Abstract
Neurons of the respiratory network in the lower brainstem express a variety of serotonin receptors (5-HTRs) that act primarily through adenylyl cyclase. However, there is one receptor family including 5-HT2A, 5-HT2B, and 5-HT2C receptors that are directed towards protein kinase C (PKC). In contrast to 5-HT2ARs, expression and function of 5-HT2BRs within the respiratory network are still unclear. 5-HT2BR utilizes a Gq-mediated signaling cascade involving calcium and leading to activation of phospholipase C and IP3/DAG pathways. Based on previous studies, this signal pathway appears to mediate excitatory actions on respiration. In the present study, we analyzed receptor expression in pontine and medullary regions of the respiratory network both at the transcriptional and translational level using quantitative RT-PCR and self-made as well as commercially available antibodies, respectively. In addition we measured effects of selective agonists and antagonists for 5-HT2ARs and 5-HT2BRs given intra-arterially on phrenic nerve discharges in juvenile rats using the perfused brainstem preparation. The drugs caused significant changes in discharge activity. Co-administration of both agonists revealed a dominance of the 5-HT2BR. Given the nature of the signaling pathways, we investigated whether intracellular calcium may explain effects observed in the respiratory network. Taken together, the results of this study suggest a significant role of both receptors in respiratory network modulation.
Introduction
Immunohistochemical and electrophysiological studies carried out over the previous twenty years have provided considerable evidence that serotonin (5-HT) released from caudal medullary raphé nuclei modulates respiratory network discharges in bulbar and spinal regions [1]–[8]. Subsequent research set out to determine which subtypes of 5-HT receptors (5-HTRs) are operative as pharmacological targets for a potential therapy to treat centrally caused breathing disturbances [9]–[17]. Those studies revealed that 5-HT1A, 5-HT2A/C, and 5-HT4(a) receptors modulate respiratory network discharge properties. These receptors represent only a fraction of the 5-HTR subtypes that modulate excitability of CNS neurons through various signaling pathways.
Amongst the 5-HTR family 5-HT2Rs include 5-HT2A, 5-HT2B, and 5-HT2CR isoforms that couple preferentially to Gq/11-proteins. The resulting activation of phospholipase C (PLC) increases hydrolysis of inositol phosphates and elevates cytosolic Ca2+ [18], [19]. 5-HT2Rs are located post-synaptically [20]–[22], and there is evidence that they modulate neurotransmission at various central and peripheral synaptic sites [23], [24].
5-HT2ARs stimulate PLC, leading to activation of protein kinase C (PKC), and increased excitability in bulbar respiratory neurons [25]–[27]. Earlier studies demonstrated PKC pathway-mediated modulation of the respiratory pattern [26] and excitation of respiratory neurons by activation of 5-HT2ARs [25], [27]. Beside direct modulation of the respiratory motor pattern, 5-HT2ARs may have a key role in the induction of long-term facilitation of phrenic nerve activity in response to intermittent hypoxia [28]–[31].
5-HT2BRs have been implicated in anxiety, schizophrenia, autism, migraine, and spreading depression [32]. In addition, 5-HT2BR-dependent serotonin uptake influences the plasma serotonin level [33]. 5-HT2BRs are also important regulators of embryonic development; inactivation of the 5-HT2BR gene leads to partial embryonic and early neonatal death in mice [34]. In the respiratory network, it has been shown that 5-HT2BRs enhance rhythmic motor discharge activity recorded in neonatal mice in vitro [35].
Until now, there were no detailed descriptions published of 5-HT2BR distribution in the brainstem with expression data only available for the neocortex, cerebellum, dorsal hypothalamus, and medial amygdala [36].
In the present report, we provide a detailed account of the expression and distribution of 5-HT2BRs and 5-HT2ARs in the ponto-medullary respiratory network including respiratory motor population of the cervical spinal cord and brainstem. Using a monospecific anti-5-HT2BR-antibody prepared in our laboratory and a commercially available 5-HT2AR antibody, we show that 5-HT2BRs and 5-HT2ARs are expressed in neurons of the pre-Bötzinger complex (pre-BötC), an essential kernel of the respiratory network associated with the primary rhythmogenesis [37]–[40].
Furthermore, our study also demonstrates that both receptors affect discharge properties in the phrenic motor output.
Materials and Methods
The experimental procedures were performed in accordance with European Community and National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The Ethics Committee of the Georg-August-University, Göttingen, Germany approved the study and assigned the approval ID T1108 to this work.
Antibody generation
The polyclonal antibodies against the rat 5-HT2BR were generated by immunizing three New Zealand White rabbits (Charles River) with a 16mer peptide derived from the second intracellular loop of the rat 5-HT2BR amino acid sequence (NH2-CAISLDRYIAIKKPIQ-COOH; NCBI-Accession No.: NP_058946). For immunization purposes, peptides were coupled to keyhole limpet hemocyanin (KLH) according to standard protocols. The rabbits were immunized with 300 µg of KLH-coupled peptide in Hunter's adjuvant (TiterMax Gold, Sigma) five times (28-days-intervall). The resulting antiserum was affinity-purified against the immunizing peptide.
Western Blot detection of 5-HT2BR protein
Brain stem tissue isolated from both male Sprague-Dawley rats and male C57BL/6J mice were resuspended in 200 µl cell lysis buffer (50 mM Tris/HCl, pH 8.0, 150 mM NaCl, 2% w/v SDS, 1% NP40, 0.5% Na-Deoxycholate) supplemented with protease inhibitor cocktail (Sigma). Protein content was determined using a Lowry assay for high SDS concentration according to manufacturer's instructions (BioRad). 40 µg of total protein of each sample were boiled in 5× Laemmli buffer (250 mM Tris/HCl, pH 6.8; 10 mM EDTA, 10% w/v SDS, 5% v/v 2-mercaptoethanol, 50% v/v glycerol, 0.5% w/v bromophenol blue) for 5 min at 95°C and then separated using a precast SDS-PAGE (Novex). Proteins were transferred to a nitrocellulose membrane. The membrane was blocked with 4% w/v BSA/TBS/0.05%Tween (pH 7.4) for 45 min at RT. 5-HT2BR protein was detected with a self-made monospecific polyclonal antibody (1∶1,000 dilution) after incubation for 3 hours at RT. After extensive washing, appropriate secondary horseradish peroxidase (HRP)-conjugated antibodies (Dianova, Hamburg, Germany) were used at a dilution of 1∶20,000 for 2 hours at RT. The visualization of the antigen-antibody reaction was performed with enhanced chemiluminescence (ECL) kit (BioRad, Germany).
Immunohistochemistry
(a) Preparation of brain tissue
Male juvenile Sprague-Dawley rats (P25–P32) were deeply anesthetized with isoflurane (1-Chloro-2,2,2-trifluoroethyl-difluoromethylether, Abbott, Wiesbaden, Germany) until they were unresponsive to painful stimuli (pressure applied to a forepaw). A thoracotomy was performed, and animals were transcardially perfused with 50 ml of 0.9% NaCl followed by 200 ml of 4% phosphate-buffered formaldehyde (10 ml/min). The brain was removed and post-fixed for 4 hours with the same fixative at 4°C, cryoprotected in 10% sucrose for 2 hours followed by 30% sucrose in 0.1 M phosphate buffer overnight at 4°C, and then frozen at −25°C. Series of 20- and 40-µm-thick brain sections were cut from cervical spinal cord to midbrain collicular level using a freezing microtome (Frigocut, Reichert-Jung, Germany).
(b) Peroxidase anti-peroxidase (PAP) staining
The endogenous peroxidase activity of free-floating sections was blocked with methanol/30% H2O2 (1∶100 dilution) for 45 min at room temperature (RT) in the dark. After washing, sections were permeabilized with 0.2% Triton X-100 for 30 min. Sections were transferred for 30 min into phosphate buffered saline (PBS; pH 7.4) containing 5% bovine serum albumin (BSA) at RT for blocking non-specific binding sites. Our homemade affinity-purified rabbit anti-5-HT2BR antibodies or mouse anti-5-HT2AR antibodies (BD Bioscience, Cat. No.: 556326, San Diego, USA) were applied at a concentration of 2–5 µg/ml in a carrier-solution of 2% BSA/PBS. Sections were incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit- or anti-mouse IgG antibodies (Dianova; 1∶4,000 diluted in 2% BSA/PBS) for 1 h at RT, washed, and subsequently incubated with freshly prepared diaminobenzidine (DAB) solution for 10 min at RT. Sections were mounted onto gelatine-coated microscope slides, dehydrated in increasing ethanol concentrations (2×50%, 2×80%, 2×99.9%), cleared with four changes of xylene, and finally coverslipped with DePeX (Serva, Germany).
(c) Immunofluorescence
Sections were permeabilized with 0.2% Triton X-100 for 30 min at RT and then washed two times with PBS (pH 7.4). Non-specific binding sites were blocked with PBS containing 5% BSA for 1 h at RT. Sections were incubated with primary antibodies (2–5 µg/ml) for 4 hours at RT. After washing, sections were incubated for 2 hours at RT in the dark with species-specific Cy2- or Cy5-conjugated secondary antibodies (Dianova, Germany; 2% BSA/PBS, antibody dilution 1∶400). Neuronal immunofluorescence was analyzed with a confocal laser-scanning microscope Meta-LSM 510 (Zeiss, Germany) using laser lines at 488 nm (Ar/Kr laser) and at 633 nm (He/Ne laser). Confocal images were processed by using overlays of two channels with the LSM 510 software provided by Zeiss. Digital images were taken at 2,048×2,048 dpi and were imported into Adobe Photoshop CS4, were digitally adjusted if necessary for brightness and contrast and were assembled into plates. Subsequent imaging procedures (cell counting) were performed using ImageJ (http://rsb.info.nih.gov/ij/).
Molecular Biology
(a) Generation of expression constructs
Brain tissue from one male rat at P11 was explanted and used for total RNA isolation with the OLS RNA kit (OLS, Germany) according to manufacturer's instructions. The total RNA was used in one-step RT-PCR (Invitrogen) using primer pairs for the 5-HT2AR gene [Htr2a, F (5′-atggaaattctttgtgaagac-3′)/R (5′-tcacacacagctaaccttttc-3′)] and 5-HT2BR gene [Htr2b, F (5′-atggcttcatcttataaaatgtc-3′)/R (5′-ctatatgtagctgacttggtcttc-3′)], respectively. The cycling program used for RT-PCR comprised of: initial reverse transcription at 55°C for 30 min followed by denaturation at 94°C for 2 min. 40 cycles of denaturation at 94°C for 15 sec, annealing at 57°C for 30 sec, and elongation at 68°C for 90 sec were concluded with a final elongation step at 68°C for 5 min. The resulting RT-PCR fragment was purified from the gel and cloned into pTarget expression vector (Promega). Sequencing validated the correct insert identity.
(b) Transfection of cell lines
Murine neuroblastoma cell line N1E-115 was obtained from ATCC and maintained at 37°C in humid atmosphere with 5% CO2 and passaged every second day. For transfection, cells were seeded 24 hours prior to transfection at a density of 100,000 cells in 4-well-plates (Nunc) on acid-washed and poly-L-lysine coated 12 mm round glass cover slips. Cells were transfected with 2 µg DNA and 2 µl Lipofectamine (Invitrogen) in 500 µl OptiMEM (Invitrogen) per well and kept under normal culture conditions for 20 hours, afterwards fresh OptiMEM replaced the medium.
(c) Detection of endogenous Htr2b in cell lines by RT-PCR
Total RNA from 107 non-transfected cells or cells transfected with 6 µg of the plasmid encoding 5-HT2BR was prepared using the OLS RNA kit. One µg of total RNA each was entered in the one-step-RT-PCR reaction, and the resulting PCR fragment was analyzed on an agarose gel. While the 5′-sequence of murine and rat Htr2b is identical, the 3′-sequences do differ. Therefore, for RT-PCR the rat forward primer was used, while the reverse primer for mouse was 5′-ctatatgtagctgacctgctcttc-3′.
(d) RT-PCR analyses of Htr2a and Htr2b of rat brain tissue
The total RNA of the cortex, hypoglossal nucleus, and pre-BötC dissected from corresponding 300-µm-thick slice preparations was isolated using GenElute™ mammalian total RNA kit (Sigma). First strand cDNA was synthesized from 1 µg total RNA using SuperScript™ first-strand synthesis system with random hexamers according to manufacturer's instructions (Invitrogen). Samples without reverse transcription (w/o RT) served as negative controls for the following PCR to exclude amplification of genomic DNA. For the PCR, specific forward and reverse primers were derived from different exons of the 5-HT2AR and 5-HT2BR cDNA to avoid amplification of genomic DNA. The cDNA sequences were obtained from the National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov/). Specificity of selected primers was tested by partial sequencing of the amplification products for their identification by SeqLab company (Göttingen, Germany). The following primer pairs were used for amplification:
5-HT2AR [Htr2a, NCBI-Accession No.: NC_005114.2; F (5′-accttgtgtgtgagtgacct-3′)/R (5′-taggccaatgctggtatagt-3′)], 5-HT2BR [Htr2b, NC_005108.2; F (5′-ctggttattctggctgtttc-3′)/R (5′-gaccacatcagcctctattc-3′)], and for β-actin [Actb, NC_005111.2; F (5′-gatatcgctgcgctcgtcgtc-3′)/R (5′-cctcggggcatcggaacc-3′)].
The PCR reaction mixture for one sample was composed of 1–2 µl cDNA, 1 µl forward primer, 1 µl reverse primer, 1 µl dNTPs (100 mM dNTP mix), 1 µl DMSO, 5 µl NH4 buffer (10×), 2 µl MgCl2 (50 mM solution), and 1 µl PANScript red DNA polymerase (PAN Biotech, Germany). The mixture was filled up to 50 µl with DEPC-treated water. The following program was used for the PCR reaction: initial denaturation at 94°C, 4 min/38×[denaturation 94°C, annealing 1 min/55°C, extension 1 min/72°C, 2 min]/final elongation 72°C, termination 10 min/4°C hold. Actb (β-Actin) was used as an internal standard for all PCR reactions.
(e) Real-time RT-PCR
The relative quantification of Htr2a and Htr2b gene expression in specific rat tissues was done by real-time RT-PCR analysis. Spinal cord, inferior olive, pre-Bötzinger complex, and parabrachial complex were dissected from corresponding 300-µm-thick cryostat sections (P32; n = 3 animals) under visual control. The total ribonucleic acid (RNA) of homogenized brain tissue was isolated using the Trizol® method according to manufacturer's instructions (GibcoBRL) and its concentration was determined using the NanoDrop ND-1000 spectrophotometer followed by its quality and integrity measurement by electrophoresis on RNA 6000 LabChip® kit (Agilent 2100 Bioanalyzer). The RNA was transcribed into the corresponding deoxyribonucleic acid (cDNA) using the iScript cDNA Synthesis Kit (BioRad). The following primer pairs were designed by using the Primer3 program (http://frodo.wi.mit.edu/primer3/):
Htr2a (NM_017254.1): F (5′-tgtcgccatccagaacccca-3′)/R (5′-gcaggcagctcccctcctta-3′); Htr2b (NM_017250.1): F (5′-agactgccgagaaccagggg-3′)/R (5′-gcggtggctgatttgctggt-3′); Hprt1 (NM_012583.2): F (5′-gtcaagcagtacagccccaaaatg-3′)/R (5′-gtttgttgttggatatgcccttgac-3′).
Gel electrophoresis revealed a single polymerase chain reaction (PCR) product, and the melting curve analysis showed a single peak for all amplification products. The PCR products were sequenced and blasted to confirm the correct identity of each amplicon. Ten-fold serial dilutions generated from cDNA of each sample were used as a reference for the standard curve calculation to determine primer efficiency. Triplicates of all real-time PCR reactions were performed in a 25 µl mixture containing 1/20 volume of the sample cDNA preparation from 250 ng total RNA, 400 nM of each primers, and 1× Fast-SYBR Green Master Mix (Applied Biosystems, USA).
The PCR-reactions were performed as follows: initial activation at 95°C for 60 s, 42 cycles of (denaturation 95°C/10 s, annealing and extension 60°C/30 s), and a final gradual increase of 0.5°C in temperature from 60°C to 90°C.
All real-time quantifications were performed using the iCycler iQ system (BioRad) and were adjusted by using the method according to Pfaffl [41].
Calcium imaging of cells recombinantly expressing 5-HT2ARs or 5-HT2BRs
The perfused brainstem preparation is, due to its thickness and need for constant perfusion not suited for microscopic analysis. Therefore, we opted to do the calcium imaging in murine neuroblastoma N1E-115 cells, where endogenous expression of 5-HT2Rs is negligible, but are known to signal via the PLC-DAG pathway [42], [43]. Another advantage of transfection is the control over which receptors (5-HT2AR, 5-HT2BR or both) are expressed in individual cells, avoiding the need for antagonists and simplifying analysis. 12–16 hours post transfection, cells were transferred to calcium-free imaging medium (130 mM NaCl, 3.5 mM KCl, 1.25 mM NaH2PO4, 24 mM NaHCO3, 1.2 mM MgSO4, 10 mM Glucose) and incubated with Fluo-4-AM (Invitrogen) at a final concentration of 5 µM for 30 min at 37°C. The Fluo-4-AM stock solution was prepared as 2 mM using 10% pluronic acid F-127 in DMSO (Sigma) and was diluted just before use. After incubation, cells were washed with calcium-free medium and Fluo-4-AM was allowed to hydrolyze for another 30 min in the presence of probenicid to avoid leeching of fluorescent probe from the cell.
For calcium imaging, Fluo-4-AM loaded cells were transferred to a recording chamber equipped with an inverted Olympus microscope (IX71) with appropriate filters (515 nm beam splitter and a 535/50 band-pass filter) and a triggered LED light source (PreciseExcite, CoolLED) with 465 nm excitation. Images were taken for 300 µsec at 1 sec intervals. After recording baseline fluorescence, cells were stimulated with 1000 nM serotonin (Sigma) in calcium-free medium. This concentration was chosen based on a dose-response curve giving a linear response for serotonin stimulation between 500 and 1500 nM. For all experiments, a 10×, 1.0 NA objective (Olympus) was used.
To compare calcium measurements between experiments, we calculated the apparent fluorescent intensity F/F0 by dividing the fluorescent intensity (F) at every time point by the average fluorescence recorded before stimulation (F0). Data were statistically analyzed with Student's t-test and presented as mean ± standard deviation (s. d.).
Perfused brainstem preparation of rats
(a) Perfused brainstem preparation of rats
The experiments on the perfused brainstem preparation [44] were performed on male Sprague-Dawley rats (P25–P32, 90–150 g) that were housed under a 12 h light/dark cycle, with food and water provided ad libitum.
Animals were deeply anesthetized with halothane until they were unresponsive to a forepaw pinch, decerebrated at the pre-collicular level and cerebellectomized, bisected below the diaphragm, and the skin was removed. The upper body was placed into a recording chamber and perfused retrogradely via the thoracic aorta with ACSF (containing in mM: MgSO4 1.25; KH2PO4 1.25; KCl 5; NaCl 125; CaCl2 2.5; NaHCO3 25; glucose 10, 1.25% Ficoll and aerated with carbogen (5% CO2/95% O2; pH 7.35 at 30°C). The perfusate was collected, filtered twice and re-circulated. Norcuronium-bromide (0.5 mg/200 ml) was added for muscle relaxation. The perfusion pressure was set to 45 to 55 mm Hg.
(b) Phrenic nerve signal processing
A silver wire immersed in bath solution within a capillary suction electrode picked up phrenic nerve activity representing the respiratory motor output to the diaphragm and inspiratory rib cage muscles. Phrenic nerve signals were amplified 2,000–5,000×, filtered (low-pass, 7,000 Hz cutoff frequency; high pass, 8 Hz) and integrated (time constant, 100 ms). The processed signals were digitized by a PowerLab 8/30 microprocessor and stored using LabChart 7 software (ADInstruments, Australia).
Drugs added to the perfusate for specific pharmacological manipulation of 5-HT2Rs were purchased from Tocris Bioscience, Ellisville, USA: 5-HT2AR agonist TCB-2 and 5-HT2AR antagonist Altanserin hydrochloride, 5-HT2BR agonist BW 723C86 and 5-HT2BR antagonist LY 272015.
(c) Analysis of phrenic nerve discharge properties
Discharges of a representative one-minute duration were measured in the absence of (control) and after intra-arterial perfusion with ACSF containing a 5-HT2A or 5-HT2B receptor agonist or antagonist. Measurements of drug effect were made at 5-minute intervals. The peak of the integrated discharge (mV) was used as an estimator of discharge intensity and normalized to the control, which was set to 100%. Discharge frequency (bursts per minute) was calculated from the integrated signals. Values (mean ± standard error of the mean) for amplitude and frequency were calculated from consecutive discharges that occurred over one minute during control and when drug effects were maximal.
All statistical tests (paired t-test) for pharmacological experiments were performed using GraphPad Prism version 5.0d for MacOS X.
Test drugs
5-HT receptor ligands tested for effects on phrenic nerve discharge properties were purchased from Tocris Bioscience, USA: TCB-2 (5-HT2AR agonist), Altanserin hydrochloride (5-HT2AR antagonist), BW 723C86 (5-HT2BR agonist), LY 272015 (5-HT2BR antagonist).
Results
Production and characterization of monospecific anti-5-HT2BR antibodies
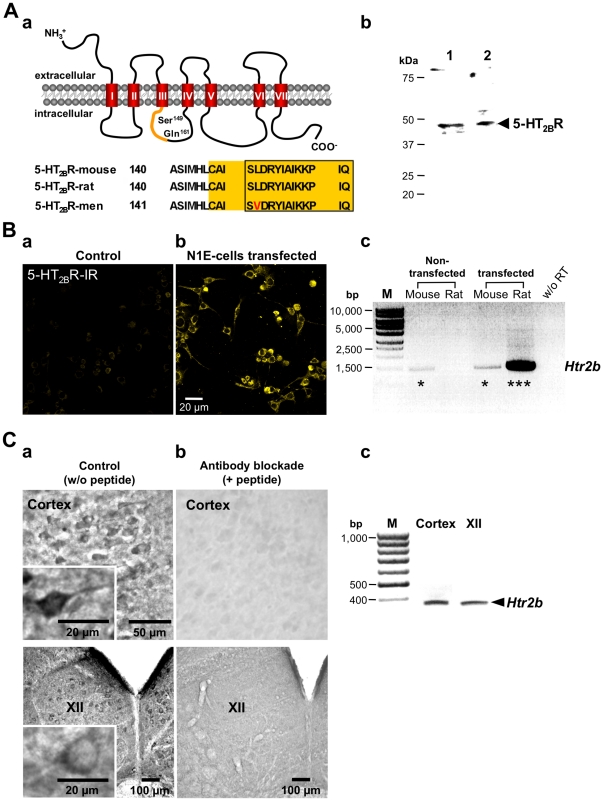
The peptide for immunization was derived from the second intracellular loop of the rat 5-HT2BR-sequence (NH2-CAISLDRYIAIKKPIQ-COOH; fig. 1Aa). The specificity of the monospecific polyclonal anti-5-HT2BR antibody was tested in three different test systems: Immunoblot analysis (n = 3) of both mouse and rat brainstem lysate revealed a specific band at 48 kDa, which is in accordance with the predicted relative molecular mass of the receptor (fig. 1Ab). Murine neuroblastoma cells recombinantly expressing rat 5-HT2BR (fig. 1B) were used for specificity testing of the antibody, while the neocortex and the hypoglossal nucleus (XII) was selected to test immunohistochemistry in tissue (fig. 1C) based on previous positive results reported by Duxon [36].
Figure 1. Verification of the anti-5-HT2BR antibody.
(A) (a) The 5-HT2BR belongs to the family of seven-transmembrane-domain receptors that are coupled to hetero-trimeric guanine-nucleotide-binding protein q (Gq). The transmembrane-domains are indicated by red cylinders (I–VII). We produced a novel monospecific polyclonal antibody against the 5-HT2BR by selecting a specific amino acid sequence (Cys146 - Gln161; NH2-CAISLDRYIAIKKPIQ-COOH) of the second intracellular loop of the rat 5-HT2BR-sequence. The peptide exhibits 100% homology in mouse. Red letters indicate mismatches in the human-sequence (L→V). (b) Immunoblot analysis of mouse (1) or rat (2) brainstem lysate revealed a specific band at about 48 kDa that corresponds with the predicted relative molecular mass of the 5-HT2BR. (B) 5-HT2BR expression in non-transfected (a) and transfected N1E-115 cells (b). The anti-5-HT2BR antibody-dependent staining indicated a strong labeling of N1E-115 cells that had been transiently transfected with the rat 5-HT2BR (b). Non-transfected cells expressing the mouse 5-HT2BR showed a weak neuronal immunofluorescent signal that corresponds with a weak PCR signal (amplicon size 1114 bp) for the mouse 5-HT2BR-mRNA (Htr2b) (c). Samples without reverse transcription (w/o RT) served as negative controls. (C) (a, b) Immunohistochemistry. Both pyramidal neurons of the cortex and motoneurons of the hypoglossal nucleus (XII) revealed a strong 5-HT2BR immunoreactivity (-IR) (a) that was effectively blocked after pre-incubation of the antibody with a 50-fold molar excess of the peptide CAISLDRYIAIKKPIQ (+ peptide) that was used for immunization (b). Insets in (a) show labeled neurons at a higher magnification. Immunolabeling was performed using the PAP-method with diaminobenzidine as chromogen. (c) RT-PCR analysis of the rat cortex and hypoglossal nucleus. The 5-HT2BR-specific mRNA (Htr2b) was detectable in neurons within both the rat cortex and the hypoglossal nucleus (XII) (amplicon size 380 bp).
The control cells faintly expressed the mouse 5-HT2BR that is also recognized by the antibody because of sequence homology. After transfection with the rat receptor the antibody labeling revealed a strong fluorescent signal. Also, both brain regions selected showed strong 5-HT2BR reactivity (fig. 1Bc).
The anti-5-HT2BR antibody immunoreactivity on cells as well as on neurons of both regions was effectively blocked after pre-incubation of the primary antibody with a 50-fold molar excess of the peptide that was used for immunization indicating specificity. As a control, RT-PCR analysis confirmed 5-HT2BR-specific mRNA expression in cells within both regions (fig. 1Cc).
Expression analysis of 5-HT2A and 5-HT2BRs in the respiratory network
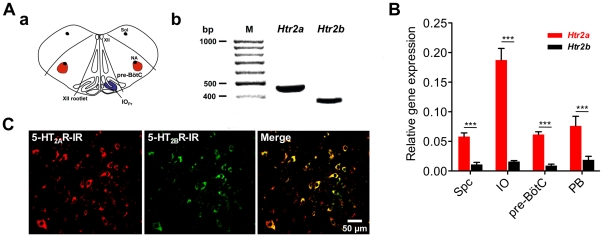
Prior to analysis of 5-HT2AR and 5-HT2BR expression at the protein level, we confirmed their expression at the RNA level. For this, we dissected specific brain stem regions (fig. 2Aa) from corresponding frozen cryostat sections of juvenile rats (P30; n = 3 in which patches of both sides of each section were combined for one sample). Conventional RT-PCR analysis revealed gene expression for 5-HT2A (Htr2a) and 5-HT2BR (Htr2b) in the pre-BötC at the RNA level (fig. 2Ab). However, relative quantification of gene expression using real-time RT-PCR analysis revealed a 5.3-fold stronger expression of the Htr2a compared to the Htr2b gene in the spinal cord (0.0582±0.0061 vs. 0.0110±0.0036), 11.8-fold in the inferior olive (0.1875±0.0196 vs. 0.0159±0.0016), 6.8-fold in the pre-BötC (0.0617±0.0046 vs. 0.0091±0.0024), and a 4.1-fold stronger one in the parabrachial complex (0.0671±0.0162 vs. 0.0188±0.0061) (fig. 2C).
Figure 2. Quantification of expression levels and co-expression of 5-HT2A and 5-HT2BRs within the pontine respiratory network.
(A) (a) shows a schematic representation of the dissected pre-BötC and its landmarks: pre-Bötzinger complex (pre-BötC), nucleus of the solitary tract (Sol), nucleus ambiguus (NA), hypoglossal nucleus (XII), principal nucleus of the inferior olive (IOPr). (b) shows the specific mRNA of both receptors detected in the pre-BötC. (B) Double labeling of 5-HT2A and 5-HT2BRs. 5-HT2A (Cy5, red) and 5-HT2BRs (Cy2, green) are strongly co-expressed in pre-BötC-neurons. Immunohistochemical analysis does not reveal the ratio of co-expressed proteins. Therefore, we performed quantitative real-time RT-PCR on four selected nuclei of the respiratory network (C). The bar diagram represents results of quantitative real-time RT-PCR analysis of 5-HT2R genes (Htr2a, Htr2b) of spinal cord (Spc), inferior olive (IO), pre-Bötzinger complex (pre-BötC), and parabrachial complex (PB). At the RNA level 5-HT2AR is significantly stronger expressed compared to Htr2b in all regions analyzed. Asterisks indicate significance (*** = p<0.001; ANOVA with Bonferroni's post hoc test).
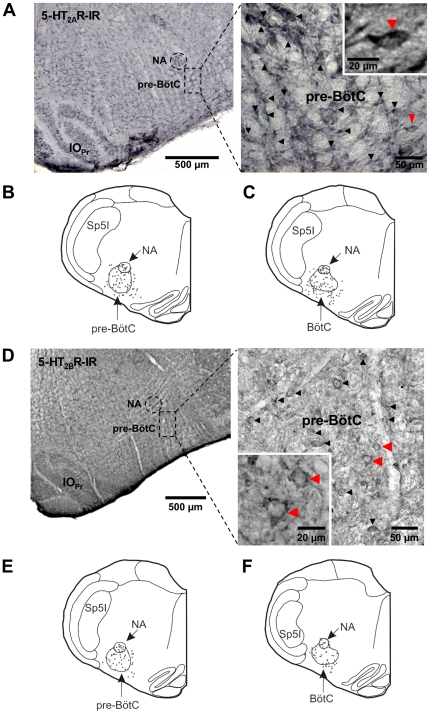
To analyze receptor expression at the protein level within the ponto-medullary respiratory network we applied our self-made monospecific polyclonal anti-5-HT2BR antibody in combination with a commercially available monoclonal anti-5-HT2AR antibody (BD Bioscience, San Diego, USA). Both receptor subtypes were expressed in crucial parts of the respiratory network such as the pre-BötC and the pontine Kölliker-Fuse nucleus (fig. 3, 4). A detailed analysis of the pre-BötC, the supposed kernel essential for the generation of the primary respiratory rhythm (Smith et al., 1991), showed a strong co-expression of 5-HT2AR and 5-HT2BR.
Figure 3. Expression patterns of 5-HT2A and 5-HT2BRs within the medullary respiratory network.
(A) 5-HT2AR expression pattern in the pre-BötC. The plots (B, C) represent 5-HT2AR immunoreactivity within the pre-BötC (B) and BötC (C). (D–F) shows the corresponding expression pattern for the 5-HT2BR. The insets show labeled neurons at a higher magnification. Abbreviations: Bötzinger complex (BötC), nucleus ambiguus (NA), pre-Bötzinger complex (pre-BötC), principal nucleus of the inferior olive (IOPr), interpolar spinal trigeminal nucleus (Sp5l).
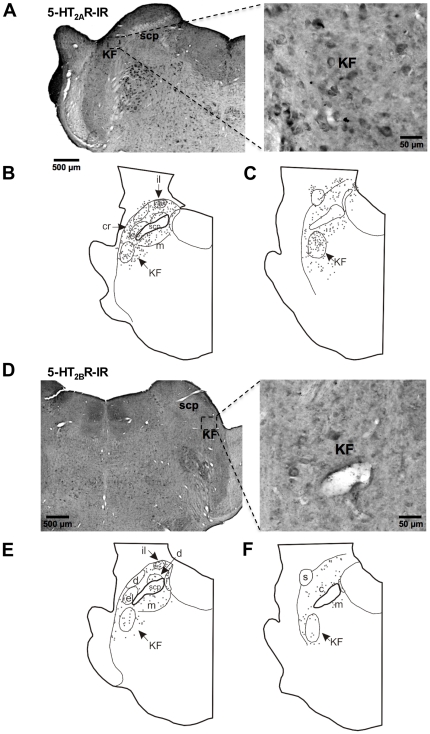
Figure 4. Expression patterns of 5-HT2A and 5-HT2BRs within the pontine respiratory network.
Both 5-HT2A and 5-HT2B receptors are abundantly expressed in neurons of the internal lateral nucleus of the parabrachial complex (B, E). Within the nucleus Kölliker-Fuse the 5-HT2BR expression is weak compared to 5-HT2ARs (A, D). Abbreviations: internal lateral nucleus of the PB (il), lateral crescent nucleus of the PB (cr), nucleus Kölliker-Fuse (KF), parabrachial complex (PB), superior cerebellar peduncle (scp).
We analyzed a total amount of 2136 5-HT2AR-immunoreactive cells (5 sections from each animal, n = 5 animals). 1345 cells (63%) of these cells also expressed the 5-HT2BR indicating strong co-expression of 5-HT2AR and 5-HT2BR (fig. 3).
In the dorsolateral pons expression of 5-HT2BR within the parabrachial complex (PB) and Kölliker-Fuse (KF) nucleus was weak, compared to the 5-HT2AR (fig. 4D–F). Contrary, the 5-HT2AR showed dense expression in the KF and lateral crescent nucleus of the PB (fig. 4A–C). Both nuclei are closely linked with respiratory control. In addition more modest expression was observed in the external lateral, central, and dorsal nuclei of the PB. Curiously, both 5-HT2AR and 5-HT2BR showed dense expression in the internal lateral nucleus (il) of the PB (fig. 4B, E), a subnucleus of the PB-complex that is still undefined in its physiological functions.
Analysis of systemic effects of 5-HT2ARs and 5-HT2BRs on respiratory activity
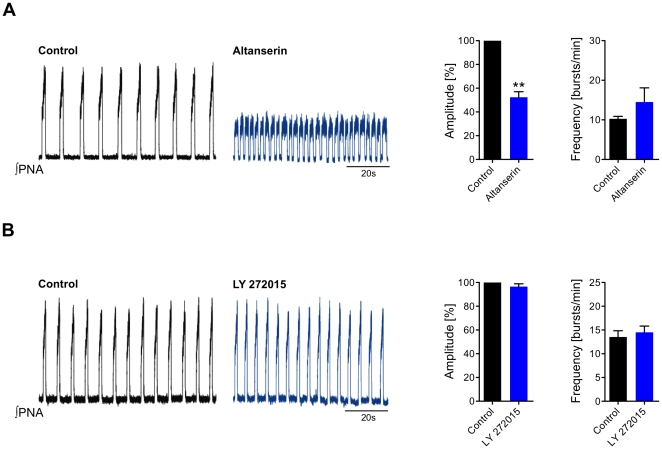
The effects of systemic application of specific antagonists for 5-HT2AR and 5-HT2BR (Altanserin hydrochloride and LY 272015, respectively) and specific agonists for 5-HT2AR and 5-HT2BR (TCB-2 and BW 723C86, respectively) on spontaneous breathing activity were investigated in male Sprague-Dawley rats using the perfused brainstem preparation [44]. We found that pharmacological manipulation of 5-HT2Rs can either change the amplitude or the frequency of the phrenic nerve activity (PNA).
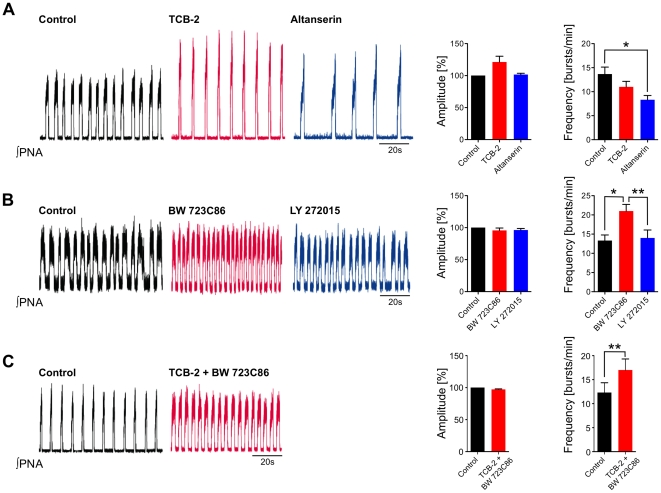
Application of the 5-HT2AR-antagonist Altanserin hydrochloride (8.0 µg/ml, n = 3) [32], [45] significantly reduced the amplitude from control of 100% to 52.34±4.72% (p<0.01), while breathing frequency increased from control of 10.25±0.63 to 14.50±3.59 bursts/min; n.s. (fig. 5A). Blockade of 5-HT2BR with the antagonist LY 272015 (2.87 µg/ml, n = 3) [35], [46] did not significantly changed both the amplitude (100% of control vs. 96.54±2.36%; n.s.) and frequency (13.50±1.32 bursts/min of control vs. 14.50±1.32 bursts/min; n.s.) (fig. 5B). Application of the 5-HT2AR-agonist TCB-2 (0.67 ng/ml, n = 5) [47] caused an increase of the amplitude (from 100% of control to 121.3±8.99; n.s.), while respiratory frequency slightly decreased (13.67±1.45 bursts/min of control vs. 11.00±1.12 bursts/min; n.s.). Administration of the 5-HT2AR-antagonist Altanserin caused a further decrease of frequency to 8.33±0.88; p<0.05; (fig. 6A). Application of the 5-HT2BR-agonist BW 723C86 (0.67 µg/ml, n = 5) [32] only caused an increase in frequency (13.33±1.45 bursts/min of control vs. 21.00±1.73 bursts/min; p<0.05), while the amplitude was not affected (from 100% of control to 95.67±3.76%; n.s.). Blockade of the 5-HT2BR using LY 272015 diminished respiratory frequency nearly to baseline level (14.00±2.08 bursts/min; p<0.01) (fig. 6B). Interestingly, the simultaneous application of both agonists together resulted only in an increase of frequency (from 12.33±2.03 to 17.00±2.31 bursts/min; p<0.01), whereas no increment of amplitude could be observed (100% of control vs. 97.33±0.67%; n.s.; fig. 6C).
Figure 5. Respiratory network responses to systemic 5-HT2AR and 5-HT2BR antagonist applications of in situ rats.
Shown are representative traces of integrated phrenic nerve activity (∫PNA) of 2 different experimental conditions. Black traces represent the ∫PNA under control conditions and blue traces indicate application of antagonists. The bar diagrams on the right give the averages of amplitude and frequency of least 3 independent experiments of each condition. Statistical analysis (paired t-test) is denoted in the panels on the right side with asterisks indicating significance (** = p<0.01). (A, B) Action of 5-HT2AR and 5-HT2BR antagonists in the perfused brainstem preparation of rat. (A) Application of the specific 5-HT2AR antagonist Altanserin (blue trace) significantly decreased phrenic nerve activity (PNA) by decreasing the amplitude. Also, a slight increase in frequency was observed. (B) Application of the 5-HT2BR antagonist LY 272015 had no discernable effect on either amplitude or frequency of ∫PNA. We interpret these findings as constitutive activity of 5-HT2AR.
Figure 6. Respiratory network responses to systemic 5-HT2AR and 5-HT2BR activation.
Shown are representative traces of integrated phrenic nerve activity (∫PNA) of 3 different experimental conditions. Black traces represent the ∫PNA under control conditions, while red traces indicate application of agonists and blue traces indicate application of antagonists. The bar diagrams on the right give the averages of amplitude and frequency of least 3 independent experiments of each condition. Statistical analysis (paired t-test) is denoted in the panels on the right side. (A–C) Action of 5-HT2AR and 5-HT2BR ligands in the perfused brainstem preparation. (A) Application of the specific 5-HT2AR agonist TCB-2 (red trace) increased phrenic nerve activity (PNA) by increase of the amplitude, and subsequent application of the specific 5-HT2AR-antagonist Altanserin (blue trace) reduced the amplitude. PNA frequency decreased below control. (B) Application of the 5-HT2BR agonist BW 723C86 increased PNA frequency. After subsequent administration of the specific 5-HT2BR-antagonist LY 272015 phrenic nerve activity returned nearly to baseline level. (C) Simultaneous application of the specific agonists TCB-2 and BW 723C86 only increased the frequency.
Calcium imaging of cells recombinantly expressing 5-HT2AR or 5-HT2BR
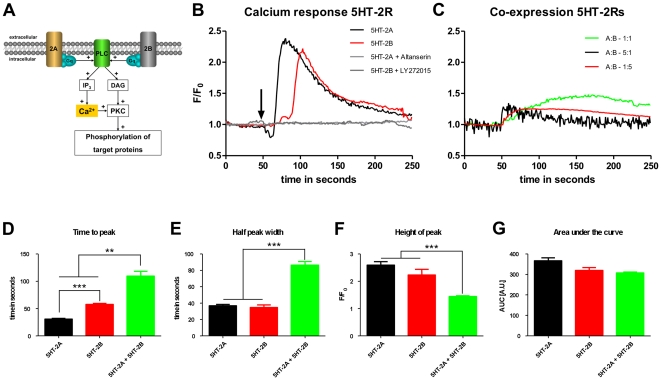
The electrophysiological recordings showed a frequency increase controlled by 5-HT2BRs when both receptors were stimulated concomitantly (fig. 5C), although both 5-HT2AR and 5-HT2BR are coupled to the same Gq-mediated signaling pathway (fig. 7A).
Figure 7. Signal transduction pathways of 5-HT2A and 5-HT2B receptors and calcium imaging.
(A) The schematic diagram illustrates convergent signaling pathways for 5-HT2Rs. (B) shows summed calcium transients recorded in N1E-115 cells transfected with either 5-HT2AR (red line) or 5-HT2BR (black line). The curves were constructed from the means of 3–5 independent experiments, averaging at least 30 individual measurements. The arrow indicates the time of stimulation. Light and dark grey curves indicate control experiments of 5-HT2AR stimulated with Altanserin and 5-HT2BR stimulated with LY272015, respectively. (C) shows summed calcium transients recorded in N1E-115 cells transfected with a stiochiometric ratio of 5-HT2AR∶5-HT2BR of 1∶1 (green line), 5-HT2AR∶5-HT2BR of 5∶1 (black line) and 5-HT2AR∶5-HT2BR of 1∶5 (red line). The bar diagrams (D–G) show statistical analysis of (D) height of peak (F/F0), (E) time to peak (s), (F) half peak width (s) and (G) area under the curve (AU) for expression of 5-HT2AR alone (black), 5-HT2BR alone (red) or expression of 5-HT2AR and 5-HT2BR together at a stiochiometric ratio of 1∶1 (green). Asterisks indicate significance (*** = p<0.001; ** = p<0.01; paired t-test). Abbreviations:, serotonin 2A receptor (2A), serotonin 2B receptor (2B), phospholipase C (PLC), inositol 1, 4, 5-triphosphate (IP3), diacylglycerol (DAG), protein kinase C (PKC), hetero-trimeric guanine-nucleotide-binding protein q (Gq), adenylyl cyclase (AC), cyclic adenosine 5′, 3′-monophosphate (cAMP), protein kinase A (PKA).
To analyze the calcium signaling of both receptors, we expressed them recombinantly either alone or together in neuroblastoma cells. To avoid artifacts, relatively low amounts of DNA were transfected to avoid overexpression.
The recombinant approach allowed us to image single cells and correlate resulting signals to a defined complement of receptors, which would have not been possible in perfused brainstem preparations.
In neuroblastoma cells expressing 5-HT2ARs and 5-HT2BRs alone, either agonist evoked a release of cytosolic Ca2+ from intracellular stores (see figure 7 and table 1) with a large initial calcium spike. While the reactions of individual cells varied slightly, the mean Ca2+ increase of cells expressing 5-HT2AR (peak F/F0 of 2.59±0.8) was similar to those expressing 5-HT2BR (peak F/F0 of 2.23±1.1). In contrast, the calcium increase was significantly faster for 5-HT2AR (time to peak 30.5±9.6) than for 5-HT2BR (time to peak 57.7±8.4). The calcium level almost returned to baseline levels within ∼35 seconds exhibiting no significant differences for both receptors, with a half-peak-width of 36.7±1.7 sec for 5-HT2AR and 34.6±3.2 for 5-HT2BR (fig. 7B).
Table 1. Effects of 5-HT2AR and 5-HT2BR agonists on Ca2+ fluorescence signals.
| Ca2+ Signal Properties | 5-HT2AR-agonist | 5-HT2BR agonist | both agonists | Unit | T-test* | T-test** |
| Onset time | 10.1±1,7 | 40.3±1.5 | 11.9±2.3 | [sec] | p, 0.001 | p, 0.01a |
| Time to peak | 30. 5±9.6 | 57.5±8.4 | 79.44±8.9 | [sec] | p, 0.01 | p, 0.01 |
| Half peak width | 36.7±1.7 | 34.6±3.2 | 86.32±5.0 | [sec] | p, 0.500 | p, 0.001 |
| Height of peak (F/F0) | 2.59±0.8 | 2.23±1.1 | 1.44±0.17 | [AU] | p, 0.500 | p, 0.001 |
| Area under the curve | 366.38±93.64 | 319.4±74.86 | 307.43±24.43 | [AU] | p, 0.500 | p, 0.500 |
comparing results from single transfected cells stimulated with 5-HT2AR-agonist or with 5-HT2BR agonist.
comparing results from both single transfected/stimulated cells against double transfected/double stimulated cells.
only when compared to single transfected cells stimulated with 5-HT2AR.
Co-application of 5-HT2AR and 5-HT2BR agonists had unexpected effects. The Ca2+ signal (79.44±8.9 sec) was significantly slower from onset to peak than the signals produced by either agonist alone (p<0.001), while the time of onset was similar to 5-HT2AR alone. In addition, the fluorescence signal at its peak (F/F0, 1.44±0.17) was notably smaller (p<0.001). While the duration of the calcium peak by co-stimulation of both receptors was nearly doubled (half-peak-width of 86.32±5.0 sec; p<0.001), the amount of released calcium, measured as “area under the curve”, was very similar no matter if the receptors are expressed alone or together.
As our real-time PCR analysis showed that 5-HT2AR and 5-HT2BR are expressed in different amounts, with 5-HT2AR being in 5- to 10-fold excess, we also transfected N1E cells with DNA ratios of 5-HT2AR to 5-HT2BR of 5∶1 and 1∶5, respectively. Regardless of the DNA ratios, the presence of 5-HT2BR always produced slow but wide calcium transients (as shown for 5-HT2AR and 5-HT2BR in fig. 7C).
Discussion
This study reveals the locations of 5-HT2AR and 5-HT2BR in regions of the ponto-medullary respiratory network, including sites where the receptors are co-expressed. We demonstrate that agonist activation of the receptors evokes dramatic changes in discharge activity recorded from the phrenic motor output, and that activation of each type of receptor has distinctive effects on phrenic nerve discharge intensity and duration. Through the use of selective receptor antagonists, we found that only the 5-HT2AR constitutively modulates phrenic motor output. In neuroblastoma cells transfected with 5-HT2AR and 5-HT2BR, we discovered distinctly different calcium signal kinetics when each type of 5-HT receptor was activated. We also uncovered unexpected effects on signal amplitude and time course when 5-HT2AR and 5-HT2BR are coactivated.
In the paragraphs to follow, we discuss each of these aspects in turn, along with their physiological implications for respiratory motor output modulation.
Distribution of 5-HT2B and 5-HT2A receptors in regions of the ponto-medullary respiratory network
Within the medulla and pons, functionally defined respiratory regions provide input to cranial motoneurons controlling the airways, and to spinal motoneurons activating inspiratory and expiratory pump muscles. A variety of neurotransmitters and modulators involved in respiratory control have been identified in many of these respiratory related compartments (reviewed by Alheid and McCrimmon [48]). In all these regions, 5-HT2AR and coupled protein kinase dependent signaling pathways have been identified functionally and anatomically [49]. Until now, however, the distribution of 5-HT2BRs had not been investigated, and nothing had been known about their functional importance to respiratory control. Our study shows that 5-HT2AR and 5-HT2BR are co-localized in the Kolliker-Fuse and Parabrachial regions of the Pons, and in the BötC and pre-BötC of the ventral medulla with an approximate 5-fold stronger expression of 5-HT2AR in all regions.
Respiratory neurons in the PB and KF constitute the pontine respiratory group. A variety of respiratory neuronal types are found in this region [50], [51], which receives axonal projections from the ventral respiratory column, and from the nucleus of the solitary tract (NTS). The NTS itself is a receiving station for pulmonary afferents from the lungs and upper airways. Based on 5-HT2AR and 5-HT2B R co-expression patterns, our study would predict contributions by both types of receptors to respiratory modulation within the KF-PB complex, with a modulatory role played by 5-HT2BRs.
Based on both in vitro and in vivo [52], [53] studies, the pre-BötC was identified as a medullary region essential for respiratory rhythm generation. The region may play a prominent role in inspiratory phase control, although the pre-BötC contains populations of neurons that exhibit a variety of respiratory related discharge patterns (Schwarzacher et al., 1995). Previously, 5-HT1A, 5-HT2A, 5-HT4, and 5-HT7 receptors have been identified in the pre-BötC by immuno-labeling [8], [12], [17]. The BötC houses a prominent population of expiratory neurons that provide widespread inhibitory projections within the ventral respiratory column (VRC), targeting both inspiratory and expiratory bulbospinal neurons as well as respiratory-related cranial motoneurons [54]. Some expiratory BötC neurons also send axon collaterals to the spinal cord, reaching at least as far as the phrenic motor nucleus [48], [55]. Our present study of 5-HT2AR and 5-HT2BR co-expression in the pre-BötC and BötC suggests that, as in the pontine respiratory group, 5-HT2AR modulation is predominant.
Differential effects of 5-HT2A and 5-HT2B receptor agonists and antagonists on phrenic nerve discharge properties
Activation of 5-HT2ARs by the selective, CNS-permeable agonist TCB-2 [47], [56] increased PNA discharge amplitude but not frequency, whereas the 5-HT2AR antagonist Altanserin decreased both amplitude and frequency: in fact, discharge frequency decreased below control level. Although speculative, we suggest that 5-HT2AR agonists target two functionally different populations of respiratory neurons: bulbospinal inspiratory neurons, leading to increased phrenic motor output, and propriobulbar inspiratory phase-regulating neurons that determine discharge frequency. Altanserin's capacity to reduce discharge frequency below control levels indicates that constitutive 5-HT2AR activation is substantial in inspiratory phase regulating neurons.
The 5-HT2BR agonist BW 723C68 increased only frequency, indicating that only neurons involved in discharge rate regulation were affected. However, the antagonist LY 272015 changed neither amplitude nor frequency. This suggests that in our in situ perfused brainstem preparation, 5-HT2BRs though present and activated by BW 723C68 were not constitutively activated.
When 5-HT2AR and 5-HT2BR agonists were given concurrently, a frequency increase that exceeded the singular effects of either agonist occurred. This is an expected outcome if frequency-controlling neurons are preferred targets for 5-HT2BR modulation in the ponto-medullary respiratory compartment.
5-HT2B and 5-HT2A receptors effects on Ca2+ signaling
Because 5-HT2A and 5-HT2B receptors both utilize an intracellular signal pathway that leads to a buildup of cytoplasmic Ca2+, we used the Ca2+ signal as a measure of receptor activation by TCB-2 and by BW 723C68. Neuroblastoma cells, for reasons presented earlier, are advantageous for measuring the magnitude and kinetics of intracellular Ca2+ fluctuations. Nonetheless, we acknowledge that there are limitations in relating Ca2+ signals detected in neuroblastoma cells to discharge properties recorded from the phrenic motor output, and we interpret our results with this caveat in mind.
Agonist activation of 5-HT2A receptors produced a Ca2+ signal that was rapid in onset, somewhat faster in time course to peak and larger in magnitude than the Ca2+ transient produced by 5-HT2B receptor activation. Another characteristic difference was an initial small dip in the signal due to 5-HT2A receptors, whereas a small Ca2+ transient preceded the predominant 5-HT2BR dependent signal. Their respective signal profiles may reflect differences in dynamic interactions involving receptor activation, cell membrane Ca-channel openings as well as Ca2+ release and uptake processes in intracellular organelles. A detailed interpretation of Ca2+ signals recorded from the respiratory rhythmic rodent slice preparation can be found in published studies by Keller and coworkers [57], [58]. Those studies illustrate Ca2+ signals that are proportional to respiratory discharge intensity. Assuming that Ca2+ signaling in neuroblastoma cells can be equated with signaling in cells of the brainstem respiratory network, we interpret our findings as follows. The faster and larger Ca2+ signal may reflect more efficient 5-HT2AR coupling to the PLC-DAG-PKC signal pathway, or to the spatial arrangement of receptor, Ca2+-channel, and organelles involved in Ca2+ release and uptake. We can offer no ready explanation for the slowing and diminution of the Ca2+ signal when 5-HT2A and 5-HT2B receptors co-expressed in neuroblastoma cells were coactivated. Perhaps different isoforms of DAG or PKC activated by 5-HT2AR and 5-HT2BR compete for phosphorylation sites on membrane calcium channels and storage sites and interact negatively.
Conclusion
Taking together the results from all sets of experiments (distribution, co-expression, phrenic nerve activity, and Ca2+ signaling), we formulate the following working hypothesis: 5-HT2AR is the dominant receptor governing respiratory control as evidenced by its stronger expression and constitutive activity. 5-HT2BR, being present in all respiratory nuclei analyzed and co-expressed with 5-HT2AR in many cells, although at a much reduced level, may act as a dose-dependent modulator. If more serotonin is released than needed to activate all 5-HT2ARs, 5-HT2BRs become activated. This would allow the system to regulate the respiratory rhythm by controlling serotonin release. Activation of spare 5-HT2BR has the strongest effect on respiratory frequency. This regulation could, at least in part, be due to Ca2+ signaling, as the presence of 5-HT2BR changes the kinetics of the Ca2+ signaling observed for both receptors alone, without altering the overall amount of mobilized calcium.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the DFG Research Center Molecular Physiology of the Brain (CMPB; FZT 103). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dick TE, Berger AJ. Axonal projections of single bulbospinal inspiratory neurons revealed by spike-triggered averaging and antidromic activation. J Neurophysiol. 1985;53:1590–603. doi: 10.1152/jn.1985.53.6.1590. [DOI] [PubMed] [Google Scholar]
- 2.Holtman JR, Jr, Dick TE, Berger AJ. Involvement of serotonin in the excitation of phrenic motoneurons evoked by stimulation of the raphe obscurus. J Neurosci. 1986;6:1185–93. doi: 10.1523/JNEUROSCI.06-04-01185.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lalley PM. Serotoninergic and non-serotoninergic responses of phrenic motoneurones to raphe stimulation in the cat. J Physiol. 1986;380:373–85. doi: 10.1113/jphysiol.1986.sp016291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Millhorn DE, Hökfelt T, Verhofstad AA, Terenius L. Individual cells in the raphe nuclei of the medulla oblongata in rat that contain immunoreactivities for both serotonin and enkephalin project to the spinal cord. Exp Brain Res. 1989;75:536–42. doi: 10.1007/BF00249904. [DOI] [PubMed] [Google Scholar]
- 5.Holtman JR, Jr, Marion LJ, Speck DF. Origin of serotonin-containing projections to the ventral respiratory group in the rat. Neuroscience. 1990;37:541–52. doi: 10.1016/0306-4522(90)90422-z. [DOI] [PubMed] [Google Scholar]
- 6.Lalley PM, Benacka R, Bischoff AM, Richter DW. Nucleus raphe obscurus evokes 5-HT-1A receptor-mediated modulation of respiratory neurons. Brain Res. 1997;747:156–9. doi: 10.1016/s0006-8993(96)01233-4. [DOI] [PubMed] [Google Scholar]
- 7.Richter DW, Lalley PM, Pierrefiche O, Haji A, Bischoff AM, et al. Intracellular signal pathways controlling respiratory neurons. Respir Physiol. 1997;110:113–123. doi: 10.1016/s0034-5687(97)00077-7. [DOI] [PubMed] [Google Scholar]
- 8.Kubin L, Volgin DV. Developmental profiles of neurotransmitter receptors in respiratory motor nuclei. Respir Physiol Neurobiol. 2008;164:64–71. doi: 10.1016/j.resp.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lalley PM, Bischoff AM, Richter DW. Serotonin 1A-receptor activation suppresses respiratory apneusis in the cat. Neurosci Lett. 1994;172:59–62. doi: 10.1016/0304-3940(94)90662-9. [DOI] [PubMed] [Google Scholar]
- 10.El-Khatib MF, Kiwan RA, Jamaleddine GW. Buspirone treatment for apneustic breathing in brain stem infarct. Respir Care. 2003;48:956–958. [PubMed] [Google Scholar]
- 11.Wilken B, Lalley P, Bischoff AM, Christen HJ, Behnke J, et al. Treatment of apneustic respiratory disturbance with a serotonin-receptor agonist. J Pediatr. 1997;130:89–94. doi: 10.1016/s0022-3476(97)70315-9. [DOI] [PubMed] [Google Scholar]
- 12.Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, et al. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science. 2003;301:226–229. doi: 10.1126/science.1084674. [DOI] [PubMed] [Google Scholar]
- 13.Stettner GM, Zanella S, Hilaire G, Dutschmann M. 8-OH-DPAT suppresses spontaneous central apneas in the C57BL/6J mouse strain. Respir Physiol Neurobiol. 2008;161:10–15. doi: 10.1016/j.resp.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Yamauchi M, Ocak H, Dostal J, Jacono FJ, Loparo KA, et al. Post-sigh breathing behavior and spontaneous pauses in the C57BL/6J (B6) mouse. Respir Physiol Neurobiol. 2008a;162:117–125. doi: 10.1016/j.resp.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamauchi M, Dostal J, Kimura H, Strohl KP. Effects of buspirone on posthypoxic ventilatory behavior in the C57BL/6J and A/J mouse strains. J Appl Physiol. 2008b;105:518–526. doi: 10.1152/japplphysiol.00069.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guenther U, Manzke T, Wrigge H, Dutschmann M, Zinserling J, Putensen C, et al. The counteraction of opioid-induced ventilatory depression by the serotonin 1A-agonist 8-OH-DPAT does not antagonize antinociception in rats in situ and in vivo. Anesth Analg. 2009;108:1169–76. doi: 10.1213/ane.0b013e318198f828. [DOI] [PubMed] [Google Scholar]
- 17.Manzke T, Dutschmann M, Schlaf G, Mörschel M, Koch UR, et al. Serotonin targets inhibitory synapses to induce modulation of network functions. Philos Trans R Soc Lond B Biol Sci. 2009;364:2589–602. doi: 10.1098/rstb.2009.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, et al. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 19.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 20.Palacios JM, Waeber C, Hoyer D, Mengod G. Distribution of serotonin receptors. Ann N Y Acad Sci. 1990;600:36–52. doi: 10.1111/j.1749-6632.1990.tb16871.x. [DOI] [PubMed] [Google Scholar]
- 21.Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 22.Vergé D, Calas A. Serotoninergic neurons and serotonin receptors: gains from cytochemical approaches. J Chem Neuroanat. 2000;18:41–56. doi: 10.1016/s0891-0618(99)00050-2. [DOI] [PubMed] [Google Scholar]
- 23.Wright IK, Garratt JC, Marsden CA. Effects of a selective 5-HT2 agonist, DOI, on 5-HT neuronal firing in the dorsal raphe nucleus and 5-HT release and meta-bolism in the frontal cortex. Br J Pharmacol. 1990;99:221–222. doi: 10.1111/j.1476-5381.1990.tb14683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garratt JC, Kidd EJ, Wright IK, Marsden CA. Inhibition of 5-hydroxytryptamine neuronal activity by the 5-HT agonist, DOI. Eur J Pharmacol. 1991;199:349–355. doi: 10.1016/0014-2999(91)90499-g. [DOI] [PubMed] [Google Scholar]
- 25.Lalley PM, Bischoff AM, Schwarzacher SW, Richter DW. 5-HT2 receptor-controlled modulation of medullary respiratory neurones in the cat. J Physiol. 1995;487:653–661. doi: 10.1113/jphysiol.1995.sp020907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haji A, Pierrefiche O, Lalley PM, Richter DW. Protein kinase C pathways modulate respiratory pattern generation in the cat. J Physiol. 1996;494:297–306. doi: 10.1113/jphysiol.1996.sp021492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onimaru H, Shamoto A, Homma I. Modulation of respiratory rhythm by 5-HT in the brainstem-spinal cord preparation from newborn rat. Pflugers Arch. 1998;435:485–494. doi: 10.1007/s004240050543. [DOI] [PubMed] [Google Scholar]
- 28.Kinkead R, Bach KB, Johnson SM, Hodgeman BA, Mitchell GS. Plasticity in respiratory motor control: intermittent hypoxia and hypercapnia activate opposing serotonergic and noradrenergic modulatory systems. Comp Biochem Physiol A Mol Integr Physiol. 2001;130:207–218. doi: 10.1016/s1095-6433(01)00393-2. [DOI] [PubMed] [Google Scholar]
- 29.Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lovett-Barr MR, Mitchell GS, Satriotomo I, Johnson SM. Serotonin-induced in vitro long-term facilitation exhibits differential pattern sensitivity in cervical and thoracic inspiratory motor output. Neuroscience. 2006;142:885–892. doi: 10.1016/j.neuroscience.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 31.Mahamed S, Mitchell GS. Simulated apnoeas induce serotonin-dependent respiratory long-term facilitation in rats. J Physiol. 2008;586:2171–2181. doi: 10.1113/jphysiol.2007.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kennett GA, Wood MD, Bright F, Cilia J, Piper DC, et al. In vitro and in vivo profile of SB 206553, a potent 5-HT2C/5-HT2B receptor antagonist with anxiolytic-like properties. Br J Pharmacol. 1996;117:427–434. doi: 10.1111/j.1476-5381.1996.tb15208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callebert J, Esteve JM, Hervé P, Peoc'h K, Tournois C, et al. Evidence for a control of plasma serotonin levels by 5-hydroxytryptamine(2B) receptors in mice. J Pharmacol Exp Ther. 2006;317:724–731. doi: 10.1124/jpet.105.098269. [DOI] [PubMed] [Google Scholar]
- 34.Nebigil CG, Choi DS, Dierich A, Hickel P, Le Meur M, et al. Serotonin 2B receptor is required for heart development. Proc Natl Acad Sci. 2000;97:9508–9513. doi: 10.1073/pnas.97.17.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Günther S, Maroteaux L, Schwarzacher SW. Endogenous 5-HT2B receptor activation regulates neonatal respiratory activity in vitro. J Neurobiol. 2006;66:949–961. doi: 10.1002/neu.20253. [DOI] [PubMed] [Google Scholar]
- 36.Duxon MS, Flanigan TP, Reavley AC, Baxter GS, Blackburn TP, et al. Evidence for the expression of the 5-HT2B receptor protein in the rat central nervous system. Neuroscience. 1997;76:323–329. doi: 10.1016/s0306-4522(96)00480-0. [DOI] [PubMed] [Google Scholar]
- 37.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Connelly CA, Dobbins EG, Feldman JL. Pre-Botzinger complex in cats: respiratory neuronal discharge patterns. Brain Res. 1992;590:337–340. doi: 10.1016/0006-8993(92)91118-x. [DOI] [PubMed] [Google Scholar]
- 39.Schwarzacher SW, Smith JC, Richter DW. Pre-Bötzinger complex in the cat. J Neurophysiol. 1995;73:1452–1461. doi: 10.1152/jn.1995.73.4.1452. [DOI] [PubMed] [Google Scholar]
- 40.Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bedlack RS, Wei M, Fox SH, Gross E, Loew LM. Distinct electric potentials in soma and neurite membranes. Neuron. 1999;13(5):1187–1193. doi: 10.1016/0896-6273(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 43.Xu C, Loew LM. Activation of Phospholipase C Increases Intramembrane Electric Fields in N1E-115 Neuroblastoma Cells. Biophys J. 2003;84(6):4144–4156. doi: 10.1016/S0006-3495(03)75139-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paton JF. A working heart-brainstem preparation of the mouse. J Neurosci Methods. 1996;65:63–8. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- 45.Sietnicks A. Involvement of 5-HT2 receptors in the LSD- and L-5-HTP-induced suppression of lordotic behavior in the female rat. J Neural Transm. 1985;61:65–80. doi: 10.1007/BF01253052. [DOI] [PubMed] [Google Scholar]
- 46.Audia JE, Evrard DA, Murdoch GR, Droste JJ, Nissen JS, et al. Potent, selective tetrahydro-beta-carboline antagonists of the serotonin 2B (5-HT2B) contractile receptor in the rat stomach fundus. J Med Chem. 1996;39:2773–80. doi: 10.1021/jm960062t. [DOI] [PubMed] [Google Scholar]
- 47.McLean TH, Parrish JC, Braden MR, Marona-Lewicka D, Gallardo-Godoy A, et al. 1-Aminomethylbenzocycloalkanes: conformationally restricted hallucino-genic phenethylamine analogues as functionally selective 5-HT2A receptor agonists. J Med Chem. 2006;49:5794–803. doi: 10.1021/jm060656o. [DOI] [PubMed] [Google Scholar]
- 48.Alheid, McCrimmon The chemical neuroanatomy of breathing. Respir Physiol Neurobiol. 2008;164:3–11. doi: 10.1016/j.resp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fay R, Kubin L. Pontomedullary distribution of 5-HT2A receptor-like protein in the rat. J Comp Neurol. 2000;418:323–45. [PubMed] [Google Scholar]
- 50.Ezure K, Tanaka I. Distribution and medullary projection of respiratory neurons in the dorsolateral pons of the rat. Neuroscience. 2006;141:1011–1023. doi: 10.1016/j.neuroscience.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 51.Song G, Yu Y, Poon CS. Cytoarchitecture of pneumotaxic integration of respiratory and nonrespiratory information in the rat. J Neurosci. 2006;26(1):300–310. doi: 10.1523/JNEUROSCI.3029-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pierrefiche O, Schwarzacher SW, Bischoff AM, Richter DW. Blockade of synaptic inhibition within the pre-Botzinger complex in the cat suppresses respiratory rhythm generation in vivo. J Physiol. 1998;509:245–254. doi: 10.1111/j.1469-7793.1998.245bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feldman JL, Janczewski WA. Point:Counterpoint: The parafacial respiratory group (pFRG)/pre-Botzinger complex (preBotC) is the primary site of respiratory rhythm generation in the mammal. Counterpoint: the preBötC is the primary site of respiratory rhythm generation in the mammal. J Appl Physiol. 2006;100(6):2096–2097; discussion 2097–2098. doi: 10.1152/japplphysiol.00119.2006. [DOI] [PubMed] [Google Scholar]
- 54.Jiang C, Lipski J. Extensive monosynaptic inhibition of ventral respiratory group neurons by augmenting neurons in the Bötzinger complex in the cat. Exp Brain Res. 1990;81(3):639–648. doi: 10.1007/BF02423514. [DOI] [PubMed] [Google Scholar]
- 55.Tian GF, Peever JH, Duffin J. Bötzinger-complex expiratory neurons monosynaptically inhibit phrenic motoneurons in the decerebrate rat. Exp Brain Res. 1998;122(2):149–156. doi: 10.1007/s002210050502. [DOI] [PubMed] [Google Scholar]
- 56.Fox MA, French HT, Laporte JL, Blackler AR, Murphy DL. The serotonin 5-HT(2A) receptor agonist TCB-2: a behavioral and neurophysiological analysis. Psychopharmacology. 2009 doi: 10.1007/s00213-009-1694-1. DOI 10.1007/s00213-009-1694-1. [DOI] [PubMed] [Google Scholar]
- 57.Lips MB, Keller BU. Activity-related calcium dynamics in motoneurons of the nucleus hypoglossus from mouse. J Neurophysiol. 1999;82:2936–2946. doi: 10.1152/jn.1999.82.6.2936. [DOI] [PubMed] [Google Scholar]
- 58.Ladewig T, Keller BU. Simultaneous patch-clamp recording and calcium imaging in a rhythmically active neuronal network in the brainstem slice preparation from mouse. Pflugers Arch. 2000;440:322–332. doi: 10.1007/s004240000277. [DOI] [PubMed] [Google Scholar]