Abstract
Purpose
We compared risk factors for high disease- and treatment-related symptom burden over 15 weeks of therapy in medically underserved patients with advanced non–small-cell lung cancer and in patients treated at a tertiary cancer center.
Patients and Methods
We monitored symptom severity weekly during chemotherapy. Patients were recruited from a tertiary cancer center (n=101) and three public hospitals treating the medically underserved (n=80). We used a composite symptom-severity score and group-based trajectory analysis to form two groups: one with consistently more severe symptoms and another with less severe symptoms. We examined predictors of group membership.
Results
Seventy percent of the sample (n=126) reported low symptom-severity levels that decreased during therapy; 30% (n=55) had consistently severe symptoms throughout the study. In multivariate analysis, patients with good performance status being treated in public hospitals were significantly more likely than patients treated at the tertiary cancer center to be in the high-symptom group (odds ratio, 5.6; 95% CI, 2.1 to 14.6; P =.001) and to report significantly higher symptom interference (P =.001). Other univariate predictors of high-symptom group membership included variables associated with being medically underserved (eg, having less education, being single, and being nonwhite). No group differences by ethnicity were observed in the public hospitals. Medically underserved patients were less likely to receive adequate pain management.
Conclusion
Patients with advanced lung cancer and good performance status treated at public hospitals were more likely than those treated at a tertiary cancer center to experience substantial symptoms during chemotherapy.
INTRODUCTION
Patients with advanced non–small-cell lung cancer (NSCLC) experience multiple distressing symptoms, which collectively impose a symptom burden that is highly disruptive to physical and emotional functioning.1 Because patients with NSCLC have limited survival, a reasonable treatment goal is reducing pain and other symptoms while increasing quality of life and functioning.2
The location in which patients with advanced cancer are treated has been found to be highly predictive of the adequacy of pain control. Sites primarily treating ethnic nonwhites and medically underserved patients have been associated with greater risk for severe pain and less adequate pain management.3–5 However, many studies reporting these disparities, including our own, have inherent limitations, such as cross-sectional design, which precludes detection of efforts to improve symptom management over time; patient samples that are often heterogeneous as to cancer type and stage; and lack of control for cancer stage and type of therapy, even though medically underserved patients often present initially with more advanced disease6,7 and will likely have greater disease-related symptom burden. Further, these studies did not assess distressing nonpain symptoms that patients with advanced NSCLC report.
To address these limitations, we conducted a prospective longitudinal study to measure symptom severity weekly during chemotherapy in patients with advanced NSCLC. To examine potential disparities in symptom management, we enrolled patients from a tertiary cancer center and from three public hospitals treating medically underserved patients with cancer. Group-based trajectory modeling was used to identify patients with consistently more severe symptoms during chemotherapy and to examine the association of high-symptom group membership with treatment setting and other factors.
PATIENTS AND METHODS
Study Participants
Patients were consecutively recruited between January 2004 and December 2008 from the thoracic medical oncology clinic of a tertiary cancer center in Houston, TX, and the general oncology clinics of three public hospitals (two in Houston, one in Miami, FL) providing care for medically underserved (noninsured/underinsured and/or low-income) patients,8 most of whom are nonwhite. Eligible patients had advanced (stage IIIB-IV) NSCLC and were scheduled to receive either intravenous chemotherapy or the oral tyrosine kinase inhibitor erlotinib.
All patients gave informed consent to participate. The study was approved by the institutional review boards of the participating institutions.
Study Procedures
To assess the severity and impact of symptoms, patients completed the M. D. Anderson Symptom Inventory (MDASI)9,10 before commencing chemotherapy (baseline assessment) and weekly thereafter for 15 weeks. The MDASI assesses 13 common cancer-related symptoms (ie, pain, fatigue, nausea, vomiting, dry mouth, shortness of breath, lack of appetite, difficulty remembering, drowsiness, disturbed sleep, sadness, distress, and numbness) over the previous 24 hours. One lung cancer–specific symptom, coughing, was added to the MDASI for this study.10 Each symptom is rated on an 11-point scale, with 0 being “not present” and 10 being “as bad as you can imagine.” Previous research has shown that MDASI symptom ratings collected from various cultural and language groups can be interpreted in a similar way in oncology practice and clinical research, with culture and language having only modest impacts on symptom ratings.11
To measure symptom impact on functioning, the MDASI contains six items measuring symptom interference with activity dimensions (ie, walking ability, general activity, normal work) and affective dimensions (ie, relations with other people, enjoyment of life, mood) of life during the past 24 hours.9,10 Interference items are rated on an 11-point scale, with 0 being “did not interfere” and 10 being “interfered completely.” The mean of the items constitutes a composite interference score.
Patients completed the MDASI during clinic visits or, when no treatment visit was scheduled during the week, by phone in response to calls from a computerized interactive voice response system. If the system failed to reach the patient, a research nurse called the patient to obtain symptom ratings. Cell phones were provided to participants who lacked a home telephone.
Study staff recorded ratings of Eastern Cooperative Oncology Group performance status (ECOG PS),12 recorded comorbid conditions,13 and calculated the Pain Management Index (PMI) at baseline.4 The PMI compares the severity of patient-reported pain with the appropriateness of the analgesics prescribed for it: the higher the PMI value, the better the pain management. A negative PMI value indicates analgesia of insufficient potency to manage the patient’s reported pain level. The PMI was used in this study as a measure of potential practice differential in symptom management between the tertiary cancer center and public hospitals.
Statistical Analysis
Descriptive statistics—mean, standard deviation (SD), 95% CI, and proportions—were used to describe patient characteristics by treatment setting. For purposes of this analysis, patients treated in the three public hospitals were aggregated into one subsample representing medically underserved patients with cancer. All reported P values are two-tailed and considered significant if lower than .05.
Calculating Propensity Scores
Previous studies indicate that poorer ECOG PS,9,14 a history of prior treatment, and advanced disease10 can be associated with higher symptom severity. We sought to lessen the bias of potential site differences relative to these known factors by conducting a logistic regression to calculate propensity scores for cancer stage, ECOG PS, and prior treatment.15 These scores were used as weights in the group-based trajectory modeling.
Determining Group Membership
Group-based trajectory analysis incorporating propensity-score weights was used to categorize patients into groups according to the level and trajectory of symptom severity they experienced (either high or low) over time. The analysis was based on the arithmetic average of the six symptoms rated most severe overall during the study. We were interested in estimating regression models for each of the two groups within our sample (unlike longitudinal mixed-effects modeling where only a single mean is modeled). We determined a priori that we would fit two groups, a high-symptom group and a low-symptom group, on the basis of both a desire for simplicity and clinical usefulness.
We specified a censored normal distribution as the general distributional form of our composite symptom-severity scores. We retained the most parsimonious solution, which in this case was one model with both an intercept and a linear term and another model with an intercept-only term.16 The final model was selected on the basis of model fit and interpretability.
Predictors of Group Membership
Univariate analyses were conducted to determine potential predictors of high-symptom versus low-symptom group membership. Fisher’s exact tests for categorical variables and t-tests for continuous variables were used for preliminary screening of potential predictors of group membership. A variable was considered a potential predictor if the P value associated with the test result was lower than .05.
After screening potential predictors in the univariate analysis, we performed a multivariate logistic regression analysis where predictors were introduced in a stepwise fashion,17 which allowed us to examine the effect of the predictor variables simultaneously. We also examined potential interactions for the weighted variables (eg, cancer stage, ECOG PS, prior treatment) as predictors of group membership.
Effect of Dropouts and Missing Data
The analyses presented above were based on all available data at each assessment time point. To determine the effect of patient dropout during the study, we performed the analyses on the subset of patients who completed the entire study. We established a variable indicating whether a patient dropped out at any time before the end of the study. We then examined whether there was significant interaction between treatment setting and this dropout indicator variable.
We also examined the interaction of group membership with dropout, as patients whose participation ended prematurely were liable to be more symptomatic.
Group Differences in Symptom Interference
As a measure of symptom impact on daily functioning, we fitted linear mixed models18 using the predictor variables from the multivariate analysis, with mean interference score as the dependent variable.
RESULTS
Sample Characteristics
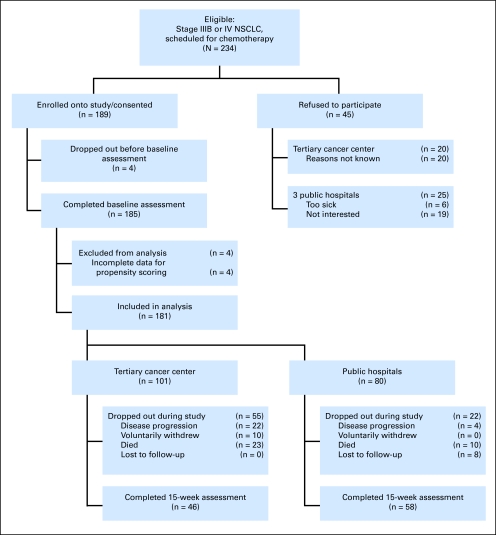
Of 234 eligible patients approached to participate in the study, 189 were enrolled; four withdrew before chemotherapy commenced, such that 185 completed the baseline assessment. Four of these did not provide complete information on the variables needed for propensity scoring and were excluded from analysis. Of the remaining 181 patients, 101 were recruited from the tertiary cancer center and 80 from the public hospitals. Of the 77 participants who did not complete the entire study (median time on study, 10 weeks), eight were lost to follow-up, 10 withdrew voluntarily, 26 discontinued treatment because of disease progression, and 33 died (Fig 1).
Fig 1.
Flow of participants through the study. NSCLC, non–small-cell lung cancer.
Table 1 presents patient and clinical characteristics of the sample by treatment site (tertiary v public) and by group (high v low symptom). Most of the patients at the tertiary center were non-Hispanic whites, whereas most at the public hospitals were not. A greater proportion of patients at the tertiary cancer center had stage IV disease. No differences were seen for type of chemotherapy regimen. We found no difference between high- and low- symptom groups in percentages of patients responding to the MDASI either by interactive voice response system or paper and pencil.
Table 1.
Sample Characteristics by Treatment Site and by Symptom Burden Group (n=181)
| Characteristic | Total Sample (N=181) | Treatment Site |
P | Symptom Burden |
P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tertiary (n=101) |
Public (n=80) |
Low (n=126) |
High (n=55) |
||||||||
| No. | % | No. | % | No. | % | No. | % | ||||
| Mean age, years | 60.0 | 61.6 | 58.0 | .01 | 60.0 | 59.9 | .92 | ||||
| Standard deviation | 8.8 | 9.0 | 8.3 | 8.9 | 8.8 | ||||||
| Sex | |||||||||||
| Men | 115 | 66 | 65 | 49 | 61 | .57 | 78 | 62 | 37 | 67 | .49 |
| Women | 66 | 35 | 35 | 31 | 39 | 48 | 38 | 18 | 33 | ||
| Marital status | |||||||||||
| Married | 114 | 83 | 82 | 31 | 39 | .001 | 89 | 71 | 25 | 45 | .001 |
| Unmarried | 67 | 18 | 18 | 49 | 61 | 37 | 29 | 30 | 55 | ||
| Education level | |||||||||||
| High school degree or less | 103 | 38 | 38 | 65 | 81 | .001 | 63 | 50 | 40 | 73 | .01 |
| Greater than high school | 77 | 62 | 62 | 15 | 19 | 62 | 50 | 15 | 27 | ||
| Job status | |||||||||||
| Employed outside home | 37 | 24 | 24 | 13 | 16 | .001 | 32 | 26 | 5 | 9 | .001 |
| Homemaker | 11 | 8 | 8 | 3 | 4 | 10 | 8 | 1 | 2 | ||
| Retired | 55 | 43 | 43 | 12 | 15 | 41 | 33 | 14 | 25 | ||
| Medical leave or disabled | 54 | 22 | 22 | 32 | 40 | 26 | 21 | 28 | 51 | ||
| Unemployed/other | 22 | 2 | 2 | 20 | 25 | 15 | 12 | 7 | 13 | ||
| Ethnicity | |||||||||||
| Asian/other | 1 | 0 | 1 | 1 | .001 | 0 | 0 | 1 | 2 | .001 | |
| Black non-Hispanic | 43 | 7 | 7 | 36 | 45 | 22 | 17 | 21 | 38 | ||
| Hispanic | 26 | 1 | 1 | 25 | 31 | 17 | 13 | 9 | 16 | ||
| White non-Hispanic | 111 | 93 | 92 | 18 | 23 | 87 | 69 | 24 | 44 | ||
| Cancer stage | |||||||||||
| IIIB | 34 | 7 | 7 | 27 | 34 | .001 | 19 | 15 | 15 | 27 | .06 |
| IV | 147 | 94 | 93 | 53 | 66 | 107 | 85 | 40 | 73 | ||
| ECOG PS (baseline) | |||||||||||
| 0 | 33 | 16 | 16 | 17 | 21 | .03 | 26 | 21 | 7 | 13 | .008 |
| 1 | 75 | 52 | 51 | 23 | 29 | 60 | 47 | 15 | 27 | ||
| 2 | 66 | 30 | 30 | 36 | 45 | 36 | 29 | 30 | 54 | ||
| 3 | 6 | 3 | 3 | 3 | 4 | 3 | 2 | 3 | 6 | ||
| 4 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | ||
| Chemotherapy regimens | |||||||||||
| Platinum + paclitaxel or docetaxel | 89 | 46 | 46 | 43 | 58 | .13 | 59 | 49 | 30 | 57 | .73 |
| Platinum + other chemotherapy | 45 | 29 | 29 | 16 | 22 | 34 | 28 | 11 | 21 | ||
| Single-agent chemotherapy | 28 | 20 | 20 | 8 | 11 | 20 | 17 | 8 | 15 | ||
| Erlotinib | 12 | 5 | 5 | 7 | 9 | 8 | 7 | 4 | 8 | ||
| Previous treatment | |||||||||||
| Chemotherapy | 45 | 27 | 27 | 18 | 23 | 27 | 21 | 18 | 33 | ||
| Surgery | 22 | 19 | 19 | 3 | 4 | 15 | 12 | 7 | 13 | ||
| Radiation | 52 | 36 | 36 | 16 | 20 | 30 | 24 | 22 | 40 | ||
| Treatment na|fkve | 94 | 43 | 43 | 51 | 64 | .007 | 67 | 70 | 27 | 49 | .63 |
| Charlson comorbidity score (0-37) | |||||||||||
| 0-1 | 129 | 68 | 68 | 61 | 80 | .09 | 87 | 71 | 42 | 79 | .27 |
| 2+ | 47 | 32 | 32 | 15 | 20 | 36 | 29 | 11 | 21 | ||
| Treatment site | |||||||||||
| Tertiary hospital | 101 | 101 | 100 | .001 | 82 | 65 | 19 | 35 | .001 | ||
| Public hospitals* | 80 | 80 | 100 | 44 | 35 | 36 | 65 | ||||
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PS, performance status.
Includes all participants treated at any of the three public hospitals.
Group Identification: High Versus Low Symptom Trajectory
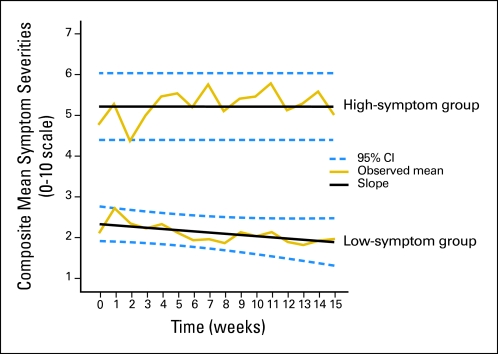
In the initial analysis, the six most severe MDASI symptoms overall were pain, fatigue, disturbed sleep, shortness of breath, drowsiness, and coughing, based on the rank-ordered grand mean of each symptom over the study period. A component score of these six symptoms (with propensity-score weighting) was used in the group-based trajectory modeling. Figure 2 illustrates the two group trajectories, with a linear development pattern by symptom severity that best met our criteria of interpretability and model fit. For the high-symptom group, severity was consistently moderate to severe over the time of the study, while severity decreased (P = .02) for the low-symptom group. Nineteen percent (19 of 101) of patients from the tertiary cancer center and 45% (36 of 80 [18 of 45, eight of 13, 10 of 22]) of patients from the public hospitals were in the high-symptom group (Table 1).
Fig 2.
Composite mean symptom severities for top six symptoms (0 to 10 scale) for two-group trajectories, all available measurements for up to 15 weeks or 4 cycles of chemotherapy (n=181). The 126 patients in the low-symptom group (70%) started with low symptom severity (intercept, 2.33 on the M. D. Anderson Symptom Inventory’s 0 to 10 scale) and reported decreasing symptom severity over time (slope, −0.03; P =.02). In contrast, the 55 patients in the high-symptom group (30%) started with high symptom severity (intercept, 5.20) that remained flat throughout the course of their treatment. Dashed lines represent the pointwise 95% CI.
Predictors of Group Membership
Univariate analysis: Potential predictors by group.
Preliminary screening using univariate analyses showed that ECOG PS, treatment site, education level, marital status, ethnicity, and job status were potentially significant predictors of group membership (Table 1). As expected, the high-symptom group had poorer performance status than the low-symptom group (odds ratio [OR], 3.5; 95% CI, 1.8 to 6.9; P =.001). Other major predictors of membership in the high-symptom group included being treated at a public hospital (OR, 3.5; 95% CI, 1.8 to 6.9; P =.001) and various factors associated with medically underserved patients, including having lower education (OR, 2.6; 95% CI, 1.3 to 5.2; P =.01), being single (OR, 2.9; 95% CI, 1.5 to 5.5; P =.001), and nonwhite ethnicity (OR, 2.8; 95% CI, 1.4 to 5.4; P= .002).
There were no significant differences between symptom burden groups in chemotherapy regimen, prior treatment, or number of comorbid conditions (Table 1). Subanalysis of the high-symptom group at the public hospitals only revealed no significant difference in the proportions of patients who were (39%) or were not (46%) non-Hispanic white.
Multivariate analysis: Predictors of group membership.
The six significant predictors of group classification (ie, ECOG PS, treatment site, education level, marital status, ethnicity, job status) identified in the univariate analysis were entered with a stepwise selection approach into the multivariate logistic regression model. Because we were interested in treatment setting differences, we included all possible first-order interactions between site and the five other significant univariate predictors. Second-order and higher-order interaction terms were not statistically significant and were therefore excluded.
The interaction between treatment site (tertiary v public hospital) and ECOG PS was the only significant predictor of classification into either the high- or low-symptom group. Patients with good performance status being treated in public hospitals were significantly more likely than patients treated at the tertiary cancer center to be in the high-symptom group (OR, 9.3; 95% CI, 3.1 to 28.1; P= .001).
Effect of Dropouts and Missing Data
Multivariate analysis revealed no significant interaction between treatment setting and the dropout variable, indicating no differential effect of dropout by treatment setting.
We also fitted the group-based trajectory models with a complete-case analysis (104 patients who completed the full 15-week study). The best-fitting model continued to be the two-group trajectory with one group having only an intercept and another having a linear term and an intercept, and it produced similar results: a high-symptom group whose symptom severity remained high over time, and a low-symptom group whose symptom severity decreased over time (data not shown). None of the 104 patients switched severity group membership.
Group Differences in Symptom Interference
We examined changes in the symptom interference scores longitudinally between baseline and the end of 15 weeks of treatment for the two groups as a measure of how symptoms interfered with daily functioning. Results from linear mixed modeling showed interaction between treatment setting and ECOG PS. When performance status was good (ECOG PS < 2), patients treated at the tertiary cancer center rated interference approximately 1.5 points lower (on a 0 to 10 scale) than patients from public hospitals (P =.001). There were no differences by treatment setting for those with poorer performance status (ECOG PS ≥ 2; P =.21). In addition, we found no significant site-by-dropout interaction.
Other Outcomes
The PMI was computed for 142 patients who reported pain and/or were taking analgesics. At baseline, negative PMI scores were derived for a significantly smaller proportion of patients treated at the tertiary cancer center than at the public hospitals (27% v 49%; P =.009). The proportion of patients with negative PMI scores at the three public hospitals ranged from 46% to 60% at baseline.
DISCUSSION
Approximately 70% of the sample (the low-symptom group) had relatively low levels of NSCLC-related symptoms when they began chemotherapy and demonstrated a slight decrease in symptom severity throughout the study. The remaining 30% (the high-symptom group) reported symptoms of moderate or greater severity throughout the study. To put these differences into clinical perspective, previous studies have shown that symptom severity scores of ≥ 5 on a 0 to 10 scale have a disproportionately greater impact on function.4,19 As shown in Figure 2, composite symptom severity scores were higher than 5 at almost all time points for the high-symptom group. As expected, the high-symptom group also consistently reported significantly higher symptom interference with functioning.
At the public hospitals, patients were approximately nine times more likely to be in the high-symptom group than patients treated at the tertiary cancer center, but only if they had good performance status. Although there was a trend for patients with poor performance status to be in the high-symptom group when treated in the public hospitals rather than the tertiary cancer center, the difference was not significant. Patients with poor performance status may be identified by staff as having greater risk for high symptoms and may therefore be more likely to receive aggressive pain management.4 Our results are consonant with reports documenting that medically underserved patients with cancer are at greater risk for higher pain severity and less adequate pain management, while extending these findings to other symptoms associated with lung cancer. Other variables associated with being treated at hospitals caring for underserved patients (eg, unmarried, less educated, nonwhite) were also significantly more characteristic of the high-symptom group.
The study was designed to control for typical patient discrepancies limiting cancer outcome studies of medically underserved patients, including initial presentation with late-stage disease6,7 and unequal use of primary treatment and adjuvant regimens.20,21 Although ethnicity was confounded by treatment site, we found little difference in high-symptom group membership at the public hospitals when the proportion of patients self-identifying as non-Hispanic white (39%) was compared with black/Hispanic patients (46%).
Several factors may be associated with higher symptom burden in public hospitals. First, we found significant differences between the tertiary cancer center and public hospitals in the adequacy of pain management at baseline, with a significantly lower proportion of patients at the public hospitals receiving analgesics appropriate to the severity of their pain. Second, compared with patients at tertiary centers, patients at public hospitals often have greater constraints on time, transportation, and funding, and less access to symptom management resources such as specialized care, pain and supportive care drugs (especially opioids for pain management),22,23 and family logistical and emotional support; they often are unable to communicate with health care providers who could aid in managing symptoms.24 Even when patients remained in this study long enough for their symptom levels to be managed, there was no evidence of improvement for those with high symptom severity.
This study has several limitations. First, patients treated in a tertiary cancer center came from one institution; thus, generalization of our results to a comparison of other public hospitals with other tertiary cancer centers, or even among centers of each type, is not warranted. Also, community settings, where most cancer patients are treated, were not sampled. Second, the representation of patients treated at public hospitals is based on a composite of three institutions. However, 45% of the patients from each of these three institutions belonged to the high-symptom group, compared with fewer than 20% at the tertiary cancer center. Third, we did not monitor adherence to symptom control and supportive care offered by the public hospitals to study patients. For example, patients may have been prescribed appropriate symptom control therapies, but were unable to follow the symptom management plan.
Correcting the discrepancies in symptom control reported here will require a multifaceted approach, including increasing the priority given to symptom management in the medically underserved,25 especially patients with late-stage disease. The high prevalence of NSCLC, together with its grim prognosis and the high level of symptoms it generates, indicate that symptom management is paramount in any calculation of treatment benefit. Although treatment site was not a significant factor in the adequacy of symptom management for patients with poor performance status, it had a significant effect on patients with better performance status—suggesting that clinicians in every cancer treatment facility should assess symptom severity and institute appropriate management for every patient, even for those who appear to be doing well.
Large, multicenter longitudinal studies designed to measure adequacy of symptom management over time should include samples from a broader selection of public hospitals, tertiary cancer centers, and community clinics. Such studies could benefit from the use of cell or home phones for repeated monitoring of symptoms outside the clinic, the method used here. Future research should include a wider examination of health service factors that may contribute to differences in symptom severity.
Acknowledgment
We thank Marilyn Morrissey, MPH, who provided protocol management, and Linda L. McCrory, RN, Araceli Garcia-Gonzalez, MD, PhD, Katherine Ramsey Gilmore, CCRP, and Maria G. Sanchez, RN, for patient recruitment and data collection at the tertiary cancer center and public hospitals.
Footnotes
Supported by Grant No. NIH/NCI R01 CA026582 from the National Cancer Institute, National Institutes of Health (C.S.C.); and the University Cancer Foundation.
Presented at the 46th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 4-8, 2010.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Charles S. Cleeland, Xin Shelley Wang, Guadalupe R. Palos, Stephen P. Richman, Charles Lu
Financial support: Charles S. Cleeland
Administrative support: Jeanie F. Woodruff
Provision of study materials or patients: Guadalupe R. Palos, Arlene Nazario, Garrett R. Lynch, Charles Lu
Collection and assembly of data: Guadalupe R. Palos, Arlene Nazario, Garrett R. Lynch, Gary M. Mobley
Data analysis and interpretation: Charles S. Cleeland, Tito R. Mendoza, Xin Shelley Wang, Jeanie F. Woodruff, Kai-Ping Liao
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Cleeland CS. Symptom burden: Multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monographs. 2007;37:16–21. doi: 10.1093/jncimonographs/lgm005. [DOI] [PubMed] [Google Scholar]
- 2.Ocaña A, Amir E, Vera F, et al. Addition of bevacizumab to chemotherapy for treatment of solid tumors: Similar results but different conclusions. J Clin Oncol. 2011;29:254–256. doi: 10.1200/JCO.2010.32.0275. [DOI] [PubMed] [Google Scholar]
- 3.Bernabei R, Gambassi G, Lapane K, et al. Management of pain in elderly patients with cancer. SAGE Study Group: Systematic Assessment of Geriatric Drug Use via Epidemiology. JAMA. 1998;279:1877–1882. doi: 10.1001/jama.279.23.1877. [DOI] [PubMed] [Google Scholar]
- 4.Cleeland CS, Gonin R, Hatfield AK, et al. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med. 1994;330:592–596. doi: 10.1056/NEJM199403033300902. [DOI] [PubMed] [Google Scholar]
- 5.Cleeland CS, Portenoy RK, Rue M, et al. Does an oral analgesic protocol improve pain control for patients with cancer? An intergroup study coordinated by the Eastern Cooperative Oncology Group. Ann Oncol. 2005;16:972–980. doi: 10.1093/annonc/mdi191. [DOI] [PubMed] [Google Scholar]
- 6.Sloane D. Cancer epidemiology in the United States: Racial, social, and economic factors. Methods Mol Biol. 2009;471:65–83. doi: 10.1007/978-1-59745-416-2_4. [DOI] [PubMed] [Google Scholar]
- 7.Patel UA, Lynn-Macrae A, Rosen F, et al. Advanced stage of head and neck cancer at a tertiary-care county hospital. Laryngoscope. 2006;116:1473–1477. doi: 10.1097/01.mlg.0000227448.71894.8c. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Department of Health and Human Services, Health Resources and Services Administration: Shortage designation: Medically underserved areas & populations http://bhpr.hrsa.gov/shortage/muaguide.htm.
- 9.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: The M. D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Mendoza TR, Wang XS, Lu C, et al. Measuring the symptom burden of lung cancer: The validity and utility of the lung cancer module of the M. D. Anderson Symptom Inventory. Oncologist. 2011;16:217–227. doi: 10.1634/theoncologist.2010-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang XS, Cleeland CS, Mendoza TR, et al. Impact of cultural and linguistic factors on symptom reporting by patients with cancer. J Natl Cancer Inst. 2010;102:732–738. doi: 10.1093/jnci/djq097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 13.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 14.Wang XS, Shi Q, Lu C, et al. Prognostic value of symptom burden for overall survival in patients receiving chemotherapy for advanced nonsmall cell lung cancer. Cancer. 2010;116:137–145. doi: 10.1002/cncr.24703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leslie RS, Ghomrawi H. The use of propensity scores and instrumental variable methods to adjust for treatment selection bias. Presented at the SAS Global Forum 2008; March 16, 2008; San Antonio TX. [Google Scholar]
- 16.Jones BL, Nagin DS, Roeder K, et al. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods & Research. 2001;29:374–393. [Google Scholar]
- 17.Hosmer DW, Lemeshow S. Applied logistic regression (ed 2) New York, NY: Wiley; 2000. [Google Scholar]
- 18.Fitzmaurice GM, Laird NM, Ware JH, et al. Applied longitudinal analysis. Hoboken, NJ: Wiley-Interscience; 2004. [Google Scholar]
- 19.Serlin RC, Mendoza TR, Nakamura Y, et al. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 20.Holmes L, Jr, Chan W, Jiang Z, et al. Impact of androgen deprivation therapy on racial/ethnic disparities in the survival of older men treated for locoregional prostate cancer. Cancer Control. 2009;16:176–185. doi: 10.1177/107327480901600210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terplan M, Smith EJ, Temkin SM, et al. Race in ovarian cancer treatment and survival: A systematic review with meta-analysis. Cancer Causes Control. 2009;20:1139–1150. doi: 10.1007/s10552-009-9322-2. [DOI] [PubMed] [Google Scholar]
- 22.Morrison RS, Wallenstein S, Natale DK, et al. “We don’t carry that”–failure of pharmacies in predominantly nonwhite neighborhoods to stock opioid analgesics. N Engl J Med. 2000;342:1023–1026. doi: 10.1056/NEJM200004063421406. [DOI] [PubMed] [Google Scholar]
- 23.Darby K, Davis C, Likes W, et al. Exploring the financial impact of breast cancer for African American medically underserved women: A qualitative study. J Health Care Poor Underserved. 2009;20:721–728. doi: 10.1353/hpu.0.0176. [DOI] [PubMed] [Google Scholar]
- 24.Ok H, Marks R, Allegrante JP, et al. Perceptions of health care provider communication activity among American cancer survivors and adults without cancer histories: An analysis of the 2003 Health Information Trends Survey (HINTS) Data. J Health Commun. 2008;13:637–653. doi: 10.1080/10810730802412172. [DOI] [PubMed] [Google Scholar]
- 25.Patrick DL, Ferketich SL, Frame PS, et al. National Institutes of Health State-of-the-Science Conference statement: Symptom management in cancer: Pain, depression, and fatigue, July 15-17, 2002. J Natl Cancer Inst. 2003;95:1110–1117. doi: 10.1093/jnci/djg014. [DOI] [PubMed] [Google Scholar]