Introduction
The insulin-like growth factor-1 receptor (IGFR1) is frequently overexpressed in a broad range of tumors. Its signaling has been shown to be involved in tumorigenesis and metastatic potential of multiple cancers.1 Drugs targeting this pathway began to be developed in the late 1990s, including monoclonal antibodies to IGFR1.2 Cixutumumab (IMC-A12; ImClone Systems, Bridgewater, NJ), a fully humanized monoclonal IgG1 antibody, binds to IGFR1 with high affinity, inhibiting its activation.3
Because IGFR1-targeted therapy is relatively new, the potential toxicities are not fully understood. IGF-1 has many physiologic roles that could potentially be compromised by the inhibition of its receptor. One of these roles is to enhance epidermal proliferative potential as well as keratinocyte cell migration.4 Therefore, potential dermatologic adverse effects can be anticipated when IGFR1 function is inhibited. An in-depth review of the literature did not reveal any previous reports that have addressed this issue. In this article, we describe four patients who were enrolled onto clinical trials using cixutumumab and subsequently developed skin and nail abnormalities.
Case Reports
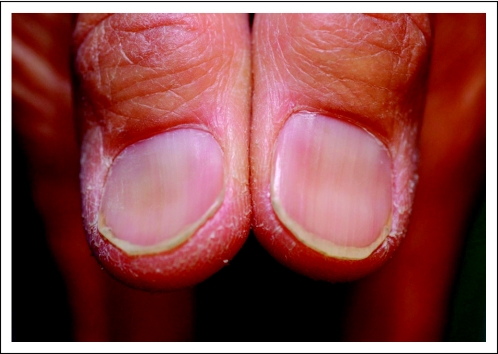
A 59-year-old Asian man known to have chronic hepatitis B had a resected, well-differentiated hepatocellular carcinoma and two local recurrences that were treated with bland transarterial embolization. When his disease progressed, however, the patient was enrolled onto a clinical trial evaluating cixutumumab in patients with advanced hepatocellular carcinoma (A Phase II Study of IMC-A12 [NSC742460] in Hepatocellular Carcinoma). The patient tolerated therapy well, and continued with stable disease for more than 2 years. The patient noted brittle nails and axillary hair thinning about 14 months after the start of therapy. Evaluation by a dermatologist revealed grade 1 koilonychia (Fig 1), xerosis, and body hair thinning. Laboratory parameters revealed that total iron (Fe) and total iron-binding capacity (TIBC) were elevated to 224 mcg/dL and 524 mcg/dL, respectively, whereas the transferrin saturation index was normal at 43%. These results are best explained by the fact that the patient was already receiving iron sulfate supplementation. The patient continues to receive cixutumumab.
Fig 1.
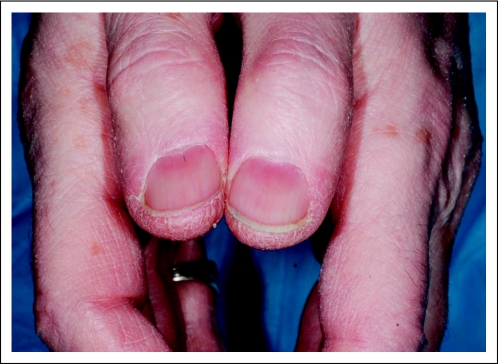
A 77-year-old man was diagnosed at age 63 years with adenocarcinoma of the prostate (Gleason score 8) with a pretreatment prostate-specific antigen of 28 ng/mL and lymph node involvement. After treatment with gonadotropin-releasing hormone agonist and multiple lines of antiandrogens, the cancer was deemed refractory to hormone therapy. The patient was enrolled onto successive clinical trials, during which he incurred repeated biochemical relapse, until he joined a clinical trial that combined cixutumumab and temsirolimus (Phase I/II Trial of Anti-IGF-IR Monoclonal Antibody IMC-A12 Plus mTOR Inhibitor Temsirolimus [CCI-779] in Metastatic Castration- Resistant Prostate Cancer). Three months after the start of this therapy, the patient developed a pruritic rash across the flanks and subtle nail changes. Evaluation by a dermatologist revealed grade 1 xerosis and grade 1 koilonychia (Fig 2). The patient's iron studies were all within normal reference ranges (Fe, 107 mcg/dL; TIBC, 349 mcg/dL; transferrin saturation index, 31%). The patient has not received any iron supplementation and remains enrolled onto the study at this time.
Fig 2.
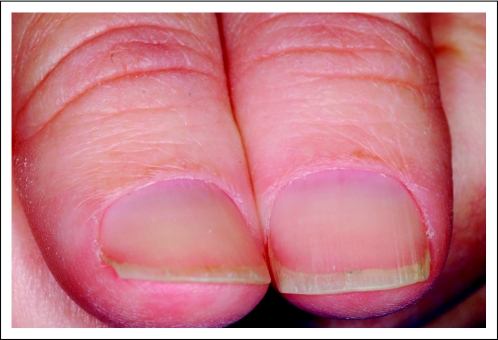
A 48-year-old woman was diagnosed at age 43 years with leiomyosarcoma involving the distal left fibula, for which she underwent wide excision. Two years later, the patient was found to have lung metastases and then bone metastases, for which she was treated with different lines of chemotherapy and palliative radiation to the sites of bone metastases. On additional progression of disease, the patient was enrolled onto a clinical trial evaluating the combination of temsirolimus and cixutumumab (A Phase II Study of Temsirolimus [CCI-779, NSC 683864] and IGF-1 Receptor Antibody Cixutumumab [IMC-A12, NSC 742460] in Patients With Metastatic Sarcomas). Three months after the start of therapy, the patient began experiencing dermatologic problems including paronychia, loss of cuticles, dry skin, pruritus, and decreased hair growth. Evaluation by a dermatologist revealed grade 2 paronychia and grade 2 xerosis (Fig 3). The patient was also evaluated for microcytic anemia and was found to have an iron deficiency (Fe, 27 mcg/dL; TIBC, 373 mcg/dL; transferrin saturation index, 7%) that was subsequently treated with intravenous iron administration. Five months after enrollment onto the trial, the patient was found to have a new brain metastasis for which she underwent resection and radiation therapy. The disease ultimately progressed and the patient died as a result of her illness.
Fig 3.
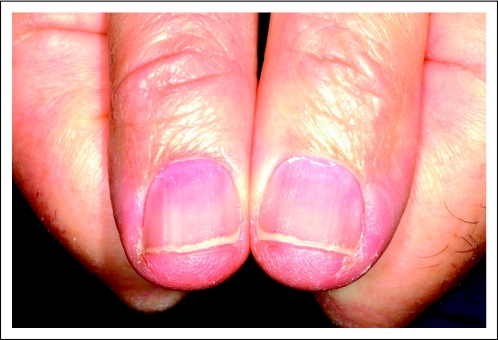
A 60-year-old man was diagnosed at age 36 years with chondrosarcoma of the sternum for which he underwent partial sternal resection with bone graft reconstruction. At age 52, he was found to have bilateral calcified lung nodules. Expectant management was chosen given the indolent nature of the disease. Five years later, enlargement of the lung nodules was noted along with new bony lesions in multiple sites. The latter were treated palliatively. At age 59, the patient was enrolled onto a randomized trial of the tyrosine kinase inhibitor brivanib versus a placebo, but the disease progressed. The patient, whose tumor tested positive for IGFR1 expression, subsequently enrolled onto the same clinical trial of cixutumumab and temsirolimus as the third patient described (the 48-year-old woman originally diagnosed with leiomyosarcoma). A rash developed over the back and chest approximately 3 weeks after the start of therapy. Evaluation by a dermatologist revealed a grade 2 rash consisting of multiple papules and pustules. A punch biopsy was obtained that showed culture-negative suppurative folliculitis. Treatment consisted of doxycycline at a dose of 100 mg twice per day and steroid cream, and significant clinical improvement was observed. The patient was also found to have grade 1 xerosis and grade 2 nail abnormalities that consisted of flattened nails with subungual proximal dyschromia and paronychia (Fig 4). The patient's iron studies were all within normal reference ranges (Fe, 66 mcg/dL; TIBC, 337 mcg/dL; transferrin saturation index, 20%). The patient has not received any iron supplementation and remains enrolled onto the study at this time. He required a nail avulsion because of progressive paronychia despite conservative measures.
Fig 4.
Discussion
IGFR1 is an attractive treatment target for multiple neoplasms.1,5 Inhibition of its action can be achieved by monoclonal antibodies, tyrosine kinase inhibitors, and small interfering RNA molecules.6,7
Cixutumumab binds avidly to IGFR1 and inhibits its activation and downstream signaling by mediating internalization and degradation of this receptor. In addition to being a mitogen, IGF-1 is a mediator of the growth-promoting action of growth hormone, and a metabolic regulator with insulin-like activity; inhibiting it would theoretically lead to many adverse effects including dermatologic effects.8
In vitro, IGFR1 was found to enhance epidermal proliferative potential as well as keratinocyte migration.4 Therefore, dermatologic adverse effects can be anticipated when IGFR1 function is inhibited. IGF-1 is locally produced by dermal papilla cells. It interacts with its receptor, which is predominantly expressed in basal keratinocytes, leading to regulation of the progenitor cell population responsible for the regeneration of the epidermis.9 Genetic experiments showed that eliminating IGFR1 in skin results in a disrupted epidermis wherein keratinocytes exhibit accelerated differentiation and decreased proliferation.10 Moreover, in vitro skin cultures treated with anti-IGFR1 antibodies show focal necrotic areas; this suggests that IGFR1 has a critical role in cell survival.11
These experimental findings shed some light on a number of disease states. Skin sections from patients with diabetes showed absent expression of IGF-1 within the basal layer and fibroblasts. This was found to contribute to the defective wound healing seen in patients with diabetes mellitus.12 Moreover, a rare autosomal recessive hereditary disease called pycnodysostosis is associated with a decrease in circulating IGF-1 levels and a phenotype that includes short stature, bone abnormalities, delayed eruption of teeth, and dysplastic nails.8,13
In view of these pathologic entities, it is reasonable to believe that the inhibition of IGFR1 using cixutumumab in the patients described created an acquired form of disease that affected the hair and nails, in particular. Other causes of koilonychia should be explored and excluded before this hypothesis can be endorsed. The single most important factor associated with koilonychia is iron deficiency anemia.14 Three of the four patients had normal iron studies. Koilonychia has also been reported in other states: idiopathic, hereditary, traumatic, or related to fungal infection, psoriasis, or even polycythemia vera.15,16 None of these inciting factors were present in our patients.
In three of the four patients, a combination of cixutumumab with mammalian target of rapamycin (mTOR) inhibitor was used. This raises the question of a possible interaction between the two classes of drugs, be it a simple additive effect or a potentiation. In fact, mTOR inhibitors have been independently linked to various dermatologic toxicities. In a recent review of the subject,17 the cutaneous and mucosal effects associated with mTOR inhibitors included acne-like dermatitis, pruritus, exanthema, mouth ulcers, and onychopathy. Types of onychopathy included longitudinal ridging, distal onycholysis, splinter hemorrhages, transverse leukonychia, and periungual disorders. These adverse dermatologic events were dose dependent and most were reversible.17 In light of these findings, an interaction between IGFR1 and mTOR inhibitors that contributes to the described dermatologic manifestations cannot be ruled out. Combination studies are needed to establish the validity of such an interaction.
In conclusion, to our knowledge, the case reports presented provide the first clinical evidence that supports the risk of dermatologic toxicity when using a drug that inhibits IGFR1. However, the experience with such investigational drugs is still limited, and studies in larger patient populations will be needed to establish the true spectrum and rate of adverse events (especially low-frequency events) associated with this class of agents. Therefore, awareness of the potential dermatologic adverse effects is important, but additional studies and longer follow-up are needed to know the clinical value and the need for dermatologic examination in patients who are being treated with inhibitors of IGFR1.
ACKNOWLEDGMENT
Supported by Grant No. 3U01CA069856-15S1, National Cancer Institute (NCI) ACTNOW funding award, Early Clinical Trials of New Anti-Cancer Agents With Phase I Emphasis (D.R.), NCI Grants No. CA148260, CA008747, American Society of Clinical Oncology Foundation-NCI Clinical Team Leader Award (R.G.M.), and Grant No. NO1-CM62206, NCI Phase II Contract (Memorial Sloan-Kettering Cancer Center; G.K.A., R.G.M.)
Footnotes
Clinical trial information can be found for the following: NCT00639509.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Mario E. Lacouture, ImClone Systems (C), Pfizer (C); Robert G. Maki, Pfizer (C); Ghassan K. Abou-Alfa, ImClone Systems (C) Stock Ownership: None Honoraria: None Research Funding: Ghassan K. Abou-Alfa, ImClone Systems Expert Testimony: None Other Remuneration: None
REFERENCES
- 1.Tao Y, Pinzi V, Bourhis J, et al. Mechanisms of disease: Signaling of the insulin-like growth factor 1 receptor pathway—Therapeutic perspectives in cancer. Nat Clin Pract Oncol. 2007;4:591–602. doi: 10.1038/ncponc0934. [DOI] [PubMed] [Google Scholar]
- 2.Gualberto A, Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: Early clinical trial results and future directions. Oncogene. 2009;28:3009–3021. doi: 10.1038/onc.2009.172. [DOI] [PubMed] [Google Scholar]
- 3.Rowinsky EK, Youssoufian H, Tonra JR, et al. IMC-A12, a human IgG1 monoclonal antibody to the insulin-like growth factor I receptor. Clin Cancer Res. 2007;13(suppl 18):5549s–5555s. doi: 10.1158/1078-0432.CCR-07-1109. [DOI] [PubMed] [Google Scholar]
- 4.Hyde C, Hollier B, Anderson A, et al. Insulin-like growth factors (IGF) and IGF-binding proteins bound to vitronectin enhance keratinocyte protein synthesis and migration. J Invest Dermatol. 2004;122:1198–1206. doi: 10.1111/j.0022-202X.2004.22527.x. [DOI] [PubMed] [Google Scholar]
- 5.Riedemann J, Macaulay VM. IGF1R signalling and its inhibition. Endocr Relat Cancer. 2006;13(suppl 1):S33–S43. doi: 10.1677/erc.1.01280. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Ji QS, Mulvihill M, et al. Inhibition of the IGF-I receptor for treatment of cancer: Kinase inhibitors and monoclonal antibodies as alternative approaches. Recent Results Cancer Res. 2007;172:59–76. doi: 10.1007/978-3-540-31209-3_5. [DOI] [PubMed] [Google Scholar]
- 7.Bohula EA, Playford MP, Macaulay VM. Targeting the type 1 insulin-like growth factor receptor as anti-cancer treatment. Anticancer Drugs. 2003;14:669–682. doi: 10.1097/00001813-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Soliman AT, Ramadan MA, Sherif A, et al. Pycnodysostosis: Clinical, radiologic, and endocrine evaluation and linear growth after growth hormone therapy. Metabolism. 2001;50:905–911. doi: 10.1053/meta.2001.24924. [DOI] [PubMed] [Google Scholar]
- 9.Stachelscheid H, Ibrahim H, Koch L, et al. Epidermal insulin/IGF-1 signalling control interfollicular morphogenesis and proliferative potential through Rac activation. EMBO J. 2008;27:2091–2101. doi: 10.1038/emboj.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadagurski M, Yakar S, Weingarten G, et al. Insulin-like growth factor 1 receptor signaling regulates skin development and inhibits skin keratinocyte differentiation. Mol Cell Biol. 2006;26:2675–2687. doi: 10.1128/MCB.26.7.2675-2687.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tavakkol A, Varani J, Elder JT, et al. Maintenance of human skin in organ culture: Role for insulin-like growth factor-1 receptor and epidermal growth factor receptor. Arch Dermatol Res. 1999;291:643–651. doi: 10.1007/s004030050469. [DOI] [PubMed] [Google Scholar]
- 12.Blakytny R, Jude EB, Martin Gibson J, et al. Lack of insulin-like growth factor 1 (IGF1) in the basal keratinocyte layer of diabetic skin and diabetic foot ulcers. J Pathol. 2000;190:589–594. doi: 10.1002/(SICI)1096-9896(200004)190:5<589::AID-PATH553>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 13.Rothenbühler A, Piquard C, Gueorguieva I, et al. Near normalization of adult height and body proportions by growth hormone in pycnodysostosis. J Clin Endocrinol Metab. 2010;95:2827–2831. doi: 10.1210/jc.2009-2531. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi T, Yamashita K, Hatao K. Incidence of koilonychia and atrophy of the lingual papillae in a patient with iron-deficiency anemia. Int J Hematol. 2010;91:161–162. doi: 10.1007/s12185-010-0505-0. [DOI] [PubMed] [Google Scholar]
- 15.Kumar G, Vaidyanathan L, Stead LG. Images in emergency medicine: Koilonychia, or spoon-shaped nails, is generally associated with iron-deficiency anemia. Ann Emerg Med. 2007;49:243, 250. doi: 10.1016/j.annemergmed.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Stone OJ. Clubbing and koilonychia. Dermatol Clin. 1985;3:485–490. [PubMed] [Google Scholar]
- 17.Campistol JM, de Fijter JW, Flechner SM, et al. mTOR inhibitor-associated dermatologic and mucosal problems. Clin Transplant. 2010;24:149–156. doi: 10.1111/j.1399-0012.2010.01232.x. [DOI] [PubMed] [Google Scholar]