Abstract
Background
Cognitive, global and functional instruments have been extensively investigated for correlations with neuropathological changes such as neurofibrillary tangles (NFTs), plaques, and synapse loss in the brain.
Objective
Our objective is to correlate the functional, global and cognitive decline assessed clinically with the neuropathological changes observed in a large prospectively characterized cohort of mild cognitive impairment (MCI) and Alzheimer’s disease (AD).
Methods
We examined 150 subjects (16 MCI and 134 AD) that were prospectively assessed and longitudinally followed to autopsy. MCI subjects clinically met Petersen criteria for single or multi-domain amnestic MCI. AD subjects clinically met NINCDS-ADRDA criteria for probable or possible AD. All subjects received the Functional Assessment Staging (FAST), the Global Deterioration Scale (GDS), and the Mini Mental State Examination (MMSE) ante-mortem. Plaque and tangle counts were gathered for hippocampus, entorhinal cortex, frontal, temporal and parietal cortices. Braak staging was performed as well.
Results
The GDS, FAST and MMSE correlated with plaque counts in all regions. The GDS, FAST and MMSE correlated with tangle counts in in all regions. The three instruments also correlated with the Braak score. The MMSE and GDS correlate better than the FAST in most regions.
Conclusions
Accumulation of neuropathology appears to correlate with functional, global, and cognitive decline as people progress from MCI through AD. In our study, both tangle and plaque accumulation correlated to clinical decline but when AD is considered alone, the correlations are not as robust.
Keywords: Neuropathology, Alzheimer’s disease, plaques, tangles, staging, cognition
INTRODUCTION
Although AD has been investigated for more than 100 years, it was not until the 1960’s that quantitative measures of the disease progression and severity in relation to function and neuropathology were made by Blessed and his colleagues. The Blessed Dementia Scale was found to be significantly correlated with senile plaques, neurofibrillary tangles (NFTs) and the progression of dementia [1]. Later studies using the Blessed scale also found a high correlation between neurofibrillary tangles of the cerebral cortex and symptoms of AD type dementia and also concluding that density of NFTs was more predictive of AD than senile plaques (SPs) [2, 3]. As with the Blessed scale, the MMSE and Clinical Dementia Rating (CDR) have been used to correlate cognitive symptoms of dementia with neuropathology [4–6]. Recently, this discussion became more relevant when a case series by Holmes and Nicoll reported clearance of amyloid and plaques in a group receiving the active immunotherapy AN1792 but the group continued to dement and the autopsy showed little change in other pathological findings [7].
There are very few reported comparisons of the GDS to neuropathology of senile plaques and NFTs [8]. Whereas the MMSE appears to be quite sensitive in earlier stages of AD, when the focus is on cognitive decline, the GDS seems to be a more appropriate choice for other aspects of decline such as global functioning [9]. As with the GDS, a limited amount of research has been done using the FAST for this purpose. One study compared the FAST and GDS to loss of hippocampal volume [10] and found that regional hippocampal volume correlated inversely with increasing FAST and GDS scores. Another study addressed neuronal loss and neurofibrillary changes [11] In that study, significant correlations were noted between the FAST and the total number of neurons and the percentage of neurons with neurofibrillary changes in CA1, CA4, and the subiculum. Neither study compared clinical pathology to Braak staging [12]. No studies have been done to date that correlate the FAST to plaque formation.
The goal of this study is to examine the relationship between functional decline as measured by the FAST, global decline as measured by the GDS, and cognitive decline as measured by the MMSE score and neuropathology changes (senile plaques and NFTs). We hypothesize a correlation between worsening cognitive, functional and global decline and increasing neuropathological changes.
METHODS
Participants
150 subjects were selected from a larger sample of 728 subjects prospectively evaluated as participants of the Banner-Sun Health Research Institute Brain Donation Program between 1/1/97 and 12/31/07. After consent, patients received medical, neurological, and neuropsychological assessments, and eventually underwent post-mortem neuropathological analysis. The mean interval between last neuropsychological assessment and death was 12.5 ± 8.7 months (S.D.) for the total sample. There was no difference identified in the MCI group (13.9± 14.5 months).
All 150 subjects selected that had complete clinical, neuropsychological and pathological data to evaluate. The sample included 16 MCI (MMSE range 24–29, FAST 2–3, GDS 2–3) and 134 AD (MMSE 0–23, FAST 4-7c, GDS 4–7). Included were 131 clinically diagnosed and autopsy-confirmed AD patients diagnosed by National Institute on Aging (NIA) [13, 14] criteria for definite or probable AD who also met NINCDS-ADRDA criteria for a clinical diagnosis of probable or possible AD [15]. The 578 subjects that were excluded had missing clinical or pathological data or had a primary diagnosis other than AD including dementia with Lewy bodies [16]. Vascular dementia [17], Parkinson’s disease dementia [18], FTD [19], etc.
MCI subjects were diagnosed clinically according to Petersen criteria for single or multi-domain amnestic MCI [20]. These MCI (n = 16) subjects had subjective complaints of memory loss and objective impairment in memory, but the magnitude of the cognitive and related deficits was insufficient for a diagnosis of dementia or AD [15, 21]. Only MCI subjects who came to autopsy prior to conversion to dementia were included in this sample.
Global Assessment
Subjects were evaluated globally utilizing the Global Deterioration Scale (GDS) [22, 23]. The GDS has seven ordinal stages (1–7) on a scale starting with Stage 1 (no cognitive decline) and ending with Stage 7 (very severe cognitive decline). The GDS incorporates both cognitive and functional aspects of aging and dementia [24, 25]. These were administered by the study clinician.
Functional Assessment
Patients were functionally assessed by utilizing the Functional Assessment Staging procedure (FAST) [26]. It is used to assess functional decline in AD. Patients who are functionally more impaired also show continuing increments in cognitive loss. The FAST contains 16 stages. Stage 1 marks no difficulties for the patient while Stage 7(f) describes the patient who is unable to hold his/her head up [22]. The latter eleven stages subdivide the FAST in the late stages of 6 and 7. These were administered by the study clinician.
Cognitive Assessments
The Mini Mental State Examination was administered as a measure of cognitive status [27]. This was administered by the clinical coordinator.
Neuropathological Examination
Pathological assessment was performed at the Civin Laboratory for Neuropathology at Banner-Sun Health Research Institute (SHRI). The average post-mortem interval was approximately 3 hours. Sections from paraffin blocks were cut at 5 μm and stained with hematoxylin and eosin (H & E). Paraffin sections from the anterior cingulate gyrus, entorhinal cortex, middle frontal gyrus, middle temporal gyrus, inferior parietal lobule and anterior medulla were stained immunohistochemically for α-synuclein (LB509 monoclonal antibody) to identify Lewy bodies and Lewy-related neurites. Sections from frozen blocks were stained with Campbell-Switzer, Gallyas and Thioflavine S methods for plaques, tangles and other inclusions. Large 4 × 3 cm frozen sections containing coronal planes through most of the frontal, temporal, parietal and occipital lobes, were stained with H & E and Luxol Fast Blue to detect cerebral white matter rarefaction (leukoaraiosis). Additional immunohistochemical procedures were used as needed, including those for ubiquitin to detect intraneuronal inclusions of motor neuron disease with dementia and αB-crystallin and phosphorylated neurofilament to detect swollen neurons in corticobasal degeneration. For all stains except H & E and Luxol Fast Blue, both positive and negative control sections were processed with every batch of slides.
Densities of plaques and neurofibrillary tangles were determined in the hippocampus, entorhinal cortex, temporal lobe, parietal lobe, and frontal lobe using CERAD [14] criteria and rated on a scale of 0 (none) to 3 (frequent). Totals of the five areas had a range with a possible maximum score of 15. Both neuritic (large and encompassing neurites) and diffuse (more minute and not surrounding neurites) plaques were included in plaque density ratings. Braak staging was performed according to Braak and Braak [12] involving evaluation of NFT progression.
Statistical Analysis
The demographics of the sample are found in Table 1. In order to graph and analyze FAST sub-stage scores of 6(a)–6(e) and 7(a)–7(f), 6(a) was converted to 6.0, 6(b) to 6.2, 6(c) to 6.4 and so forth [28]. AD/MCI subjects were analyzed together to encompass a full range of cognitive and functional impairment. MCI subjects were not analyzed separately as the sample size was too small to make any observation seem valid. Density scores of plaques and tangles in tissue from the hippocampus, entorhinal cortex, temporal lobe, parietal lobe, frontal lobe and a total of these scores were correlated with the FAST, GDS, and MMSE using Spearman’s rho. The nonparametric Spearman rho statistic was used for the correlations because the FAST and GDS scales were ordinal and because the scores for the MMSE were not normally distributed. All analyses were conducted using either Microsoft Excel or SPSS (SPSS, Chicago, IL).
Table 1.
Demographics and Clinical Assessments
| Characteristic | N=150 |
|---|---|
| Age at death (years)(mean ± SEM) | 83.6 ± 0.7 |
| Gender (% female) | 48.3 |
| Education (years)(mean ± SEM) | 14.7 ± 0.2 |
| MMSE (mean ± SEM) | 17.0 ± 1.3 Range (0–29) |
| FAST (mean ± SEM) | 5.1 ± 0.2 Range (2-7c) |
| GDS (mean ± SEM) | 4.8 ± 0.2 Range (2–7) |
SEM, standard error of the mean;
MMSE, Mini Mental State Examination;
FAST, Functional Assessment Staging;
GDS, Global Deterioration Scale.
RESULTS
For statistical purposes, MCI subjects were added to the AD subject group since amnestic MCI is widely considered a prodromal condition and to broaden the spectrum of the AD disease process observation window. No significant differences in age or brain weight were found between AD and MCI subjects. All patients were Caucasian and a approximately half were female. The mean age of the cohort at the time of death was 83.6 years. The mean disease duration was 7.95 ± 5.18 years. The demographics are summarized in Table 1.
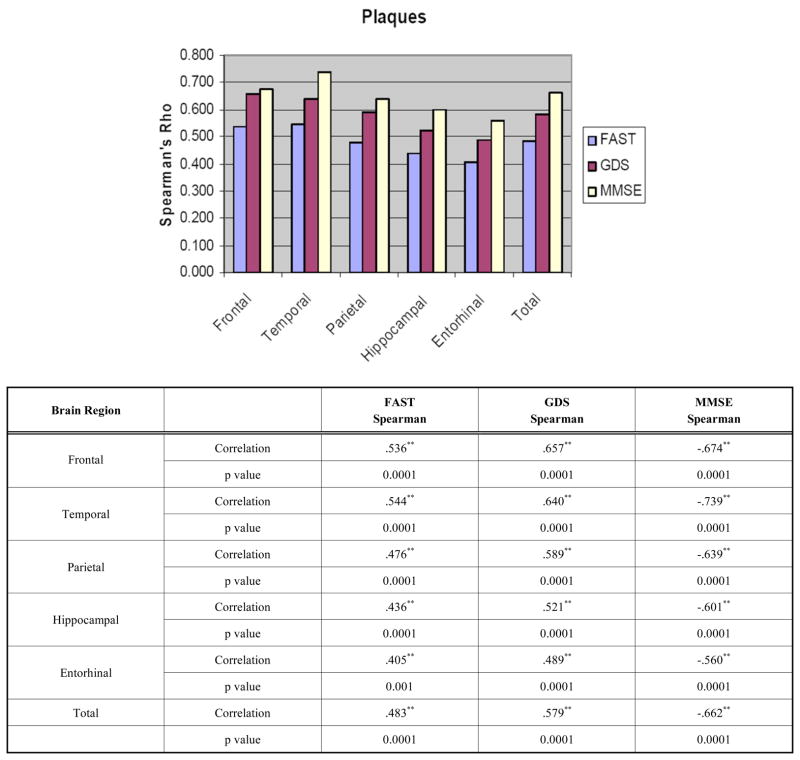
When the entire sample was examined, the FAST correlated significantly with plaque counts in the frontal, temporal, parietal, hippocampal and entorhinal cortices (Fig. 1). The data are also presented in a tabulated manner with Fig. (1). The GDS correlated significantly with plaque counts in all brain regions investigated. Similarly, the MMSE correlated with plaque counts in all brain regions investigated. (Fig. 1). There were no significant differences between the FAST, GDS and MMSE in their respective correlations to regional plaque counts. Correlations were better in the neo-cortices than the hippocampal and entorhinal cortices.
Fig. (1).
Correlations between Clinical Assessments and Plaque Densities shown graphically and tabulated. *p <0.05, ** p <0.01.
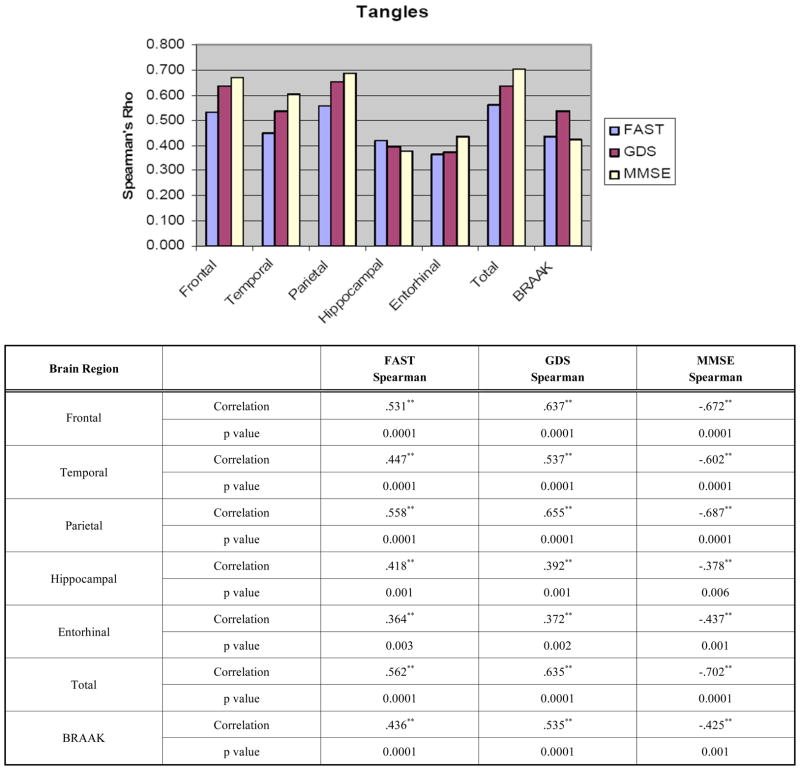
For NFTs, significant correlations were observed for all regions and the FAST, GDS and MMSE scores (Fig. 2). The data are also presented in a tabulated manner with Fig. (2). The FAST, GDS, and MMSE correlated strongly with tangle counts in the neocortex but less robustly entorhinal cortex and the hippocampus. For Braak staging, all three measures showed significant correlations. There were no differences between the groups in terms of correlations with regional tangle counts or Braak staging.
Fig. (2).
Correlations between Clinical Assessments and Neurofibrillary Tangles and Braak Stage presented graphically and tabulated.
*p <0.05, ** p <0.01.
Because of concerns about the possibility of dilution of correlation when combining AD and MCI, we repeated correlations in the AD subjects alone. These data are tabulated in Table 2. As can be seen, tangles continue to correlate quite well with MMSE, GDS, and FAST in the neocortex but not in the archicortex (hippocampus and entorhinal cortex). Plaque correlations are considerably weaker by region but not diluted entirely. Again, they appear more robust in neo-cortex than in the archicortex. The FAST does not correlate significantly with total plaque counts.
Table 2.
Table 2a. Correlations between Clinical Assessments and Plaque Densities for AD Only
| Brain Region | FAST Spearman | GDS Spearman | MMSE Spearman | |
|---|---|---|---|---|
| Frontal | Correlation | .30** | .43** | −.36** |
| p value | <0.05 | <0.001 | 0.02 | |
| Temporal | Correlation | .24** | .36** | −.41** |
| p value | 0.06 | 0.006 | <0.01 | |
| Parietal | Correlation | .21** | .0.34** | −.32** |
| p value | 0.12 | <0.01 | 0.04 | |
| Hippocampal | Correlation | .26** | .33** | −.34** |
| p value | <0.05 | 0.01 | <0.05 | |
| Entorhinal | Correlation | .09** | .18** | −.15** |
| p value | 0.50 | 0.17 | 0.33 | |
| Total | Correlation | .22** | .32** | −.30** |
| p value | 0.10 | 0.01 | 0.05 |
DISCUSSION
Though this topic has been investigated extensively, our study has several important contributions. First, it is the first study to look at the interaction between cognitive, functional, and global decline by different anatomical regions in AD. Second, it is the largest study of its kind to date; incorporating several clinical instruments, and is the first study of its kind to incorporate MCI subjects. Third, unlike other clinical pathological correlations, we find that cognitive, functional and global measures correlate to plaque counts. In this study, we find that global (GDS), cognitive (MMSE) and functional levels (FAST) in subjects ranging from MCI through the spectrum of AD severity correlate with accumulation of AD pathology in the neocortical areas of the brain. The major finding of the study is that the more post-mortem AD pathology, the worse the cognitive, functional, and global decline.
There has been continued debate about the role of senile plaques and NFTs in the progression of AD. In general, NFT counts have been shown to be a slightly better predictor of functional, global, and cognitive functioning than plaques, especially in the hippocampus and entorhinal cortex. Some have found that senile plaques are the primary correlate with AD progression [29–32]. Others have supported NFTs as more accurate predictors of clinical symptoms [3–5, 12, 33–38]. Still others suggest other markers such as synapse or neuron counts correlating with cognitive decline[39, 40]. It has also been suggested that there is a dependent relationship between the two pathologies in correlating with AD progression [41]. Discrepancies may reflect differences in staining and sampling techniques performed [29, 32]. In contrast to the majority of studies finding superior correlation with tangle pathology, we find that plaque correlations with cognitive, functional, and global decline were similar in extent to NFT correlations in all areas of the brain when AD and MCI are considered together. We find that tangle counts correlate better than plaque counts when AD is considered alone and our tangle count correlations are similar to those which have been previously reported. That finding more closely approximates what other investigators have found. Also, in contrast to other studies finding that AD dementias are associated with an increase in hippocampal neuropathology, the hippocampus and entorhinal plaque count correlations were lowest with all three instruments but better with tangle correlations. This may reflect the prior observations that plaques and tangles have different distributions throughout the brain.
One strength of this study was the ability to analyze pathology in many regions providing more details about plaques and NFTs as indicators of AD progression. Another strength of this study is the inclusion of MCI subjects as MCI progresses to AD much faster than age-matched individuals. MCI progresses to AD at a rate of 10% – 15% per year compared to cognitively intact individuals who convert at a rate of 1% – 2% per year [42, 43]. In some studies, it has been found that up to 80% of MCI subjects will convert to AD [20, 44, 45]. Yet another strength of this study is the use of data that has been prospectively collected rather than in a post hoc manner. This may increases the quality of the data being obtained. Using prospective data helps to identify the strength of the association between clinical measures and pathological changes by specific regions of the brain. The subjects were also similar in several ways including ethnic background, education, and age which may result in minimizing variability. On the basis of this report, future studies should take into consideration other parameters besides cognitive scales and should investigate several brain regions. Weaknesses of this study include possible methodological insensitivities of the quantification techniques.
Further research should include an even larger number of MCI subjects. This would help to clarify further whether a continuous progression in neuropathology correlates with AD clinical progression. However, few MCI subjects expire in this phase and thus autopsy tissue is difficult to acquire. Overall this study contributes to identifying the interaction between pathological changes in the brain and the clinical changes that occur as progress from early cognitive changes to Alzheimer’s disease. On the basis of this report, future studies should take into consideration other parameters besides cognitive scales and should investigate several brain regions.
Table 2b.
Correlations between Clinical Assessments and Tangle Densities for AD Only
| Brain Region | FAST Spearman | GDS Spearman | MMSE Spearman | |
|---|---|---|---|---|
| Frontal | Correlation | .40** | .51** | −.058** |
| p value | 0.002 | 0.0001 | 0.0001 | |
| Temporal | Correlation | .31** | .41** | −.46** |
| p value | 0.02 | 0.0013 | 0.002 | |
| Parietal | Correlation | .45** | .55** | −.62** |
| p value | 0.0004 | 0.0001 | 0.0001 | |
| Hippocampal | Correlation | .29** | .27** | −.19** |
| p value | <0.05 | <0.05 | 0.21 | |
| Entorhinal | Correlation | .18** | .22** | −.22** |
| p value | 0.16 | 0.10 | 0.15 | |
| Total | Correlation | .41** | .49** | −.54** |
| p value | 0.0013 | 0.0001 | 0.0002 |
p <0.05,
p <0.01.
Footnotes
This study was supported by NIA P30s AG 019610, NIA P30 AG 08051, NIA RO1 AG 03051 and the Banner-Sun Health Research Institute.
CONFLICT OF INTEREST
None of the authors report any conflict of interest pertaining to this study
References
- 1.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatr. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 2.Crystal H, Dickenson D, Fuld P, Masur D, Scott R, Mehler M, et al. Clinico-pathological studies in dementia: nondemented subjects with pathologically confirmed Alzheimer’s disease. Neurology. 1988;38:1682–7. doi: 10.1212/wnl.38.11.1682. [DOI] [PubMed] [Google Scholar]
- 3.Arriagada PV, Growden JH, Hedley-Whyte ET, Hyman B. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–9. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 4.Giannakopoulos P, Herrman FR, Busiere T, Bouras C, Kovari E, Perl DP, et al. Tangle and neuron numbers, but not amyloid loads, predict cognitive status in Alzheimer’s disease. Neurology. 2003;60:1495–500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- 5.Bierer LM, Hof PR, Purohit DP, Carlin L, Schmeidler J, Davis KL, et al. Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer’s disease. Arch Neurol. 1995;52(1):81–8. doi: 10.1001/archneur.1995.00540250089017. [DOI] [PubMed] [Google Scholar]
- 6.von Gunten A, Kovari E, Bussere T, Rivara CB, Gold G, Bouras C, et al. Cognitive impact of neuronal pathology in the entorhinal cortex and CA1 field in Alzheimer’s disease. Neurobiol Aging. 2006;27(2):270–7. doi: 10.1016/j.neurobiolaging.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, et al. Long-term effects of Abeta42 immunization in Alzheimer’s disease: follow-up of a randomized, placebo-controlled phase I trial. Lancet. 2008;372(9634):216–23. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 8.Thal DR, Arendt T, Waldmann G, Holzer M, Zedlick D, Rub U, et al. Progression of neurofibrillary changes and PHF-tau in end-stage Alzheimer’s disease is different from plaque and cortical microglial pathology. Neurobiol Aging. 1998;19(6):517–25. doi: 10.1016/s0197-4580(98)00090-6. [DOI] [PubMed] [Google Scholar]
- 9.Kraemer HC, Taylor JL, Tinklenberg JR, Yesavage JA. The stages of Alzheimer’s disease a reappraisal. Dement Geriatr Cogn Disord. 1998;9(6):299–308. doi: 10.1159/000017081. [DOI] [PubMed] [Google Scholar]
- 10.Bobinski M, Wegiel J, Wisniewski HM, Tarnawski M, Reisberg B, Mlodzik B, et al. Atrophy of hippocampal formation subdivisions correlates with stage and duration of Alzheimer Disease. Dementia. 1995;6:205–10. doi: 10.1159/000106948. [DOI] [PubMed] [Google Scholar]
- 11.Bobinski M, Wegiel J, Tarnawski M, Bobinski M, Reisberg B, de Leon MJ, et al. Relationships between regional neuronal loss and neurofibrillary changes in the hippocampal formation and duration and severity of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56(4):414–20. doi: 10.1097/00005072-199704000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 13.Khachaturian ZS. Diagnosis of Alzheimer’s disease. Arch Neurol. 1985;42:1097–104. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 14.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41(4):479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 15.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 16.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113–24. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 17.Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43(2):250–60. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 18.Emre M. Dementia associated with Parkinson’s disease. Lancet Neurol. 2003;2(4):229–37. doi: 10.1016/s1474-4422(03)00351-x. [DOI] [PubMed] [Google Scholar]
- 19.Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, et al. Consortium for Frontotemporal Lobar Degeneration. Neuropathologic diagnostic and nosologic criteria for fronto-temporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114(1):5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- 22.Reisberg B, Franssen E, Bobinski M, Auer S, Monteiro I, Boksay I, et al. Overview of methodological issues for pharmacological trials in mild, moderate, and severe Alzheimer’s disease. Int Psychogeriatr. 1996;8(2):159–93. doi: 10.1017/s1041610296002566. [DOI] [PubMed] [Google Scholar]
- 23.Reisberg B, Sclan SG, Franssen E, Kluger A, Ferris S. Dementia staging in chronic care populations. Alzheimer Dis Assoc Dis. 1994;8(Supp1):s188–s205. [PubMed] [Google Scholar]
- 24.Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139(9):1136–9. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 25.Cohen-Mansfield J, Reisberg B, Bonnema J, Berg L, Dastoor D, Pfeffer RI, et al. Staging methods for the assessment of dementia: perspectives. J Clin Psychiatry. 1996;57:190–8. [PubMed] [Google Scholar]
- 26.Sclan SG, Reisberg B. Functional Assessment Staging (FAST) in Alzheimer’s disease: reliability, validity, and ordinality. Int Psychogeriatr. 1992;4(Supp1):55–69. doi: 10.1017/s1041610292001157. [DOI] [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein S, McHugh PRJ. Mini-Mental State” a practical method for grading the cognitive state of patients for the clinician. Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 28.Monteiro IM, Boksay I, Auer SR, Torossian C, Sinaiko E, Reisberg B. Reliability of routine clinical instruments for the assessment of Alzheimer’s disease administered by telephone. J Geriatr Neurol. 1998;11:18–24. doi: 10.1177/089198879801100105. [DOI] [PubMed] [Google Scholar]
- 29.Cummings BJ, Cotman CW. Image analysis of beta-amyloid load in Alzheimer’s disease and relation to dementia severity. Lancet. 1995;346:1524–8. doi: 10.1016/s0140-6736(95)92053-6. [DOI] [PubMed] [Google Scholar]
- 30.Cummings BJ, Pike CJ, Shankle R, Cotman CW. Beta-amyloid deposition and other measures of neuropathology predict cognitive status in Alzheimer’s Disease. Neurbiol Aging. 1996;17(6):921–33. doi: 10.1016/s0197-4580(96)00170-4. [DOI] [PubMed] [Google Scholar]
- 31.Naslund J, Haroutunian V, Mohs R, Davis KL, Davies KL, Greengard P, et al. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA. 2000;283(12):1571–7. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- 32.Bussiere T, Friend PD, Sadeghi N, Wicinski B, Lin GI, Bouras C, et al. Stereologic assessment of the total cortical volume occupied by amyloid deposits an dits relationship with cognitive status in aging and Alzheimer’s disease. Neouroscience. 2002;112(1):75–91. doi: 10.1016/s0306-4522(02)00056-8. [DOI] [PubMed] [Google Scholar]
- 33.Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques, and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol Aging. 1991;12(4):295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- 34.McKee AC, Kosik KS, Kowall NW. Neuritic pathology and dementia in Alzheimer’s disease. Ann Neurol. 1991;30:156–65. doi: 10.1002/ana.410300206. [DOI] [PubMed] [Google Scholar]
- 35.Haroutunian V, Purohit D, Perl D, Marin D, Khan K, Lantz M, et al. Neurofibrillary tangles in nondemented elderly subjects and mild Alzheimer disease. Arch Neurol. 1999;56:713–8. doi: 10.1001/archneur.56.6.713. [DOI] [PubMed] [Google Scholar]
- 36.Guizollet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol. 2003;60(5):729–36. doi: 10.1001/archneur.60.5.729. [DOI] [PubMed] [Google Scholar]
- 37.Bennett DA, Schneider JA, Wilson RW, Bienas JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61:378–84. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- 38.Berg L, McKeel DWFr, Miller FP, Storandt M, Rubin EH, Morris FC, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998;55(3):326–35. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- 39.Gomez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cotex neurons occurs in very mild Alzheimer’s Disease. J Neurosci. 1996;16(14):4491–500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 41.Ingelsson M, Fukumoto H, Newell KL, Growdon JH, Hedley-Whyte ET, Frosch MP, et al. Early Abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology. 2004;62(6):925–31. doi: 10.1212/01.wnl.0000115115.98960.37. [DOI] [PubMed] [Google Scholar]
- 42.Peterson RC, Doody R, Kurtz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 43.Tierney MC, Szalal JP, Snow WG, Fisher RH, Noves A, Nadon G, et al. Prediction of probably Alzheimer’s disease in memory-impaired patients: a prospective longitudinal study. Neurology. 1996;46:661–5. doi: 10.1212/wnl.46.3.661. [DOI] [PubMed] [Google Scholar]
- 44.Morris JC. Mild cognitive impairment and preclinical Alzheimer’s disease. Geriatrics. 2005;(Suppl):9–14. [PubMed] [Google Scholar]
- 45.Storandt M, Grant EA, Miller JP, Morris JC. Rates of progression in mild cognitive impairment and early Alzheimer’s disease. Neurology. 2002;59(7):1034–41. doi: 10.1212/wnl.59.7.1034. [DOI] [PubMed] [Google Scholar]