Abstract
Purpose
Identifying strong markers of prognosis are critical to optimize treatment and survival outcomes in patients with malignant recurrent glioma. We investigated the prognostic significance of exercise behavior and functional capacity in this population.
Patients and Methods
Using a prospective design, 243 patients with WHO grades 3 to 4 recurrent malignant glioma and Karnofsky performance status (KPS) ≥ 70 completed a self-administered questionnaire that assessed exercise behavior and performed a 6-minute walk test (6MWT) to assess functional capacity. Cox proportional models were used to estimate the risk of all-cause mortality according to 6MWT distance (6MWD; < 390 meters, 390-489 meters, > 489 meters) and exercise behavior (metabolic equivalent [MET] –h/wk) adjusted for KPS and other important clinical factors.
Results
Median follow-up was 27.43 months. During this period, 149 deaths were recorded (61% of the total sample). Exercise behavior was an independent predictor of survival (P = .0081). Median survival was 13.03 months for patients reporting < 9 MET-h/wk relative to 21.84 months for those reporting ≥ 9 MET-h/wk. Exercise behavior added incremental prognostic value beyond that provided by KPS, age, sex, grade, and number of prior progressions (P < .001). Compared with patients reporting < 9 MET-h/wk, the adjusted hazard ratio for mortality was 0.64 (95% CI, 0.46 to 0.91) for patients reporting ≥ 9 MET-h/wk. Functional capacity was not an independent predictor of prognosis.
Conclusion
Exercise behavior is a strong independent predictor of survival that provides incremental prognostic value to KPS as well as traditional markers of prognosis in malignant recurrent glioma.
INTRODUCTION
Malignant recurrent glioma is a major challenge in the oncology setting, with median survival of only 4 to 6 months.1,2 Several factors, including age, performance status (PS), tumor grade and histology, and number of prior progressions, are strong independent predictors of survival in this population.3 Of these factors, PS scoring, either assessed by Karnofsky performance scale (KPS) or Eastern Cooperative Oncology Group (ECOG) scoring systems, is consistently a robust independent prognostic factor.3,4 Thus, physical functioning plays an integral role in modulation of treatment and disease pathophysiology in malignant glioma. Current subjective PS scoring systems, however, fail to fully characterize physical functioning and lack the sensitivity to accurately discriminate between individuals with good (ie, KPS > 70; ECOG 0 to 1) PS.5 Alternative clinical tools that provide more sensitive and objective assessments of physical functioning may allow for more accurate prognostication and inform therapeutic intervention.
Several methods are available to clinicians that provide objective determinations of physical functioning in the oncology setting.6 Of these, a 6-minute walk test (6MWT) is a simple and clinically feasible method to evaluate functional capacity and is a robust predictor of mortality in numerous clinical settings.7–10 Our group previously demonstrated the clinical utility of the 6MWT in patients with recurrent glioma,5 although the prognostic importance of the 6MWT in the oncology setting outside of a small preliminary study in advanced lung cancer11 is not known.
A major determinant of functional capacity is exercise behavior. Several epidemiologic studies suggest, in general, that self-reported regular exercise (eg, ≥ 9 metabolic equivalent [MET] –h/wk, equivalent to brisk walking for 30 minutes on 5 d/wk) is associated with a 15% to 61% reduction in the risk of cancer-specific death and all-cause mortality after a diagnosis of operable breast,12,13 colorectal,14,15 and prostate cancer.16 Importantly, prior studies examining the association between exercise behavior and survival in patients with cancer have not evaluated functional capacity by using objective methods; therefore, it is not known whether functional capacity provides prognostic information beyond exercise behavior. Similarly, it is not known whether functional capacity and/or exercise behavior add incremental prognostic value beyond PS measures or other traditional markers of prognosis. Here, we sought to investigate the independent relationship between functional capacity, exercise behavior, and survival in adults with recurrent malignant glioma. We also investigated whether these parameters added prognostic information beyond KPS and other traditional (eg, age, tumor grade) markers of prognosis in this population.
PATIENTS AND METHODS
Patients and Setting
Patients with histologically confirmed WHO grades 3 to 4 malignant glioma (ie, glioblastoma multiforme, anaplastic astrocytoma, or anaplastic oligodendroglioma) with recurrent disease, who had received or were receiving salvage therapy, and who were presenting to the Preston Robert Tisch Brain Tumor Center at Duke University Medical Center were eligible. Patients were eligible regardless of treatment (receiving v not receiving treatment) and disease (active v stable disease) status. Additional eligibility criteria included the following: legal age (ie, older than 18 years), KPS ≥ 70 at study entry, primary attending oncologist approval, ability to read and understand English, and no contraindications to a 6MWT according to American Thoracic Society recommendations.17 The Duke University Medical Center institutional review board approved the study, and written informed consent was obtained from all participants before initiation of study procedures.
Functional Capacity
Functional capacity was assessed by using a 6MWT in a measured corridor according to American Thoracic Society guidelines.17 Briefly, patients were instructed to walk at their fastest pace and to cover the longest possible distance over 6 minutes under the supervision of an American College of Sports Medicine–certified exercise specialist. During exercise, oxyhemoglobin saturation and heart rate were monitored continuously by using pulse oximetry (BCI, Hand-Held Pulse Oximeter, Waukesha, WN). The age- and sex-predicted 6MWD was calculated from the equation provided by Gibbons et al.18
Exercise Behavior
Exercise behavior was assessed by self-report using the leisure score index of the Godin Leisure-Time Exercise Questionnaire.19 The leisure score index contains three questions that assess the average frequency of mild, moderate, and strenuous intensity exercise during free time in a typical week. In this study, participants reported their average weekly exercise since their primary adjuvant treatment consultation. We also asked for average duration within each exercise intensity category. To calculate our primary outcome of total exercise behavior, the frequency of exercise sessions per week within each intensity category was multiplied by the average reported duration, weighted by an estimate of the MET, summed across all intensities and expressed as average MET-hours per week. The standard MET weightings and examples for each of the exercise intensities are as follows: mild (3 METs; eg, easy walking, yoga), moderate (5 METs; eg, brisk walking, tennis), and strenuous (9 METs; eg, running, vigorous swimming).
Clinical Parameters and Follow-Up
Patient characteristics and medical therapy data were abstracted from medical records. PS was assessed by using the KPS scale and was assessed at the time of study enrollment by the attending oncologist. Follow-up survival data was obtained through July 2010.
Statistical Analysis
Descriptive statistics were reported for clinical parameters and study outcomes. The Cox proportional hazards model was used to examine the effect of functional capacity and exercise behavior on survival. A likelihood ratio test was used in the context of the Cox model to assess the contribution of walk distance and exercise behavior in predicting survival beyond that provided by KPS (< 90 v ≥ 90) alone as well as the combination of age (younger than 45 years v 45 years or older), sex, grade (III v IV), the number of prior disease progressions (< 2 v ≥ 2), and KPS. Functional capacity was categorized via an unbiased tertile split as defined by the 6MWD (ie, < 390 meters, 390 to 489 meters, > 489 meters). The median value of the 6MWD within each category was used as a predictor for linear trend in analyses. Exercise behavior was analyzed as MET-hours per week (< 9 MET-h/wk v ≥ 9 MET-h/wk) on the basis of prior work.12,13 Survival time was defined as the time between assessment of functional capacity and death; for patients remaining alive, survival was censored at the time of last follow-up. A two-sided significance level of .05 was used for all statistical tests. All statistical analyses were conducted by using SAS, version 9.2 (SAS Institute, Cary, NC).
RESULTS
Participant recruitment took place between May 2007 and April 2009. During this period, 243 patients were registered and completed all study procedures. In brief, 1,528 patients were screened for study eligibility during the study period. Of these, 374 (24%) met inclusion criteria, and 243 (65%) agreed to participate and completed all study procedures. Major reasons for noneligibility were no prior disease progressions (n = 559), low-grade glioma (n = 314), and tumor type (n = 88). Major reasons for study refusal were not interested (n = 51), no time (n = 25), and schedule conflict (n = 10).
Clinical Characteristics
Patient characteristics are presented in Table 1. The mean ± standard deviation age was 49 ± 12 years (range, 20 to 77 years), and 68% were men. Sixty-nine percent were diagnosed with glioblastoma, and the median KPS was 90 (range, 70 to 100). Median time to study enrollment from recurrent diagnosis was 20 months (range, 3 to 241 months). The mean 6MWD was 448 meters (range, 102 to 825 meters), which is equivalent to 38% ± 17% less than that predicted for age and sex. No adverse events were observed during the 6MWT. The mean ± standard deviation exercise behavior was 14 ± 19 MET-h/wk. Twenty-six percent of patients were currently meeting national exercise guidelines (ie, ≥150 min/wk of moderate to vigorous intensity exercise), whereas 24% reported no exercise behavior.
Table 1.
Demographic and Clinical Characteristics of Participants
| Variable | Grade of Disease |
|||||
|---|---|---|---|---|---|---|
| All Patients |
III |
IV |
||||
| No. | % | No. | % | No. | % | |
| Total patients | 243 | 100 | 76 | 31 | 167 | 69 |
| Age, years | ||||||
| Mean | 49 | 44 | 52 | |||
| SD | 12 | 11 | 11 | |||
| < 45 | 82 | 34 | 40 | 53 | 42 | 25 |
| ≥ 45 | 161 | 66 | 36 | 47 | 125 | 75 |
| Male sex | 165 | 68 | 51 | 67 | 114 | 68 |
| Karnofsky performance status | ||||||
| 70 | 11 | 5 | 4 | 5 | 7 | 4 |
| 80 | 77 | 32 | 21 | 28 | 56 | 34 |
| 90 | 124 | 51 | 43 | 57 | 81 | 49 |
| 100 | 31 | 13 | 8 | 11 | 23 | 14 |
| Status of disease at study entry | ||||||
| Active | 46 | 19 | 14 | 18 | 32 | 19 |
| Stable | 197 | 81 | 62 | 82 | 135 | 81 |
| No. of prior treatments | ||||||
| 1 | 22 | 9 | 4 | 5 | 18 | 11 |
| 2 | 76 | 31 | 25 | 33 | 51 | 31 |
| 3 | 77 | 32 | 19 | 25 | 58 | 35 |
| ≥ 4 | 68 | 28 | 28 | 37 | 40 | 24 |
| No. of prior progressions | ||||||
| 0 | 17 | 7 | 7 | 9 | 10 | 6 |
| 1 | 134 | 55 | 37 | 49 | 97 | 58 |
| 2 | 59 | 24 | 12 | 16 | 47 | 28 |
| ≥ 3 | 33 | 14 | 20 | 26 | 13 | 8 |
| Receiving salvage therapy | ||||||
| Yes | 212 | 87 | 67 | 88 | 145 | 87 |
| No | 31 | 13 | 9 | 12 | 22 | 13 |
| Receiving anti-VEGF therapy | ||||||
| Yes | 121 | 50 | 36 | 47 | 85 | 51 |
| No | 122 | 50 | 40 | 53 | 82 | 49 |
| Receiving decadron | ||||||
| Unknown | 2 | 1 | 2 | 3 | 0 | 0 |
| Yes | 70 | 29 | 17 | 22 | 53 | 32 |
| No | 171 | 70 | 57 | 75 | 114 | 68 |
| Receiving anti-epileptic medication | ||||||
| Yes | 183 | 75 | 59 | 78 | 124 | 74 |
| No | 60 | 25 | 17 | 22 | 43 | 26 |
| Functional capacity (6MWD), meters | ||||||
| Mean | 448 | 441 | 451 | |||
| SD | 135 | 137 | 134 | |||
| Range | 102-825 | 102-751 | 182-825 | |||
| % below age-sex predicted | ||||||
| Mean | 38 | 39 | 37 | |||
| SD | 17 | 19 | 15 | |||
| Exercise behavior, MET-hrs/wk | ||||||
| Mean | 14 | 16 | 14 | |||
| SD | 19 | 26 | 16 | |||
Abbreviations: SD, standard deviation; VEGF, vascular endothelial growth factor; 6MWD, 6-minute walk distance; MET, metabolic equivalent.
Survival Analysis
Median follow-up was 27.43 months. During this period, 149 deaths were recorded (61% of the total sample). The overall median survival time for the entire sample from study entry was 15.76 months (95% CI, 12.70 to 20.92 months).
Functional capacity.
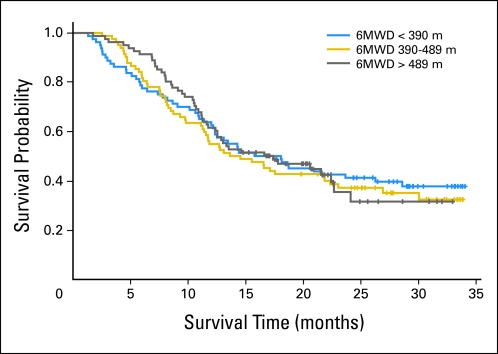
The median 6MWD within each tertile was 314.25 meters (range, 102 to 385.5 meters), 434.5 meters (range, 390 to 489 meters), and 567.5 meters (range, 493 to 825 meters). There was no independent association between the 6MWD and survival (Ptrend = .977; Table 2; Fig 1). Specifically, the corresponding median survival among tertiles was 16.91 months, 14.08 months, and 17.37 months, respectively. The 6MWD did not provide incremental prognostic information beyond traditional markers of prognosis (Ptrend = .870; Table 2). Compared with patients achieving a 6MWD of less than 390 meters, the adjusted hazard ratio (HR) for all-cause mortality was 1.02 (95% CI, 0.68 to 1.51) for a 6MWD of 390 to 489 meters, and it was 0.97 (95% CI, 0.63 to 1.48) for a 6MWD of greater than 489 meters.
Table 2.
Association Between Functional Capacity and Survival
| Analysis | 6-Minute Walk Distance (meters) |
Likelihood Ratio Ptrend | ||
|---|---|---|---|---|
| < 390 | 390-489 | ≥ 489 | ||
| No. of events | 49 | 53 | 47 | |
| No. at risk | 80 | 82 | 81 | |
| Walk distance, meters | ||||
| Median | 314.25 | 434.5 | 567.5 | |
| Range | 102-385.5 | 390-489 | 493-825 | |
| Survival, months | ||||
| Median | 16.91 | 14.08 | 17.37 | |
| Range | 12.17-28.59 | 11.25-22.34 | 11.74-22.66 | |
| Unadjusted HR | Referent | 1.10 | 1.01 | .977 |
| 95% CI | Referent | 0.75 to 1.63 | 0.67 to 1.51 | |
| HR adjusted for KPS | Referent | 1.15 | 1.15 | .509 |
| 95% CI | Referent | 0.78 to 1.7 | 0.76 to 1.73 | |
| Adjusted HR* | Referent | 1.02 | 0.97 | .870 |
| 95% CI | Referent | 0.68 to 1.51 | 0.63 to 1.48 | |
NOTE. Functional capacity measured with the 6-minute walk distance.
Abbreviations: HR, hazard ratio; KPS, Karnofsky performance status.
Adjusted for age, sex, grade, number of prior progressions, and KPS.
Fig 1.
Association between 6-minute walk distance (SMWD) and survival.
Exercise behavior.
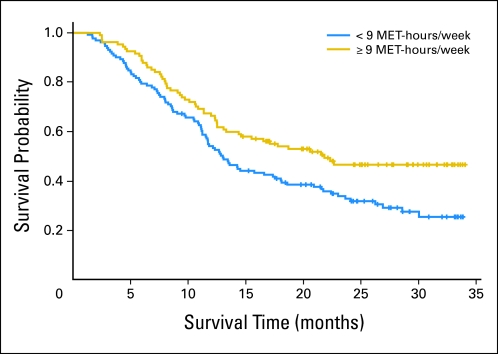
The association between exercise behavior (ie, < 9 MET-h/wk v ≥ 9 MET-h/wk), and survival is presented in Table 3. Exercise behavior was an independent predictor of survival (P = .008; Table 3; Fig 2). Median survival was 13.03 months (95% CI, 11.25 to 17.37 months) for those reporting less fewer than 9 MET-h/wk compared with 21.84 months (95% CI, 13.32 to ∞ months) for those reporting ≥ 9 MET-h/wk. Exercise behavior provided incremental prognostic information beyond KPS alone (P < .001; Table 3) and plus other traditional markers of prognosis (P < .001; Tables 3 and 4). Compared with patients reporting fewer than 9 MET-h/wk, the adjusted HR for mortality was 0.64 (95% CI, 0.46 to 0.91) for patients reporting ≥ 9 MET-h/wk.
Table 3.
Association Between Exercise Behavior and Survival
| Analysis | Exercise Behavior (MET-h/wk) |
Likelihood Ratio P | |
|---|---|---|---|
| < 9 | ≥ 9 | ||
| No. of events | 91 | 55 | |
| No. at risk | 131 | 107 | |
| Survival, months | |||
| Median | 13.03 | 21.84 | |
| Range | 11.25 to 17.37 | 13.32 to ∞* | |
| Unadjusted HR | Referent | 0.64 | .008 |
| 95% CI | Referent | 0.46 to 0.89 | |
| HR adjusted for KPS | Referent | 0.68 | < .001 |
| 95% CI | Referent | 0.48 to 0.95 | |
| Adjusted HR† | Referent | 0.64 | < .001 |
| 95% CI | Referent | 0.46 to 0.91 | |
NOTE. Exercise behavior measured as MET-h/wk.
Abbreviations: MET, metabolic equivalent; HR, hazard ratio; KPS, Karnofsky performance status.
Upper value was out of range.
Adjusted for age, sex, grade, number of prior progressions, and KPS.
Fig 2.
Association between exercise behavior (metabolic equaivalent [MET]–h/wk) and survival.
Table 4.
Multivariate Cox Regression Analysis
| Variable | Reduced Model |
Full Model |
||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Age ≥ 45 years | 1.26 | 0.86 to 1.86 | 1.12 | 0.75 to 1.68 |
| Male sex | 1.75 | 1.20 to 2.54 | 1.74 | 1.19 to 2.53 |
| Grade IV | 2.80 | 1.82 to 4.29 | 2.93 | 1.89 to 4.54 |
| No. of prior progressions ≥ 2 | 1.23 | 0.88 to 1.73 | 1.28 | 0.91 to 1.80 |
| KPS < 90 | 1.78 | 1.27 to 2.48 | 1.72 | 1.22 to 2.41 |
| Exercise behavior ≥ 9 MET-h/wk | — | 0.64 | 0.46 to 0.91 | |
Abbreviations: HR, hazard ratio; KPS, Karnofsky performance status; MET, metabolic equivalent.
DISCUSSION
Recurrent malignant glioma is one of the greatest challenges in the oncology setting; thus, identification of accurate markers of prognosis to optimize treatment and survival outcomes in this population is of major clinical importance. To this end, several research groups have evaluated the prognostic importance of nontraditional markers that may complement current markers to optimize clinical outcome. For example, Meyers et al20 reported that cognitive function but not activities of daily living (ADLs) was an independent predictor of survival in 80 patients with recurrent glioma after adjustment for age, KPS, histology, and time since diagnosis. In contrast, we found that exercise behavior was a strong independent predictor of survival. ADLs and exercise behavior are often considered to be synonymous; however, these measures evaluate different aspects of physical functioning. ADLs evaluate the patient's ability to bathe, feed, and more, whereas exercise is defined as a planned, structured, and repetitive physical activity performed in leisure (ie, nonoccupation) time. Together, these results indicate that basic ADLs appear to be well preserved in patients with recurrent glioma and, as a result, do not provide prognostic value, whereas exercise behavior successfully discriminates mortality risk. Of interest, exercise behavior provided incremental prognostic information beyond KPS, yet KPS remained a strong predictor of mortality in multivariate analysis. This suggests that exercise behavior and KPS are not redundant. In fact, this evidence demonstrates that KPS and exercise behavior provide complementary mortality risk prediction information in recurrent glioma. Collectively, our findings provide strong preliminary evidence that a simple assessment of self-reported exercise behavior is an important new component of patient evaluation that, in turn, may improve prognostication.
The clinical significance of exercise behavior in this study extends prior work that indicates that, in general, regular exercise is associated with significant reductions in the risk of death after a diagnosis of breast, prostate, or colorectal cancer.14,15,21,22 A point of contention in studies investigating the association between exercise and clinical outcome after a cancer diagnosis is whether higher levels of exercise simply reflect lower disease burden and/or symptomology (ie, reverse causation) as opposed to a direct biologic effect. Intriguingly, we found here that functional capacity was not prognostic, implying that it is the ability to exercise that may be predictive of clinical outcome in this setting. However, increasing evidence suggests that exercise modulates a range of systemic (host) factors (eg, metabolic and sex-steroid hormone concentrations, immune surveillance/cytokine/angiogenic factors, and products of oxidation) that, in turn, may alter ligand availability in the tumor microenvironment with subsequent effects on relevant cell signaling pathways.23 Hypothesis-driven, translational studies are required to unravel this complex relationship to optimize the safety and efficacy of exercise in the oncology setting. Regardless, increasing exercise behavior levels after a cancer diagnosis is an important clinical goal. Randomized trials demonstrate that structured exercise training is a safe and well-tolerated therapy associated with significant improvements in several clinically relevant outcomes, such as cardiorespiratory fitness, quality of life, and fatigue in patients with cancer both during and after primary adjuvant therapy.24,25 Behavioral strategies, such as telephone counseling, print-based materials,26 step pedometers,27 and oncologist-based advice,28 are effective strategies to increase exercise behavior in patients with cancer, although whether the magnitude of change in exercise behavior observed (approximately 30 to 90 min/wk) confers favorable changes in clinically relevant outcomes remains to be determined.
An important finding was that functional capacity, as measured by a 6MWT, was not associated with survival in patients with recurrent glioma. The 6MWD is a strong independent predictor of mortality in a wide range of clinical populations,7–10 including non–small-cell lung cancer.11 The 6MWT was originally developed for patients with heart failure, although the value has been additionally established in a wide range of clinical populations. However, our results indicate that the clinical utility of the 6MWT may not extend to recurrent glioma. A potential explanation is that patients with recurrent glioma may present with neurologic impairment that limits their ability to adequately perform a walking test; thus, such a test does not provide an accurate assessment of the cardiovascular limits to exercise tolerance. This may also explain why self-reported exercise behavior was prognostic, whereas the 6MWT was not. In addition, a 6MWT does not provide an assessment of cardiorespiratory fitness or the mechanisms of exercise intolerance.6 As such, alternative functional performance measures that are not limited by neurologic impairments as well as those that provide an objective measure of cardiorespiratory fitness may provide better alternatives. One tool that addresses these issues is a cycle ergometry–based cardiopulmonary exercise test to assess peak oxygen consumption.6 In addition, measures of skeletal muscle size (eg, computed tomography, magnetic resonance imaging) and/or strength (eg, isokinetic dynamometry) also may provide important complementary information, given the high incidence of steroid myopathy in patients with glioma. Our group has demonstrated the safety and feasibility of these assessments in patients with primary glioma.29 Future research investigating the prognostic significance of these methodologies as well as change in functional performance outcomes across time in recurrent malignant glioma as well as in other malignances are warranted.
This study has important limitations. We recognize that we have only demonstrated correlation, and not causation, between exercise behavior and survival. Also, given our eligibility criteria, our results are only generalizable to patients with a KPS ≥ 70. On the basis of our study design, it was not possible to delineate whether higher levels of exercise simply reflect lower disease burden and/or symptomology or a direct exercise-induced biologic effect. Prospective, randomized trials are required to answer this important question. In summary, exercise is a strong independent predictor of survival that provides incremental prognostic value beyond traditional markers of prognosis in recurrent glioma. Additional studies investigating the association between exercise, functional performance measures, and clinical outcome after a cancer diagnosis are warranted.
Footnotes
Supported by National Institutes of Health Grants No. CA143254, CA142566, CA138634, CA133895, and CA125458 (all, L.W.J.) and by funds from George and Susan Beischer.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Lee W. Jones
Administrative support: Miranda West
Provision of study materials or patients: Whitney E. Hornsby, Miranda West, Annick Desjardins, James J. Vredenburgh, Emily Waner
Collection and assembly of data: Whitney E. Hornsby, Diane R. Fels, Miranda West, Annick Desjardins, James J. Vredenburgh, Emily Waner, Allan H. Friedman, Katherine B. Peters
Data analysis and interpretation: Emily Ruden, April D. Coan, James E. Herndon II, Whitney E. Hornsby, Henry S. Friedman, Lee W. Jones
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Ballman KV, Buckner JC, Brown PD, et al. The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro Oncol. 2007;9:29–38. doi: 10.1215/15228517-2006-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamborn KR, Yung WK, Chang SM, et al. Progression-free survival: An important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10:162–170. doi: 10.1215/15228517-2007-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu W, Lamborn KR, Buckner JC, et al. Joint NCCTG and NABTC prognostic factors analysis for high-grade recurrent glioma. Neuro Oncol. 2010;12:164–172. doi: 10.1093/neuonc/nop019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carson KA, Grossman SA, Fisher JD, et al. Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials. J Clin Oncol. 2007;25:2601–2606. doi: 10.1200/JCO.2006.08.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones LW, Cohen RR, Mabe SK, et al. Assessment of physical functioning in recurrent glioma: Preliminary comparison of performance status to functional capacity testing. J Neurooncol. 2009;94:79–85. doi: 10.1007/s11060-009-9803-x. [DOI] [PubMed] [Google Scholar]
- 6.Jones LW, Eves ND, Haykowsky M, et al. Cardiorespiratory exercise testing in clinical oncology research: Systematic review and practice recommendations. Lancet Oncol. 2008;9:757–765. doi: 10.1016/S1470-2045(08)70195-5. [DOI] [PubMed] [Google Scholar]
- 7.Lederer DJ, Arcasoy SM, Wilt JS, et al. Six-minute-walk distance predicts waiting list survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:659–664. doi: 10.1164/rccm.200604-520OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lettieri CJ, Nathan SD, Browning RF, et al. The distance-saturation product predicts mortality in idiopathic pulmonary fibrosis. Respir Med. 2006;100:1734–1741. doi: 10.1016/j.rmed.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Flaherty KR, Andrei AC, Murray S, et al. Idiopathic pulmonary fibrosis: Prognostic value of changes in physiology and six-minute-walk test. Am J Respir Crit Care Med. 2006;174:803–809. doi: 10.1164/rccm.200604-488OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cote CG, Pinto-Plata V, Kasprzyk K, et al. The 6-min walk distance, peak oxygen uptake, and mortality in COPD. Chest. 2007;132:1778–1785. doi: 10.1378/chest.07-2050. [DOI] [PubMed] [Google Scholar]
- 11.Kasymjanova G, Correa JA, Kreisman H, et al. Prognostic value of the six-minute walk in advanced non-small cell lung cancer. J Thorac Oncol. 2009;4:602–607. doi: 10.1097/JTO.0b013e31819e77e8. [DOI] [PubMed] [Google Scholar]
- 12.Holmes MD, Chen WY, Feskanich D, et al. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 13.Irwin ML, Smith AW, McTiernan A, et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: The health, eating, activity, and lifestyle study. J Clin Oncol. 2008;26:3958–3964. doi: 10.1200/JCO.2007.15.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: Findings from CALGB 89803. J Clin Oncol. 2006;24:3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 15.Meyerhardt JA, Giovannucci EL, Ogino S, et al. Physical activity and male colorectal cancer survival. Arch Intern Med. 2009;169:2102–2108. doi: 10.1001/archinternmed.2009.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenfield SA, Stampfer MJ, Giovannucci E, et al. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011;29:726–732. doi: 10.1200/JCO.2010.31.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 18.Gibbons WJ, Fruchter N, Sloan S, et al. Reference values for a multiple repetition 6-minute walk test in healthy adults older than 20 years. J Cardiopulm Rehabil. 2001;21:87–93. doi: 10.1097/00008483-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–146. [PubMed] [Google Scholar]
- 20.Meyers CA, Hess KR, Yung WK, et al. Cognitive function as a predictor of survival in patients with recurrent malignant glioma. J Clin Oncol. 2000;18:646–650. doi: 10.1200/JCO.2000.18.3.646. [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: Meta-analysis of published studies. Med Oncol. doi: 10.1007/s12032-010-9536-x. 10.1007/s12032-010-9536-x [epub ahead of print on April 22, 2010] [DOI] [PubMed] [Google Scholar]
- 22.Meyerhardt JA, Giovannucci EL, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 23.Thompson HJ, Wolfe P, McTiernan A, et al. Wheel running-induced changes in plasma biomarkers and carcinogenic response in the 1-methyl-1-nitrosourea-induced rat model for breast cancer. Cancer Prev Res (Phila) 2010;3:1484–1492. doi: 10.1158/1940-6207.CAPR-10-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 25.Jones LW, Liang Y, Pituskin EN, et al. Effect of exercise training on peak oxygen consumption in patients with cancer: A meta-analysis. Oncologist. 2011;16:112–120. doi: 10.1634/theoncologist.2010-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morey MC, Snyder DC, Sloane R, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW—A randomized controlled trial. JAMA. 2009;301:1883–1891. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vallance JK, Courneya KS, Plotnikoff RC, et al. Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors. J Clin Oncol. 2007;25:2352–2359. doi: 10.1200/JCO.2006.07.9988. [DOI] [PubMed] [Google Scholar]
- 28.Jones LW, Courneya KS, Fairey AS, et al. Effects of an oncologist's recommendation to exercise on self-reported exercise behavior in newly diagnosed breast cancer survivors: A single-blind, randomized controlled trial. Ann Behav Med. 2004;28:105–113. doi: 10.1207/s15324796abm2802_5. [DOI] [PubMed] [Google Scholar]
- 29.Jones LW, Mourtzakis M, Peters KB, et al. Changes in functional performance measures in adults undergoing chemoradiation for primary malignant glioma: A feasibility study. Oncologist. 2010;15:636–647. doi: 10.1634/theoncologist.2009-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]