Abstract
Purpose
This multicenter randomized trial was designed to test whether melanoma-associated helper peptides augment CD8+ T-cell responses to a melanoma vaccine and whether cyclophosphamide (CY) pretreatment augments CD4+ or CD8+ T-cell responses to that vaccine.
Patients and Methods
In all, 167 eligible patients with resected stage IIB to IV melanoma were randomly assigned to four vaccination study arms. Patients were vaccinated with 12 class I major histocompatibility complex–restricted melanoma peptides (12MP) to stimulate CD8+ T cells and were randomly assigned to receive a tetanus helper peptide or a mixture of six melanoma-associated helper peptides (6MHP) to stimulate CD4+ T cells. Before vaccination, patients were also randomly assigned to receive CY pretreatment or not. T-cell responses were assessed by an ex vivo interferon gamma ELISpot assay. Clinical outcomes and toxicities were recorded.
Results
Vaccination with 12MP plus tetanus induced CD8+ T-cell responses in 78% of patients and CD4+ T-cell responses to tetanus peptide in 93% of patients. Vaccination with 12MP plus 6MHP induced CD8+ responses in 19% of patients and CD4+ responses to 6MHP in 48% of patients. CY had no significant effect on T-cell responses. Overall 3-year survival was 79% (95% CI, 71% to 86%), with no significant differences (at this point) by study arm.
Conclusion
Melanoma-associated helper peptides paradoxically decreased CD8+ T-cell responses to a melanoma vaccine (P < .001), and CY pretreatment had no immunologic or clinical effect. Prior work showed immunologic and clinical activity of 6MHP alone. Possible explanations for negative effects on CD8 responses include modulation of homing receptor expression or induction of antigen-specific regulatory T cells.
INTRODUCTION
Most peptide-based cancer vaccines have incorporated class I major histocompatibility complex (MHC) –restricted peptides to activate CD8+ cytotoxic T lymphocytes. However, activation of tumor-specific CD4+ helper T cells may also be critical for elimination of tumor.1–3 Adoptive therapy with CD4+ T cells can induce tumor protection or regression,1,2 and depletion of CD4+ T cells inhibits vaccine-induced protective immunity.4 Immune responses to pathogens integrate cytotoxic T lymphocyte responses to epitopes presented by class I MHC molecules and CD4+ T-cell responses to epitopes presented by class II MHC molecules.5 CD4+ cells can activate dendritic cells for heightened antigen presentation. Th1 CD4+ cells produce a cytokine milieu critical for induction of immune-mediated tumor destruction.6,7 CD4+ T-cell responses are also implicated in establishing immunologic memory.8 In patients with HIV, induction of CD4+ responses to HIV antigens augments HIV-specific CD8+ T-cell reactivity.9 Despite the acknowledged importance of CD4+ helper T cells in active immunotherapy, there has been a paucity of experience, in patients who have cancer, with tumor-associated peptides that stimulate CD4+ T-cell (helper peptides), the investigation of which has been limited to pilot or nonrandomized studies with only a few peptides.10–12
In prior studies,13–17 we included an HLA-DR–restricted helper peptide from tetanus toxoid in melanoma vaccines, which activated CD4+ T cells nonspecifically. Class II MHC-restricted helper peptides from melanoma-associated proteins now promise to activate CD4+ T cells in a melanoma-specific manner.18 In a phase I/II clinical trial, we found that a mixture of six melanoma helper peptides (6MHP) derived from cancer-testis antigens (CTAs) and melanocytic differentiation proteins (MDPs) was immunogenic in more than 80% of patients and was associated with durable clinical responses (12%) and durable stable disease (12%).19 This study was designed to address whether melanoma-associated helper peptides augment induction and persistence of CD8+ T-cell responses to a multipeptide melanoma vaccine administered in the adjuvant setting to melanoma patients with a predicted high risk for recurrence.
A second question addressed in this study was whether pretreatment with cyclophosphamide (CY) enhanced CD4+ or CD8+ T-cell responses to a multipeptide vaccine. CY doses lower than those used for tumor lysis have been reported to augment immune responses in mice and humans20–24 through several potential mechanisms.23,25–31 Prior studies20,24,29,30,32,33 have tested immunomodulatory properties of 75 to 1,000 mg/m2 CY with varied results. For patients with melanoma, CY pretreatment was associated with augmented delayed-type hypersensitivity responses to an autologous melanoma cell vaccine in nonrandomized studies.20,21 However, studies of the effects of CY on immune responses to vaccines have been limited by small sample size, nonrandomized designs, and/or lack of defined antigens. The largest study is with 300 mg/m2 dosing, which we evaluated.
In this article, we report findings from a multicenter randomized trial designed to test whether melanoma-associated helper peptides augment CD8+ T-cell responses to a melanoma vaccine and whether CY pretreatment augments CD4+ or CD8+ T-cell responses.
PATIENTS AND METHODS
Patients
Eligible patients had American Joint Committee on Cancer (AJCC; 6th edition) (34) stage IIB to IV melanoma from cutaneous, mucosal, or unknown primary sites, rendered clinically free of disease by surgery or stereotactic radiosurgery. Inclusion criteria included expression of HLA-A1, -A2, or -A3; expression of HLA-DR1, -DR4, -DR11, -DR13, or -DR15; age 18 years and older; Eastern Cooperative Oncology Group (ECOG) performance status 0 to 1; adequate liver and renal function; and ability to give informed consent. Exclusion criteria included ocular melanoma; pregnancy; cytotoxic chemotherapy, interferon, or radiation within the preceding 4 weeks; multiple brain metastases; use of steroids; class III to IV heart disease; or severe autoimmune disease. Patients provided informed consent, and the study was approved by the institutional review board and the US Food and Drug Administration.
Vaccine Composition
Patients received a vaccine comprising 12 melanoma peptides restricted by HLA-A1, -A2, or -A3 (12MP), as described,16 plus peptides to stimulate helper T cells. Vaccines with 12MP plus the tetanus peptide AQYIKANSKFIGITEL13 are called MELITAC 12.1; vaccines incorporating 12MP plus a mixture of 6MHP19 are called MELITAC 12.6. Peptide sequences are provided in the Data Supplement.
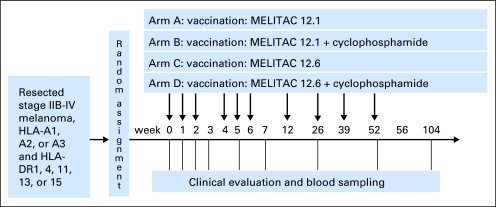
Each vaccine was administered as 2 mL of stable water-in-oil emulsion prepared by the two-syringe method and consisting of 100 μg of each of the 12 melanoma peptides, 190 μg of each helper peptide, and 1 mL Montanide ISA-51 VG adjuvant (Seppic, Paris, France/Fairfield, NJ).35 A drop of each emulsion was tested for stability in water before injection. Vaccines were administered, half subcutaneously and half intradermally, on days 1, 8, 15, 29, 36, and 43 and then at 3, 6, 9, and 12 months (Fig 1).
Fig 1.
Mel44 protocol schema. MELITAC 12.1, vaccines with 12 class I major histocompatibility complex–restricted melanoma peptides (12MP) plus the tetanus peptide AQYIKANSKFIGITEL; MELITAC 12.6, vaccines incorporating 12MP plus a mixture of six melanoma-associated helper peptides (6MHP).
Clinical Trial Design
This was an open-label, multicenter phase I/II study with random assignment to MELITAC 12.1 or MELITAC 12.6, with or without CY pretreatment (Fig 1). Primary goals were to test safety and immunogenicity. The study was designed to assess a partial ordering of immune response magnitudes among the four study arms, specifically arm D (12.6 + CY) greater than B (12.1 + CY) or C (12.6), and B or C greater than A (12.1). The study included interim safety assessments. Sample size determination was based on differences in cumulative immune response measured in the peripheral blood mononuclear cells (PBMCs) over six vaccines compared with baseline. We were interested in detecting at least a 30% increase in the four main comparisons of interest. The study was designed to test these comparisons at the two-sided 2.5% significance level (10% overall) with 90% power at the alternative, requiring 40 participants per arm (total, 160 eligible). Maximum accrual was adjusted up to 173 participants to allow for 5% ineligibility and 3% overenrollment. Patients were accrued at three participating institutions, were stratified by HLA type and institution (Table 1), and were randomly assigned within strata with various block sizes. Randomization lists were generated by the study statisticians and stored in the database, with arm assignment being released to the study coordinator only at the time of registration. The study design is presented schematically in Figure 1.
Table 1.
Demographics and Clinical Characteristics of Eligible Patients
| Characteristic | Study Arm |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A(n = 41) |
B(n = 41) |
C(n = 42) |
D(n = 43) |
All(N = 167) |
||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Sex | ||||||||||
| Male | 28 | 68 | 25 | 61 | 29 | 69 | 30 | 70 | 112 | 67 |
| Race | ||||||||||
| Black/African American | 0 | 0 | 0 | 0 | 0 | |||||
| Hispanic | 1 | 0 | 1 | 0 | 2 | 1.2 | ||||
| Hospital | ||||||||||
| Fox Chase Cancer Center | 9 | 6 | 7 | 6 | 28 | |||||
| MD Anderson Cancer Center | 12 | 13 | 13 | 14 | 52 | |||||
| University of Virginia | 20 | 22 | 22 | 23 | 87 | |||||
| Disease status | ||||||||||
| Initial diagnosis | 17 | 20 | 24 | 24 | 85 | |||||
| Recurrence | 24 | 21 | 18 | 19 | 82 | |||||
| Stage at enrollment | ||||||||||
| IIB | 2 | 2 | 5 | 4 | 13 | |||||
| IIC | 2 | 3 | 3 | 2 | 10 | |||||
| IIIA | 4 | 8 | 5 | 4 | 21 | |||||
| IIIB | 11 | 10 | 11 | 18 | 50 | |||||
| IIIC | 10 | 10 | 9 | 8 | 37 | |||||
| IV | 12 | 8 | 9 | 7 | 36 | |||||
| ECOG PS | ||||||||||
| 0 | 34 | 39 | 40 | 38 | 151 | |||||
| 1 | 7 | 2 | 2 | 5 | 16 | |||||
| Median age, years | 59 | 60 | 55 | 58 | 58 | |||||
| HLA expression | ||||||||||
| HLA-A1 | 12 | 13 | 13 | 14 | 52 | |||||
| HLA-A2 | 23 | 21 | 22 | 19 | 85 | |||||
| HLA-A3 | 15 | 17 | 20 | 20 | 72 | |||||
| HLA-A31 | 4 | 1 | 0 | 1 | 6 | |||||
NOTE. Total enrollment for study arms A through D was 41, 43, 42, and 44, respectively.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PS, performance status.
Toxicity Assessment and Stopping Rules
The trial was monitored continuously for treatment-related adverse events by using National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Toxicities were recorded by patients in a daily toxicity diary and reviewed weekly by interview with a study clinician. Protocol treatment was to be discontinued for all grade 3, ocular grade 1, allergic grade 2, or higher, unexpected treatment-related adverse events. Patients with disease progression requiring other therapy were removed from treatment.
End Points
The primary end point was the maximum cumulative circulating CD8+ T cell response to 12MP measured by ELISpot assay over the first six vaccines (to day 50). This is reported both as a binary measure of immune response (yes/no) and a continuous measure based on fold-increase over background corrected for any prevaccine reactivity. Secondary end points included CD8+ T-cell responses to 12MP during and after booster vaccines (> day 50 to 2 years), T-cell response to helper peptides, disease-free survival, and overall survival.
Collection of PBMCs
At times shown in Figure 1, peripheral blood (120 mL week 0, then 75 mL) was drawn into heparinized tubes, and 20 mL was drawn for serum studies. Blood drawn at other participating institutions was shipped in insulated containers at near room temperature for overnight delivery. PBMCs were isolated by using Ficoll gradient centrifugation and were cryopreserved in 10% dimethylsulfoxide/90% serum by the Biorepository and Tissue Research Facility at the University of Virginia.
ELISpot Assays
ELISpot assays (for interferon gamma) were performed on PBMCs collected through day 50 (weeks 0, 1, 2, 3, 5, and 7). Assays were performed directly ex vivo after cryopreservation (direct ELISpot), as reported17,36 (details in Data Supplement). Patients were designated immunologic responders if vaccine antigen-specific responses represented increases of at least 0.02% of CD4+ or CD8+ T cells over negative control and were at least two-fold above the negative control and above any prevaccine response, and if standard deviations of the antigen-reactive responses and negative controls did not overlap. These criteria match those we used previously17 and are in the range of other published criteria (Data Supplement). Interassay coefficients of variation (CVs) were calculated for normal donor PBMC responses to the pool of 32 peptides from cytomegalovirus, Epstein-Barr virus, and influenza proteins,37 testing high and low responders in each assay. For two high-responder donors (mean spots per 100,000 cells: 233, 485), CVs were 15% and 7%, respectively (weighted mean, 10%). For two low-responder donors (mean spots per 100,000 cells: 29, 96), CVs were 47% and 28%, respectively (weighted mean, 34%).
Primary analyses were performed on the basis of eligible patients. For hypothesis testing, patients who discontinued protocol therapy early were considered to have immune response failures if no response was observed in evaluable samples. Immune response rates were calculated on the basis of the proportion of patients whose data met the stated criteria. Point estimates and 95% CIs were calculated for summary parameters. Differences between study arms by binary measures of immune response and measures of cumulative response were assessed by χ2 tests and Kruskal-Wallis tests, respectively. Evaluation of immune response persistence to year 2 is not yet complete and will be reported in the future. The product-limit method was used to estimate overall survival and disease-free survival.
RESULTS
Eligibility Review
In all, 170 patients were enrolled beginning in March 2005 until meeting the accrual goal in January 2008. Three (1.8%) were ineligible on post review (one thrombocytopenia, one tumor progression before first treatment, and one insufficient time after radiation therapy). These patients had been randomly assigned to arms B (two) and D (one). Immune responses were evaluable for 161 (96%) of the 167 eligible patients. Patient demographics and clinical presentations were similar across study arms (Table 1).
CD8 T-Cell Responses to Class I MHC-Restricted Peptides: Direct ELISpot Assay
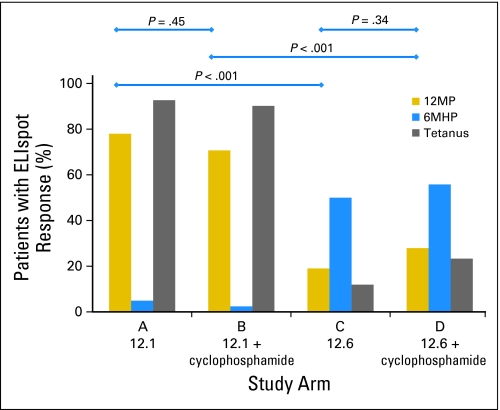
Immune responses were induced by 11 of the 12 peptides by day 50. Patients in arm A or B (MELITAC 12.1 with or without CY) had the highest immune response rates for nine of the peptides (Table 2). Rates of immune response to at least one peptide restricted by each HLA allele were also highest in arms A and B: 69%, 83%, and 68%, for HLA-A1, -A2, and -A3, respectively (Table 2). CD8+ T-cell response rates to the 12MP mixture were 78% and 71% for arms A and B, respectively (P = .45, not significant), and 19% and 28% for arms C and D, respectively (P = .34, not significant; Fig 2 and Table 2). For this end point, the predicted ordering of D more than (C or B) more than A was the opposite of what was observed. For most peptides tested, the observed ordering was effectively A = B more than C = D, with marked decreases in reactivity observed in arms C and D compared with arms A and B (P < .001 for both).
Table 2.
Percent of Patients (167 evaluable patients) With Immune Responses to Each Peptide or to Peptide Mixtures (direct ELISpot data through day 50)
| Individual Class I MHC Peptides* | Source Protein | Study Arm |
Pairs of Study Arms |
||||
|---|---|---|---|---|---|---|---|
| A (12.1)(n = 41) | B (12.1 + Cy)(n = 41) | C (12.6)(n = 42) | D (12.6 + Cy)(n = 43) | A+B (12.1)(n = 82) | C+D (12.6)(n = 85) | ||
| HLA-A1 | |||||||
| DAEKSDICTDEY | Tyrosinase | 42 | 54 | 23 | 14 | 48 | 19 |
| EADPTGHSY | MAGE-A1 | 8 | 8 | 8 | 0 | 8 | 4 |
| EVDPIGHLY | MAGE-A3 | 25 | 15 | 8 | 0 | 20 | 4 |
| SSDVIPIGTY | Tyrosinase | 8 | 0 | 0 | 7 | 4 | 4 |
| HLA-A2 | |||||||
| GLYDGMEHL | MAGE-A10 | 39 | 48 | 9 | 11 | 43 | 10 |
| IMDQVPFSV | gp100 | 78 | 57 | 18 | 21 | 68 | 20 |
| YLEPGPVTA | gp100 | 9 | 0 | 5 | 0 | 5 | 2 |
| YMDGTMSQV | Tyrosinase | 4 | 0 | 5 | 5 | 2 | 5 |
| HLA-A3 | |||||||
| ALLAVGATK | gp100 | 42 | 47 | 5 | 0 | 44 | 2 |
| ASGPGGGAPR | NY-ESO-1 | 16 | 6 | 0 | 0 | 11 | 0 |
| LIYRRRLMK | gp100 | 0 | 0 | 0 | 0 | 0 | 0 |
| SLFRAVITK | MAGE-A1 | 53 | 59 | 5 | 14 | 53 | 10 |
| Mixtures of class I MHC peptides | |||||||
| Maximum of HLA-A1 peptides | 42 | 69 | 23 | 21 | 56 | 22 | |
| Maximum of HLA-A2 peptides | 83 | 57 | 23 | 26 | 70 | 24 | |
| Maximum of HLA-A3 peptides | 68 | 59 | 5 | 14 | 64 | 10 | |
| Maximum of all 12 peptides | 73 | 68 | 17 | 26 | 71 | 21 | |
| 12MP mixture | 78 | 71 | 19 | 28 | 74 | 24 | |
| Helper peptides | |||||||
| 6MHP mixture | 2 | 0 | 48 | 56 | 1 | 52 | |
| Tetanus peptide | 93 | 90 | 12 | 23 | 91 | 18 | |
NOTE. Bold values represent the highest immune response rates per study arm (or pair of study arms).
Abbreviations: Cy, cyclophosphamide; MAGE, melanoma-associated gene; MHC, major histocompatibility complex; 6MHP, mixture of six melanoma-associated helper peptides; 12MP, 12 class I MHC-restricted melanoma peptides.
Immune response rates for individual peptides or for groups of peptides restricted by an HLA allele are based on the subset of patients expressing that HLA allele.
Fig 2.
Immune response rates for 12 class I major histocompatibility complex–restricted melanoma peptides (12MP), a mixture of six melanoma-associated helper peptides (6MHP), and tetanus peptide by ELISpot assay are shown for each study arm. These are based on evaluation of peripheral blood mononuclear cells at weeks 1, 2, 3, 5, and 7 (through day 50). P values for the response to 12MP are noted for four pairs of study arms. 12.1 [MELITAC 12.1], vaccines with 12MP plus the tetanus peptide AQYIKANSKFIGITEL; 12.6 [MELITAC 12.6], vaccines incorporating 12MP plus 6MHP.
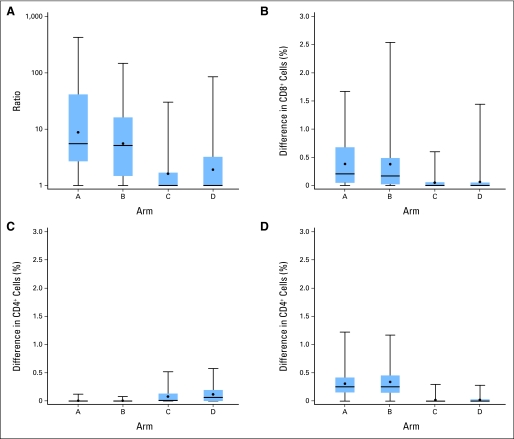
Magnitudes of CD8+ T-cell responses to 12MP are shown in Figure 3, both by fold-increase (Fig 3A) and by percent of CD8+ cells (Fig 3B). For arms A and B, these values were equivalent at approximately 5× and 0.2% of CD8+ cells, but for arms C and D, median values were only 1×, and 0% of CD8+ cells. Differences across all four arms were significant (P < .001) for both analyses. For arms A and B, 75th percentile values were approximately 0.5% to 0.7%, and the highest responses exceeded 1% (Fig 3A). Summaries of raw ELISpot data are provided in the Data Supplement.
Fig 3.
The magnitudes of immune responses to 12 class I major histocompatibility complex–restricted melanoma peptides (12MP) by patient, grouped by study arm. Box plots represent the magnitude of ELISpot reactivity to the 12MP mixture, mixture of six melanoma-associated helper peptides (6MHP), or tetanus peptide through day 50. Boxes represent 25th through 75th percentile values, with mean (black circle) and median (horizontal line) marked, and with maximum and minimum values at the ends of the stems. Maximum responses to the 12MP pool per patient are plotted as fold-increase over baseline (A) and as increase in percent of CD8+ cells (B). Maximum responses to the 6MHP pool (C) and tetanus peptide (D) are plotted as increase in percent of CD4+ cells responding to each peptide in interferon gamma ELISpot assays. The mean background reactivity across all assays was 19.3 spots (95% CI, 15.9 to 22.7 spots). CD8+ cells and CD4+ cells on average represented 20% (95% CI, 19% to 21%) and 36.3% (95% CI, 34.5% to 38.2%) of peripheral blood mononuclear cells, respectively. Thus, on average, 100 spots per well above negative controls represent approximately 0.25% of CD8+ cells.
CD4+ T-Cell Responses to Tetanus Helper Peptide and 6MHP: Direct ELISpot Assay
Th1 CD4+ T-cell responses were measured by direct interferon gamma ELISpot assay. Immune responses to tetanus peptide were detected in 91% of patients vaccinated with tetanus peptide (average across arms A and B) and in only 18% of patients vaccinated with 6MHP (arms C and D; Fig 2 and Table 2). Responses to 6MHP were detected in only 1% of patients vaccinated with tetanus peptide (arms A and B) but in 52% of patients vaccinated with 6MHP (arms C and D; Fig 2 and Table 2). CD4+ T-cell response magnitudes were greater to tetanus peptide (medians, approximately 0.25% for arms A and B; Fig 3C) than to 6MHP (medians < 0.1% for arms C and D; Fig 3D). Differences across all four arms were significant (P < .001). However, there is no evidence of association between immune responses and relapse-free or overall survival.
Summary of Clinical Toxicities
Treatment-related adverse events are detailed in the Data Supplement for 170 treated patients and were similar to those from our prior peptide-based trials.16,17,19 There was one treatment-related grade 4 toxicity (hypoglycemia; < 1%), but no treatment-related deaths or deaths on study. Fourteen patients (8%) had unexpected treatment-related grade 3 adverse events that required reporting to the institutional review board: four (arm A), seven (arm B), two (arm C), and one (arm D). They included injection site ulceration (n = 10); injection site reaction/induration (n = 7); and auditory toxicity, lymphopenia, vomiting, hypoglycemia, muscle pain, pain (not otherwise specified), dyspnea, pneumonitis, and cytokine release syndrome (one each). Treatment-related autoimmune toxicities were reported in 10 patients (6%; details and study completion rates in Data Supplement).
Clinical Outcome
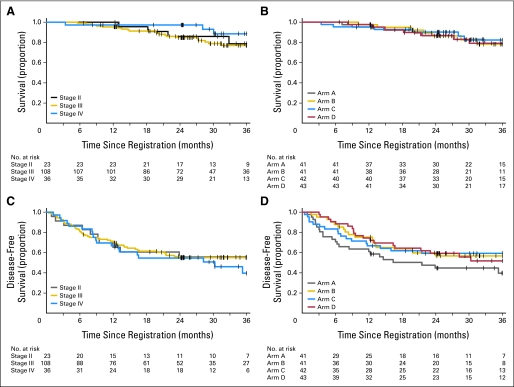
For the entire patient cohort, estimates were approximately 88% (95% CI, 81% to 92%) for 2-year overall survival and 56% (95% CI, 48% to 63%) for 2-year disease-free survival and were 79% (95% CI, 71% to 86%) for 3-year overall survival and 52% (95% CI, 44% to 60%) for 3-year disease-free survival. There were no significant differences in outcome by study arm at this early follow-up. In a global test across the four study arms, there was no difference in overall survival (P = .95) or disease-free survival (P = .26). Kaplan-Meier curves are shown in Figure 4.
Fig 4.
Clinical outcome. Overall survival by stage (A) and study arm (B) and disease-free survival by stage (C) and study arm (D) are shown as Kaplan-Meier curves from the time of study enrollment.
DISCUSSION
Cancer immunotherapy is coming of age, with a cancer vaccine approved for prostate cancer,38 improved survival with CTLA4 (CD152) blockade for melanoma,39 clinical benefit of adding a peptide vaccine to high-dose interleukin-2 (IL-2),40 and durable responses to adoptive T-cell therapy for melanoma and other cancers.41 These treatments increase T-cell activation, and most target defined T-cell antigens. In addition, most effective immune therapies can also induce severe toxicity. Cancer vaccines offer the potential to activate T cells with specificity and low morbidity to bring benefits of immune therapy to patients treated in the adjuvant setting.
We previously found that vaccination with either 12MP or 6MHP induced antitumor CD8+16,17 and CD4+ T cells,19 respectively. In this study, we tested the hypothesis that adding 6MHP to 12MP would increase the magnitude and persistence of CD8+ T-cell responses to 12MP, but it paradoxically decreased CD8+ T-cell responses compared with MELITAC 12.1 vaccination. Adding 12MP did not appear to inhibit helper T-cell responses: CD4+ T-cell response rates to tetanus peptide exceeded 90%, and response rates to 6MHP were approximately 50%. The latter result is comparable with the 57% immune response rate in PBMCs when vaccinating with 6MHP alone.19 However, responses to 6MHP were lower than responses to tetanus peptide. One might conclude that the negative effect of 6MHP simply reflects a weak helper T-cell response. However, in a separate study, we found that vaccination with 12MP + 6MHP led to lower CD8+ T-cell responses than vaccination with 12MP alone.42
Possible explanations for the observed negative effect as measured in PBMCs may include induction of antigen-specific regulatory T cells, Th2 cytokine induction by 6MHP, increased homing of T cells to tumor deposits, or sequestration of T cells at the vaccine site.43 Prior vaccination with 6MHP has induced Th1-dominant responses and was not associated with increases in total regulatory T cells.19 If clinical outcome were improved with addition of 6MHP, it might suggest that melanoma-specific helper T-cell responses support better homing to tumor; however, these patients had no clinically evident disease at study entry, and at current early follow-up, a survival effect has not been observed. There is a weak trend to shorter disease-free survival for patients on arm A (MELITAC 12.1 alone), leaving open some possibility that there may be a clinical impact with longer follow-up. Future studies to define the mechanism of this observation will include characterizing cytokines and chemokines produced in the vaccine site microenvironment and draining nodes, changes in antigen-specific regulatory T cells, and differences in homing receptors expressed on T cells induced by each vaccination strategy.
In addition to testing the effect of melanoma-associated helper peptides, this study also evaluated the effect of CY pretreatment. CY provided no detectable improvement in CD4+ or CD8+ T-cell responses (Figs 2 and 3) or in clinical outcome (Fig 4). In preclinical studies, immunopotentiation has been reported with CY administered 1 to 7 days before vaccination.44–47 Prior experience in humans suggested that CY increased immunogenicity when administered 3 days before a cell-based vaccine, but those studies were nonrandomized and were limited by semiquantitative immunologic end points.20,21,24 Other human experience failed to identify changes in regulatory T cells with CY treatment,48 and recent data identified negative effects of CY (200 mg/m2 or greater) pretreatment on cellular immune responses to a breast cancer cell vaccine.33 This study was unique in evaluating effects of CY on immune responses to defined antigens as well as effects on both CD4+ and CD8+ responses. Other doses or timing of CY pretreatment may have effects different from those observed in this study; however, the dose and timing here is in the range of what has been previously tested. Thus, this study and others challenge the value of CY as a vaccine adjuvant for induction of CD8+ or Th1 CD4+ responses.
In summary, this study supports the immunogenicity of 12MP for CD8+ T cells and both tetanus peptide and 6MHP for CD4+ T cells. However, the combination of 6MHP with 12MP paradoxically reduced the circulating CD8+ T-cell response, and CY (300 mg/m2) pretreatment had no measurable effect on CD8+ or CD4+ responses. Clinical outcome was not increased by adding melanoma-associated helper peptides or by adding CY, although there is a trend to shorter disease-free survival in patients who received neither (arm A). Nonetheless, the immunogenicity of 6MHP in this study, and its clinical activity in prior work,19 support further investigation of vaccines by using melanoma-associated helper peptides, and the interaction of CD8+ and CD4+ T-cell responses needs to be understood. We plan to pursue whether the combination vaccination induces antigen-specific regulatory T cells, regulatory cytokines, or homing receptors that may alter homing to tumor, vaccine sites, or other peripheral tissues.
Supplementary Material
Acknowledgment
We thank Patrice Neese and Carmel Nail for administering vaccines and for recording and managing toxicities; Donna Deacon for vaccine preparation; Cheryl Murphy, Kelly Smith, Nadejda Galeassi, and Kim Underwood for outstanding ELISpot assay work; and Elizabeth Coleman for assistance in data management and data entry for the ELISpot assays. We also thank clinical research coordinators Jennifer Hodges, University of Virginia (UVA), Emily Jackson (UVA), and Kristin Shaller-Padavic (Fox Chase Cancer Center). The regulatory and auditing work was supported and overseen by a team including Robyn Fink, Elizabeth Woodson, Sarah Lewis, Christine Schulte, Scott Boerner, Erin Farris, Beverely Turner, and Kim Underwood.
Glossary Terms
- Cancer-testis antigen (CTA):
Proteins expressed on the surface of cancer and testicular cells capable of eliciting an immune response outside of the immunologically shielded testis.
- CD4:
The surface antigen that characterizes CD4+ T lymphocytes, CD4 is associated with the T-cell receptor and major histocompatibility complex, necessary for antigen recognition.
- CTLA4 (CD152):
Receptor on activated T cells that binds B7 molecules with a higher affinity than CD28, downregulating T-cell responses by inhibiting CD28 signaling.
- ELISpot:
Enzyme-linked immunospot that is exquisitely sensitive to assay minute amounts of mediators that are produced by cells. Typically, cells are deposited on a membrane coated with an antibody specific for a given protein. The protein of interest is captured directly around the secreting cell and is detected with an antibody specific for a different epitope. Coupled with colorimetry, the cells are visualized by specialized plate readers. Thus, the molecule is assayed before it is diluted in the supernatant, captured by receptors of adjacent cells, or degraded.
- Helper peptide:
A peptide that binds to class II MHC molecules and thereby creates an epitope recognized by CD4+ helper T cells.
- Melanocytic differentiation protein (MDP):
Protein expressed by melanocytes and by melanoma cells, typically functioning as part of the melanin synthetic pathway. Examples include tyrosinase, gp100, and MART-1/MelanA.
- Th1:
A categorization of helper (CD4+) T-cell responses, manifested typically by production of cytokines, including interferon gamma, interleukin-2, and tumor necrosis factor α, and with functional importance in supporting generation of cytotoxic T-cell responses.
- Th2:
A categorization of helper (CD4+) T-cell responses, manifested typically by production of cytokines, including interleukin-4, interleukin-5, and interleukin-10, and with functional importance in supporting generation of B-cell and antibody responses.
Footnotes
Supported by Grants No. NIH R01 CA118386 from the National Institutes of Health/National Cancer Institute (C.L.S); No. NIH/NCI P30 CA44579 from the University of Virginia (UVA) Cancer Center Support Clinical Trials Office, Biorepository and Tissue Research Facility, Flow Cytometry Core, and Biomolecular Core Facility; No. NIH M01 RR00847 from the UVA General Clinical Research Center; and by philanthropic support from the Commonwealth Foundation for Cancer Research, Alice and Bill Goodwin, Frank and Jane Batten, the James and Rebecca Craig Foundation, George S. Suddock, Richard and Sherry Sharp, and the Patients and Friends Research Fund of the UVA Cancer Center.
Presented in part at the 24th Annual Meeting of the International Society for the Biological Therapy of Cancer, Washington, DC, October 29-30, 2009.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00118274.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Craig L. Slingluff Jr, GlaxoSmithKline (C), immatics biotechnologies (C); Merrick I. Ross, Genentech (C), GlaxoSmithKline (C) Stock Ownership: None Honoraria: Merrick I. Ross, Genentech, GlaxoSmithKline Research Funding: Craig L. Slingluff Jr, GlaxoSmithKline Expert Testimony: None Other Remuneration: Craig L. Slingluff Jr, GlaxoSmithKline
AUTHOR CONTRIBUTIONS
Conception and design: Craig L. Slingluff Jr, Gina R. Petroni, Kimberly A. Chianese-Bullock, William W. Grosh
Financial support: Craig L. Slingluff Jr.
Administrative support: Craig L. Slingluff Jr, Kimberly A. Chianese-Bullock, William W. Grosh
Provision of study materials or patients: Craig L. Slingluff Jr, Merrick I. Ross, Naomi B. Haas, Margaret von Mehren, William W. Grosh
Collection and assembly of data: Craig L. Slingluff Jr, Merrick I. Ross, Naomi B. Haas, Margaret von Mehren
Data analysis and interpretation: Craig L. Slingluff Jr, Gina R. Petroni, Kimberly A. Chianese-Bullock, Mark E. Smolkin
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Hunder NN, Wallen H, Cao J, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn M, Sugawara H, McGowan P, et al. CD4+ T cell clones specific for the human p97 melanoma-associated antigen can eradicate pulmonary metastases from a murine tumor expressing the p97 antigen. J Immunol. 1991;146:3235–3241. [PubMed] [Google Scholar]
- 3.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kayaga J, Souberbielle BE, Sheikh N, et al. Anti-tumour activity against B16–F10 melanoma with a GM-CSF secreting allogeneic tumour cell vaccine. Gene Ther. 1999;6:1475–1481. doi: 10.1038/sj.gt.3300961. [DOI] [PubMed] [Google Scholar]
- 5.Weiss WR, Sedegah M, Berzofsky JA, et al. The role of CD4+ T cells in immunity to malaria sporozoites. J Immunol. 1993;151:2690–2698. [PubMed] [Google Scholar]
- 6.Hung K, Hayashi R, Lafond-Walker A, et al. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsui S, Ahlers JD, Vortmeyer AO, et al. A model for CD8+ CTL tumor immunosurveillance and regulation of tumor escape by CD4 T cells through an effect on quality of CTL. J Immunol. 1999;163:184–193. [PubMed] [Google Scholar]
- 8.Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol. 1998;10:588–594. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 9.Lichterfeld M, Kaufmann DE, Yu XG, et al. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J Exp Med. 2004;200:701–712. doi: 10.1084/jem.20041270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phan GQ, Touloukian CE, Yang JC, et al. Immunization of patients with metastatic melanoma using both class I- and class II-restricted peptides from melanoma-associated antigens. J Immunother. 2003;26:349–356. doi: 10.1097/00002371-200307000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong R, Lau R, Chang J, et al. Immune responses to a class II helper peptide epitope in patients with stage III/IV resected melanoma. Clin Cancer Res. 2004;10:5004–5013. doi: 10.1158/1078-0432.CCR-04-0241. [DOI] [PubMed] [Google Scholar]
- 12.Knutson KL, Schiffman K, Disis ML. Immunization with a HER-2/neu helper peptide vaccine generates HER-2/neu CD8 T-cell immunity in cancer patients. J Clin Invest. 2001;107:477–484. doi: 10.1172/JCI11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slingluff CL, Jr, Yamshchikov G, Neese P, et al. Phase I trial of a melanoma vaccine with gp100(280–288) peptide and tetanus helper peptide in adjuvant: Immunologic and clinical outcomes. Clin Cancer Res. 2001;7:3012–3024. [PubMed] [Google Scholar]
- 14.Slingluff CL, Jr, Petroni GR, Yamshchikov GV, et al. Clinical and immunologic results of a randomized phase II trial of vaccination using four melanoma peptides either administered in granulocyte-macrophage colony-stimulating factor in adjuvant or pulsed on dendritic cells. J Clin Oncol. 2003;21:4016–4026. doi: 10.1200/JCO.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Slingluff CL, Jr, Petroni GR, Yamshchikov GV, et al. Immunologic and clinical outcomes of vaccination with a multiepitope melanoma peptide vaccine plus low-dose interleukin-2 administered either concurrently or on a delayed schedule. J Clin Oncol. 2004;22:4474–4485. doi: 10.1200/JCO.2004.10.212. [DOI] [PubMed] [Google Scholar]
- 16.Slingluff CL, Jr, Petroni GR, Chianese-Bullock KA, et al. Immunologic and clinical outcomes of a randomized phase II trial of two multipeptide vaccines for melanoma in the adjuvant setting. Clin Cancer Res. 2007;13:6386–6395. doi: 10.1158/1078-0432.CCR-07-0486. [DOI] [PubMed] [Google Scholar]
- 17.Slingluff CL, Jr, Petroni GR, Olson WC, et al. Effect of granulocyte/macrophage colony-stimulating factor on circulating CD8+ and CD4+ T-cell responses to a multipeptide melanoma vaccine: Outcome of a multicenter randomized trial. Clin Cancer Res. 2009;15:7036–7044. doi: 10.1158/1078-0432.CCR-09-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novellino L, Castelli C, Parmiani G. A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer Immunol Immunother. 2005;54:187–207. doi: 10.1007/s00262-004-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slingluff CL, Jr, Petroni GR, Olson W, et al. Helper T-cell responses and clinical activity of a melanoma vaccine with multiple peptides from MAGE and melanocytic differentiation antigens. J Clin Oncol. 2008;26:4973–4980. doi: 10.1200/JCO.2008.17.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berd D, Maguire HC, Jr, Mastrangelo MJ. Induction of cell-mediated immunity to autologous melanoma cells and regression of metastases after treatment with a melanoma cell vaccine preceded by cyclophosphamide. Cancer Res. 1986;46:2572–2577. [PubMed] [Google Scholar]
- 21.Berd D, Maguire HC, Jr, Mastrangelo MJ. Potentiation of human cell-mediated and humoral immunity by low-dose cyclophosphamide. Cancer Res. 1984;44:5439–5443. [PubMed] [Google Scholar]
- 22.Proietti E, Greco G, Garrone B, et al. Importance of cyclophosphamide-induced bystander effect on T cells for a successful tumor eradication in response to adoptive immunotherapy in mice. J Clin Invest. 1998;101:429–441. doi: 10.1172/JCI1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machiels JP, Reilly RT, Emens LA, et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–3697. [PubMed] [Google Scholar]
- 24.Sahasrabudhe DM, deKernion JB, Pontes JE, et al. Specific immunotherapy with suppressor function inhibition for metastatic renal cell carcinoma. J Biol Response Mod. 1986;5:581–594. [PubMed] [Google Scholar]
- 25.Matar P, Rozados VR, Gervasoni SI, et al. Th2/Th1 switch induced by a single low dose of cyclophosphamide in a rat metastatic lymphoma model. Cancer Immunol Immunother. 2002;50:588–596. doi: 10.1007/s00262-001-0237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matar P, Rozados VR, González AD, et al. Mechanism of antimetastatic immunopotentiation by low-dose cyclophosphamide. Eur J Cancer. 2000;36:1060–1066. doi: 10.1016/s0959-8049(00)00044-7. [DOI] [PubMed] [Google Scholar]
- 27.Schiavoni G, Mattei F, Di Pucchio T, et al. Cyclophosphamide induces type I interferon and augments the number of CD44(hi) T lymphocytes in mice: Implications for strategies of chemoimmunotherapy of cancer. Blood. 2000;95:2024–2030. [PubMed] [Google Scholar]
- 28.Awwad M, North RJ. Cyclophosphamide (Cy)-facilitated adoptive immunotherapy of a Cy-resistant tumour: Evidence that Cy permits the expression of adoptive T-cell mediated immunity by removing suppressor T cells rather than by reducing tumour burden. Immunology. 1988;65:87–92. [PMC free article] [PubMed] [Google Scholar]
- 29.Berd D, Mastrangelo MJ. Effect of low dose cyclophosphamide on the immune system of cancer patients: Depletion of CD4+, 2H4+ suppressor-inducer T-cells. Cancer Res. 1988;48:1671–1675. [PubMed] [Google Scholar]
- 30.Hoon DS, Foshag LJ, Nizze AS, et al. Suppressor cell activity in a randomized trial of patients receiving active specific immunotherapy with melanoma cell vaccine and low dosages of cyclophosphamide. Cancer Res. 1990;50:5358–5364. [PubMed] [Google Scholar]
- 31.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155:1063–1074. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berd D, Maguire HC, Jr, Mastrangelo MJ. Impairment of concanavalin A-inducible suppressor activity following administration of cyclophosphamide to patients with advanced cancer. Cancer Res. 1984;44:1275–1280. [PubMed] [Google Scholar]
- 33.Emens LA, Asquith JM, Leatherman JM, et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: A chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009;27:5911–5918. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 35.Slingluff CL, Petroni GR, Smolkin ME, et al. Immunogenicity for CD8+ and CD4+ T cells of 2 formulations of an incomplete Freund's adjuvant for multipeptide melanoma vaccines. J Immunother. 2010;33:630–638. doi: 10.1097/CJI.0b013e3181e311ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chianese-Bullock KA, Irvin WP, Jr, Petroni GR, et al. A multipeptide vaccine is safe and elicits T-cell responses in participants with advanced stage ovarian cancer. J Immunother. 2008;31:420–430. doi: 10.1097/CJI.0b013e31816dad10. [DOI] [PubMed] [Google Scholar]
- 37.Currier JR, Kuta EG, Turk E, et al. A panel of MHC class I restricted viral peptides for use as a quality control for vaccine trial ELISPOT assays. J Immunol Methods. 2002;260:157–172. doi: 10.1016/s0022-1759(01)00535-x. [DOI] [PubMed] [Google Scholar]
- 38.Higano CS, Schellhammer PF, Small EJ, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 39.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartzentruber DJ, Lawson D, Richards J, et al. A phase III multi-institutional randomized study of immunization with the gp100:209-217(210M) peptide followed by high-dose IL-2 compared with high-dose IL-2 alone in patients with metastatic melanoma. J Clin Oncol. 2009;27(suppl):463s. abstr CRA9011. [Google Scholar]
- 41.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slingluff CL, Jr, Lee SJ, Chianese-Bullock KA, et al. First report of a randomized phase II trial of multi-epitope vaccination with melanoma peptides for cytotoxic T cells and helper T cells in patients with metastatic melanoma: An Eastern Cooperative Oncology Group Study (E1602) J Clin Oncol. 2010;28(suppl):613s. abstr 8508. [Google Scholar]
- 43.Overwijk W, Hailemichael Y, Dai Z, et al. Peptide/incomplete Freund adjuvant emulsion depots are a graveyard for tumor antigen-specific CD8+ T cells. J Immunother. 2009;32:971. [Google Scholar]
- 44.Salem ML, Kadima AN, El-Naggar SA, et al. Defining the ability of cyclophosphamide preconditioning to enhance the antigen-specific CD8+ T-cell response to peptide vaccination: Creation of a beneficial host microenvironment involving type I IFNs and myeloid cells. J Immunother. 2007;30:40–53. doi: 10.1097/01.cji.0000211311.28739.e3. [DOI] [PubMed] [Google Scholar]
- 45.Ercolini AM, Ladle BH, Manning EA, et al. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med. 2005;201:1591–1602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu JY, Wu Y, Zhang XS, et al. Single administration of low dose cyclophosphamide augments the antitumor effect of dendritic cell vaccine. Cancer Immunol Immunother. 2007;56:1597–1604. doi: 10.1007/s00262-007-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+ CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 48.Audia S, Nicolas A, Cathelin D, et al. Increase of CD4+ CD25+ regulatory T cells in the peripheral blood of patients with metastatic carcinoma: A Phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4+ CD25+ T lymphocytes. Clin Exp Immunol. 2007;150:523–530. doi: 10.1111/j.1365-2249.2007.03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.