Abstract
Severe hypoxia can lead to injury and mortality in vertebrate or invertebrate organisms. Our research is focused on understanding the molecular mechanisms that lead to injury or adaptation to hypoxic stress using Drosophila as a model system. In this current study, we employed the UAS–Gal4 system to dissect the protective role of Hsp70 in specific tissues in vivo under severe hypoxia. In contrast to an over-expression in tissues like muscles, heart and brain, we found that over-expression of Hsp70 in hemocytes of flies provides a remarkable survival benefit to flies exposed to severe hypoxia for days. Furthermore, these flies were not only tolerant to severe hypoxia but also to other stresses such as oxidant stress (e.g., paraquat feeding or hyperoxia). Interestingly we observed that the better survival with Hsp70 over-expression in hemocytes under hypoxia or oxidant stress is causally linked to reactive oxygen species (ROS) reduction in whole flies. We also show that hemocytes are a major source of ROS generation, leading to injury during hypoxia and their elimination results in a better survival under hypoxia. Hence, our study identified a protective role of Hsp70 in Drosophila hemocytes which is linked to ROS reduction in the whole flies and thus helps in their remarkable survival during oxidant or hypoxic stress.
Keywords: Hsp70, hemocytes, reactive oxygen species, hypoxia, oxidative stress
Introduction
Whether hypoxia is present during normal conditions (high altitude) or during pathologic states (e.g., obstructive sleep apnea, and sickle cell anemia), it challenges vertebrate as well as invertebrate organisms. Various studies in animal models have examined experimentally the effects of hypoxia (intermittent, IH, or constant, CH) on specific tissues such as heart, brain, and kidneys [1-5]. These studies have demonstrated that the response to low O2 is not only dependent on intensity and duration of the stimulus but also on the paradigm used. For example, CH and IH are very different in their effect on growth, proliferation, and neuronal injury [6, 7]. In our recent study, we focused on gene expression changes associated with severe and short term constant or intermittent hypoxia using Drosophila as a model system [8]. Our microarray analysis has identified distinct responses to IH and CH in terms of gene expression. Multiple gene families were up- or down-regulated in response to these stresses. During IH, biological processes primarily involved in multi-drug resistance and defense responses were over-represented [8]. In contrast, there were several gene families that were over-represented in CH treated flies, such as those involved in the response to unfolded proteins, chitin, lipid, carboxylic acid, amino acid-metabolic processes, and immunity [8]. Indeed, the heat shock protein family was the most up-regulated group in CH and this was exclusive to this treatment.
The protective role of stress-inducible-proteins such as Hsp70 during low oxygen conditions such as ischemia or hypoxia, have been studied extensively in vivo as well as in vitro models [9-27]. A number of in vitro studies have shown increased survival in cardiac myocytes and astrocytes under hypoxic conditions after heat pre-conditioning [10, 11, 14, 22]. Although, the exact mechanism(s) of protection is not totally clear, Hsp70 has been known to involve anti-apoptotic, anti-necrotic or chaperonic function [9, 28]. Recently there is accumulating evidence that suggests that the cytoprotective effect of Hsp70 is related to the modulation of cellular redox status and reduction of intracellular ROS [15, 29-31]. Besides its intracellular protective functions, heat shock proteins participate in immunoregulatory mechanisms [32-34]. Extracellular or membrane bound HSP can elicit an immune response thereby linking innate and adaptive immune systems[32].
In order to study the functional role of heat shock proteins in hypoxia tolerance in our previous study we tested a) the survival of P-element lines in which Hsp70 was upregulated under hypoxia and b) the role of Hsp70 over-expression in specific tissues on survival under hypoxia utilizing progenies of crosses with specific GAL4 drivers[8]. We found that the up regulation of Hsp70 in P-elements remarkably increased survival under hypoxia and the over-expression of Hsp70 in heart and hemocytes together (Hand-Gal4) as well as mushroom bodies in brain provided a survival benefit to adult flies when exposed to longer periods of hypoxia(days)[8]. In our current study we are focusing on the role of Hsp70 over-expression in hemocytes under hypoxia. Hemocytes are the phagocytes of invertebrates and found in the hemolymph of flies. In larvae, circulating hemocytes engulf bacteria and apoptotic cells. During the larval stage, they also play an important role in adult and pupae structural remodeling involving both their phagocytotic (apoptotic cells) as well as their immune function[35]. A previous study by Charroux and Royet 2009, has shown that during this developmental process, phagocytosis may not be as critical as immune-protection, since normal developmental rates were observed when flies devoid of hemocytes were reared under axenic conditions[36]. In Drosophila, hemocytes are only produced during two stages of their life cycle, once during embryogenesis and again during the larval stage from lymph glands [35]. Adult flies do not produce hemocytes but contain a mixture of embryonic and lymph gland derived hemocytes[35]. They provide cellular defense in flies[37, 38] and their function ranges from phagocytosis of biotic targets such bacteria or apoptotic bodies, to rapid transient release of antimicrobial peptides, to clotting as well as activation of phenoloxidase cascade[37, 39]. Previous studies have demonstrated several classes of hemocytes in Drosophila: prohemocyte, plasmatocytes, podocytes, lamellocytes, crystal cells and secretory cells [40]. On the basis of expression of blood cell antigens, Ando et al, defined 4 cell types of hemocyte populations – plasmatocytes, crystal cells, lamellocytes and precursor cells[40]. However, according to recent nomenclature, the major cell types of hemocytes are plasmatocytes, lamellocytes and crystal cells [41-43]. The most abundant are round-shaped plasmatocytes which comprise 95% of circulating hemocytes and resemble macrophages primarily removing foreign material and apoptotic cells by phagocytosis. Crystal cells are characterized by crystal-like inclusions in the cytoplasm and are involved in melanin deposition in wounds and around foreign objects. The third type is a class of large flat cells, the lamellocytes, which appear when the larvae are infected by parasitoid wasps. These cells participate in the encapsulation of the parasites. Hemocytes are known to generate reactive oxygen intermediates (ROI) and reactive nitrogen intermediates (RNI) as respiratory bursts when triggered by stimuli such as infection [44]. Our hypothesis is that Hsp70 over-expression in hemocytes lowers ROS levels which, in turn, provide survival advantage to flies exposed to hypoxia or other oxidative stresses.
Materials and methods
Drosophila stocks
Wild type Canton S (CS), yw, UAS-Hsp70, RNAi-Hsp70 and Gal4 driver stocks were obtained from the Drosophila Stock center (Bloomington, Indiana, USA). UAS-hid and reaper stocks were generous gifts from Dr. Tian Xu’s Lab (Yale University School of Medicine, New Haven, CT). Flies were maintained on standard-cornmeal Drosophila medium in an incubator at temperature of 25°C and 30-50% humidity. Based on the previous study by Charroux and Royet 2009, the F1-Progeny of flies in which hemocytes were eliminated by hid or rpr were reared on antibiotic food (ampicillin-500 μg/ml, tetracycline-50 μg/ml, rifamycin-200 μg/ml)[36].
Feeding flies N-Acetylcysteine and Ascorbic acid
Fresh stock solution of (20mg/ml in H20) of N-acetyl-L-cysteine (Sigma) (Brack et al. 1997[45]) was prepared weekly and stored at 4°C and diluted before use. The dry food (0.35g) was mixed with 1.5 ml of water in the vial (control food) or 1.5 ml of NAC dilution (10mg/ml). The survival of NAC-fed wild type flies versus regular food fed wild type flies was tested under hypoxia (1.5% O2) and hyperoxia (90% O2). In another set of experiments, wild type flies were fed ascorbic acid (0.36mM) and tested under 20mM paraquat as described in Bonilla et al., 2006[46]. Fresh stock solution of ascorbic acid was made and mixed with dry food. Flies were fed with food containing ascorbic acid (Sigma) or regular food for 5 days and then their survival was tested in vials containing 22-mm filter paper disk soaked in 20mM paraquat in 5% sucrose solution for 24 hours.
Survival of flies under 1.5% O2 CH
In order to over-express Hsp70 in the hemocytes the UAS line for heat shock 70Bbb (yw; P{y[+t7.7]=Mae-UAS.6.11}Hsp70Bbb[UY2168]) was crossed with Hemese-GAL4(He-Gal4-Hemese is a hemocyte specific transmembrane protein). In order to knock-down Hsp70 in the hemocytes, RNAi stock of Hsp70Bbb (y[1] v[1]; P{y[+t7.7] v[+t1.8]=TRiP.JF03215}attP2) was crossed with Hemese-GAL4. For elimination of hemocytes UAS-hid and rpr stocks were crossed with He-Gal4 driver. The F1 progeny (3-4 day old) of these crosses were tested under 1.5 % O2 CH for over a period until the controls flies were dead. Flies were exposed in specially designed computerized chambers which can modulate the level of O2 using a combination of Oxygen (O2) and Nitrogen (N2) with Oxycycler hydraulic system (Model A44x0, BioSpherix, Redfield, NY) and ANA-Win2 Software (Version 2.4.17, Watlow Anafaze, CA). For CH, the O2 level was maintained at 1.5% O2 continuously. Unpaired student t-tests were used to calculate significant differences in the percentage survival of experimental (Hsp70 over-expression in hemocytes, hemocytes depleted progeny) as compared to the controls (CS, yw) under each treatment.
Measuring ROS levels in flies
We used 2,7 dichlorofluorescein di-acetate method of ROS detection described by Lee et al [47] to measure ROS levels at normoxia as well as after hypoxia or oxidative stress treatments. Flies were homogenized with 500μl of PBST (PBS containing 0.1% Tween 20), and then 100 μl of each supernatant was transferred into a 96-well plate. 2, 7 dichlorofluorescein (Invitrogen) was added to each sample with a final concentration of 50 μm. The fluorescent intensity was measured at excitation 485nm, emission 640nm and quantified using fluorescent microplate reader (Biotek, VT). Fluorescent intensities are normalized by protein levels and initial control values. Each experiment was done with three replicates of 30 flies each. The oxidation-insensitive counterpart of dichlorofluorescein dye (C369, Invitrogen) was used as a control to ensure that the changes in the fluorescence seen with oxidation-sensitive dye DCFH were solely due to changes in ROS production. In order to compare the release of ROS from hemocytes between the controls and the Hsp70 over-expression lines, we isolated hemocytes from larvae fed with either 20mM paraquat solution or sucrose solution for 24 hours. Hemolymph was bled from the late wandering larvae (larvae unexposed and exposed to 20mM paraquat, n=50) to isolate hemocytes following the protocol by [48]in 100ul PBS in a 96-well microplate. 2, 7 dichlorofluorescein was added to each sample with a final concentration of 50 μm. The percent relative ROS levels was calculated as a ratio of ROS mean intensity of exposed cells: mean intensity of unexposed cells [49].
Oxidative stress resistance test in flies
a) Paraquat treatment
To study the effect of oxidative stress, 3-5 day old adult flies were exposed to 5mM paraquat (Sigma) for 12 days. The flies were kept in vials containing 22-mm filter paper disk soaked in 5mM paraquat in 5% sucrose solution. Flies were fed daily with 100 μl of fresh paraquat with sucrose. Unpaired student t-tests were used to calculate significant differences in the percentage survival of experimental (Hsp70 over-expression in hemocytes) as compared to the controls (CS, yw) under each treatment.
b) Hyperoxia treatment
Adult flies (3-5 day old) were exposed to 90% O2 for 9 days (until controls were dead). Unpaired student t-tests were used to calculate significant differences in the percentage survival of experimental (Hsp70 over-expression in hemocytes) as compared to the controls (CS, yw) under each treatment.
Immunofluorescent Analysis and hemocyte imaging
We used a Green Fluorescent Protein (GFP) labeled Hemese-Gal4 driver to observe hemocytes in adult flies. Adults flies and larvae of F1-progeny in which Hsp70 is over-expressed in hemocytes (UAS Hsp70/ UAS-GFP He-Gal4) and in which hemocytes were eliminated by hid (UAS-hid/UAS-GFP He-Gal4) or reaper (UAS-rpr/UAS-GFPHe-Gal4) were viewed under fluorescent microscopy (Carl Zeiss, Thornwood, NY). For hemolymph analysis, larvae from each genotype were bled on a slide, and viewed under a microscope (Carl Zeiss, Thornwood, NY).
Results
Over-expression of Hsp70 in hemocytes and heart provides survival advantage
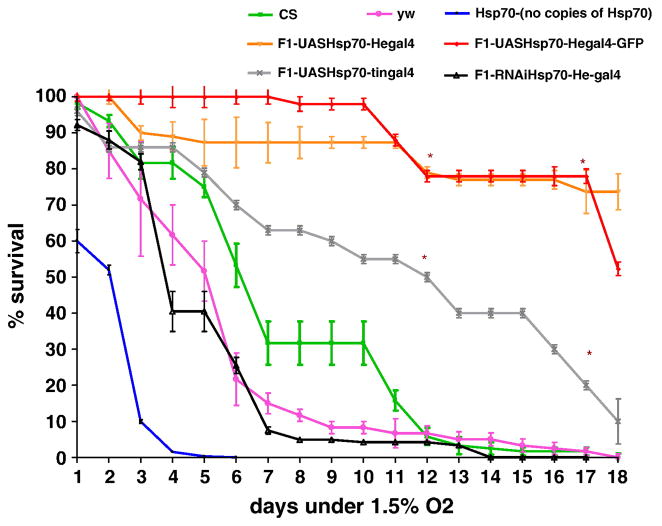
Comparing gene expression after acute constant hypoxia to that after intermittent hypoxia in flies, we have previously shown that Hsp70 was upregulated by several folds during constant hypoxia (CH) [8] and P-element lines with Hsp70 over-expression had a remarkable survival advantage during hypoxia. In order to further dissect the mechanisms of protection of Hsp70, we first over-expressed it in various tissues utilizing the UAS-Gal4 system, and subjected the F1 progeny to severe hypoxia by exposing them to 1.5% O2 CH. In our previous study, in order to examine the tissue-specificity of Hsp70 over-expression we expressed it ubiquitously(da-Gal4) as well as in various cell types and tissues such as neurons (elav-Gal4), glial cells (Eaat1-Gal4), brain(P{GawB}c739-Gal4), cardioblasts, pericardial cells and lymph gland (hand-Gal4). We found that over-expression in brain (c739-Gal4), cardioblasts, pericardial cells and lymph gland (hand-Gal4) provided survival advantage to flies under hypoxia[8]. We are extending this study further to explore the effect of Hsp70 over-expression specifically in hemocytes and heart under severe hypoxia. We found that over-expression of Hsp70 in hemocytes (using Hemese-Gal4 driver or He-Gal4, Hemese is a hemocyte specific transmembrane protein [50]) provides a major survival advantage during hypoxia. Indeed, after a 12-day exposure to 1.5% O2, the flies over-expressing Hsp70 in hemocytes had a 80% survival, as compared to a 10% survival in controls (unpaired t-test, P<0.001). Even, after 18 days of this severe hypoxia exposure, these lines with Hsp70 over-expression had a survival of 75% when all the controls had already died (Figure 1). Over-expression of Hsp70 in hemocyte specific driver lines (He-Gal4, He-Gal4-UAS-GFP) had similar effects as shown in Figure 1. The separate UAS control flies as well as He-Gal4 drivers alone had similar survival to controls. Hence, the survival benefit in the F1 progeny was due to Hsp70 over-expression in hemocytes. Flies with no copies of Hsp70 or with knock-down of Hsp70Bbb in hemocytes survived poorly even when compared to controls (Figure 1 and [8]). In order to confirm that hemocytes are still present in the F1 progeny in which Hsp70 was over-expressed, we viewed the flies (UAS-Hsp70/UAS-GFP-He-Gal4) under fluorescent microscopy (Figure 2). We demonstrate that hemocytes are present in both larvae and adult flies, whereas no signal was detected in controls at either stages of development. The flies that over-expressed Hsp70 in the heart (using tin-Gal4) also survived better than the controls (P<0.05, unpaired t-test). For example, after 12 days of hypoxia exposure, the F1-progeny over-expressing Hsp70 in the heart had a survival of 40% as compared to the controls which had less than 10% survival (P<0.05, Figure 1).
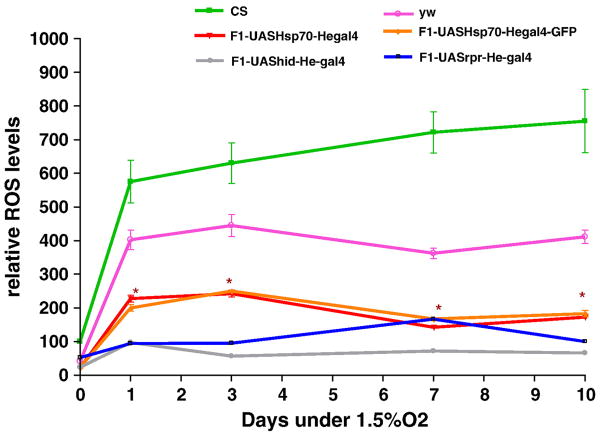
Figure 1.
Survival of adult flies exposed to severe hypoxia (1.5% O2). Over-expression of Hsp70 in hemocytes significantly increased survival as compared to controls. Each point represents the average of at least three tests for each line and error bars represent the standard errors. * with unpaired t-test P<0.05 with controls vs. the genotype.
Figure 2. Visualization of hemocytes through GFP hemese marker and comparison among phenotypes.
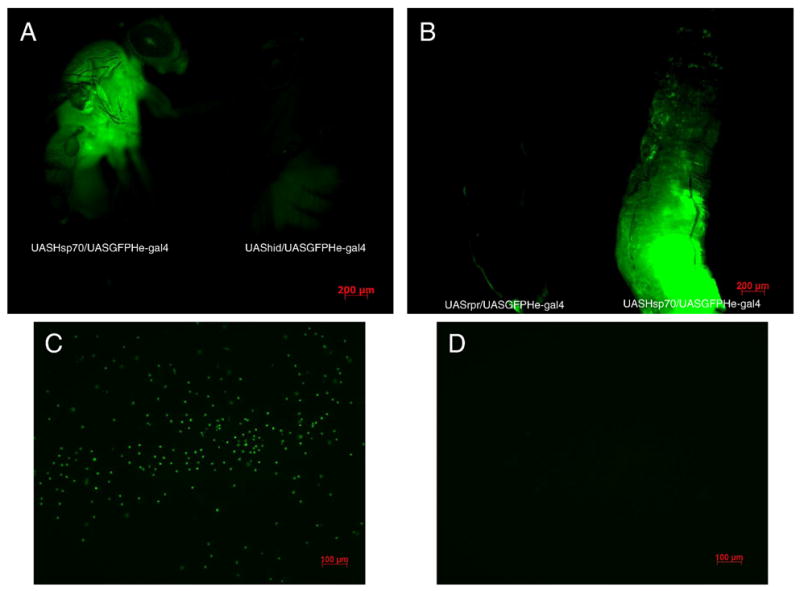
A - F1 progeny adults in which Hsp70 is over-expressed (UAS-Hsp70/He-Gal4GFP) in hemocytes GFP signal confirms the presence of hemocytes whereas expression of apoptotic gene hid gives rise to adults (F1-UAShid/heGal4GFP) devoid of GFP signal suggesting hemocytes are eliminated, image taken with both flies on the same field at 4x magnification.
B - F1 progeny larvae in which Hsp70 is over-expressed (UAS-Hsp70/He-Gal4GFP) in hemocytes GFP signal confirms the presence of hemocytes whereas expression of apoptotic gene rpr gives rise to larvae (F1-UASrpr/heGal4GFP) devoid of GFP signal suggesting hemocytes are eliminated, image taken with both larvae on the same field at 4x magnification.
C - GFP tagged hemocytes when larvae of F1- UAS-Hsp70/He-Gal4GFP are bled on the slide, taken at 10x magnification at same GFP exposure time and intensity between groups.
D - Shows lack of GFP signal and hence hemocytes when larvae of F1-UAShid/heGal4GFP are bled on the slide suggesting that these are devoid of mature hemocytes taken at 10x magnification at same GFP exposure time and intensity between groups.
How Hsp70 over-expression protects fly tissues is not clear at present. A number of hypotheses with some experimental evidence have been advanced such as chaperonic function to prevent misfolding of proteins [51, 52], anti-apoptotic activity [28] or protection against free-radical injury[29]. In this work we test the importance of Hsp70 in lowering ROS generated during hypoxia and other oxidative stresses and hypothesize that Hsp70 prevents free radicals injury associated with acute constant hypoxia. Severe hypoxia such as 1.5% O2 could generate free-radicals [53-55], and hence one of the potentially important mechanism(s) of protection of Hsp70 could be its effects on ROS.
Over-expression of Hsp70 in hemocytes significantly lowered ROS levels
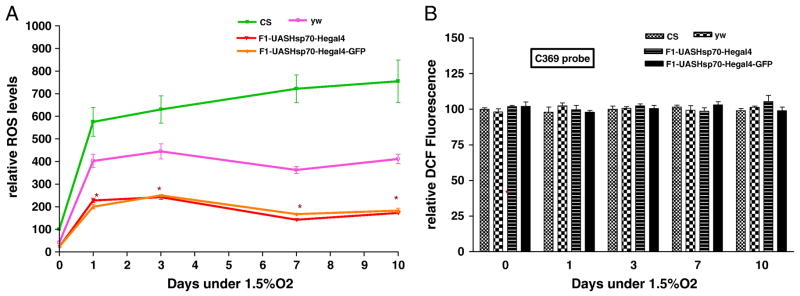
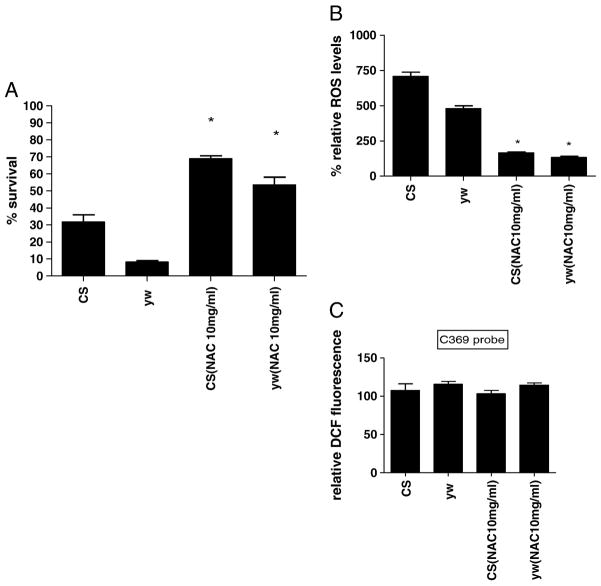
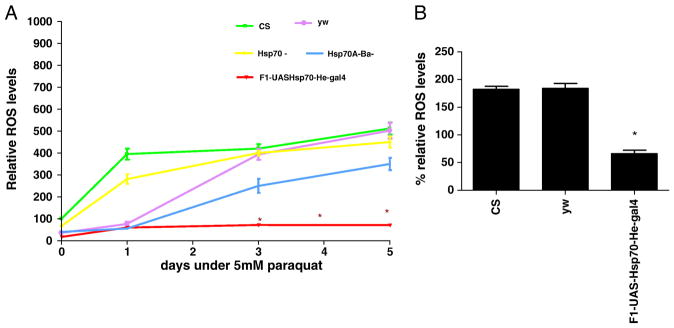
Acute hypoxia is associated with free radical generation and injury [53-55]. An imbalance between ROS production and antioxidant defenses can lead to oxidative stress and injury. Although there are data suggesting that Hsp70 protects against oxidative stress, the mechanisms of its protection are unknown. Figure 3a show that Hsp70 over-expression in hemocytes significantly lowers the relative ROS levels of flies. After one day of exposure at a constant 1.5% O2, hemocyte-specific Hsp70 over-expression lines had significantly lower ROS levels (3 times lower) as compared to controls (P<0.05, unpaired t-test). With continued hypoxia exposure we observed a remarkable difference in the relative ROS levels between controls and Hsp70 over-expression lines. For instance, after 10 days of severe hypoxia, ROS levels of CS control were 6 times greater as compared to the F1-progeny of UAS-Hsp70 and He-Gal4 (P<0.001, paired t-test). This strongly suggests that the reduction of ROS levels in these flies could be a major mechanism that prevents free radical injury, which in turn provides a survival advantage to hemocyte-specific Hsp70 over-expression during acute hypoxia (Figure 1). To confirm that our measurements in DCFH oxidation were not related to DCFH uptake, efflux or ester cleavage, we repeated the experiments with an oxidation-insensitive analogue of DCFH (C369). Figure 3b shows that there were no significant differences between controls as well as Hsp70 over-expression lines when tested with oxidation-insensitive probe. Hence the observed differences between control flies (CS and yw) and Hsp70 over-expression lines under hypoxia are attributed to changes in ROS levels, as measured by the oxidation-sensitive dye. Furthermore, when control flies were fed an ROS inhibitor such as NAC, the flies had significantly better survival under hypoxia as well as much lower ROS levels as compared to control flies that were fed regular food (Figure 4). For example, after 9 days under severe hypoxia (1.5% O2), NAC-fed CS flies had double the survival rate as compared to the CS flies fed with regular food (Figure 4a) (p<0.001, unpaired t-test). Similar results were seen for yw NAC-fed flies as well (Figure 4a). This suggests that the mortality of flies under hypoxia is related to increased ROS levels under such conditions (Figure 4b, c).
Figure 3.
Figure 3a – Relative ROS levels after 1.5% O2. Up regulation of Hsp70 in hemocytes reduced the ROS levels significantly. Each point represents the average of at least three tests for each line and error bars represent the standard errors. * with unpaired t-test P<0.05 with controls vs. the genotype.
Figure 3b- Measurement of Fluorescence with oxidation-insensitive probe of DCFH (C369) as a control under 1.5% O2. Each bar represents the average of at least three tests for each line and error bars represent the standard errors.
Figure 4.
Effect of feeding an ROS inhibitor NAC to CS and yw control flies under severe hypoxia (1.5% O2).
Figure 4a - Survival of adult flies after 9 days exposure to 1.5% O2. Flies that were fed NAC survived more than twice when compared to flies fed control food. * with unpaired t-test P<0.05 between the NAC fed food and regular food fed flies.
Figure 4b - Relative ROS levels after 1.5% O2. Each bar represents the average of at least three tests for each line and error bars represent the standard errors. * with unpaired t-test P<0.05 between the NAC fed food vs. regular food fed flies.
Figure 4c - Measurement of Fluorescence with oxidation-insensitive probe of DCFH (C369) as a control under 1.5% O2. Each bar represents the average of at least three tests for each line and error bars represent the standard errors.
Hsp70 over-expression is protective during oxidative stress
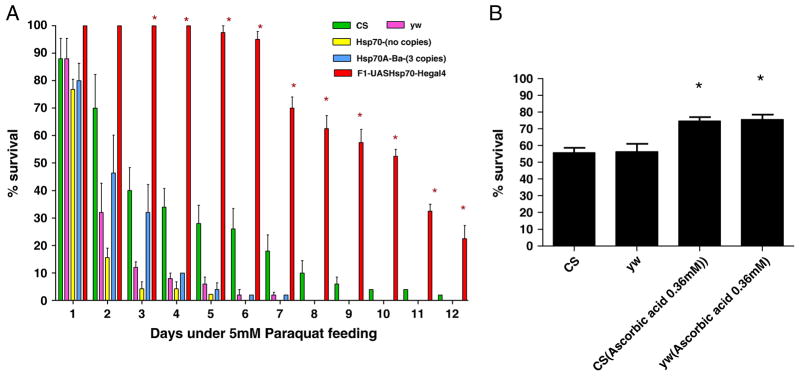
In order to reinforce our hypothesis of the protective effect of Hsp70 against oxidative injury, we tested the survival of flies under conditions that generate large amounts of free radicals such as paraquat or hyperoxia treatment. First, we found that the stocks that had no copies of Hsp70 were much more susceptible to paraquat as compared to controls (Figure 5a). In fact, the stock with no copies of Hsp70 was dead after 5 days of 5mM paraquat exposure at which point the CS control have a survival rate of 30%. Second, we observed that flies with the over-expression of Hsp70 in hemocytes had > 90% survival rate after 6 days of paraquat which was triple that of controls. Hence, Hsp70 over-expression increases survival rates of flies in paraquat. Flies fed ascorbic acid also had significantly increased survival as compared with flies that were fed regular food without ascorbic acid, suggesting that the mortality is causally linked to an increase in ROS levels (Figure 5b). Indeed, the ROS levels of the flies over-expressing Hsp70 only in hemocytes had significantly lower ROS levels in whole flies as compared to the controls (P<0.05)(Figure 6a). Even after a single day of paraquat exposure, there were apparent differences in the ROS levels between controls and hemocyte-specific Hsp70 lines. The CS control had 5 times higher relative levels of ROS as compared to hemocyte-Hsp70 over-expression lines (P<0.005, unpaired t-test). We isolated hemocytes to study the ROS generation directly in vitro in 96-well plate. We observed that hemocytes isolated from larvae in which Hsp70 was over-expressed generated half the ROS levels when larvae were fed paraquat (20mM), as compared to controls (P<0.05) (Figure 6b). No significant differences were observed in oxidation-insensitive counterpart of DCFH (C369) between the control flies (CS, yw) and the Hsp70 over-expression flies.
Figure 5.
Figure 5a - Survival of adult flies exposed to 5mM Paraquat. Over-expression of Hsp70 in hemocytes significantly increased survival as compared to controls. Each bar represents the average of at least three tests for each line and error bars represent the standard errors. * With unpaired t-test P<0.05 with controls vs. the genotype.
Figure 5b - Effect of feeding flies ascorbic acid under paraquat treatment. Survival of adult flies exposed to 20mM Paraquat. Each bar represents the average of at least three tests for each line and error bars represent the standard errors. * With unpaired t-test P<0.05 with control food fed flies vs.ascorbic acid fed flies.
Figure 6.
Figure 6a - Relative ROS levels after 5mM paraquat treatment. Upregulation of Hsp70 in hemocytes reduced the ROS levels significantly. Each point represents the average of at least three tests for each line and error bars represent the standard errors. * With unpaired t-test P<0.05 with controls vs. the genotype.
Figure 6b - % relative ROS levels after 24 hours 20mM Paraquat treatment of larvae. Hemocytes isolated from Hsp70 over-expression generated significantly less ROS as compared to controls. Each bar represents the average of at least three tests for each line and error bars represent the standard errors. * With unpaired t-test P<0.05 with controls vs. the genotype.
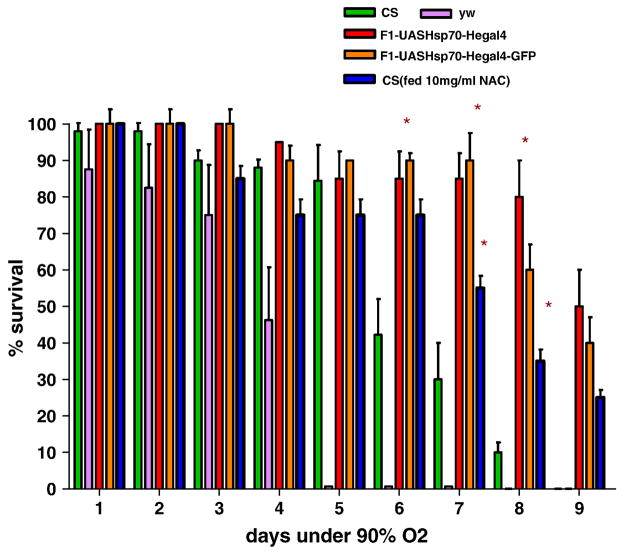
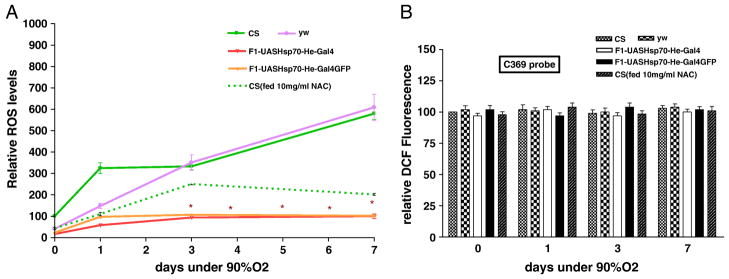
When we exposed flies to hyperoxia (90%O2) for a week, we observed a similar trend in the survival and relative ROS levels. After 7 days of hyperoxia treatment, flies over-expressing Hsp70 in hemocytes had still a survival rate of 95% as compared to 30% survival in CS (P <0.05- t-test) (Figure 7). Even when both the controls were dead, Hsp70 over-expression lines had a moderate survival as depicted in figure 7. ROS levels after hyperoxia were also consistent with our hypothesis: Hsp70 over-expression in hemocytes was associated with lower ROS levels during oxidative stress and improved survival (Figure 8a). During hyperoxia, ROS levels increased quickly in CS controls by three days, and both CS and yw had 3-fold greater levels than the Hsp70-over-expression lines. The much lower levels of ROS in the Hsp70 flies were maintained for the whole period of exposure (Figure 8a). No significant differences were observed with the oxidation-insensitive probe C369 of Dichlorofluorescein (Figure 8b). In addition, when the control CS flies were fed with an ROS inhibitor (NAC), flies had significantly lower ROS levels and survived much better than those fed with regular food (Figure 7, 8).
Figure 7.
Survival of adult flies exposed to hyperoxia (90%O2) for 7 days. Over-expression of Hsp70 in hemocytes significantly increased survival as compared to controls. Each bar represents the average of at least three tests for each line and error bars represent the standard errors. * With unpaired t-test P<0.05 with controls vs. the genotype.
Figure 8.
Figure 8a- Relative ROS levels after hyperoxia treatment. Upregulation of Hsp70 in hemocytes reduced the ROS levels significantly. Each point represents the average of at least three tests for each line and error bars represent the standard errors. * With unpaired t-test P<0.05 with controls vs. the genotype.
Figure 8b-Measurement of Fluorescence with oxidation-insensitive probe of DCFH (C369) as a control under 90% O2. . Each bar represents the average of at least three tests for each line and error bars represent the standard errors
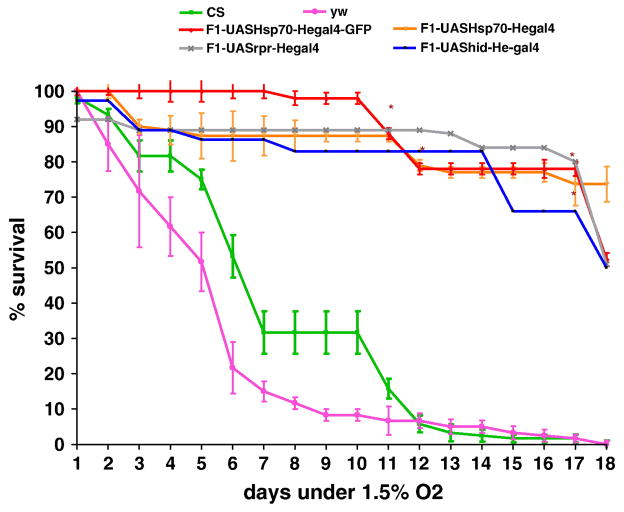
Depletion of hemocytes has a similar effect on survival and ROS levels as Hsp70 over-expression in hemocytes
Cellular defense in flies primarily comprises of hemocyte mediated immune responses such as phagocytosis and encapsulation [37, 56]. At the initiation of phagocytosis, hemocytes are known to generate reactive oxygen intermediates (ROI) such as O2- and H2O2 [57, 58]. Certain stresses such as heavy metals exposure or hypoxia have been known to enhance ROS generation in hemocytes, thereby mortality in invertebrates [59-61]. In order to understand the mechanism(s) by which Hsp70 over-expression in hemocytes lowers ROS levels in adult flies, we hypothesized that hemocytes release ROS under hypoxia or hyperoxia, which, in turn, can be detrimental to survival in flies. Indeed, when we tested flies in which hemocytes are removed (cell specific ablation by UAS-hid or reaper and He-Gal4 driver), they had a similar survival as lines over-expressing Hsp70 in hemocytes under severe hypoxia (Figure 9). In order to verify that hemocytes were eliminated, we viewed the F1 adult flies, larvae (UAS-hid/ UAS-GFPHe-Gal4) and hemocytes isolated from larvae on a slide under fluorescent microscopy. We did not observe any GFP positive cells in the adult flies (number of adults tested n=45) as well as larvae in which hemocytes were eliminated (number of larvae dissected n=45) (Figure 2). Similar results were obtained with F1- UAS-rpr/UASGFPHe-Gal4 (n=95). These lines (F1-UAS-reaper or hid X He-Gal4) had a survival of 85% even after 12 days of 1.5% O2 which was significantly higher than the controls (10%) (P<0.05, unpaired t-test). Their survival under hypoxia is similar to Hsp70 over-expression lines in hemocytes as shown is Figure 9. Interestingly, ROS levels of the lines in which hemocytes are eliminated (F1 – lines of UAS-hid, reaper, and He-Gal4) were significantly lower than controls under hypoxia (Figure 10). Even after 10 days of hypoxia, flies without hemocytes (F1-UAS-hid-he-Gal4, UAS-reaper-he-Gal4) had ROS levels that were 7 times lower than those in CS controls (P<0.05). No significant differences were observed with the oxidation-insensitive probe C369 of Dichlorofluorescein.
Figure 9.
Survival of adult flies exposed to hypoxia (1.5% O2). Flies in which hemocytes are depleted by crossing either hid or reaper have similar increased survival as Hsp70 over-expression lines in hemocytes. Each point represents the average of at least three tests for each line and error bars represent the standard errors. * With unpaired t-test P<0.05 with controls vs. the genotype.
Figure 10.
Relative ROS levels under 1.5% O2.Flies in which hemocytes are killed by reaper or hid have lower ROS levels than controls. Each point represents the average of at least three tests for each line and error bars represent the standard errors. * With unpaired t-test P<0.05 with controls vs. the genotype.
Discussion
Using gene expression analysis and functional assays, we have recently shown that an up regulation of Hsp70 remarkably improves survival of flies under severe hypoxia [8]. In this current study, we employed the UAS–Gal4 system to further dissect the protective role of Hsp70 in specific tissues in vivo under similar conditions. We found that Hsp70 plays an important role in various tissues during such conditions. First, we found that Hsp70 over-expression in heart leads to better survival than controls in severe hypoxia. Surprisingly, we also observed that Hsp70 over-expression in hemocytes provides even further survival benefit under such a stress. On the other hand, flies with no copies of Hsp70 (Hsp70-) or in which Hsp70Bbb was knocked down in hemocytes survived significantly less as compared to CS control under 1.5% O2, suggesting that Hsp70 plays an important role in hypoxia tolerance. Interestingly, when we over-expressed Hsp70 in hemocytes, flies were not only tolerant to severe hypoxia but also to other stresses such as oxidant stress using paraquat feeding or hyperoxia. To explore the possible link between Hsp70 over-expression, oxidant stress and hypoxia, we examined the relation between Hsp70 and ROS generation.
Our data have demonstrated that over expression of Hsp70 in hemocytes lowered ROS levels in whole flies under hypoxia. This suggested to us that a) hemocytes may be a major source of ROS generation and this may cause injury and increased mortality in flies during hypoxia and b) there is a major potential link between Hsp70 and ROS. There are several invertebrate studies that have monitored the effect of various stresses such as hypoxia, pH, hypercapnia or heavy metals on ROS generation in hemocytes [57, 58, 61, 62]. Similarly in mammalian systems, the change of ROS generation and phagocytic activity of macrophages under hypoxic stress have been extensively studied [63-66]. For example, in mammalian macrophages studies [63, 66], hypoxia significantly increased ROS production and phagocytosis whereas in another study in Crab population hypercapnic hypoxia decreased ROI production and the phagocytic activity of hemocytes. The conclusions that can be drawn from these studies vary depending on the duration and severity of hypoxia. In our study, we obtained in vivo evidence that hemocytes generate ROS during severe hypoxia by studying the effect of elimination of hemocytes using UAS-hid or reaper X He-Gal4 crosses. Indeed, we found that flies in which hemocytes are eliminated have significantly lower ROS levels and survive much better than controls during hypoxia. In addition, since over-expression of Hsp70 in hemocytes considerably lowers ROS levels of flies during hypoxia and provides a survival benefit, we believe that this protective effect of Hsp70 can be linked to lowering ROS generation by hemocytes in such conditions. Similar studies have demonstrated a protective role of heat shock proteins under hypoxic conditions [9, 14, 17, 25, 30, 31] or in pre-conditioning experiments in myocytes. However, this is the first study showing a close link between hemocytes and a survival advantage when Hsp are over-expressed in hemocytes or when hemocytes are eliminated from the whole fly. One of the potential damaging effects of severe hypoxia is the generation of reactive oxygen species [53-55]. This prompted us to investigate the effect of Hsp70 over-expression using other well known free radical generating paradigms such as hyperoxia. Hsp70 over-expression in hemocytes protected flies under hyperoxia as well paraquat feeding. This is intriguing since hypoxia and hyperoxia are very different physiological processes[67] and cells have distinct responses to both stresses. However, it is possible that some of the underlying mechanisms leading to injury are similar, such as the signaling pathways of ROS generation. Indeed, we found that the survival of flies, under hypoxia and hyperoxia, was linked to ROS generation since flies, fed an anti-oxidant such as NAC, had lower ROS levels and a remarkable improvement in survival as compared to flies fed regular food. Interestingly, we found that Hsp70 over-expression in hemocytes during larval stages had similar effect as adults in lowering the ROS levels after exposure to paraquat, suggesting that similar pathways may be involved in protecting the larvae from oxidative stress.
How Hsp70 up regulation in hemocytes lowers ROS levels in whole flies is not clear at this time. There is evidence that shows that heat shock proteins can selectively protect mitochondria against oxidative injury by preventing alterations in mitochondrial membrane potential and cristae formation [68]. This is a potentially important mechanism since mitochondria is the main source of ROS generation during oxidative stress conditions such as hypoxia or hyperoxia[67]. Heat shock proteins also have an ability to protect cells and tissues from damaging effects of inflammation[69]. Some of the potential mechanism(s) that can explain the protective role of Hsp70 include the inhibition of NADPH oxidase activity and, reducing TNF- levels and its cytotoxic effects.[69].
In summary, in this study we examined the role of Hsp70 over-expression in certain tissue under severe hypoxia using Drosophila as the model system. This study has demonstrated that a) Hsp70 over-expression in heart and hemocytes provides significant survival benefit under hypoxia, b) the improved survival by Hsp70 up regulation in hemocytes is linked to ROS reduction in whole flies, and c) hemocytes may be a major source of ROS under hypoxia and hence their elimination lowers ROS levels and increases survival.
Acknowledgments
We sincerely thank Dr. Tian Xu, Dr. José Carlos Pastor-Pareja and Dr. Rolf Bodmer for sharing fly stocks, Dr. Dan Zhou for suggestions and Ms. Orit Gavrialov, Ms. Ying Lu-Bo, and Ms. Mary Hsiao for technical assistance. This work has been supported by NIH grant (RO1 NS037756) to GGH.
List of Abbreviations
- CH
Constant hypoxia
- UAS
Upstream activating sequence
- He
Hemese
- CS
Canton S
- yw
yellow white
- Hsp70
Heat shock protein 70
- ROS
reactive oxygen species
- rpr
reaper
- hid
head involution defective
- GFP
Green Fluorescent protein
- DCFH
Dichlorofluorescein
- NAC
N- Acetylcysteine
References
- 1.Douglas RM, Miyasaka N, Takahashi K, Latuszek-Barrantes A, Haddad GG, Hetherington HP. Chronic intermittent but not constant hypoxia decreases NAA/Cr ratios in neonatal mouse hippocampus and thalamus. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1254–1259. doi: 10.1152/ajpregu.00404.2006. [DOI] [PubMed] [Google Scholar]
- 2.Fan C, Iacobas DA, Zhou D, Chen Q, Lai JK, Gavrialov O, Haddad GG. Gene expression and phenotypic characterization of mouse heart after chronic constant or intermittent hypoxia. Physiol Genomics. 2005;22:292–307. doi: 10.1152/physiolgenomics.00217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu XQ, Haddad GG. Decreased neuronal excitability in hippocampal neurons of mice exposed to cyclic hypoxia. J Appl Physiol. 2001;91:1245–1250. doi: 10.1152/jappl.2001.91.3.1245. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Zhu HF, Xia R, Zhou ZN, Zhu PH. Effect of intermittent and continuous hypoxia on ryanodine receptors of rat heart. Life Sci. 2003;73:2151–2160. doi: 10.1016/s0024-3205(03)00599-x. [DOI] [PubMed] [Google Scholar]
- 5.Zhou D, Wang J, Zapala MA, Xue J, Schork NJ, Haddad GG. Gene expression in mouse brain following chronic hypoxia: role of sarcospan in glial cell death. Physiol Genomics. 2008;32:370–379. doi: 10.1152/physiolgenomics.00147.2007. [DOI] [PubMed] [Google Scholar]
- 6.Farahani R, Kanaan A, Gavrialov O, Brunnert S, Douglas RM, Morcillo P, Haddad GG. Differential effects of chronic intermittent and chronic constant hypoxia on postnatal growth and development. Pediatr Pulmonol. 2008;43:20–28. doi: 10.1002/ppul.20729. [DOI] [PubMed] [Google Scholar]
- 7.Kanaan A, Farahani R, Douglas RM, Lamanna JC, Haddad GG. Effect of chronic continuous or intermittent hypoxia and reoxygenation on cerebral capillary density and myelination. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1105–1114. doi: 10.1152/ajpregu.00535.2005. [DOI] [PubMed] [Google Scholar]
- 8.Azad P, Zhou D, Russo E, Haddad GG. Distinct mechanisms underlying tolerance to intermittent and constant hypoxia in Drosophila melanogaster. PLoS One. 2009;4:e5371. doi: 10.1371/journal.pone.0005371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giffard RG, Xu L, Zhao H, Carrico W, Ouyang Y, Qiao Y, Sapolsky R, Steinberg G, Hu B, Yenari MA. Chaperones, protein aggregation, and brain protection from hypoxic/ischemic injury. J Exp Biol. 2004;207:3213–3220. doi: 10.1242/jeb.01034. [DOI] [PubMed] [Google Scholar]
- 10.Bergeron M, Mivechi NF, Giaccia AJ, Giffard RG. Mechanism of heat shock protein 72 induction in primary cultured astrocytes after oxygen-glucose deprivation. Neurol Res. 1996;18:64–72. doi: 10.1080/01616412.1996.11740380. [DOI] [PubMed] [Google Scholar]
- 11.Brar BK, Stephanou A, Wagstaff MJ, Coffin RS, Marber MS, Engelmann G, Latchman DS. Heat shock proteins delivered with a virus vector can protect cardiac cells against apoptosis as well as against thermal or hypoxic stress. J Mol Cell Cardiol. 1999;31:135–146. doi: 10.1006/jmcc.1998.0857. [DOI] [PubMed] [Google Scholar]
- 12.Das DK, Maulik N. Cardiac genomic response following preconditioning stimulus. Cardiovasc Res. 2006;70:254–263. doi: 10.1016/j.cardiores.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 13.Date T, Mochizuki S, Belanger AJ, Yamakawa M, Luo Z, Vincent KA, Cheng SH, Gregory RJ, Jiang C. Expression of constitutively stable hybrid hypoxia-inducible factor-1alpha protects cultured rat cardiomyocytes against simulated ischemia-reperfusion injury. Am J Physiol Cell Physiol. 2005;288:C314–320. doi: 10.1152/ajpcell.00374.2004. [DOI] [PubMed] [Google Scholar]
- 14.Gray CC, Amrani M, Yacoub MH. Heat stress proteins and myocardial protection: experimental model or potential clinical tool? Int J Biochem Cell Biol. 1999;31:559–573. doi: 10.1016/s1357-2725(99)00004-7. [DOI] [PubMed] [Google Scholar]
- 15.Guo S, Wharton W, Moseley P, Shi H. Heat shock protein 70 regulates cellular redox status by modulating glutathione-related enzyme activities. Cell Stress Chaperones. 2007;12:245–254. doi: 10.1379/CSC-265.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heads RJ, Yellon DM, Latchman DS. Differential cytoprotection against heat stress or hypoxia following expression of specific stress protein genes in myogenic cells. J Mol Cell Cardiol. 1995;27:1669–1678. doi: 10.1016/s0022-2828(95)90722-x. [DOI] [PubMed] [Google Scholar]
- 17.Lee JE, Yenari MA, Sun GH, Xu L, Emond MR, Cheng D, Steinberg GK, Giffard RG. Differential neuroprotection from human heat shock protein 70 overexpression in in vitro and in vivo models of ischemia and ischemia-like conditions. Exp Neurol. 2001;170:129–139. doi: 10.1006/exnr.2000.7614. [DOI] [PubMed] [Google Scholar]
- 18.Liu JC, He M, Wan L, Cheng XS. Heat shock protein 70 gene transfection protects rat myocardium cell against anoxia-reoxygeneration injury. Chin Med J (Engl) 2007;120:578–583. [PubMed] [Google Scholar]
- 19.Matsumori Y, Hong SM, Aoyama K, Fan Y, Kayama T, Sheldon RA, Vexler ZS, Ferriero DM, Weinstein PR, Liu J. Hsp70 overexpression sequesters AIF and reduces neonatal hypoxic/ischemic brain injury. J Cereb Blood Flow Metab. 2005;25:899–910. doi: 10.1038/sj.jcbfm.9600080. [DOI] [PubMed] [Google Scholar]
- 20.Meerson FZ, Malyshev I, Zamotrinskii AV, Vorontsova E. Cardioprotective effects of adaptation to restraint stress and hypoxia. Kardiologiia. 1992;32:43–48. [PubMed] [Google Scholar]
- 21.Mestril R, Chi SH, Sayen MR, O’Reilly K, Dillmann WH. Expression of inducible stress protein 70 in rat heart myogenic cells confers protection against simulated ischemia-induced injury. J Clin Invest. 1994;93:759–767. doi: 10.1172/JCI117030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papadopoulos MC, Sun XY, Cao J, Mivechi NF, Giffard RG. Over-expression of HSP-70 protects astrocytes from combined oxygen-glucose deprivation. Neuroreport. 1996;7:429–432. doi: 10.1097/00001756-199601310-00013. [DOI] [PubMed] [Google Scholar]
- 23.Shinohara T, Takahashi N, Kohno H, Yamanaka K, Ooie T, Wakisaka O, Murozono Y, Taniguchi Y, Torigoe Y, Hara M, Shimada T, Saikawa T, Yoshimatsu H. Mitochondria are targets for geranylgeranylacetone-induced cardioprotection against ischemia-reperfusion in the rat heart. Am J Physiol Heart Circ Physiol. 2007;293:H1892–1899. doi: 10.1152/ajpheart.00493.2007. [DOI] [PubMed] [Google Scholar]
- 24.Williamson CL, Dabkowski ER, Dillmann WH, Hollander JM. Mitochondria protection from hypoxia/reoxygenation injury with mitochondria heat shock protein 70 overexpression. Am J Physiol Heart Circ Physiol. 2008;294:H249–256. doi: 10.1152/ajpheart.00775.2007. [DOI] [PubMed] [Google Scholar]
- 25.Xu L, Dayal M, Ouyang YB, Sun Y, Yang CF, Frydman J, Giffard RG. Chaperonin GroEL and its mutant D87K protect from ischemia in vivo and in vitro. Neurobiol Aging. 2006;27:562–569. doi: 10.1016/j.neurobiolaging.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 26.Yeh CH, Hsu SP, Yang CC, Chien CT, Wang NP. Hypoxic preconditioning reinforces HIF-alpha-dependent HSP70 signaling to reduce ischemic renal failure-induced renal tubular apoptosis and autophagy. Life Sci. 86:115–123. doi: 10.1016/j.lfs.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 27.Zhang K, Zhao T, Huang X, Liu ZH, Xiong L, Li MM, Wu LY, Zhao YQ, Zhu LL, Fan M. Preinduction of HSP70 promotes hypoxic tolerance and facilitates acclimatization to acute hypobaric hypoxia in mouse brain. Cell Stress Chaperones. 2009;14:407–415. doi: 10.1007/s12192-008-0094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Didelot C, Schmitt E, Brunet M, Maingret L, Parcellier A, Garrido C. Heat shock proteins: endogenous modulators of apoptotic cell death. Handb Exp Pharmacol. 2006:171–198. doi: 10.1007/3-540-29717-0_8. [DOI] [PubMed] [Google Scholar]
- 29.Ilangovan G, Venkatakrishnan CD, Bratasz A, Osinbowale S, Cardounel AJ, Zweier JL, Kuppusamy P. Heat shock-induced attenuation of hydroxyl radical generation and mitochondrial aconitase activity in cardiac H9c2 cells. Am J Physiol Cell Physiol. 2006;290:C313–324. doi: 10.1152/ajpcell.00362.2005. [DOI] [PubMed] [Google Scholar]
- 30.Chong KY, Lai CC, Lille S, Chang C, Su CY. Stable overexpression of the constitutive form of heat shock protein 70 confers oxidative protection. J Mol Cell Cardiol. 1998;30:599–608. doi: 10.1006/jmcc.1997.0623. [DOI] [PubMed] [Google Scholar]
- 31.Xu L, Voloboueva LA, Ouyang Y, Emery JF, Giffard RG. Overexpression of mitochondrial Hsp70/Hsp75 in rat brain protects mitochondria, reduces oxidative stress, and protects from focal ischemia. J Cereb Blood Flow Metab. 2009;29:365–374. doi: 10.1038/jcbfm.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joly AL, Wettstein G, Mignot G, Ghiringhelli F, Garrido C. Dual Role of Heat Shock Proteins as Regulators of Apoptosis and Innate Immunity. Journal of Innate Immunity. 2010;2: 238–247. doi: 10.1159/000296508. [DOI] [PubMed] [Google Scholar]
- 33.Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–476. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- 34.Asea A. Chaperokine-induced signal transduction pathways. Exerc Immunol Rev. 2003;9: 25–33. [PMC free article] [PubMed] [Google Scholar]
- 35.Holz A, Bossinger B, Strasser T, Janning W, Klapper R. The two origins of hemocytes in Drosophila. Development. 2003;130:4955–4962. doi: 10.1242/dev.00702. [DOI] [PubMed] [Google Scholar]
- 36.Charroux B, Royet J. Elimination of plasmatocytes by targeted apoptosis reveals their role in multiple aspects of the Drosophila immune response. Proc Natl Acad Sci U S A. 2009;106:9797–9802. doi: 10.1073/pnas.0903971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lavine MD, Strand MR. Insect hemocytes and their role in immunity. Insect Biochem Mol Biol. 2002;32:1295–1309. doi: 10.1016/s0965-1748(02)00092-9. [DOI] [PubMed] [Google Scholar]
- 38.Williams MJ. Drosophila hemopoiesis and cellular immunity. J Immunol. 2007;178:4711–4716. doi: 10.4049/jimmunol.178.8.4711. [DOI] [PubMed] [Google Scholar]
- 39.Jiravanichpaisal P, Lee BL, Soderhall K. Cell-mediated immunity in arthropods: Hematopoiesis, coagulation, melanization and opsonization. Immunobiology. 2006;211:213–236. doi: 10.1016/j.imbio.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 40.Kurucz E, Vaczi B, Markus R, Laurinyecz B, Vilmos P, Zsamboki J, Csorba K, Gateff E, Hultmark D, Ando I. Definition of Drosophila hemocyte subsets by cell–type specific antigens. Acta Biol Hung. 2007;58(Suppl):95–111. doi: 10.1556/ABiol.58.2007.Suppl.8. [DOI] [PubMed] [Google Scholar]
- 41.Markus R, Laurinyecz B, Kurucz E, Honti V, Bajusz I, Sipos B, Somogyi K, Kronhamn J, Hultmark D, Ando I. Sessile hemocytes as a hematopoietic compartment in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2009;106:4805–4809. doi: 10.1073/pnas.0801766106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meister M, Lagueux M. Drosophila blood cells. Cell Microbiol. 2003;5:573–580. doi: 10.1046/j.1462-5822.2003.00302.x. [DOI] [PubMed] [Google Scholar]
- 43.Stofanko M, Kwon SY, Badenhorst P. Lineage tracing of lamellocytes demonstrates Drosophila macrophage plasticity. PLoS One. 5:e14051. doi: 10.1371/journal.pone.0014051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nappi AJ, Vass E. Cytotoxic reactions associated with insect immunity. Phylogenetic Perspectives on the Vertebrate Immune System. 2001;484:329–348. doi: 10.1007/978-1-4615-1291-2_33. [DOI] [PubMed] [Google Scholar]
- 45.Brack C, Bechter-Thuring E, Labuhn M. N-acetylcysteine slows down ageing and increases the life span of Drosophila melanogaster. Cell Mol Life Sci. 1997;53:960–966. doi: 10.1007/PL00013199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonilla E, Medina-Leendertz S, Villalobos V, Molero L, Bohorquez A. Paraquat-induced oxidative stress in drosophila melanogaster: effects of melatonin, glutathione, serotonin, minocycline, lipoic acid and ascorbic acid. Neurochem Res. 2006;31:1425–1432. doi: 10.1007/s11064-006-9194-8. [DOI] [PubMed] [Google Scholar]
- 47.Lee KS, Iijima-Ando K, Iijima K, Lee WJ, Lee JH, Yu K, Lee DS. JNK/FOXO-mediated neuronal expression of fly homologue of peroxiredoxin II reduces oxidative stress and extends life span. J Biol Chem. 2009;284:29454–29461. doi: 10.1074/jbc.M109.028027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zettervall CJ, Anderl I, Williams MJ, Palmer R, Kurucz E, Ando I, Hultmark D. A directed screen for genes involved in Drosophila blood cell activation. Proc Natl Acad Sci U S A. 2004;101:14192–14197. doi: 10.1073/pnas.0403789101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salnikow K, Su W, Blagosklonny MV, Costa M. Carcinogenic metals induce hypoxia-inducible factor-stimulated transcription by reactive oxygen species-independent mechanism. Cancer Res. 2000;60:3375–3378. [PubMed] [Google Scholar]
- 50.Kurucz E, Zettervall CJ, Sinka R, Vilmos P, Pivarcsi A, Ekengren S, Hegedus Z, Ando I, Hultmark D. Hemese, a hemocyte-specific transmembrane protein, affects the cellular immune response in Drosophila. Proc Natl Acad Sci U S A. 2003;100:2622–2627. doi: 10.1073/pnas.0436940100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 52.Walter S, Buchner J. Molecular chaperones--cellular machines for protein folding. Angew Chem Int Ed Engl. 2002;41:1098–1113. doi: 10.1002/1521-3773(20020402)41:7<1098::aid-anie1098>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 53.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Desireddi JR, Farrow KN, Marks JD, Waypa GB, Schumacker PT. Hypoxia increases ROS signaling and cytosolic Ca(2+) in pulmonary artery smooth muscle cells of mouse lungs slices. Antioxid Redox Signal. 12:595–602. doi: 10.1089/ars.2009.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91:807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- 56.Kedra E. The role of hemocytes in the defensive reactions in insect. Wiad Parazytol. 2003;49:311–312. [PubMed] [Google Scholar]
- 57.Boyd JN, Burnett LE. Reactive oxygen intermediate production by oyster hemocytes exposed to hypoxia. J Exp Biol. 1999;202(Pt 22):3135–3143. doi: 10.1242/jeb.202.22.3135. [DOI] [PubMed] [Google Scholar]
- 58.Moss B, Allam B. Fluorometric measurement of oxidative burst in lobster hemocytes and inhibiting effect of pathogenic bacteria and hypoxia. Journal of Shellfish Research. 2006;25:1051–1057. [Google Scholar]
- 59.Dailianis S. Production of superoxides and nitric oxide generation in haemocytes of mussel Mytilus galloprovincialis (Lmk.) after exposure to cadmium: a possible involvement of Na(+)/H(+) exchanger in the induction of cadmium toxic effects. Fish Shellfish Immunol. 2009;27:446–453. doi: 10.1016/j.fsi.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 60.Gomez-Mendikute A, Cajaraville MP. Comparative effects of cadmium, copper, paraquat and benzo[a]pyrene on the actin cytoskeleton and production of reactive oxygen species (ROS) in mussel haemocytes. Toxicol In Vitro. 2003;17:539–546. doi: 10.1016/s0887-2333(03)00093-6. [DOI] [PubMed] [Google Scholar]
- 61.Xian JA, Wang AL, Ye CX, Chen XD, Wang WN. Phagocytic activity, respiratory burst, cytoplasmic free-Ca(2+) concentration and apoptotic cell ratio of haemocytes from the black tiger shrimp, Penaeus monodon under acute copper stress. Comp Biochem Physiol C Toxicol Pharmacol. 152:182–188. doi: 10.1016/j.cbpc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 62.Tanner CA, Burnett LE, Burnett KG. The effects of hypoxia and pH on phenoloxidase activity in the Atlantic blue crab, Callinectes sapidus. Comp Biochem Physiol A Mol Integr Physiol. 2006;144:218–223. doi: 10.1016/j.cbpa.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 63.Anand RJ, Gribar SC, Li J, Kohler JW, Branca MF, Dubowski T, Sodhi CP, Hackam DJ. Hypoxia causes an increase in phagocytosis by macrophages in a HIF-1alpha-dependent manner. J Leukoc Biol. 2007;82:1257–1265. doi: 10.1189/jlb.0307195. [DOI] [PubMed] [Google Scholar]
- 64.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kundu N, Zhang S, Fulton AM. Sublethal oxidative stress inhibits tumor cell adhesion and enhances experimental metastasis of murine mammary carcinoma. Clin Exp Metastasis. 1995;13:16–22. doi: 10.1007/BF00144014. [DOI] [PubMed] [Google Scholar]
- 66.Rydberg EK, Salomonsson L, Hulten LM, Noren K, Bondjers G, Wiklund O, Bjornheden T, Ohlsson BG. Hypoxia increases 25-hydroxycholesterol-induced interleukin-8 protein secretion in human macrophages. Atherosclerosis. 2003;170:245–252. doi: 10.1016/s0021-9150(03)00302-2. [DOI] [PubMed] [Google Scholar]
- 67.Cataldi A. Cell responses to oxidative stressors. Curr Pharm Des. 16:1387–1395. doi: 10.2174/138161210791033969. [DOI] [PubMed] [Google Scholar]
- 68.Polla BS, Kantengwa S, Francois D, Salvioli S, Franceschi C, Marsac C, Cossarizza A. Mitochondria are selective targets for the protective effects of heat shock against oxidative injury. Proc Natl Acad Sci U S A. 1996;93:6458–6463. doi: 10.1073/pnas.93.13.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jacquier-Sarlin MR, Fuller K, Dinh-Xuan AT, Richard MJ, Polla BS. Protective effects of hsp70 in inflammation. Experientia. 1994;50:1031–1038. doi: 10.1007/BF01923458. [DOI] [PubMed] [Google Scholar]