5a protein (r=0.633, P<0.001), while Nuc-pYStat3 did not correlate with nuclear Stat5b protein (r=0.190, P=0.104; Figure 3C).
Figure 3.
AQUA analysis reveals Stat5 and Stat3 are correlated in human breast cancer. A. Immunofluorescent staining of human breast cancer tissues using pY-Stat3(red) or pY-Stat5(red), Cytokeratin(green), and Dapi(blue). Representative correlative images of normal tissue showing high expression of nuclear pY-Stat5 and pY-Stat3, Case 1 showing moderate expression of nuclear pY-Stat5 and pY-Stat3, and Case 2 showing no expression of nuclear pY-Stat5 and pY-Stat3. B. AQUA quantification of nuclear pY-Stat3 and pY-Stat5 protein levels in normal, DCIS, invasive ductal carcinomas, and lymph node metastases (left panel). Correlation analyses of nuclear pY-Stat5 and pY-Stat3 proteins in human breast cancer samples (right panel). C. Correlation analyses of nuclear Stat5a or b and pY-Stat3 proteins in human breast cancer samples.
Discussion
The present study indicates, based on the largest clinical breast cancer material examined so far, that levels of nuclear localized, tyrosine phosphorylated Stat3 (Nuc-pYStat3) are of limited broad prognostic value in breast cancer. The new information is unexpected in light of extensive experimental evidence of progression-promoting roles of Stat3 in breast cancer. However, the data helps resolve contradictory data from four previous reports on the prognostic value of Stat3 based on smaller breast cancer materials. Dolled-Filhart and colleagues [18] detected a modest favorable prognostic value of nuclear localized, tyrosine phosphorylated Stat3 in tumors of breast cancer of patients with node -negative disease, while Sheen-Chen and colleagues found that levels of total cellular Stat3 protein in breast cancer patients correlated with decreased 5 year survival [20]. The present study, like those of Berclaz and colleagues [17] as well as Yamashita and colleagues [19] did not detect independent prognostic value of Stat3. In our patient cohort, levels of Nuc-pYStat3 did not correlate with favorable prognosis within node-negative patients, although a modest favorable prognosis was detected in patients with low grade tumors in univariate analysis. However, this effect was not retained in multivariate analysis. It should be noted that differences in prognostic value of Stat3 between the different reports may be due to a combination of different readouts for Stat3 as well as differences in patient cohorts and length of clinical follow-up. The present study used the same phosphor-Stat3 antibody as that used by Dolled-Filhart [18], while the three other studies used Stat3 protein expression as the readout. The possibility remains that Stat3 has prognostic value in certain molecular subsets of breast cancer and this will be a subject of future investigation.
The present study unexpectedly revealed that levels of Nuc-pYStat3 are reduced over progression from normal breast epithelia to invasive and metastatic breast cancer. In this regard, Nuc-pYStat3 paralleled closely the pattern of Nuc-pYStat5, both markers displaying the highest levels within normal epithelia and the lowest in lymph node metastases. In contrast, Diaz and colleagues reported higher levels of Nuc-pYStat3 in tumors compared to nearby normal epithelia but the effect was not quantified [16]. A possible explanation for this apparent discrepancy is that nearby normal epithelia used in their study has lower Nuc-pYStat3 due to a cancer field effect than unmatched normal breast epithelia used in our study. We also observed that within invasive breast cancers, levels of Nuc-pYStat3 and Nuc-pYStat5 were positively correlated as determined by quantitative im-munofluorescence. This correlation might be explainable predominantly by a correlation between Nuc-pYStat3 and Stat5a but not Stat5b. While a positive association between total protein levels of total Stat3 and Stat5 proteins was previously noted in breast cancer based on pathologist scoring of diaminobenzidine chromogen staining [19], this is the first report of a positive correlation between levels of Nuc-pYStat3 and Nuc-pYStat5 in breast cancer.
Recent studies have indicated that Stat5b and Stat5a play non-redundant roles in breast cancer [23], as Stat5a may promote differentiation of breast cancer cell lines [27-29] while Stat5b may promote proliferation and invasive characteristics [30, 31]. The fact that Nuc-pYStat3 levels are correlated with nuclear levels of Stat5a but not Stat5b protein raises the possibility that pro-differentiation effects of Stat5 may obscure the effects of Stat3 in breast cancer cells. Stat5 is a favorable prognostic marker in breast cancer [19, 21, 26], and tumors with combined Stat3 and Stat5 activation were reportedly more well-differentiated than those with Stat3 activation alone [32]. Intriguingly, Stat5-induced gene transcription was reported to be dominant over Stat3 [32]. Therefore, it is possible that when Stat3 is activated in combination with Stat5, the pro-differentiation effects of Stat5 mask the tumor progression-promoting effects of Stat3.
Our analyses suggest that there is limited broad prognostic value of Nuc-pYStat3 in breast cancer, despite clearly established roles of Stat3 in progression of breast cancer based on numerous experimental studies. Future studies will need to explore the prognostic value of Nuc-pYStat3 within molecularly defined subtypes of breast cancer, and also incorporate strategies to independently measure Stat3α and Stat3β, isoforms of Stat3 that may have very different functions [33]. The possibility of context-dependent, dual tumor promoting and tumor suppressive effects of Stat3 in breast cancer also needs to be considered [34].
Acknowledgments
Supported by US National Institutes of Health grants R01-CA101841 and R01-CA118740 to H.R., and NCI Support Grant 1P30CA56036-08 to the Kimmel Cancer Center. Furthermore, this project is funded, in part, under a Commonwealth University Research Enhancement (CURE) Program grant with the Pennsylvania Department of Health. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions.
References
- 1.Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 2.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 3.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 4.Garcia R, Yu CL, Hudnall A, Catlett R, Nelson KL, Smithgall T, Fujita DJ, Ethier SP, Jove R. Constitutive activation of Stat3 in fibroblasts transformed by diverse oncoproteins and in breast carcinoma cells. Cell Growth Differ. 1997;8:1267–1276. [PubMed] [Google Scholar]
- 5.Sartor CI, Dziubinski ML, Yu CL, Jove R, Ethier SP. Role of epidermal growth factor receptor and STAT-3 activation in autonomous proliferation of SUM-102PT human breast cancer cells. Cancer Res. 1997;57:978–987. [PubMed] [Google Scholar]
- 6.Li L, Shaw PE. Autocrine-mediated activation of STAT3 correlates with cell proliferation in breast carcinoma lines. J Biol Chem. 2002;277:17397–17405. doi: 10.1074/jbc.M109962200. [DOI] [PubMed] [Google Scholar]
- 7.Burke WM, Jin X, Lin HJ, Huang M, Liu R, Reynolds RK, Lin J. Inhibition of constitutively active Stat3 suppresses growth of human ovarian and breast cancer cells. Oncogene. 2001;20:7925–7934. doi: 10.1038/sj.onc.1204990. [DOI] [PubMed] [Google Scholar]
- 8.Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, Cox CE, Falcone R, Fairclough R, Parsons S, Laudano A, Gazit A, Levitzki A, Kraker A, Jove R. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene. 2001;20:2499–2513. doi: 10.1038/sj.onc.1204349. [DOI] [PubMed] [Google Scholar]
- 9.Ranger JJ, Levy DE, Shahalizadeh S, Hallett M, Muller WJ. Identification of a Stat3-dependent transcription regulatory network involved in metastatic progression. Cancer Res. 2009;69:6823–6830. doi: 10.1158/0008-5472.CAN-09-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng GZ, Zhang WZ, Sun M, Wang Q, Coppola D, Mansour M, Xu LM, Costanzo C, Cheng JQ, Wang LH. Twist is transcriptionally induced by activation of STAT3 and mediates STAT3 oncogenic function. J Biol Chem. 2008;283:14665–14673. doi: 10.1074/jbc.M707429200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbieri I, Pensa S, Pannellini T, Quaglino E, Maritano D, Demaria M, Voster A, Turkson J, Cavallo F, Watson CJ, Provero P, Musiani P, Poli V. Constitutively active Stat3 enhances neu-mediated migration and metastasis in mammary tumors via upregulation of Cten. Cancer Res. 2010;70:2558–2567. doi: 10.1158/0008-5472.CAN-09-2840. [DOI] [PubMed] [Google Scholar]
- 12.Ling X, Arlinghaus RB. Knockdown of STAT3 expression by RNA interference inhibits the induction of breast tumors in immunocompetent mice. Cancer Res. 2005;65:2532–2536. doi: 10.1158/0008-5472.CAN-04-2425. [DOI] [PubMed] [Google Scholar]
- 13.Zhao W, Jaganathan S, Turkson J. A cell permeable Stat3 SH2 domain mimetic inhibits Stat3 activation and induces antitumor cell effects in vitro. J Biol Chem. 2010 doi: 10.1074/jbc.M110.154088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song H, Wang R, Wang S, Lin J. A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proc Natl Acad Sci U S A. 2005;102:4700–4705. doi: 10.1073/pnas.0409894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin L, Hutzen B, Zuo M, Ball S, Deangelis S, Foust E, Pandit B, Ihnat MA, Shenoy SS, Kulp S, Li PK, Li C, Fuchs J, Lin J. Novel STAT3 phosphorylation inhibitors exhibit potent growth-suppressive activity in pancreatic and breast cancer cells. Cancer Res. 2010;70:2445–2454. doi: 10.1158/0008-5472.CAN-09-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diaz N, Minton S, Cox C, Bowman T, Gritsko T, Garcia R, Eweis I, Wloch M, Livingston S, Seijo E, Cantor A, Lee JH, Beam CA, Sullivan D, Jove R, Muro-Cacho CA. Activation of stat3 in primary tumors from high-risk breast cancer patients is associated with elevated levels of activated SRC and survivin expression. Clin Cancer Res. 2006;12:20–28. doi: 10.1158/1078-0432.CCR-04-1749. [DOI] [PubMed] [Google Scholar]
- 17.Berclaz G, Altermatt HJ, Rohrbach V, Siragusa A, Dreher E, Smith PD. EGFR dependent expression of STAT3 (but not STAT1) in breast cancer. Int J Oncol. 2001;19:1155–1160. doi: 10.3892/ijo.19.6.1155. [DOI] [PubMed] [Google Scholar]
- 18.Dolled-Filhart M, Camp RL, Kowalski DP, Smith BL, Rimm DL. Tissue microarray analysis of signal transducers and activators of transcription 3 (Stat3) and phospho-Stat3 (Tyr705) in node-negative breast cancer shows nuclear localization is associated with a better prognosis. Clin Cancer Res. 2003;9:594–600. [PubMed] [Google Scholar]
- 19.Yamashita H, Nishio M, Ando Y, Zhang Z, Hamaguchi M, Mita K, Kobayashi S, Fujii Y, Iwase H. Stat5 expression predicts response to endocrine therapy and improves survival in estrogen receptor-positive breast cancer. Endocr Relat Cancer. 2006;13:885–893. doi: 10.1677/erc.1.01095. [DOI] [PubMed] [Google Scholar]
- 20.Sheen-Chen SM, Huang CC, Tang RP, Chou FF, Eng HL. Prognostic value of signal transducers and activators of transcription 3 in breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:2286–2290. doi: 10.1158/1055-9965.EPI-08-0089. [DOI] [PubMed] [Google Scholar]
- 21.Nevalainen MT, Xie J, Torhorst J, Bubendorf L, Haas P, Kononen J, Sauter G, Rui H. Signal transducer and activator of transcription-5 activation and breast cancer prognosis. J Clin Oncol. 2004;22:2053–2060. doi: 10.1200/JCO.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 22.LeBaron MJ, Crismon HR, Utama FE, Neilson LM, Sultan AS, Johnson KJ, Andersson EC, Rui H. Ultrahigh density microarrays of solid samples. Nat Methods. 2005;2:511–513. doi: 10.1038/nmeth772. [DOI] [PubMed] [Google Scholar]
- 23.Tran TH, Utama FE, Lin J, Yang N, Sjolund AB, Ryder A, Johnson KJ, Neilson LM, Liu C, Brill KL, Rosenberg AL, Witkiewicz AK, Rui H. Prolactin inhibits BCL6 expression in breast cancer through a Stat5a-dependent mechanism. Cancer Res. 2010;70:1711–1721. doi: 10.1158/0008-5472.CAN-09-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 25.Cox DR, Hinkley DV, Reid N, Snell EJ. London ; New York: Chapman and Hall; 1991. Statistical theory and modelling : in honour of Sir David Cox, FRS. [Google Scholar]
- 26.Amy R, Peck AKW, Chengbao Liu, Ginger A Stringer, Alexander C Klimowicz, Edward Pequignot, Boris Freydin, Thai H Tran, Ning Yang, Anne L Rosenberg, Jeffrey A Hooke, Albert J Kovatich, Marja T Nevalainen, Craig D Shriver, Terry Hyslop, Guido Sauter, David L Rimm, Anthony M Magliocco, Hallgeir Rui. Loss of Nuclear Localized and Tyrosine Phosphorylated Stat5 in Breast Cancer Predicts Poor Clinical Outcome and Increased Risk of Antiestrogen Therapy Failure. JCO In Press. [DOI] [PMC free article] [PubMed]
- 27.Sultan AS, Xie J, LeBaron MJ, Ealley EL, Nevalainen MT, Rui H. Stat5 promotes homotypic adhesion and inhibits invasive characteristics of human breast cancer cells. Oncogene. 2005;24:746–760. doi: 10.1038/sj.onc.1208203. [DOI] [PubMed] [Google Scholar]
- 28.Sultan AS, Brim H, Sherif ZA. Co-overexpression of Janus kinase 2 and signal transducer and activator of transcription 5a promotes differentiation of mammary cancer cells through reversal of epithelial-mesenchymal transition. Cancer Sci. 2008;99:272–279. doi: 10.1111/j.1349-7006.2007.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Q, Wang HY, Liu X, Wasik MA. STAT5A is epigenetically silenced by the tyrosine kinase NPM1-ALK and acts as a tumor suppressor by reciprocally inhibiting NPM1-ALK expression. Nat Med. 2007;13:1341–1348. doi: 10.1038/nm1659. [DOI] [PubMed] [Google Scholar]
- 30.Fox EM, Bernaciak TM, Wen J, Weaver AM, Shupnik MA, Silva CM. Signal transducer and activator of transcription 5b, c-Src, and epidermal growth factor receptor signaling play integral roles in estrogen-stimulated proliferation of estrogen receptor-positive breast cancer cells. Mol Endocrinol. 2008;22:1781–1796. doi: 10.1210/me.2007-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernaciak TM, Zareno J, Parsons JT, Silva CM. A novel role for signal transducer and activator of transcription 5b (STAT5b) in beta1-integrin-mediated human breast cancer cell migration. Breast Cancer Res. 2009;11 doi: 10.1186/bcr2341. R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker SR, Nelson EA, Zou L, Chaudhury M, Signoretti S, Richardson A, Frank DA. Reciprocal effects of STAT5 and STAT3 in breast cancer. Mol Cancer Res. 2009;7:966–976. doi: 10.1158/1541-7786.MCR-08-0238. [DOI] [PubMed] [Google Scholar]
- 33.Maritano D, Sugrue ML, Tininini S, Dewilde S, Strobl B, Fu X, Murray-Tait V, Chiarle R, Poli V. The STAT3 isoforms alpha and beta have unique and specific functions. Nat Immunol. 2004;5:401–409. doi: 10.1038/ni1052. [DOI] [PubMed] [Google Scholar]
- 34.Ecker A, Simma O, Hoelbl A, Kenner L, Beug H, Moriggl R, Sexl V. The dark and the bright side of Stat3: proto-oncogene and tumor-suppressor. Front Biosci. 2009;14:2944–2958. doi: 10.2741/3425. [DOI] [PubMed] [Google Scholar]