Abstract
Allergic asthma is a dysregulation of the immune system which leads to the development of Th2 responses to innocuous antigens (allergens). Some infections and microbial components can re-direct the immune response toward the Th1 response, or induce regulatory T cells to suppress the Th2 response, thereby inhibiting the development of allergic asthma. Since Acinetobacter baumannii infection can modulate lung cellular and cytokine responses, we studied the effect of A. baumannii in modulating airway eosinophilia in a mouse model of allergic asthma. Ovalbumin (OVA)-sensitized mice were treated with live A. baumannii or phosphate buffered saline (PBS), then intranasally challenged with OVA. Compared to PBS, A. baumannii treatment significantly reduced pulmonary Th2 cytokine and chemokine responses to OVA challenge. More importantly, the airway inflammation in A. baumannii-treated mice was strongly suppressed, as seen by the significant reduction of the proportion and the total number of eosinophils in the bronchoalveolar lavage fluid. In addition, A. baumannii-treated mice diminished lung mucus overproduction and pathology. However, A. baumannii treatment did not significantly alter systemic immune responses to OVA. Serum OVA-specific IgE, IgG1 and IgG2a levels were comparable between A. baumannii- and PBS-treated mice, and tracheobronchial lymph node cells from both treatment groups produced similar levels of Th1 and Th2 cytokines in response to in vitro OVA stimulation. Moreover, it appears that TLR-4 and IFN-γ were not directly involved in the A. baumannii-induced suppression of airway eosinophilia. Our results suggest that A. baumannii inhibits allergic airway inflammation by direct suppression of local pulmonary Th2 cytokine responses to the allergen.
Introduction
Allergic asthma is a chronic, reversible airway inflammatory disease of significant public health importance. Although the exact mechanism is not clear, allergic asthma appears to result from allergen specific type 2 T helper (Th2) lymphocyte proliferation with concomitant excessive production of Th2 cytokines interleukin (IL)-4, IL-5, IL-13 and/or IL-25 [1]. The allergen-specific Th2-like immune responses include secretion of allergen specific IgE, overproduction of bone marrow eosinophils, airway eosinophilia, mucus secretion by goblet cells, and smooth muscle contraction, all collectively contributing to airway hyperreactivity [2], [3].
The gene-environment interaction seems to modulate the aberrant immune responses to allergens, which leads to the development and perpetuation of asthma [2]. In the last several decades, the incidence of asthma has increased rapidly in both developed and developing countries [4], with the estimated number of asthmatic patients increasing from about 130 million people in the mid-1990s to 330 million in 2008 [5], [6]. However, there are notable disparities in the prevalence of asthma between developed and developing countries, and between urban and rural areas of the same country [5]. It is postulated by the hygiene hypothesis that improved living conditions (such as better hygiene and reduced incidences of infectious diseases) in industrialized countries and urban areas may somewhat contribute to the development of allergic asthma [5], [7].
According to the hygiene hypothesis, neonatal and early childhood exposure to certain microbes and their products may shift the immune response toward a Th1 phenotype, or activate regulatory T cells and enhance IL-10 production, and thus suppress the aberrant allergen-specific Th2 responses and alleviate or inhibit the development of clinical symptoms of allergic asthma [8]–[11]. This idea is supported by many experimental and clinical studies with several microbes and their products [8]–[17].
Acinetobacter baumannii is a gram-negative, extracellular bacterium that causes nosocomial and community-acquired pneumonia and other infections [18]. Previous studies in our and other laboratories have shown that intranasal (i.n.) administration of A. baumannii induces acute bronchopneumonia characterized with neutrophil infiltration at the first 72 h after infection [19], [20], followed by macrophages and lymphocytes infiltration, and rapid clearance of the bacteria ∼4 days after infection. Although allergic asthma is primarily mediated by Th2-like immune responses, factors of the innate immune system can play important roles in disease initiation and progression. For example, as the ligand of TLR4, LPS co-administration with allergens was found to either inhibit or exacerbate the severity of asthmatic responses in mice [15]. Adoptive transfer of resident alveolar macrophages also inhibited the airway hyperresponsiveness to OVA challenge in rats [21]. Since A. baumannii lung infection significantly modulates the host innate immune response, we examined the effect of A. baumannii infection/treatment of ovalbumin (OVA)-sensitized mice on the development of airway eosinophilia and associated pulmonary pathology upon subsequent OVA challenge, using a mouse model of OVA-induced allergic asthma. Our results showed that A. baumannii infection suppressed both OVA-specific Th1 and Th2 cytokine responses and the expression of eotaxins in the lung, through a TLR-4 and IFN-γ-independent mechanism. More importantly, the infection suppressed airway eosinophilia and associated lung pathology. The results of this study emphasize the importance of infection-associated innate immune responses in the regulation of the development of allergic asthma.
Materials and Methods
Mice
Six- to 8-week-old female C57BL/6 mice were purchased from Charles River Laboratories (St. Constant, Quebec, Canada). Female B6.129S7-Ifngtm1Ts/J (IFN-γ−/−), C57BL/10ScNJ (TLR4−/−) and C57BL/10SnJ (TLR4+/+) mice of a similar age were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Mice were housed under specific pathogen-free conditions in the Animal Resources, Institute for Biological Sciences, National Research Council Canada (Ottawa) and given free access to sterile water and ovalbumin (OVA)-free mouse chow. The mice were housed and used in accordance with the recommendations of the Canadian Council on Animal Care Guide to the Care and Use of Experimental Animals. This study and all animal care/use protocols were approved (ID # 2006.20 and 2007.15) by the Institute for Biological Sciences (National Research Council Canada) Animal Care Committee.
Airway eosinophilia induction and A. baumannii inoculation
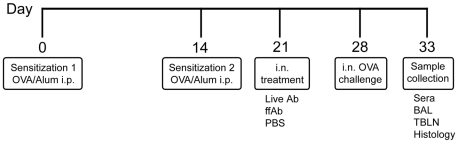
Airway eosinophilia in the mouse model was induced as described before [17] and is illustrated in Figure 1. Briefly, mice were sensitized by intraperitoneal (i.p) injection of 2 µg OVA (Sigma Chemical Co., St Louis, MO, USA) admixed in 100 µl alum (Pierce Laboratories, Rockford, IL, USA) at day 0 and 14. Seven days later (day 21), mice were treated by intranasal (i.n.) administration (50 µl volume) of phosphate-buffered saline (PBS), live A. baumanii (∼108 CFU) in PBS, or formalin-fixed A. baumannii (ffAb) in PBS (∼108 CFU). Seven days post-treatment (day 28), the mice were intranasally challenged with 100 µg OVA in 50 µl of PBS. Five days after OVA challenge (day 33), the mice were sacrificed and samples collected for various assays as indicated below. For live A. baumannii treatment, fresh inocula were prepared for each experiment from a frozen stock culture of A. baumannii (ATCC 17961, American Type Culture Collection, Manassas, VA) as described previously [20]. The actual treatment concentration in each experiment was determined by plating 10-fold serial dilutions on brain heart infusion (BHI) agar supplemented with 50 µg/ml streptomycin. Our previous studies showed that, at this infection dose (∼108 CFU), A. baumannii is generally cleared from the lungs in 4 days [20]. For obtaining the formalin-fixed A. baumannii cells, the cells were fixed by overnight incubation at 37°C in 10% neutral buffered formalin while gently rotating the culture vessel, followed by 3× washing and centrifugal harvesting in PBS.
Figure 1. Experimental protocol for the study.
Mice were sensitized by i.p. administration of 2 µg ovalbumin admixed with 100 µl alum at day 0 and 14. At day 21, the mice were treated by i.n. administration (50 µl volume) of PBS, live A. baumannii (∼108 CFU) or formalin-fixed (ff) A. baumannii (∼108 CFU). At day 28, mice were intranasally challenged with 100 µg OVA in 50 µl PBS or 50 µl PBS alone, as described in Material and Methods. Five days after challenge (day 33), mice were sacrificed for sample collection.
Bronchoalveolar lavage (BAL) and histopathology
Mice were sacrificed five days after i.n. OVA challenge. Sera were separated and stored at −80°C for antibody assays. The lungs were lavaged five times with 1 ml PBS supplemented with 3 mM EDTA and 1% fetal bovine serum [20]. Total lavage cell numbers were enumerated using a haemocytometer, and differential cell counts were determined on cytospin preparations stained with Hema-3 stain set (Fisher Scientific, Middletown, VA, USA). BAL fluid was centrifuged at 3,000×g for 7 min, and supernatants were collected and stored at −80°C. In some experiments, the lungs were removed immediately after lavage and immersed in 10% neutral buffered formalin. The tissues were processed by standard paraffin embedding methods (Department of Pathology and Laboratory Medicine, University of Ottawa, Ottawa, Canada), sectioned (4 µm thick), and stained with haematoxylin and eosin (H&E) or periodic acid-Schiff (PAS) for histopathological evaluation. In some experiments, the lungs from each mouse were minced into small pieces using scissors, and homogenized in 2 ml of saline supplemented with Complete® protease inhibitors (Roche Diagnostics Canada, Laval, QC, Canada). The supernatant was collected by centrifugation (3,000×g for 7 min) and stored at −80°C until used for cytokine assay.
Total RNA isolation and real-time reverse transcriptase polymerase chain reaction (qRT-PCR) for cytokine mRNA expression
For RNA extraction, the lungs were immersed immediately in RNAlater® (Qiagen Inc., Valencia CA) and stored at −20°C. Total RNA was extracted using TRIzol (Invitrogen Canada, Burlington). Relative abundance of cytokine mRNA in lungs were evaluated using a real-time RT-PCR-based method, as described elsewhere [17]. Briefly, single stranded cDNA was prepared through reverse transcription, cytokine genes were amplified and quantified using primers and probes designed with the Primer3 program [22]. Levels of PCR products were normalized to the housekeeping gene β-2-microglobulin (β2m), and data presented as the average of relative expression values in A. baumannii-treated mice compared with those in the corresponding tissues of PBS-treated mice [17].
In vitro re-stimulation culture of tracheobronchial lymph node (TBLN) cells
To assess in vitro cytokine responses to OVA stimulation, TBLNs were collected for the preparation of single-cell suspensions (4×106 cells/ml). TBLN cells were cultured in 48-well tissue culture plates in RPMI-1640 (Sigma-Aldrich, St Louis, MO, USA) supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen, Grand Island, NY, USA), 50 µM 2-mercaptoethanol (Sigma-Aldrich), and 100 U/ml penicillin G and 100 µg/ml streptomycin (Invitrogen). The cells were either stimulated with 1 mg/ml OVA or medium alone. Culture supernatants were collected 72 h later (500×g centrifugation for 10 min) and stored at −80°C for cytokine assays.
Cytokine and chemokine assays
The levels of eotaxin-1, IFN-γ, IL-4, IL-5, IL-10, IL-12p40, and IL-13 in the BAL fluid and lung homogenate supernatants were measured by the Milliplex mouse cytokine/chemokine kit (Millipore, Billerica, MA) on a Luminex® 100IS system (Luminex Corp, Austin, TX, USA) [23]. The analysis was done in duplicate, and the cytokine concentrations were calculated against the standards using Beadview® software (ver. 1.03, Upstate). The detection limit was <2 pg/ml for IL-4, IL-10, and IL-13, 5.54 pg/mL for IL-12p40, 8.97 pg/ml for IFN-γ, and 30.9 pg/mL for IL-5, respectively. IL-17A levels in the BAL fluid and lung homogenates were determined by mouse IL-17A ELISA Ready-Set-Go kit (eBioscience, San Diego, CA, USA), and the limit of detection was 6 pg/ml.
Enzyme-linked immunosorbent assay (ELISA) for ovalbumin-specific immunoglobulin isotype
Serum OVA-specific IgE were assayed using anti-IgE mAb- (4B-39, BD Biosciences, Mississauga, Ontario, Canada) coated microtiter plates, and detected by an ELISA assay using OVA-biotin/streptavidin-horseradish peroxidase (HRP) in conjunction with TMB substrate (KPL Inc., Gaithersburg, MD, USA). Serum OVA-specific IgG1 and IgG2a were measured using OVA-coated microtiter plates (Immulon 2, Thermo Labsystems, Franklin, MA, USA) and detected using alkaline phosphatase conjugated goat anti-mouse IgG1 or IgG2a, respectively (Caltag Laboratories, Burlingame, CA, USA), in conjunction with pNPP substrate (KPL Inc.) [24].
FACS analysis of regulatory T (Treg) cells
The percentages of Treg (CD4+CD25+Foxp3+) cells in the BAL fluid were determined by FACS analysis using the One Step Staining Mouse Treg Flow™ Kit (BioLegend, San Diego, CA). Briefly, BAL cell samples were washed in PBS containing 1% BSA. Aliquots containing ∼106 cells were permeabilized using fixation/permeabilization buffer, following the manufacturer's protocol, and then stained using Alexa Fluor® 488 anti-mouse FOXP3/CD25 PE/CD4 PerCP antibody cocktail or 20 µl Alexa Fluor® 488 rat IgG2b, κ isotype control/CD25 PE/CD4 PerCP antibody cocktail for 30 min at 4°C. After staining, the cells were washed twice with the above PBS solution, and analyzed by FACS Canto II flow cytometer (BD Biosciences, San Jose, CA) using FACS Diva (BD Biosciences, San Jose, CA) software.
Statistical analyses
All parametric data are presented as mean ± standard deviation (SD) for each group. Differences between groups were analyzed by Student's t-test or by one-way and two-way ANOVA followed by the Bonferroni multiple comparison test, when appropriate. P<0.05 was considered to be statistically significant. All statistical analyses were done using GraphPad Prism software (version 4.0, GraphPad Software, San Diego, CA, USA).
Results
Acinetobacter baumannii infection inhibits airway eosinophilia and associated pulmonary pathology in OVA-sensitized/challenged mice
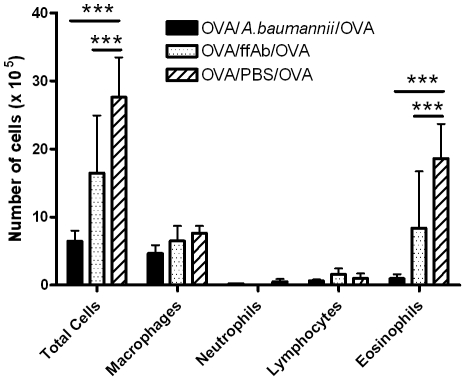
To examine the effect of A. baumannii infection on the development of airway eosinophilia and associated pulmonary pathology, groups of OVA-sensitized C57BL/6 mice were intranasally inoculated with A. baumannii (A. baumannii treatment) or PBS (PBS treatment) 3 weeks after sensitization. BAL fluid from OVA-sensitized mice that had been treated with PBS prior to challenging with OVA (OVA/PBS/OVA), had infiltration of large numbers of eosinophils and mononuclear cells, as well as some neutrophils (Figure 2). In contrast, i.n. inoculation of OVA-sensitized mice with live A. baumannii prior to challenge with OVA (OVA/A. baumannii/OVA) significantly inhibited eosinophil influx into the bronchoalveolar space (P<0.001), without significantly affecting the recruitment of the other cell types (neutrophils, macrophages or lymphocytes), as compared with PBS-treated mice (Figure 2). As expected, the BAL cells from OVA-sensitized and i.n. PBS-challenged mice (OVA/PBS/PBS) contained predominantly (99%) alveolar macrophages (negative control).
Figure 2. Inhibition of airway eosinophilia in OVA-sensitized mice by live A. baumannii.
Mice were sensitized by i.p. administration of OVA/alum on days 0 and 14, then treated with A. baumannii or PBS on day 21, and intranasally challenged with OVA on day 28, as described in Fig. 1. Five days after the i.n. OVA challenge, mice were euthanized, and their lungs were lavaged. Upper panel: Total and differential cell counts in the bronchoalveolar lavage (BAL) fluid were enumerated on cytospin preparations. Each bar represents the mean total number of respective types of cells in the BAL fluid ± SD (n = 5). The data presented represent 1 of at least 2 separate experiments with similar results. ***P<0.001. Lower panel: The BAL cells from OVA-sensitized, PBS-challenged mice (A) consist of predominantly alveolar macrophages whereas the BAL cells from PBS-treated, OVA-challenged mice (B) consist of mainly eosinophils (arrows) with a donut- or horseshoe-shaped nucleus and a pink granular cytoplasm. The majority of BAL cells from A. baumannii-treated, OVA-challenged mice (C) are large alveolar macrophages (arrowheads) with a foamy cytoplasm. HemaStat-3 staining, bar = 100 µm.
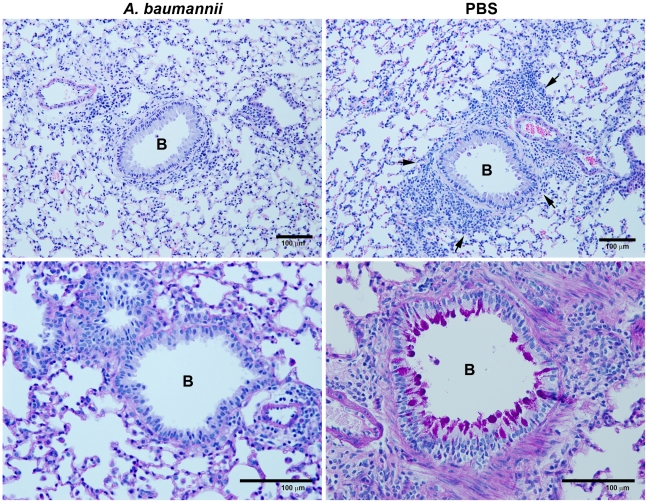
We also examined the histopathogical changes in the lungs of these mice. As shown in Figure 3, i.n. OVA challenge of the sensitized mice treated with PBS (right panel) induced significant perivascular and peribronchial infiltration of various types of inflammatory cells, especially eosinophils, whereas OVA challenge of A. baumannii-treated, sensitized mice substantially suppressed inflammatory cell infiltration and associated pulmonary pathology (left panel). Excess mucus secretion is an important pathophysiological indicator of allergic asthma. Compared with PBS-treated mice, the mice treated with A. baumannii had substantially reduced mucus production by bronchial epithelial cells (Figure 3). These results indicated that infection with A. baumannii significantly inhibited the development of allergic airway eosinophilia and associated pulmonary pathology in mice.
Figure 3. Representative lung histopathology from OVA-sensitized mice treated with A. baumannii.
The mice were sensitized and treated as described in Figure 2 and killed 5 days after the OVA challenge. Note the severe pulmonary inflammation in the areas adjacent to various sized airways in PBS-treated, OVA-sensitized/challenged mouse (arrows, top right panel) and the presence of large numbers of mucus-producing goblet cells (dark purple) (bottom right panel) whereas the inflammation, goblet cell hyperplasia and mucus production were relatively minor in the lungs of mice treated with A. baumannii (top left and bottom left panels). B = bronchus. Top panels stained with H&E; bottom panels stained with periodic acid-Schiff, Bar = 100 µm.
Several groups, including us, have previously shown that certain crude or purified bacterial components (such as lipopolysaccharide, LPS) can suppress allergic airway eosinophilia as effectively as the live bacterial infection [9], [14], [15], [17], [25], [26]. To examine this possibility, OVA-sensitized mice were treated by i.n. administration of formalin-fixed A. baumannii (ffAb) before OVA challenge, and the eosinophil responses were compared with those in mice intranasally treated with PBS or live A. baumannii. Compared to the BAL fluid from the PBS treatment group, BAL fluid from the ffAb treatment group had ∼50% lower eosinophil infiltration upon i.n. OVA challenge (Figure 4). However, the live A. baumannii infection suppressed pulmonary eosinophil responses by more than 90%, compared to the PBS control treatment. These observations indicate that although certain component(s) of A. baumannii can partially suppress allergic airway inflammation, active infection is necessary to maximize the inhibitory effect.
Figure 4. Inhibition of airway eosinophilia in OVA-sensitized mice by intranasal treatment with live or formalin-fixed A. baumannii (ffAb).
Mice were sensitized by i.p. administration with OVA/alum on days 0 and 14. Sensitized mice were i.n. treated with either 1×108 CFU live or formalin-fixed A. baumannii on day 21 and i.n. challenged with OVA on day 28. Cells in the bronchoalveolar lavage (BAL) fluid were collected 5 days after OVA challenge and differential cell types were enumerated on cytospin preparations. Each bar represents the mean total numbers of respective types of cells in the BAL fluid ± SD (n = 5). The data presented represent 1 of at least 2 separate experiments with similar results. ***P<0.001.
A. baumannii infection does not significantly alter serum OVA-specific IgE and IgG subclass levels
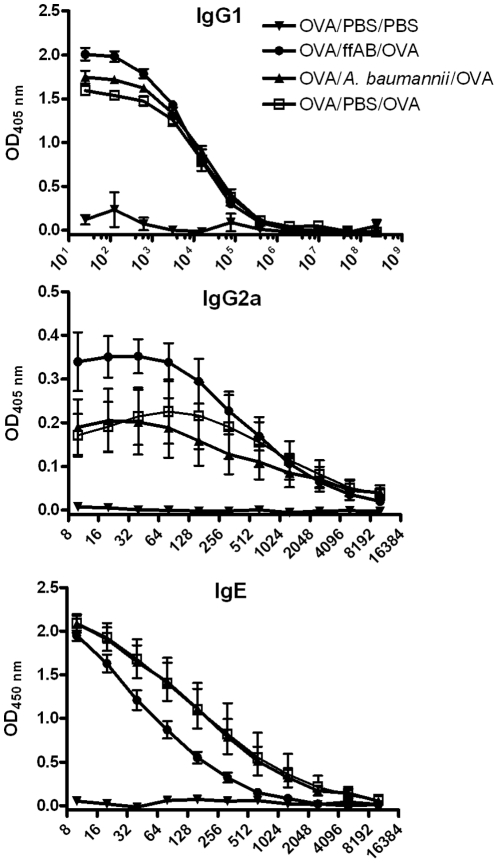
Allergic asthma is generally recognized as a Th2-dependent immune response with increased serum antigen-specific IgE and IgG1 production [1], [2]. As a first step to address the potential mechanism of A. baumannii-induced inhibition of airway eosinophilia, we examined the effect of i.n. treatment with live or formalin-fixed A. baumannii on the changes in the serum IgE and IgG subclasses (IgG1 and IgG2a). In agreement with published reports [8], [12], [14], [27], simply sensitizing mice with OVA-alum at 0 and 14 d (OVA/PBS/PBS group) induced only marginal levels of OVA-specific IgE, IgG1 and IgG2a antibodies (Figure 5). As expected, intranasal OVA challenge of the sensitized mice (OVA/PBS/OVA) induced a remarkable increase in serum OVA-specific IgG1, IgG2a, and IgE in sensitized mice. However, treatment of sensitized mice with live or formalin-fixed A. baumannii showed no significant effect on the serum OVA-specific IgE or IgG1 levels as compared with PBS treatment group (Figure 5). The A. baumannii treatment also showed no effect on the Th1-dependent OVA-specific IgG2a levels (Figure 5).
Figure 5. OVA-specific serum IgG1, IgG2a, and IgE levels in OVA-sensitized mice.
Mice were OVA-sensitized as described in Figure 2 and treated with PBS and challenged with PBS (OVA/PBS/PBS),,treated with A. baumannii and challenged with OVA (OVA/A. baumannii/OVA), treated with formalin-fixed A. baumanii and challenged with OVA (OVA/ffAb/OVA), or treated with PBS and challenged with OVA (OVA/PBS/OVA). Groups of 5 mice were euthanized five days after i.n. OVA challenge, and serum was collected. The OVA-specific IgG1, IgG2a and IgE levels were measured using ELISA. Each data point represents the mean OD value ± SD of five mice in each group.
Effect of i.n A. baumannii treatment on lung cytokine/chemokine responses in allergic mice
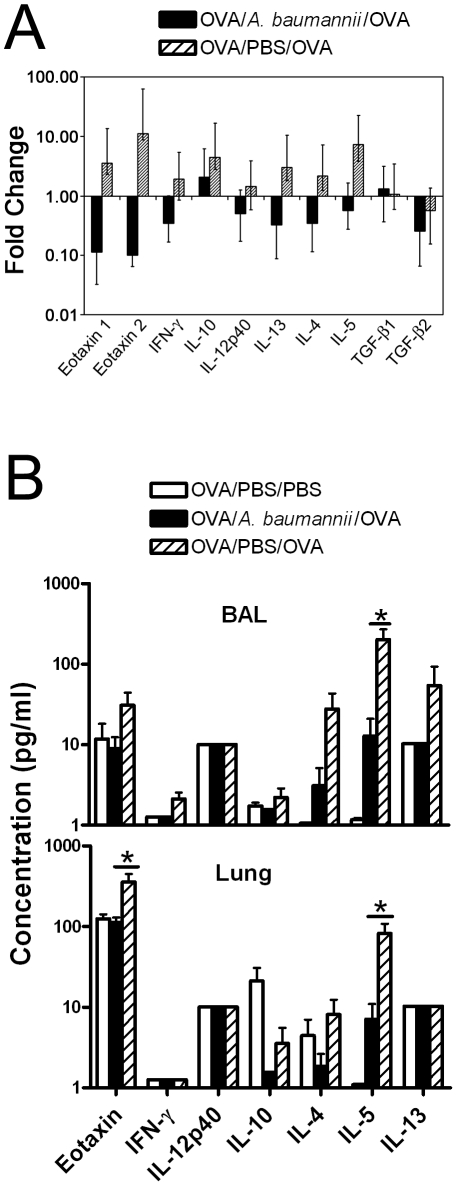
It is well recognized that allergic airway eosinophilia and inflammatory responses are regulated by multiple chemokines [28]–[31] and Th2 cytokines [32]–[34]. To examine the effect of A. baumannii on the cytokine/chemokine responses to OVA challenge in OVA-sensitized mice, the mRNA expression of cytokine IFN-γ and IL-12p40 (Th1 associated), IL-4, IL-5, and IL-13 (Th2-associated), and IL-10 (regulatory/suppressor function associated) in the lungs, as well as their corresponding protein levels in the BAL fluid and in lung homogenate supernatants were analyzed from mice killed at day 5 after i.n OVA challenge. Compared with PBS treatment, live A. baumannii treatment resulted in a substantial reduction in IL-4, IL-5 and IL-13 mRNA expression, as well as decreases in the mRNA expression of IFN-γ, IL-10, and IL-12p40 (Figure 6A). Consistent with the mRNA expression patterns, the protein levels of these cytokines were also generally lower in the lungs and BAL fluids of A. baumannii-treated mice as compared to the PBS-treated mice, although these differences were only statistically significant for IL-5 (P<0.05)(Figure 6B).
Figure 6. Cytokine responses in the lung and BAL fluid of OVA-sensitized mice following OVA challenge.
(A) Real-time PCR analysis of cytokine mRNA expression in lung tissues in OVA-sensitized and A. baumannii treated mice following i.n. OVA challenge. Mice were sensitized and treated as described in Figure 2 and were euthanized 5 days after i.n. OVA challenge. The lungs were collected for RNA extraction. Relative levels of cytokine mRNA expression were determined by real-time RT-PCR analysis as described in Materials and methods. Mouse β-2 microglobulin RNA was measured and used to calculate relative expression using the formula Rel Exp = 2−(ΔΔCT). Results shown are the average and ranges (error bars) of relative expression values determined using cDNA from A. baumannii- or PBS-treated, OVA challenged mice in relation to the corresponding expression levels in PBS challenged mice (n = 5 for all groups). (B) Effect of A. baumannii infection on cytokine levels in BAL fluid and lung homogenates in OVA-sensitized mice following i.n. OVA challenge. Mice were sensitized and treated as described in Figure 2 and were euthanized 5 days after i.n. OVA challenge. The levels of indicated cytokines in BAL fluid and in the lung homogenate supernatants were measured on a Luminex 100IS system using the Milliplex MAP mouse cytokine/chemokine detection kit (Millipore). Each bar represents the mean pg cytokine/mL ± SD (n = 5). The data are representative of two to three independent experiments. *P<0.05 compared to the PBS-treated group.
We also found that the mRNA expression of eotaxin 1 and eotaxin 2, the key chemokines in the induction of eosinophil influx into allergic tissue in mice and humans [28], [31], [32], [35], was markedly reduced in A. baumannii-infected mice, compared to the high expression levels after OVA challenge in PBS-treated mice (Figure 6A). Correspondingly, the level of eotaxin-1 in the lung homogenates and, to a lesser degree in the BAL fluid was significantly lower (P<0.05) in A. baumannii-infected mice (Figure 6B).
To further examine the potential mechanism underlying the A. baumannii-induced suppression of allergic airway eosinophilia, we compared the cytokine responses to in vitro OVA re-stimulation of TBLN cells obtained from PBS- or A. baumannii-treated, OVA sensitized mice 5 days after an i.n. OVA challenge. As can be seen in Figure 7, similar to the TBLN cells from PBS-treated mice, the TBLN cells from A. baumannii-treated mice produced comparable amounts of IFN-γ, IL-10, IL-4, IL-5 and IL-13 in response to in vitro OVA re-stimulation. The levels of IL-12p40 and eotaxin were at the lower limit of detection. As expected, stimulation of TBLN cells from both groups of mice with medium alone induced only minimal amounts of cytokine production (Figure 7).
Figure 7. Cytokine responses to in vitro re-stimulation of tracheobronchial lymph node (TBLN) cells from A. baumannii-treated mice.
Groups of OVA-sensitized C57BL/6 mice were i.n. treated with either A. baumannii or PBS 7 days before i.n. OVA challenge. Mice were killed 5 days after the challenge and their TBLNs were collected and used for in vitro culture to determine cytokine production in response to OVA stimulation. Single cell suspensions (4×106 cells/mL) were re-stimulated in vitro for 48 h with either OVA (1 mg/mL) or culture medium only. The cytokine levels in the supernatants were determined by Luminex. Data are presented as mean concentration (pg/ml) ± SD (n = 5), and are representative of two independent experiments.
TLR4 or IFN-γ is dispensable for A. baumannii-induced inhibition of airway eosinophilia
Since A. baumannii is a gram-negative bacterium that contains abundant amounts of LPS [36], we examined the potential contribution of LPS in the A. baumannii-induced inhibition of allergic airway eosinophilia, using TLR4−/− mice. In agreement with previous observations [15], [37], [38], OVA-sensitized TLR4−/− mice showed significantly higher airway eosinophil responses to i.n OVA challenge than did the OVA-sensitized wild type mice, suggesting that TLR4 signaling per se can efficiently suppress OVA-induced airway inflammation. However, A. baumannii infection significantly inhibited, and at a similar magnitude, the airway eosinophilia in both strains of mice (Figure 8). This result indicates that A. baumannii-induced suppression of allergic airway eosinophilia is independent of the TLR4 signaling pathway.
Figure 8. A. baumannii infection inhibits airway eosinophilia in OVA-challenged TLR-4−/− and IFN-γ−/− mice.
Knock out (KO) and corresponding wild-type (WT) mice were sensitized i.p. with OVA/alum on days 0 and 14 and treated with live A. baumannii or PBS as described in Fig. 2. Mice were i.n. challenged with OVA on day 28. Cells in the bronchoalveolar lavage (BAL) fluid were collected 5 days after OVA challenge and different cell types were enumerated on cytospin preparations. Each bar represents the mean total number of respective types of cells per mouse lung ± SD (n = 5). *P<0.05 and ***P<0.001.
IFN-γ is an important Th1 cytokine that down-regulates Th2 cytokine responses, and it has been implicated in the development of allergic asthma [39]–[41]. We examined whether IFN-γ plays a role in A. baumannii-induced suppression of airway eosinophilia, using IFN-γ−/− mice. In comparison with PBS-treated wild type mice, the PBS-treated IFN-γ−/− mice displayed much stronger airway eosinophilia following i.n. OVA challenge, suggesting a general role for IFN-γ in the suppression of the immune responses to OVA challenge in OVA-sensitized mice (Figure 8). However, airway eosinophilia in both IFN-γ−/− and wild type mice was largely suppressed at a comparable magnitude after A. baumannii treatment (Figure 8), suggesting that A. baumannii-induced suppression of airway eosinophilia is not mediated by IFN-γ.
Treg cells do not appear to mediate A. baumannii-induced inhibition of airway eosinophilia
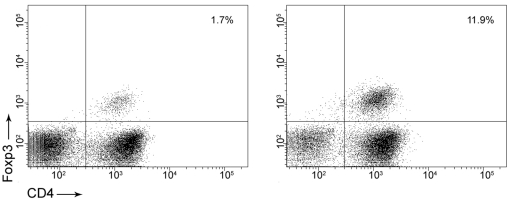
Microbes can suppress the Th2 response of allergic airway eosinophilia through an elevated regulatory T cell response [42], [43]. We compared the number of Treg cells in BAL samples by flow cytometry and found that although there was a more remarkable increase in total BAL lymphocyte number in A. baumannii-treated, allergic mice than sham-treated, allergic mice, the percentage of Treg cells (defined as CD4+CD25+Foxp3+) was in fact lower in the A. baumannii-treated, allergic mice, than in sham-treated, allergic mice (1.7% vs 11.9%, Figure 9). In addition, the total number of Treg cells in the BAL of A. baumannii-treated, allergic mice (1.1×104 Treg cells) was actually lower than the number of Treg cells in sham-treated, allergic mice (2.0×104 Treg cells) (data not shown), suggesting that A. baumannii-induced suppression of airway eosinophilia is unlikely to be mediated through Treg cell-associated suppression of Th2 responses.
Figure 9. Regulatory T cells are not increased in BAL fluid after A. baumannii treatment of OVA-sensitized mice.
BAL from A. baumannii-treated and PBS-treated, allergic mice were collected at 5 days after i.n. OVA challenge and the percentage of regulatory T cells was calculated by flow cytometry as determined by CD4+CD25+Foxp3+ staining. A representative dot plot from an A. baumannii-treated (left panel) and a sham-treated (right panel) mouse illustrates the percentage of CD4+Foxp3+ Treg cells gated on total lymphocytes in the BAL fluid.
Discussion
According to the hygiene hypothesis, exposure to microbes in early childhood and throughout the life may modulate immune responses during allergen stimulation, and prevent the development of allergic asthma. Based on this theory, several clinical and experimental studies that employed bacteria or bacterial component(s) to prevent or treat asthma have shown some promising results [9], while the mechanisms for such suppression remain largely undefined. For example, Mycobacteria spp., the predominantly studied bacteria in experimental and clinical treatment of allergic asthma [8], [12], [14], [42], may alleviate airway inflammation by inducing different immune responses to allergens. Intranasal administration of live BCG before OVA challenge suppressed IL-5 production and airway eosinophilia in an IFN-γ dependent manner [8], while subcutaneous treatment with killed M. vaccae before OVA sensitization inhibited allergic airway inflammation through the induction of a CD4+CD45RBlo regulatory T cell secreted IL-10 and TGF-β [42]. Similarly, T cell-derived IL-10 has been found to be necessary to alleviate asthmatic symptoms when dead mycobacteria or lipoglycan are administered before OVA challenge [14]. Thus, bacterial suppression of allergic airway inflammation and eosinophila may depend on the specific timing of the administration, the route of administration, and the nature of the microbes themselves. Microbes can modulate the allergic airway eosinophilia by switching a predominantly Th2 phenotype to a predominantly Th1 phenotype, or by suppressing the Th2 responses through elevated regulatory T cell response.
Acinetobacter baumannii is a ubiquitous Gram-negative, opportunistic pathogen that frequently induces both nosocomial and community-acquired infections such as pneumonia, skin infection, urinary tract infection and bacteremia [44]–[46]. A. baumannii infection has recently emerged as a major cause of nosocomial infections worldwide, likely because the bacteria can survive on the surface of medical devices such as catheters and ventalitors, as well as on the hands of hospital staff for extended periods of time, and can easily spread through water droplets in the air [18], [46]. Moreover, A. baumannii infections have become increasingly difficult to treat because of the bacteria's rapid development of resistance to multiple antibiotics [18], [47]. In this study, we found that i.n. administration of live, and to a lesser extent, formalin fixed A. baumannii significantly inhibited airway eosinophilia in mice. Live A. baumannii treatment largely suppressed pulmonary Th2 cytokines IL-4, IL-5 and IL-13 production, as well as eosinophil-chemotactic chemokine eotaxin 1 and eotaxin 2. However, levels of Th1 cytokines IL-12 and IFN-γ in the lung (BAL fluid and tissue homogenate supernatant) were not significantly altered. Moreover, TBLN cells from A. baumannii - and PBS-treated mice secreted similar amounts of cytokines upon in in vitro stimulation, and the levels of serum OVA-specific IgE, IgG1 and IgG2a were comparable. These results imply that A. baumannii infection did not change systemic immune responses to OVA from a Th2 to Th1 type, which is in contrast to several previous studies with other bacterial species such as Chlamydia spp, [48], Listeria monocytogenesis [10], and the live vaccine strain of Francisella tularensis (LVS) [17]. Since A. baumannii is an extracellular bacterium, it is likely that it may stimulate host immune responses that are different than those induced by the intracellular bacteria mentioned above. Indeed, several extracellular bacteria such as Streptococcus pneumoniae and Bordetella pertussis alleviate airway eosinophilia [25], [26]. Compared to saline treatment, treatment of mice with S. pneumoniae after OVA sensitization resulted in significant reduction of OVA-specific Th2 cytokines IL-5 and IL-13 responses by their TBLN cells without significant increases in IFN-γ production [25]. Similarly, administration of heat-killed whole cell B. pertussis during sensitization and before OVA challenge also significantly suppressed airway eosinophilia and lung inflammation, which correlated with suppressed Th2 cytokine (IL-4 or IL-5) responses without the increased Th1 cytokine (IL-12 or IFN-γ) levels [26]. It is not clear, however, whether administration of this bacterium changed the systemic immune responses. Similar to these extracellular bacteria, A. baumannii-induced inhibition of airway eosinophilia is associated with suppressed airway Th2 cytokine and chemokine production, without the enhancement of Th1 responses. However, in contrast to S. pneumoniae, A. baumannii treatment did not change the host systemic immune responses to OVA. This is probably due to the fact that pulmonary A. baumannii infection is acute and largely limited to the lungs, and the infection may not be extensive and persistent enough to modulate the systemic immune responses to OVA. Moreover, using IFN-γ−/− mice, we demonstrated that IFN-γ is not essential for A. baumannii induced suppression of allergic inflammation (Figure 8). The expression of IL-10 in lungs or upon in vitro OVA stimulation of TBLN lymphocytes from A. baumannii-treated mice also showed no differences in comparison to those of PBS-treated control mice after OVA challenge. Real-time RT-PCR results also indicated that pulmonary TGF-β production was similar between PBS- or A. baumannii-treated mice. In addition, we have demonstrated that A. baumannii-treated allergic mice appear to have fewer, rather than more, Treg cells in their BAL than do PBS-treated allergic mice (Figure 9). These results suggest that regulatory T cells may not be involved in the inhibition of allergic response by A. baumannii treatment. These findings cumulatively suggest that A. baumannii-induced inhibition of airway eosinophilia and associated pulmonary pathological changes did not involve the systemic suppression of Th2 associated factors or the elevation of Th1-specific parameters.
A. baumannii-induced alleviation of allergic airway inflammation is not an isolated observation in the Acinetobacter species, since it has been previously shown that i.n. treatment of mice with A. lwoffii F78, a non-pathogenic Acinetobacter isolate cultured from farm cowsheds, also suppressed airway eosinophilia and airway hyperresponsiveness [49]. However, unlike A. baumannii, A. lwoffii activates dendritic cells with increased expression of surface activation markers CD40, CD80, CD86 and MHCII, and induces highly Th1 polarizing immune responses including the enhanced expression of delta-4 mRNA and increased secretion of IL-12 secretion, as well as reduced mRNA expression for Jagged-1 [49]. However, there is no information on whether the inhibition is correlated with serum antibody levels/changes, or IL-4, 5, 13, and IFN-γ production. In our work, we used a clinically relevant Acinetobacter species, and showed similar suppression of the allergic airway responses. Moreover, our study indicates that alleviated airway inflammation in A. baumannii-treated mice is associated with suppressed Th2 cytokine and chemokine eotaxin expression in the lung. However, it is not entirely clear if A. lwoffii and A. baumannii utilize fundamentally different immunoregulation mechanisms in the inhibition of allergic airway eosinophilia, since the treatment regime used in A. lwoffii studies was quite different from the present study in that A. lwoffii treatment started 10 days before antigen sensitization and continued every second day throughout the whole sensitization and challenge phases of the study [49].
Our study showed that active infection with A. baumannii is not essential for the inhibition of allergic airway eosinophilia, and killed whole cells (formalin-fixed) are also capable of inhibiting the development of airway eosinophila, although to a lesser extent (Figure 4). A. baumannii is a gram-negative bacterium containing abundant LPS, and recent studies have shown that earlier childhood exposure to bacterial LPS is correlated with reduced risk of atopy such as hay fever and allergic airway diseases [50]. Treatment of mice with Escherichia coli LPS suppresses the development of airway hyperreactivity in mouse models of asthma [15], [37], [38]. Since LPS has been implicated in the regulation of allergic asthma development mainly through TLR-4 signalling pathway [51], we studied the effect of A. baumannii administration on allergic responses in TLR4−/− mice. Although PBS-treated TLR-4−/− mice showed stronger airway eosinophilia than PBS-treated wild type mice, indicating suppression of airway inflammation by TLR-4 signaling, it is interesting to note that A. baumannii treatment suppressed airway eosinophilia in both TLR4−/− and wild type mice at a similar magnitude (Figure 8). This result argues against a role for LPS in A. baumannii–induced suppression of allergy airway eosinophilia. Instead, it suggests that other components of A. baumannii may be more important in suppression of OVA-specific inflammation.
A. baumannii infection per se induces strong inflammatory responses and A. baumannii can cause substantial airway epithelial damage via secretion of enzymes and toxic products leading to oxidative stress and epithelial cell apoptosis [52]. As such, it is possible that the reduction in most cytokines and inflammatory cells assessed in A. baumannii-treated allergic mice versus PBS-treated allergic mice could be due to increased epithelial cell death. However, this is unlikely since we, and others, have previously shown that i.n. A. baumannii infection at this dose generally increases proinflammatory cytokine and chemokine responses in the lungs [19], [20]. Moreover, we also showed that formalin-killed A. baumannii, which causes little or no damage to the pulmonary epithelium, also inhibits airway eosinophilia (Figure 4), making it unlikely that epithelial cell death is responsible for the reduction in most cytokines and inflammatory cells seen in this model.
In conclusion, administration of A. baumannii to mice that had already been sensitized to OVA is capable of inhibiting airway eosinophilia and associated pulmonary pathology. Our results further support the observed role of microbes and their products on the development/outcome of the pathogenesis of allergic asthma. Future studies to examine the long term effect of treatment, the use of inactivated bacterial cells, and particularly the identification of effective bacterial component(s) should be undertaken to explore A. baumannii as a potential immunomodulator for the treatment of human allergic asthma.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the intramural research funding program (A-base) of the National Research Council Canada. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lazaar AL, Panettieri RA. Pathogenesis and treatment of asthma: recent advances. Drug Discov Today: Dis Mech. 2004;1:111–116. [Google Scholar]
- 2.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lukacs NW. Role of chemokines in the pathogenesis of asthma. Nat Rev Immunol. 2001;1:108–116. doi: 10.1038/35100503. [DOI] [PubMed] [Google Scholar]
- 4.Nauta AJ, Engels F, Knippels LM, Garssen J, Nijkamp FP, et al. Mechanisms of allergy and asthma. Eur J Pharmacol. 2008;585:354–360. doi: 10.1016/j.ejphar.2008.02.094. [DOI] [PubMed] [Google Scholar]
- 5.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, Parasites, and the Hygiene Hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 6.Finn PW, Bigby TD. Innate Immunity and Asthma. Proc Am Thorac Soc. 2009;6:260–265. doi: 10.1513/pats.200807-064RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strachan DP. Hay fever, Hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erb KJ, Holloway JW, Sobeck A, Moll H, Le Gros G. Infection of Mice with Mycobacterium bovis-Bacillus Calmette-Guerin (BCG) Suppresses Allergen-induced Airway Eosinophilia. J Exp Med. 1998;187:561–569. doi: 10.1084/jem.187.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matricardi PM, Bjorksten B, Bonini S, Bousquet J, Djukanovic R, et al. Microbial products in allergy prevention and therapy. Allergy. 2003;58:461–471. doi: 10.1034/j.1398-9995.2003.00175.x. [DOI] [PubMed] [Google Scholar]
- 10.Hansen G, Yeung VP, Berry G, Umetsu DT, DeKruyff RH. Vaccination with Heat-Killed Listeria as Adjuvant Reverses Established Allergen-Induced Airway Hyperreactivity and Inflammation: Role of CD8+ T Cells and IL-18. J Immunol. 2000;164:223–230. doi: 10.4049/jimmunol.164.1.223. [DOI] [PubMed] [Google Scholar]
- 11.Kline JN, Kitagaki K, Businga TR, Jain VV. Treatment of established asthma in a murine model using CpG oligodeoxynucleotides. Am J Physiol Lung Cell Mol Physiol. 2002;283:L170–179. doi: 10.1152/ajplung.00402.2001. [DOI] [PubMed] [Google Scholar]
- 12.Smit JJ, Van Loveren H, Hoekstra MO, Van der Kant PAA, Folkerts G, et al. Therapeutic treatment with heat-killed Mycobacterium vaccae (SRL172) in a mild and severe mouse model for allergic asthma. Eur J Pharmacol. 2003;470:193–199. doi: 10.1016/s0014-2999(03)01794-1. [DOI] [PubMed] [Google Scholar]
- 13.Jain VV, Businga TR, Kitagaki K, George CL, O'Shaughnessy PT, et al. Mucosal immunotherapy with CpG oligodeoxynucleotides reverses a murine model of chronic asthma induced by repeated antigen exposure. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1137–1146. doi: 10.1152/ajplung.00073.2003. [DOI] [PubMed] [Google Scholar]
- 14.Sayers I, Severn W, Scanga CB, Hudson J, Gros GL, et al. Suppression of allergic airway disease using mycobacterial lipoglycans. J Allergy Clin Immunol. 2004;114:302–309. doi: 10.1016/j.jaci.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez D, Keller AC, Faquim-Mauro EL, de Macedo MS, Cunha FQ, et al. Bacterial Lipopolysaccharide Signaling Through Toll-Like Receptor 4 Suppresses Asthma-Like Responses Via Nitric Oxide Synthase 2 Activity. J Immunol. 2003;171:1001–1008. doi: 10.4049/jimmunol.171.2.1001. [DOI] [PubMed] [Google Scholar]
- 16.Wohlleben G, Muller J, Tatsch U, Hambrecht C, Herz U, et al. Influenza A Virus Infection Inhibits the Efficient Recruitment of Th2 Cells into the Airways and the Development of Airway Eosinophilia. J Immunol. 2003;170:4601–4611. doi: 10.4049/jimmunol.170.9.4601. [DOI] [PubMed] [Google Scholar]
- 17.KuoLee R, Zhou H, Harris G, Zhao X, Qiu H, et al. Inhibition of airway eosinophilia and pulmonary pathology in a mouse model of allergic asthma by the live vaccine strain of Francisella tularensis. Clin Exp Allergy. 2008;38:1003–1015. doi: 10.1111/j.1365-2222.2008.02956.x. [DOI] [PubMed] [Google Scholar]
- 18.Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Micro. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 19.Knapp S, Wieland CW, Florquin S, Pantophlet R, Dijkshoorn L, et al. Differential Roles of CD14 and Toll-like Receptors 4and 2 in Murine Acinetobacter Pneumonia. Am J Respir Crit Care Med. 2006;173:122–129. doi: 10.1164/rccm.200505-730OC. [DOI] [PubMed] [Google Scholar]
- 20.van Faassen H, KuoLee R, Harris G, Zhao X, Conlan JW, et al. Neutrophils Play an Important Role in Host Resistance to Respiratory Infection with Acinetobacter baumannii in Mice. Infect Immun. 2007;75:5597–5608. doi: 10.1128/IAI.00762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Careau E, Bissonnette EY. Adoptive Transfer of Alveolar Macrophages Abrogates Bronchial Hyperresponsiveness. Am J Respir Cell Mol Biol. 2004;31:22–27. doi: 10.1165/rcmb.2003-0229OC. [DOI] [PubMed] [Google Scholar]
- 22.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 23.KuoLee R, Zhao X, Austin J, Harris G, Conlan JW, et al. Mouse model of oral infection with virulent type A Francisella tularensis. Infect Immun. 2007;75:1651–1660. doi: 10.1128/IAI.01834-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen W, Havell EA, Moldawer LL, McIntyre KW, Chizzonite RA, et al. Interleukin 1: an important mediator of host resistance against Pneumocystis carinii. J Exp Med. 1992;176:713–718. doi: 10.1084/jem.176.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preston JA, Essilfie A-T, Horvat JC, Wade MA, Beagley KW, et al. Inhibition of allergic airways disease by immunomodulatory therapy with whole killed Streptococcus pneumoniae. Vaccine. 2007;25:8154–8162. doi: 10.1016/j.vaccine.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 26.Young-Suk Kim, Keun-Sang Kwon, Dae-Ki Kim, Il-Whan Choi, Hern-Ku Lee. Inhibition of murine allergic airway disease by Bordetella pertussis. Immunology. 2004;112:624–630. doi: 10.1111/j.1365-2567.2004.01880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ennis DP, Cassidy JP, Mahon BP. Whole-cell pertussis vaccine protects against Bordetella pertussis exacerbation of allergic asthma. Immunol Letters. 2005;97:91–100. doi: 10.1016/j.imlet.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Zepeda EA, Rothenberg ME, Ownbey RT, Celestin J, Leder P, et al. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat Med. 1996;2:449–456. doi: 10.1038/nm0496-449. [DOI] [PubMed] [Google Scholar]
- 29.Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, et al. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. 2007;120:1324–1331. doi: 10.1016/j.jaci.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 30.Mould AW, Ramsay AJ, Matthaei KI, Young IG, Rothenberg ME, et al. The Effect of IL-5 and Eotaxin Expression in the Lung on Eosinophil Trafficking and Degranulation and the Induction of Bronchial Hyperreactivity. J Immunol. 2000;164:2142–2150. doi: 10.4049/jimmunol.164.4.2142. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalo JA, Lloyd CM, Kremer L, Finger E, Martinez AC, et al. Eosinophil recruitment to the lung in a murine model of allergic inflammation. The role of T cells, chemokines, and adhesion receptors. J Clin Invest. 1996;98:2332–2345. doi: 10.1172/JCI119045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pease JE, Williams TJ. Eotaxin and asthma. Curr Opin Pharmacol. 2001;1:248–253. doi: 10.1016/s1471-4892(01)00044-3. [DOI] [PubMed] [Google Scholar]
- 33.Pope SM, Zimmermann N, Stringer KF, Karow ML, Rothenberg ME. The Eotaxin Chemokines and CCR3 Are Fundamental Regulators of Allergen-Induced Pulmonary Eosinophilia. J Immunol. 2005;175:5341–5350. doi: 10.4049/jimmunol.175.8.5341. [DOI] [PubMed] [Google Scholar]
- 34.Pope SM, Fulkerson PC, Blanchard C, Akei HS, Nikolaidis NM, et al. Identification of a Cooperative Mechanism Involving Interleukin-13 and Eotaxin-2 in Experimental Allergic Lung Inflammation. J Biol Chem. 2005;280:13952–13961. doi: 10.1074/jbc.M406037200. [DOI] [PubMed] [Google Scholar]
- 35.Ponath PD, Qin S, Ringler DJ, Clark-Lewis I, Wang J, et al. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest. 1996;97:604–612. doi: 10.1172/JCI118456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacLean LL, Perry MB, Chen W, Vinogradov E. The structure of the polysaccharide O-chain of the LPS from Acinetobacter baumannii strain ATCC 17961. Carbohydr Res. 2009;344:474–478. doi: 10.1016/j.carres.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, McCusker C. Neonatal exposure with LPS and/or allergen prevents experimental allergic airways disease: development of tolerance using environmental antigens. J Allergy Clin Immunol. 2006;118:143–151. doi: 10.1016/j.jaci.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 38.Hollingsworth JW, Whitehead GS, Lin KL, Nakano H, Gunn MD, et al. TLR4 Signaling Attenuates Ongoing Allergic Inflammation. J Immunol. 2006;176:5856–5862. doi: 10.4049/jimmunol.176.10.5856. [DOI] [PubMed] [Google Scholar]
- 39.Gavett S, O'Hearn D, Li X, Huang S, Finkelman F, et al. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J Exp Med. 1995;182:1527–1536. doi: 10.1084/jem.182.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakagome K, Okunishi K, Imamura M, Harada H, Matsumoto T, et al. IFN-g Attenuates Antigen-Induced Overall Immune Response in the Airway As a Th1-Type Immune Regulatory Cytokine. J Immunol. 2009;183:209–220. doi: 10.4049/jimmunol.0802712. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida M, Leigh R, Matsumoto K, Wattie J, Ellis R, et al. Effect of Interferon-γ on Allergic Airway Responses in Interferon-{gamma}-deficient Mice. Am J Respir Crit Care Med. 2002;166:451–456. doi: 10.1164/rccm.200202-095OC. [DOI] [PubMed] [Google Scholar]
- 42.Zuany-Amorim C, Sawicka E, Manlius C, Le Moine A, Brunet LR, et al. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat Med. 2002;8:625–629. doi: 10.1038/nm0602-625. [DOI] [PubMed] [Google Scholar]
- 43.Preston JA, Thorburn AN, Starkey MR, Beckett EL, Horvat JC, et al. Streptococcus pneumoniae infection suppresses allergic airways disease by inducing regulatory T-cells. Eur Respir J. 2011;37:53–64. doi: 10.1183/09031936.00049510. [DOI] [PubMed] [Google Scholar]
- 44.Gaynes R, Edwards JR. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41:848–854. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 45.Joly-Guillou ML. Clinical impact and pathogenicity of Acinetobacter. Clin Microbiol Infect. 2005;11:868–873. doi: 10.1111/j.1469-0691.2005.01227.x. [DOI] [PubMed] [Google Scholar]
- 46.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: Emergence of a Successful Pathogen. Clin Microbiol Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fournier PE, Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis. 2006;42:692–699. doi: 10.1086/500202. [DOI] [PubMed] [Google Scholar]
- 48.Han X, Fan Y, Wang S, Yang J, Bilenki L, et al. Dendritic cells from Chlamydia-infected mice show altered Toll-like receptor expression and play a crucial role in inhibition of allergic responses to ovalbumin. Eur J Immunol. 2004;34:981–989. doi: 10.1002/eji.200324387. [DOI] [PubMed] [Google Scholar]
- 49.Debarry J, Garn H, Hanuszkiewicz A, Dickgreber N, Blümer N, et al. Acinetobacter lwoffii and Lactococcus lactis strains isolated from farm cowsheds possess strong allergy-protective properties. J Allergy Clin Immunol. 2007;119:1514–1521. doi: 10.1016/j.jaci.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 50.von Mutius E, Braun-Fahrlander C, Schierl R, Riedler J, Ehlermann S, et al. Exposure to endotoxin or other bacterial components might protect against the development of atopy. Clin Exp Allergy. 2000;30:1230–1234. doi: 10.1046/j.1365-2222.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- 51.Michel O. Role of lipopolysaccharide (LPS) in asthma and other pulmonary conditions. J Endotoxin Res. 2003;9:293–300. doi: 10.1179/096805103225002539. [DOI] [PubMed] [Google Scholar]
- 52.Smani Y, Docobo-Pérez F, McConnell MJ, Pachón J. Acinetobacter baumannii-induced lung cell death: Role of inflammation, oxidative stress and cytosolic calcium. Microb Pathog. 2011;50:224–232. doi: 10.1016/j.micpath.2011.01.008. [DOI] [PubMed] [Google Scholar]