Abstract
Aim
Chromium and cysteine supplementation can improve glucose metabolism in animal studies. This study examined the hypothesis that a cysteinate complex of chromium is significantly beneficial than either of them in lowering blood glucose and vascular inflammation markers in ZDF rats.
Methods
Starting at the age of 6 wks, ZDF rats were supplemented orally (daily gavages for 8 more wks) with saline-placebo (D) or chromium (400µg Cr/KgBW) as chromium-dinicocysteinate (CDNC), chromium-dinicotinate (CDN), or chromium-picolinate (CP) or equimolar L-cysteine (LC, img/Kg BW), and fed Purina 5008 diet for 8 wks. ZDF rats of 6 wks age before any supplementations and onset of diabetes were considered as baseline (BL).
Results
D rats showed elevated levels of fasting blood glucose, HbA1, CRP, MCP-1, ICAM-1 and oxidative stress (LP) and lower adiponectin and vitamin C, when compared to BL rats. In comparison to D group, CDNC group had significantly lower blood glucose, HbA1, CRP, MCP-1, ICAM-1 and LP and increased vitamin C and adiponectin levels. CDN, CP or LC showed significantly less or no effect on these biomarkers. Only CDNC lowered blood creatinine levels in comparison to D. While CDN and CP had no effect, activation of NFkB, Akt and GLUT-2 levels were decreased, IRS-1 activation increased in livers of CDNC-rats. CDNC effect on glycemia, NFkB, Akt and IRS-1 in liver was significantly greater compared with LC. Blood chromium levels did not differ between Cr-groups. Exogenous vitamin C supplementation significantly inhibited MCP-1 secretion in U937 monocytes cultured in high-glucose-medium.
Conclusions
CDNC is a potent hypoglycemic compound with anti-inflammatory activity apparently mediated by elevated blood vitamin C and adiponectin and inhibition of NFkB, Akt, and Glut-2 and increased IRS-1 activation in livers of type 2 diabetic rats.
Keywords: Chromium, glycemia, oxidative stress, CRP, MCP-1, ICAM-1, vitamin C, diabetes, vascular inflammation, L-cysteine
Introduction
Intensive blood glucose control dramatically reduces the devastating complications that result from poorly controlled diabetes (1, 2). While diabetes is treated with diet and insulin administration, for many patients, achieving tight glucose control is difficult with current regimens. Thus, development of a novel adjuvant therapy is needed to achieve better control of glycemia in diabetic patients.
Elevated levels of oxidative stress can potentially impair cellular glucose metabolism via a variety of mechanisms, including redox imbalance and insulin resistance (2, 3). N-acetylcysteine (NAC) and other cysteine containing compounds have been shown to lower oxidative stress in diabetic animal models (4–6). NAC supplementation in drinking water and naturally formed cysteine containing compounds in garlic and onion plants can lower blood glucose levels in streptozotocin-treated diabetic mice (4). On the other hand, in some studies chromium supplementation lowered blood levels of glucose and glycosylated hemoglobin and oxidative stress in diabetic animals and humans (7–15). This study tested the hypothesis that a new compound, chromium dinicocysteinate (CDNC), complex of chromium and L-cysteine is more efficacious than chromium dinicotinate or chromium picolinate (CP) supplementation in the improvement of glycemic control and reduction of vascular inflammation in diabetes using Zucker diabetic fatty (ZDF) rat model. This study reports that cysteinate form of chromium is significantly more efficacious compared with other chromium compounds in improving glycemia as assessed by blood glucose and glycated hemoglobin levels, and vascular inflammation as assessed by CRP, MCP-1, ICAM-1 and oxidative stress levels in ZDF rats, a model of type 2 diabetes. This effect of CDNC is apparently mediated by elevated blood levels of vitamin C and adiponectin and inhibition of NFkB, Akt, and Glut-2, and increased IRS-1 activation in the livers of ZDF rats.
Materials and Methods
All procedures followed were in accordance with the ethical standards of the institution, and approval was obtained from the institutional Animal Welfare Committee. Male Zucker diabetic rats were purchased at 5 weeks of age (200–220 g) from Charles River (Wilmington, MA) and allowed 2 days for environmental and trainer handling acclimation. The rats were housed and labeled in individual cages. Rats were assigned into various groups by computer generated randomization. Rats were fasted overnight, and then weighed. The rats were tested for hyperglycemia by measuring their blood glucose concentration. Blood for the blood glucose was obtained via tail incision and measured using an Advantage Accu-chek glucometer (Boehringer Mannheim Corp., Indianapolis, IN). Rats were gavaged with saline alone served as the diabetic control (D) group. Rats in other groups were respectively gavaged daily with 400 µg Cr (CrDNC)/kg body weight, 400 µg Cr (CrDN)/kg body weight or 400 µg Cr (CP)/kg body weight. Each rat received the appropriate dose of chromium daily for 8 weeks by oral gavage using 20G feeding needles (Popper and Sons, New Hyde Park, NY). Chromium dinicotinate (ChromeMate) and chromium dinicocysteinate were obtained from InterHealth Nutraceuticals, Inc (Benicia, CA). Chromium picolinate was obtained from Nutrition-21 (Purchase, NY). Chromium dinicotinate, chromium picolinate and chromium dinicocysteinate were obtained pure and solutions were mixed in normal saline. Each group of rats received the same dose of chromium supplementation, which was calculated based on the molecular weight and chromium content supplied on the label by the manufacturer. In additional experiments, rats were supplemented with placebo-saline (D), CDNC (400 µg Cr/kg BW) or LC (0.93 mg/Kg BW). Body weight and blood glucose concentrations were monitored weekly. The chromium or LC supplementation dose was adjusted every week according to any change in body weight to maintain a similar chromium dose per Kg BW of the rats over the entire period of the study for each group. The rats were maintained under standard housing conditions at 22 ± 2°C with 12:12-h light/dark cycles and fed with a Purina 5008 lab chow diet (Charles River, Wilmington, MA). Food intake was monitored on two separate occasions during the 8 week period to assess the consumption. At the end of 8 weeks the rats were fasted overnight then euthanized for analysis by exposure to halothane (2-bromo-2-chloro-1,1,1-trifluoroethane). Blood was collected via heart puncture with a 19½ gauge needle into EDTA vacutainer tubes. Plasma was isolated after centrifuging blood in a 4°C centrifuge at 1500 rpm for 10 minutes.
Treatment with high glucose (HG) and vitamin C
U937 (500,000 cells/ml) were treated with normal glucose (7 mM) and HG (35 mM) without and with vitamin C). Mannitol (35 mM) was used as an osmolarity control. For MCP-1 secretion studies, cells were treated at 37°C for 24 hrs. HG is known to stimulate the secretion of cytokines in monocyte cell model (16). In this study, cells were exposed to a high glucose concentration of 35 mM. Glucose concentrations as high as 50 mM have been found in the blood of patients with uncontrolled diabetes (17, 18). Thus, the glucose concentration of 35 mM used in this cell culture study is physiological. Vitamin C was prepared fresh for each experiment.
CRP, MCP-1, ICAM-1, lipid peroxidation, adiponectin and vitamin C
CRP, MCP-1, ICAM-1 and adiponectin levels in the plasma were determined by the sandwich ELISA method using commercially available kits from Fisher Thermo Scientific Co (Rockford, IL). MCP-1 in the supernatant of treated monocytes was determined by sandwich ELISA method kit obtained from R & D systems (Minneapolis, MN). All appropriate controls and standards as specified by the manufacturer’s kit were used. In the cytokine assay, control samples were analyzed each time to check the variation from plate to plate on different days of analysis. Lipid peroxidation was determined by measuring malondialdehyde (an end product of lipid peroxidation) by its reaction with thiobarbituric acid (13) and vitamin C by DTNB method (19).
Measurement of glycosylated hemoglobin (GHb) and glucose
Glycosylated hemoglobin was determined using Glyco-Tek Affinity column kits and reagents (cat# 5351) purchased from Helena Laboratories (Beaumont, TX). Glucose levels were determined using glucose oxidase measured with an Accu-check Advantage glucometer (Boehringer Manheim Corporation, Indianapolis, IN).
Liver function tests, blood cell count and blood chemistry profile
A portion of blood from rats in each group was sent to the clinical laboratory of LSUHSC-Shreveport (located in the same building) for clinical tests to determine liver and renal function and red blood cell counts. Chromium analyses were carried out by the Covance company (Madison, WI) using the elements by ICP mass spectrometry method (20) in plasma samples shipped on dry ice.
Western blotting analyses of liver homogenates
Tissues excised from the experimental rats were immediately frozen using liquid nitrogen, ground well into powders, and frozen at −70°C until further use. The frozen tissue powders (~150 mg) were resuspended in 1mL PBS containing protease inhibitors, vortexed and centrifuged at 15,000 rpm, 4°C for 10 min. The supernatants were discarded and the pellets washed once more as described above. Washed pellets were resuspended in 500µL extraction buffer (25mM Tris, 0.5mM EDTA, PMSF 0.1mM, pH 7.4) with protease inhibitors and dounce homogenized using a homogenizer and subjected to mild sonication. The tubes were centrifuged at 15,000 rpm (4°C, 30 min) and the supernatants collected. The collected supernatants were further subjected to centrifugation once more as described above and cleared of cell debris. The protein content of the supernatants was estimated using a BCA protein assay. Equal amounts of protein from each group were loaded onto SDS-polyacrylamide gel after boiling for 5 min with β-mercaptoethanol as a reducing agent. The separated proteins were transferred to a nitrocellulose membrane, blocked with 1% BSA in T-PBS (0.25% Tween-20 in PBS) and incubated overnight at 4°C with the respective primary antibodies. The next day, membranes were washed with T-PBS (8 mins, 4 cycles) and incubated with secondary antibodies conjugated with horseradish peroxidase (HRP) in 5% non-fat milk for 30 min at room temperature. The membranes were again washed with T-PBS (8 mins, 4 cycles), treated with chemiluminescence reagents for 2 min and exposed to X-ray films developed through autoradiography. A β-actin antibody was used to assess the loading equality.
p-Akt and p-NFkB antibodies were purchased from Santacruz biotechnologies (Santacruz, CA), T-Akt from Cell signaling (Danvers, MA), p-IRS-1 and T-IRS-1 from Millipore (Danvers, MA), while T-NFkB, GLUT-2 and b-actin antibodies were purchased from Abcam (Cambridge, MA). All the antibodies were rabbit polyclonal while b-actin is a mouse monoclonal antibody.
All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise mentioned. Data were analyzed statistically using one-way ANOVA among different groups using Sigma Stat 3.1 and Sigma Plot 8.02 software (Jandel Scientific, San Rafael, CA). When data passed a normality test, all groups were compared by using the Student-Newman-Keuls method. Kruskal-Wallis one way ANOVA on ranks (Dunn’s method) was used for pairwise multiple comparisons when data failed a normality test. A p value of less than 0.05 was considered significant.
Results
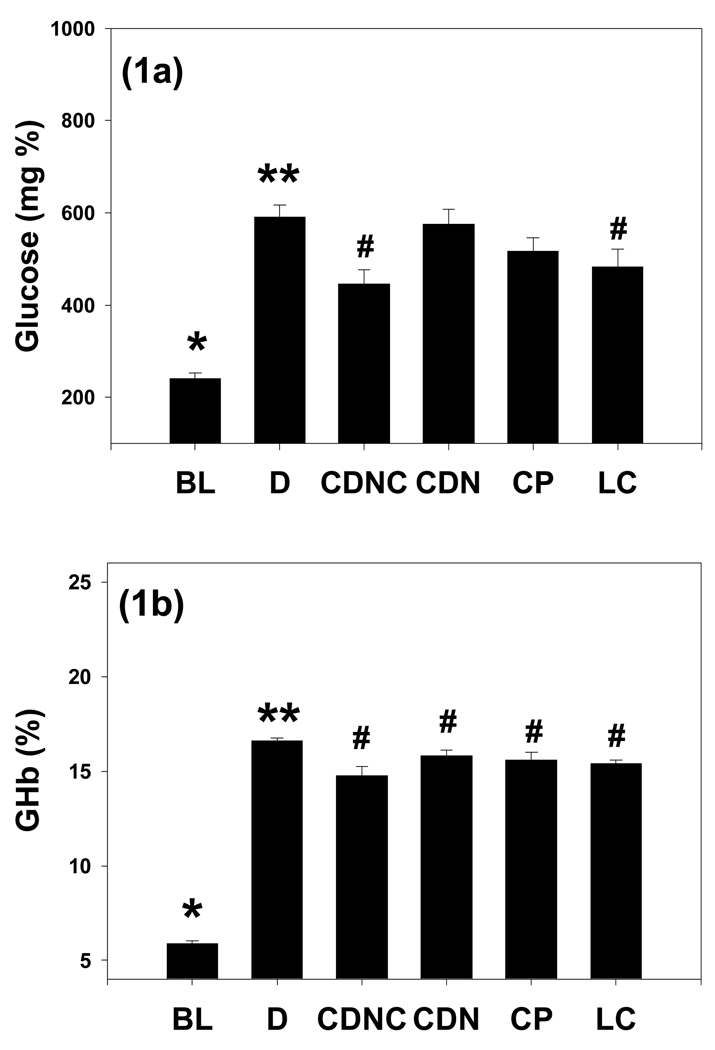
Figure 1 illustrates a significant increase in glucose levels in placebo-controls (D) compared with BL. CDNC and LC-supplemented rats showed significantly lower glucose levels compared with D. However, neither CDN nor CP had a significant effect on blood glucose in comparison to D. The CDNC group had significantly lower blood glucose levels compared with the CDN or CP groups. Similarly, CDNC and LC supplementation significantly lowered GHb levels compared with D. HbA1 levels in the CDN and CP groups were also lower compared with those of D. This suggests that among all the treatment groups, CDNC supplementation showed the most beneficial effect in decreasing glycemia levels.
Figure 1.
Effect of supplementation of chromium dinicocysteinate (CDNC), chromium dinicotinate (CDN), chromium picolinate (CP), or L-cysteine (LC) on blood glucose (a) and glycated hemoglobin (b) levels in ZDF rats. Values are Mean±SE. Rats were supplemented with saline (D, control (n=18), CDNC (n=12), CDN (n=12), CP (n=12) or LC (n=11) by gavages daily for 8 wks. BL (n=12): baseline value before the start of supplementation. Differences between values marked * versus**, **versus# are significant (p<0.05).
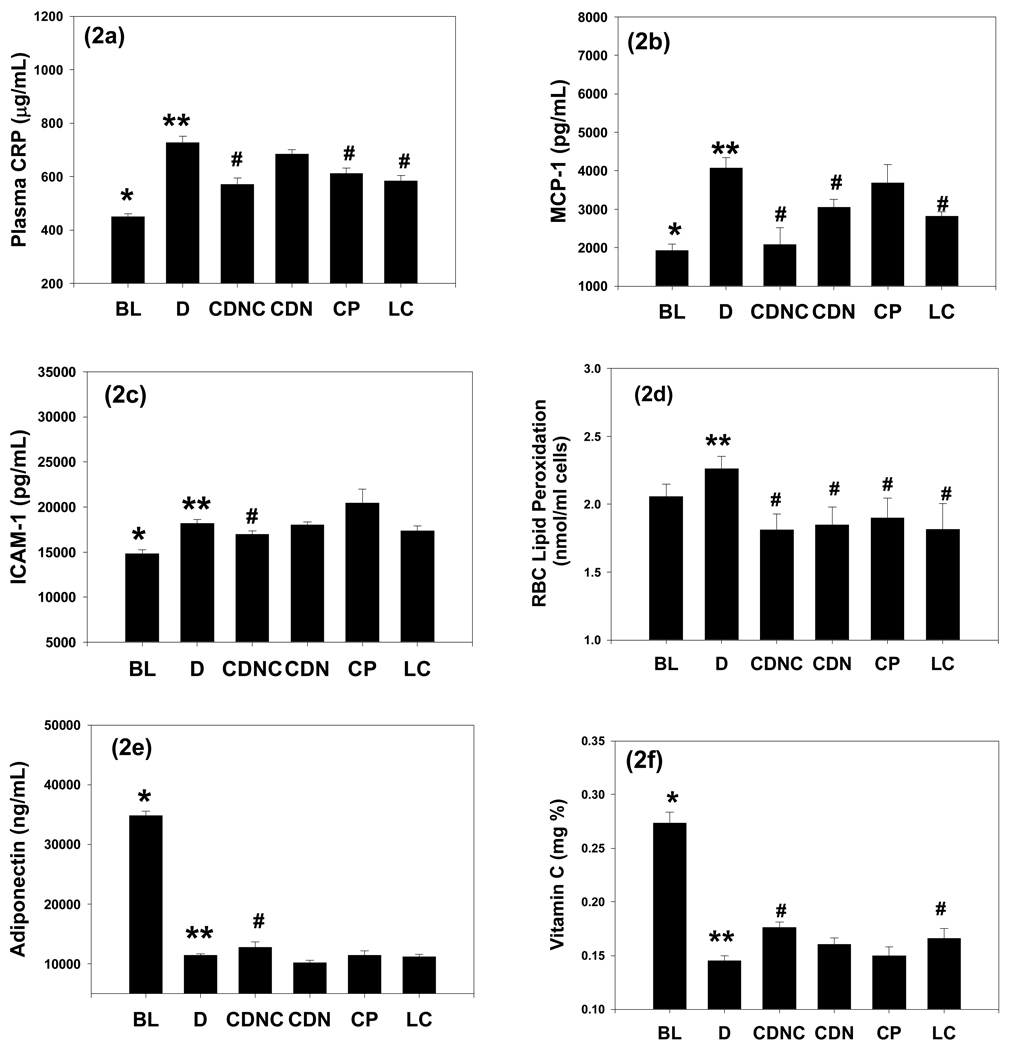
Figure 2 shows CRP, MCP-1, ICAM-1 and lipid peroxidation (LP) levels in blood at the beginning of treatment (baseline) and after supplementation with saline (D), CDN, CDNC, or CP in ZDF rats. There was a significant increase in CRP, MCP-1, and ICAM-1 levels in D compared with BL. CDNC and LC-supplemented rats showed significantly lower CRP and MCP-1 levels compared with D. CDN did have a significant effect on MCP-1 and of CP on CRP levels in comparison to D. Similarly, CDNC- supplemented rats showed significantly lower levels of ICAM-1 compared with those in the D group. However, ICAM-1 levels did not differ in either CDN or CP or LC compared with D group (figure 2c). Figure 2d illustrates that lipid peroxidation levels were lower in the CDNC, CDN or CP or LC groups compared with D. This suggests that compared to placebo (D), CDNC was most effective among all the treatment groups at lowering MCP-1, CRP, ICAM-1 and oxidative stress levels.
Figure 2.
Effect of supplementation of chromium dinicocysteinate (CDNC), chromium dinicotinate (CDN), chromium picolinate (CP) or L-cysteine (LC) on plasma CRP (a), MCP-1 (b), ICAM-1 (d) and lipid peroxidation (e), adiponectin, (f) vitamin C levels in ZDF rats. Abbreviations and number of rats in each group are as in figure 1. Differences between values marked * versus**, **versus# are significant (p<0.05).
Figure 2e illustrates blood levels of adiponectin at the beginning of treatment (baseline) and after supplementation with saline (control), CDNC or CDN or CP, or LC for 8 weeks in ZDF rats. There were significantly lower levels of adiponectin in controls compared with baseline. CDNC supplemented rats showed significantly increased levels compared with those in the D group. However, adiponectin levels did not differ in either CDN or LC compared with D group. This suggests that in comparison to the control group, CDNC supplementation was best among all the treatment groups in improving adiponectin levels in ZDF rats. There was a significant decrease in vitamin C levels in D compared with baseline (Figure 2f). CDNC and LC supplemented rats showed significant increase in vitamin C levels in comparison to D. However, CDN or CP supplementation showed no beneficial effect on vitamin C levels in comparison to D. These results suggest that among all the treatment groups, CDNC showed most increase in blood vitamin C levels in comparison to the D group.
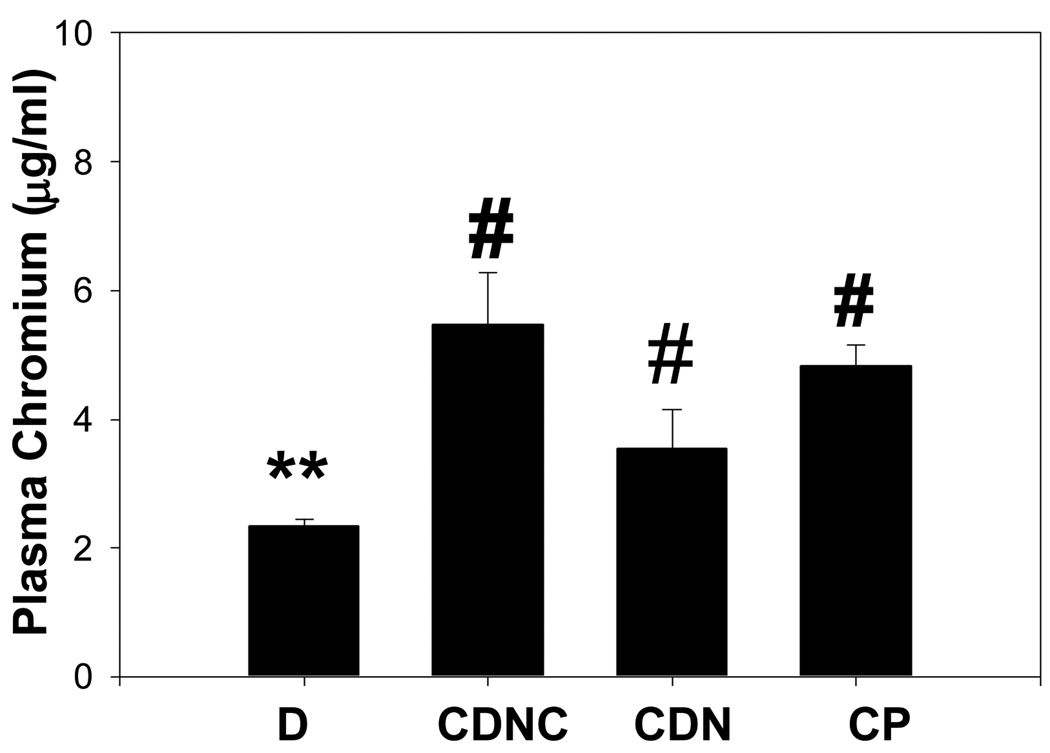
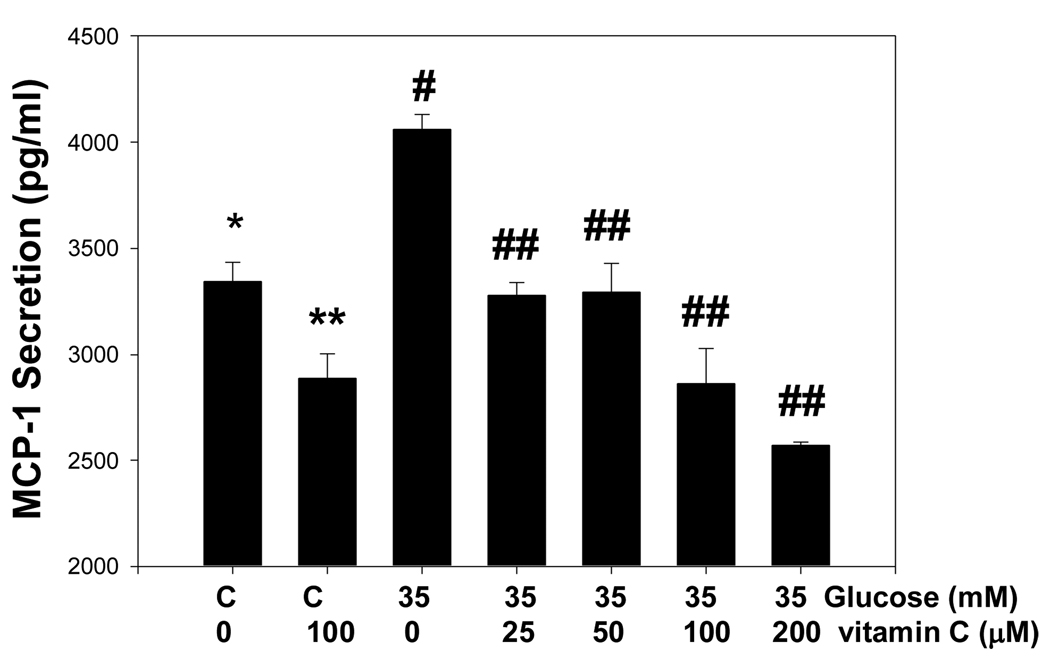
Figure 3 shows that plasma levels of chromium increased in the CDNC, CDN and CP supplemented groups compared with D; however, the plasma chromium levels were similar among the CDNC, CDN and CP groups. This suggests that the absorption of chromium is similar among the different chromium compounds used for supplementation. High glucose treatment caused significant stimulation of MCP-1 secretion in cultured monocytes (Figure 4). The MCP-1 secretion was significantly inhibited in cells supplemented with exogenous vitamin C, which suggests that elevated circulating vitamin C concentration may contribute to lowering of MCP-1 in CDNC supplemented rats. Mannitol did not have any effect on MCP-1 secretion (data not shown here).
Figure 3.
Plasma chromium levels in placebo (D, n=6) chromium dinicocysteinate (CDNC, n=6), chromium dinicotinate (CDN, n=6), and chromium picolinate (CP, n=6) supplementation in ZDF rats. Differences in values among ** vs # are significant (p<0.05).
Figure 4.
Effect of vitamin C supplementation on MCP-1 secretion by human monocytes cultured in high glucose medium for 24 hours at 37°C. Differences were significant between values *vs**, *vs#, #vs##.
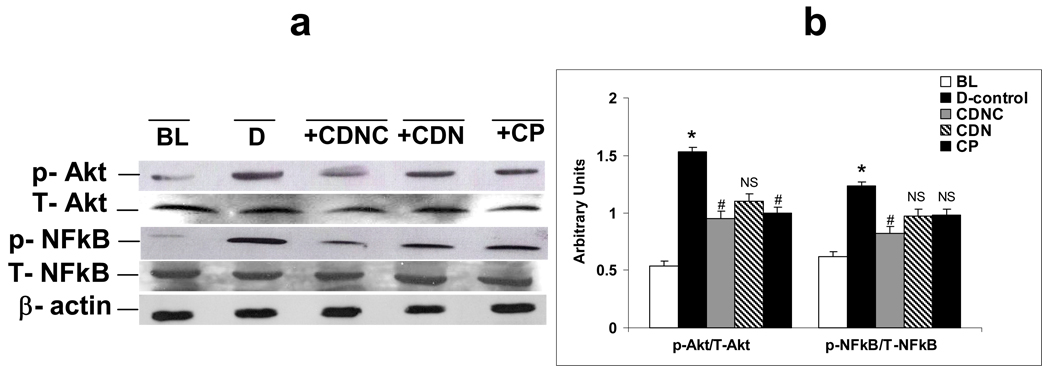
Figures 5a and 5b show phospho (Ser 473) and total Akt, phospho (Ser 276) and total NFkB levels in liver homogenates of control and experimental rats. CDNC-treated rats showed decreased levels of activated NFkB when compared to D rats. Likewise Akt, a downstream target of NFkB was phosphorylated in diabetic rats when compared to baseline controls, and CDNC administration inhibited this phosphorylation. There were no change in the expression levels of total Akt and NFkB in either D rats or in rats that received either of the chromium supplement. While CDN and CP had no effect, activation of NFkB (52%), and Akt (33%), and levels of GLUT-2 (35%), were decreased, while IRS-1 (45%) activation increased in livers of CDNC-supplemented rats in comparison to D (placebo).
Figure 5.
Effect of supplementation of chromium dinicocysteinate (CDNC), chromium dinicotinate (CDN), and chromium picolinate (CP) on total (T) and phosphorylated (p) NFkB and Akt in liver of ZDF rats. (a) Western blot represent 3 independent experiments; b) The graph represents summarized data from three experiments. Arbitrary units-ratio obtained by normalizing the intensity of individual bands against either T-Akt or T-NFkB. * - significant when compared with base line. # - significant when compared with D-control, NS-Non-significant when compared with D-control.
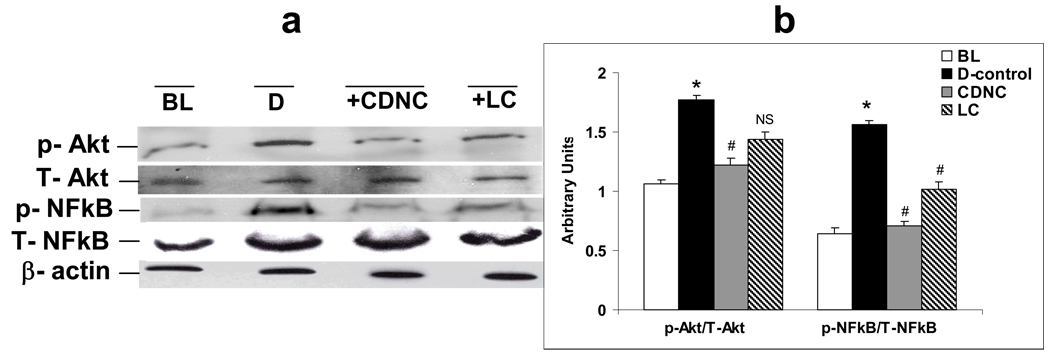
Figures 6a and 6b depicts phospho (Ser 473) and total Akt, phospho (Ser 276) and total NFkB levels in liver homogenates of D and rats treated with LC and CDNC. LC supplementation had a significant effect on the above parameters but CDNC supplementation had a much greater effect than LC alone.
Figure 6.
Effect of supplementation of saline-placebo (D), chromium dinicocysteinate (CDNC) and L-Cysteine (LC) on NFkB and Akt activation in liver of ZDF rats. (a) The western blots, and (b) bar graph summarizes data from three independent experiments. Western blotting analyses expressed as arbitrary units-ratio ratio obtained by normalizing the intensity of individual bands against either T-Akt or T-NFkB. *- significant when compared with base line. # - significant when compared with D-control, NS-Non-significant when compared with D-control.
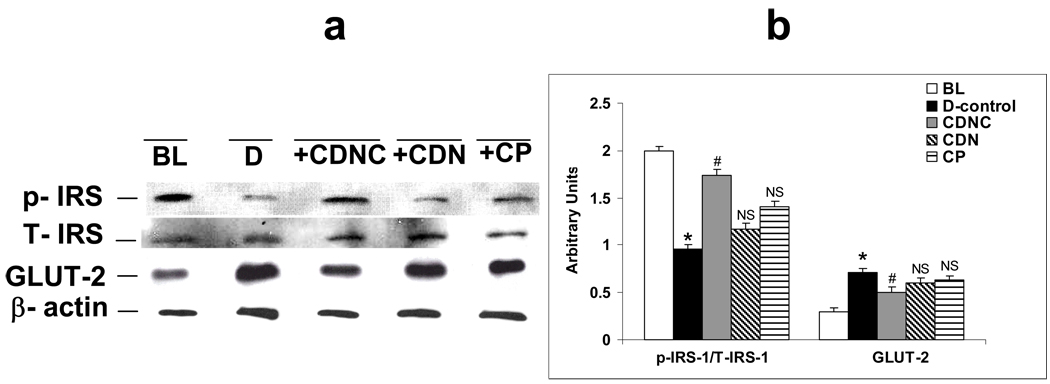
Figures 7a and 7b show the levels of IRS-1 (phospho (Tyr941) and total) and GLUT-2 in liver homogenates of control and diabetic animals supplemented with CDNC, CDN and CP respectively. In diabetic rats (D), impaired insulin signaling in the liver is marked by decreased activation of IRS-1 (p-IRS-1) and increased GLUT-2 levels were observed compared to BL. Supplementation with chromium compounds caused levels to revert nearly to those of baseline controls. However, the effects were more pronounced in CDNC-supplemented rats in comparison to those receiving CDN or CP supplements.
Figure 7.
Effect of placebo, chromium dinicocysteinate (CDNC), chromium dinicotinate (CDN), and chromium picolinate (CP) supplementation on IRS-1 activation and GLUT2 levels in liver of ZDF rats. (a) The western blot, and (b) graph summarizes data from three independent experiments. Arbitrary units-ratio obtained by normalizing the intensity of individual bands against T-IRS or β-actin. *- significant when compared with base line. # - significant when compared with D-control, NS- Non-significant when compared with D-control.
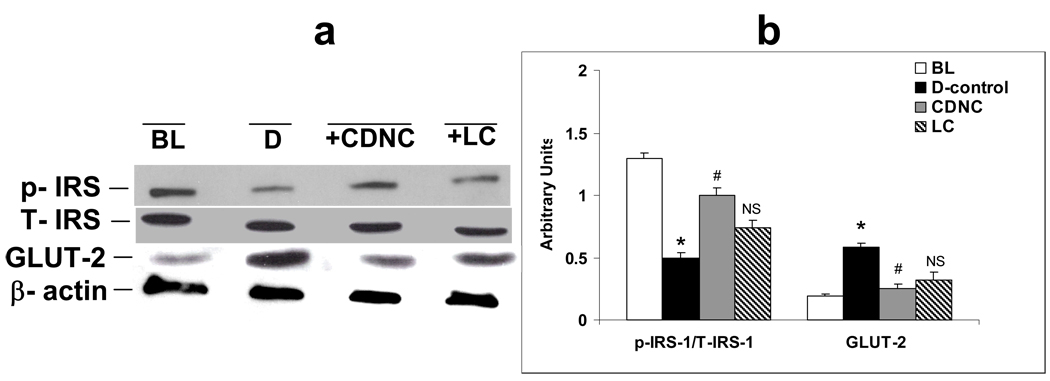
Figures 8a and 8b shows levels of IRS-1 activation and GLUT-2 in livers of LC and CDNC supplemented rats. Both LC and CDNC supplementation improved insulin signaling as shown by increased IRS-1 activation and decreased GLUT-2 levels. Further studies also found that L-cysteine supplementation alone lowered glycemia and caused inhibition of NFkB and Akt and increased IRS-1 activation in the liver but to a lesser extent compared to CDNC-supplemented rats.
Figure 8.
Effect of saline-placebo (D), chromium dinicocysteinate (CDNC) and L-Cysteine (LC) supplementation on IRS-1 activation and GLUT2 levels in liver of ZDF rats. (a) The Western blot, and (b) graph represents data from three independent experiments. Arbitrary units-ratio obtained by normalizing the intensity of individual bands against T-IRS or β-actin. *- significant when compared with base line. # - significant when compared with D-control, NS-Non-significant when compared with D-control.
Table I gives data on alanine aminotransferase (ALT), alkaline phosphatase (AP), aspartate aminotransferase (AST), blood urea nitrogen (BUN), creatinine and anion gap levels in the blood of supplemented rats. There were no changes in ALT, AST, BUN or anion gap levels in the CDNCsupplemented group in comparison to control diabetic group. The data demonstrate that neither chromium dinicotinate nor chromium dinicocysteinate supplementation seems to cause any toxicity as assessed by liver function. CDNC and LC significantly lowered blood levels of alkaline phosphatase, however, creatinine levels were significantly lower in CDNC-supplemented rats compared with control rats. Neither form of chromium compound affected hemoglobin, hematocrit or RBC counts in supplemented ZDF rats (data not given).
Table I.
Effect of CDNC, CDN, CP or LC supplementation on blood markers of liver and renal function in ZDF Rats.
| n | AP | BUN | CRT | AST | ALT | |
|---|---|---|---|---|---|---|
| units | U/L | mg/dL | mg/dL | U/L | U/L | |
| BL | 14 | 33.53±5.35* | 15.27±0.47* | 0.35±0.01* | 158.13±16.70 | 71.00±2.30* |
| D | 14 | 67.00±7.30** | 19.07±0.61** | 0.44±0.01** | 188.38±16.70 | 109.29±7.28** |
| CDNC | 12 | 35.15 ±3.95# | 18.23 ±0.64 | 0.41 ±0.00# | 171.67 ±17.48 | 99.00 ±6.03 |
| CDN | 12 | 56.18±5.53 | 18.92±0.40 | 0.45±0.02 | 155.45±11.78 | 111.83±7.52 |
| CP | 12 | 47.91±5.18 | 18.00±0.71 | 0.42±0.01 | 316.83±45.21 | 144.92±19.45 |
| LC | 6 | 36.40#±4.65 | 18.50±0.29 | 0.42±0.02 | 218.20±25.46 | 119.20±11.37 |
BL: baseline values at the start of supplementation. Each value represents the mean ± SE. Differences between * versus ** and between** and # are significant (p<0.05).
AP: Alkaline phosphatase; BUN: Blood Urea Nitrogen; CRT: Creatinine; AST: Aspartate Aminotransferase; ALT: Alanine Aminotransferase.
There was no difference in the body weights, total cholesterol, or triglyceride levels among CDNC, CDN, CP or LC supplemented ZDF rats at sacrifice (data not given). The weekly diet intake by each rat assessed at 5 and 7 weeks after beginning of supplementation was similar in all the treatment groups (data not given).
Discussion
Oxidative stress, CRP, MCP-1 and ICAM-1 levels are elevated in the blood of many diabetic patients, and are positively associated with an increase in vascular inflammation (2, 3, 21). Hyperglycemia and vascular inflammation are major risk factors that contribute to development of CVD in diabetic patients.
N-acetylcysteine (NAC) and other cysteine containing compounds have been shown to lower oxidative stress in diabetic animal models (4–6). Trivalent chromium is an essential nutrient and has been shown to lower oxidative stress and improve glucose and lipid metabolism (7, 9). It has been proposed that chromium supplementation increases the amount of a chromium-containing oligopeptide present in the insulin-sensitive cells that bind to the insulin receptor, markedly increasing the activity of the insulinstimulated tyrosine kinase and phosphorylation of insulin receptor substrate-1 and glucose transporter GLUT4 (7, 9). Chromium supplementation in the form of commercially available chromium dinicotinate (CDN) or chromium picolinate (CP) is widely used by the diabetic patient population. Subclinical chromium deficiency may be a contributor to insulin resistance and CVD, particularly in aging and diabetic populations (7, 9). Although not in all studies, some studies of diabetic patients and diabetic animals have reported decreased blood glucose and glycosylated hemoglobin levels or decreased insulin requirements after chromium supplementation (7–15).
This study demonstrates that supplementation with a novel chromium compound chromium dinicocysteinate (CDNC) markedly reduced circulating levels of glucose and glycated hemoglobin. In addition, CDNC significantly lowered blood levels of MCP-1, CRP, ICAM-1 and lipid peroxidation and increase blood vitamin C and adiponectin levels. CDNC supplementation appeared to be significantly more effective compared with CDN, CP, or LC supplementation. The beneficial effect of CDNC supplementation was also significant in the lowering of blood levels of creatinine compared with control-placebo in ZDF rats. This is a novel finding.
The glycated hemoglobin measurement reflects a mean glucose concentration over the preceding 2–3 months, in contrast to that of a one-time glucose level, which may be influenced by anesthesia or the stress of bleeding when the animals are sacrificed. There were no differences between the blood levels of hemoglobin or the RBC counts or hematocrits in diabetic rats receiving placebo or CDNC, CDN or CP supplementation, which suggests that lower glycated hemoglobin levels in CDNC-supplemented rats is not due to any change in life span of RBC.
Circulating MCP-1 and CRP levels are elevated in insulin resistant states such as obesity, impaired glucose tolerance, and type 1 and 2 diabetes (22). Studies using in vitro monocyte-endothelial adhesion assays and in vivo studies using knock-out mice for the CCR2 gene (receptor of MCP-1) and antibodies for MCP-1 have demonstrated that MCP-1 plays an important role in vascular inflammation and atherosclerotic lesion formation (22). Overexpression of MCP-1 causes inhibition of Akt and tyrosine phosphorylation in liver and skeletal muscle, macrophage recruitment, and insulin resistance in aP2-MCP-1 mice (22). Our finding of decreased levels of circulating MCP-1 in CDNC-supplemented diabetic rats is novel, and suggests that CDNC supplementation can prevent or delay the atherosclerosis associated with diabetes.
To understand the mechanism by which CDNC supplementation lowers glycemia and vascular inflammation, we investigated signal transduction pathways associated with glucose metabolism in the livers of diabetic rats. Elevated Akt phosphorylation is a primary intermediate event in insulin signaling associated with hyperglycemia. Akt phosphorylation could be a result of elevated pro-inflammatory cytokine-mediated NFkB activation, an independent independent of the conventional Akt phosphorylation event that occurs during insulin signalling.
Oxidative stress associated with diabetes activates NFkB, which then activates the insulin resistance cascade (3). Hyperglycemia is known to increase reactive oxygen species (ROS) generation and oxidative stress in diabetic rats and patients (13, 23, 24). Oxidative stress is a known activator of NFkB, which undergoes nuclear translocation and serine phosphorylation at residue 276 in its p65 subunit. It then associates with surrounding chromatin components and binds with DNA, which promotes the transcription of pro-inflammatory cytokines that mediate the insulin resistance cascade. Studies exist suggesting that NFkB activation may preceed the increased phosphorylation of Akt (ser 473) associated with diabetes (25). Increased Akt phosphorylation by CDNC as observed in this study could be mediated by high circulating levels of MCP-1 and CRP independent of activation by the PI-3 kinase pathway.
Insulin receptor substrate 1 (IRS-1) is the major substrate of insulin receptor kinase and has many tyrosine phosphorylation sites, which provide binding sites for many kinases including the 85-kDa subunit of PI3-kinase (PI3-kinase p85). Tyrosine phosphorylation of IRS-1 also binds PI3- kinase p85, thereby activating PI3-kinase (26), leading to the activation of glucose transport into cells through glucose transporters (GLUT). Impairment in IRS-1 phosphorylation and therefore a lack of downstream mediation of insulin signaling occurs in diabetes. GLUT 2 is the major glucose transporter protein involved in transport of glucose across hepatocytes, either from inside or outside depending upon the insulin stimuli. Increased expression of liver GLUT- 2 and a decrease in the IRS-1 activation in diabetic rats in our study is consistent with evidence found in the literature (27).
Supplementing diabetic rats with different chromium derivatives resulted in a mitigatory effect. All three compounds caused reduction in NFkB and Akt activation. IRS-1 was activated and GLUT-2 levels were decreased in the diabetic rats that received these supplements. Among them, CDNC supplementation showed better effects when compared to CDN or CP treatment. The effect produced by CDNC could be due to the combined effects of both chromium and L-cysteine (LC) because LC supplementation independently also inhibited NFkB activation and improved insulin signaling. When combined with chromium it produced better effects, which could explain the greater efficacy of CDNC over CDN or CP. These observations indicate that CDNC is a potent supplement that could be used to reduce the glycemia and vascular inflammation associated with diabetes.
Elevated circulating levels of ICAM-1 are linked to a higher incidence of vascular disease and diabetes (28). The significant decrease in ICAM-1 levels observed in this study suggests that CDNC supplementation not only improves glycemia but also has a positive effect on endothelial cell function. A significant reduction in blood creatinine levels in CDNC-supplemented rats compared with placebo-supplemented rats also suggests an improvement in endothelial cell function and its contribution to improved kidney function as assessed by lower blood creatinine levels in CDNC-supplemented ZDF rats.
Diabetic patients have low blood levels of vitamin C and adiponectin. Some studies have shown a link between low circulating adiponectin concentration and insulin resistance (29, 30). A number of studies have shown that vitamin C supplementation can lower oxidative stress and markers of vascular inflammation and atherosclerosis (31–34). Epidemiological studies have shown that higher plasma vitamin C predicts lower risk of stroke in general population and improvement in cognitive function in adults with type 2 diabetes (35, 36). Thus, the beneficial effect of CDNC supplementation in increasing circulating vitamin C levels is of special interest. Our cell culture studies also indicate that elevated blood vitamin C concentration may have a role in lower MCP-1 levels in CDNC or LC supplemented rats. Elevated vitamin C may result from the sparing of vitamin C in its antioxidative action by cysteine and chromium. The mechanism by which adiponectin is increased in CDNC supplemented rats is not clear. Nevertheless, elevated levels of both vitamin C and adiponectin apparently can contribute to the lowering of glycemia in CDNC supplemented rats.
The liver plays an important role in maintaining normal glucose concentrations during fasting as well as postprandially. There is evidence that measures of liver function and subclinical inflammation are related to insulin sensitivity and predict new-onset type 2 diabetes independent of classical risk factors (37). A recent study demonstrated that insulin sensitivity inversely correlated independently with elevated alkaline phosphatase activities in the blood of 472 apparently healthy men (38). A decrease in the alkaline phosphatase activities in the CDNC-supplemented group suggests an improvement in liver function which may play a role in the improved glycemia observed in CDNC-supplemented ZDF rats. Although alkaline phosphatase leaks into the blood stream in a manner similar to that of the other liver enzymes ALT and AST, AP is also present in organs such as bone and intestine. The levels of ALT and AST in blood did not differ between control and CDNC-treated groups in this study. Thus, it is not clear whether CDNC does indeed provide any protection to liver function in ZDF rats.
We also found that elevated lipid peroxidation levels in the blood of diabetic rats were abolished in rats supplemented with CDNC. Oxidative stress plays a key role in the regulatory pathway that progresses from elevated glucose to monocyte and endothelial cell activation in the enhanced vascular inflammation of diabetes (3). The inhibitory effect of CDNC on pro-inflammatory cytokine inhibition may be mediated partly by reduction in the oxidative stress pathways both due to chromium and L-cysteine (10, 39) or elevated blood vitamin C levels, as well reduction in the Akt phosphorylation associated with the insulin resistance cascade in the liver. Nevertheless, inhibition of circulating levels of CRP and MCP-1 along with the phosphorylation of Akt, can explain the observed lowering of blood glucose and glycated hemoglobin levels in CDNC-supplemented diabetic rats.
The recommended estimated adequate dietary intake range for Cr3+ for adults is 50–200µg/day (9). Human studies have mostly used 1000 µg Cr3+ per day (15). Assuming an average 70 kg body weight, this would translate to an intake of nearly 15 µg Cr3+/kg body weight. The dose of CDN or CDNC used in this study is 400 µg Cr3+/kg rat body weight, which is similar to that previously used by other investigators in chromium supplementation studies with diabetic rats or mice (7). Thus, the dose of chromium used per body weight in this rat study is higher than that used in human clinical trials. Whether any differences exist in the absorption of Cr3+ between humans and rats is not known.
CDN is a complex of chromium and the essential B-vitamin niacin, whereas CDNC is a complex of L-cysteine and CDN, and chromium picolinate is a complex of chromium and picolinate. The CDN and CP forms of chromium are consumed widely by the public in many countries. L-cysteine derivatives such as NAC can potentially scavenge oxygen radicals (39). Results of this study indicate that the presence of the cysteine molecule in CDNC helps provide better protection against hyperglycemia and the activation of signal transduction pathways associated with the vascular inflammation of diabetes. Analyses of blood chromium levels suggest that the absorption of chromium does not appear to differ among CDNC, CDN or CP-supplemented diabetic rats. No signs of toxicity were seen as a result of supplementation with any of the three chromium compounds used in this study.
In conclusion, chromium dinicocysteinate supplementation has the potential to lower blood levels of glycemia, oxidative stress, CRP, MCP-1, ICAM-1, creatinine and alkaline phosphatase, which is apparently mediated by increased vitamin C and adiponectin levels in blood, and inhibition of NFkB activation and improved insulin signaling in the liver of this diabetic animal model. The evidence that supplementation with CDNC can cause significant improvements in glycemia, the markers of vascular inflammation, and kidney function is novel, and needs to be explored in the diabetic patient population. If it works as well as this research hints that it may, then chromium dinicocysteinate supplementation could be used as an adjuvant therapy for the reduction of vascular inflammation and complications in diabetes.
Acknowledgements
The authors are supported by grants from NIDDK and the Office of Dietary Supplements of the National Institutes of Health (RO1 DK064797) and RO1 DK072433, and by InterHealth Nutraceuticals Inc. (Benicia, CA). The authors thank Ms Georgia Morgan for excellent editing of this manuscript.
References
- 1.Schaumberg DA, Glynn RJ, Jenkins AJ, Lyons TJ, Rifai N, Manson JE, Ridker PM, Nathan DM. Effect of intensive glycemic control on levels of markers of inflammation in type 1 diabetes mellitus in the diabetes control and complications trial. Circulation. 2005;111:2446–2453. doi: 10.1161/01.CIR.0000165064.31505.3B. [DOI] [PubMed] [Google Scholar]
- 2.Singh U, Devraj S, Jialal I. Vitamin E, oxidative stress and inflammation. Ann Rev Nutr. 2005;25:151–174. doi: 10.1146/annurev.nutr.24.012003.132446. [DOI] [PubMed] [Google Scholar]
- 3.Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxidant Redox Signal. 2005;7:1040–1052. doi: 10.1089/ars.2005.7.1040. [DOI] [PubMed] [Google Scholar]
- 4.Hsu CC, Yen HF, Yin MC, Tsai CM, Hsieh CH. Five cysteine-containing compounds delay diabetic deterioration in Balb/cA mice. J Nutr. 2004;134:3245–3249. doi: 10.1093/jn/134.12.3245. [DOI] [PubMed] [Google Scholar]
- 5.Liu Z, Li J, Zeng Z, Liu M, Wang M. The antidiabetic effects of cysteinyl metformin, a newly synthesized agent, in alloxan-and streptozocin-induced diabetic rats. Chem Biol Interact. 2008;173:68–75. doi: 10.1016/j.cbi.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Adachi Y, Yoshikawa Y, Sakurai H. Antidiabetic zinc(II)-N-acetyl-L-cysteine complex: evaluations of in vitro insulinomimetic and in vivo blood glucose-lowering activities. Biofactors. 2007;29:213–223. doi: 10.1002/biof.5520290405. [DOI] [PubMed] [Google Scholar]
- 7.Vincent JB. Recent advances in the nutritional biochemistry of trivalent chromium. Proc Nutr Soc. 2004;63:41–47. doi: 10.1079/PNS2003315. [DOI] [PubMed] [Google Scholar]
- 8.Hummel M, Standl E, Schnell O. Chromium in metabolic and cardiovascular disease. Horm Metabolic Disease. 2007;39:743–751. doi: 10.1055/s-2007-985847. [DOI] [PubMed] [Google Scholar]
- 9.Cefalu WT, Hu FB. Role of chromium in human health and in diabetes. Diab Care. 2004;27:2741–2751. doi: 10.2337/diacare.27.11.2741. [DOI] [PubMed] [Google Scholar]
- 10.Bartlett HE, Eperjesi F. Nutritional supplementation for type 2 diabetes: a systematic review. Ophthal. Physiol. Opt. 2008;28:503–523. doi: 10.1111/j.1475-1313.2008.00595.x. [DOI] [PubMed] [Google Scholar]
- 11.Jain SK, Kannan K. Chromium chloride inhibits oxidative stress and TNF-α secretion caused by exposure to high glucose in cultured monocytes. Biochem Biophys Res Commun. 2001;289:687–691. doi: 10.1006/bbrc.2001.6026. [DOI] [PubMed] [Google Scholar]
- 12.Rajpathak S, Rimm EB, Li T, Morris JS, Stampfer MJ, Willett WC, Hu FB. Lower toenail chromium in men with diabetes and cardiovascular disease compared with healthy men. Diabetes Care. 2004;27:2211–2216. doi: 10.2337/diacare.27.9.2211. [DOI] [PubMed] [Google Scholar]
- 13.Jain SK, Rains J, Croad J. Effect of chromium niacinate and chromium picolinate supplementation on fasting blood glucose, HbA1, TNF-α, IL-6, CRP, cholesterol, triglycerides and lipid peroxidation levels in streptozotocine treated diabetic rats. Free Radical Biol Med. 2007;43:1124–1131. doi: 10.1016/j.freeradbiomed.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain SK, Rains J, Jones K. High glucose and ketosis (acetoacetate) increases, and chromium niacinate inhibits IL-6, IL-8 and MCP-1 secretion in U937 monocytes. Antioxidant Redox Signaling. 2007;9:1581–1590. doi: 10.1089/ars.2007.1577. [DOI] [PubMed] [Google Scholar]
- 15.Anderson RA, Cheng N, Bryden NA, Polansky MM, Cheng N, Chi J, Feng J. Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes. 1997;46:1786–1791. doi: 10.2337/diab.46.11.1786. [DOI] [PubMed] [Google Scholar]
- 16.Jain SK, Rains J, Croad J. Curcumin supplementation lowers TNF-α, IL-6, IL-8 and MCP-1 secretion in high glucose-treated cultured monocytes and blood levels of TNF-α, IL-6, MCP-1, glucose and glycosylated hemoglobin in diabetic rats. Antioxidant Redox Signaling. 2009;11:241–249. doi: 10.1089/ars.2008.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Candiloros H, Muller S, Zeghari N, Donner M, Drouin P, Ziegler O. Decreased erythrocyte membrane fluidity in poorly controlled IDDM. Influence of ketone bodies. Diabetes Care. 1995;18:549–551. doi: 10.2337/diacare.18.4.549. [DOI] [PubMed] [Google Scholar]
- 18.McDonnell CM, Pedreira CC, Vadamalayan B, Cameron FJ, Werther GA. Diabetic ketoacidosis, hyperosmolarity and hypernatremia: are high carbohydrate drinks wosening initial presentation. Pediatric Diabetes. 2005;6:90–94. doi: 10.1111/j.1399-543X.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- 19.Jain SK, Wise R, Yanamandra K, Dhanireddy R, Bocchinni JA. Newborn birth weight and the maternal and cord- blood vitamin C, vitamin E and lipid peroxide levels. Mol Cell Biochem. 2008;309:217–221. doi: 10.1007/s11010-007-9638-8. [DOI] [PubMed] [Google Scholar]
- 20.Elements by ICP Spectrometry. Method 993.14. 18th Ed. Gaithersburg, MD: AOAC International; 2005. Offficail methods of analyses of AOAC International. [Google Scholar]
- 21.Donnelly R, Davis KR. Type 2 Diabetes and atherosclerosis. Diabetes, Obesity and Metabolism. 2000;2:S21–S30. doi: 10.1046/j.1463-1326.2000.00019.x. [DOI] [PubMed] [Google Scholar]
- 22.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, ManabeI I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281:26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 23.Jain SK. Hyperglycemia can cause membrane lipid peroxidation and osmotic fragility in human red blood cells. J Biol Chem. 1989;264:21340–21345. [PubMed] [Google Scholar]
- 24.Jain SK, McVie R, Duett J, Herbst JJ. Erythrocyte membrane lipid peroxidation and glycosylated hemoglobin in diabetes. Diabetes. 1989;38:1539–1543. doi: 10.2337/diab.38.12.1539. [DOI] [PubMed] [Google Scholar]
- 25.Meng F, Liu L, Chin PC, Mello SRD. Akt is a downstream target of NFkB. J Biol Chem. 2002;277:29674–29680. doi: 10.1074/jbc.M112464200. [DOI] [PubMed] [Google Scholar]
- 26.Sun XJ, Wang LM, Zhang Y, Yenush L, Myers MGJ, Glasheen E, Lane WS, Pierce JH, White MF. Role of IRS-2 in insulin and cytokine signaling. Nature (London) 1995;377:173–177. doi: 10.1038/377173a0. [DOI] [PubMed] [Google Scholar]
- 27.Slieker LJ, Sundell KL, Heath WF, Osborne HE, Bue J, Manetta J, Sportsman JR. Glucose transporter levels in tissues of spontaneously diabetic Zucker fa/fa rat (ZDF/drt) and viable yellow mouse (Avy/a) Diabetes. 1992;41:187–193. doi: 10.2337/diab.41.2.187. [DOI] [PubMed] [Google Scholar]
- 28.Kevil CC, Patel RP, Bullard DC. Essential role of ICAM-1 in mediating monocyte adhesion to aortic endothelial cells. Amer J Physiol. 2001;281:C1442–C1447. doi: 10.1152/ajpcell.2001.281.5.C1442. [DOI] [PubMed] [Google Scholar]
- 29.Swarbrick MM, Havel PJ. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metab Syndrom Related Disord. 2008;6:87–102. doi: 10.1089/met.2007.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaturu S, Daberry B, Rains J, Jain SK. Resistin and adiponectin levels in subjects with coronary artery disease and type 2 diabetes. Cytokine. 2006;34:219–223. doi: 10.1016/j.cyto.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Vega-López S, Devaraj S, Jialal I. Oxidative stress and antioxidant supplementation in the management of diabetic cardiovascular disease. J Investig Med. 2004;52:24–32. doi: 10.1136/jim-52-01-23. [DOI] [PubMed] [Google Scholar]
- 32.Jialal I, Vega GL, Grundy SM. Physiologic levels of ascorbate inhibit the oxidative modification of low density lipoprotein. Athersclerosis. 1990;82:185–191. doi: 10.1016/0021-9150(90)90039-l. [DOI] [PubMed] [Google Scholar]
- 33.Loke WM, Proudfoot JM, McKinley AJ, Croft KD. Augmentation of monocytes intracellular ascorbate in vitro protects cells from oxidative damage and inflammatory responses. Biochem Biophys Res Commun. 2006;345:1039–1043. doi: 10.1016/j.bbrc.2006.04.174. [DOI] [PubMed] [Google Scholar]
- 34.Langlois M, Duprez D, Delanghe J, De Buyzere M, Clement DL. Serum vitamin C concentration is low in peripheral arterial disease and is associated with inflammation and severity of atherosclerosis. Circulation. 2001;103:1863–1868. doi: 10.1161/01.cir.103.14.1863. [DOI] [PubMed] [Google Scholar]
- 35.Myint PK, Luben RN, Welch AA, Bingham SA, Wareham NJ, Khaw KT. Plasma vitamin C concentrations predict risk of incident stroke over 10 y in 20 649 participants of the European Prospective Investigation into Cancer Norfolk prospective population study. Amer J Clin Nutr. 2008;87:64–69. doi: 10.1093/ajcn/87.1.64. [DOI] [PubMed] [Google Scholar]
- 36.Chui MH, Greenwood CE. Antioxidant vitamins reduce acute meal-induced memory deficits in adults with type-2 diabetes. Nutrition Research. 2008;28:423–429. doi: 10.1016/j.nutres.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 37.Ohlson LO, Larsson B. Risk factors for type 2 diabetes mellitus: thirteen and one-half years of follow-up of the participants in a study of Swedish men born in 1913. Diabetologia. 1988;31:798–805. doi: 10.1007/BF00277480. [DOI] [PubMed] [Google Scholar]
- 38.Godsland IF, Johnston DG. Co-associations between insulin sensitivity and measures of liver function, subclinical inflammation, and hematology. Metabolism Clinical and Experimental. 2008;57:1190–1197. doi: 10.1016/j.metabol.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Dröge W. Oxidative stress and ageing: is ageing a cysteine deficiency syndrome? Phil Trans Royal Soc. 2005;360:2355–2372. doi: 10.1098/rstb.2005.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]