Abstract
Exposure of erythrocytes to reduced oxygen (O2) tension activates the heterotrimeric G protein Gi, resulting in accumulation of cAMP and release of ATP. The mechanism by which exposure of erythrocytes to reduced O2 tension activates Gi is not known. Here we investigate the hypothesis that, in rabbit erythrocytes, ATP release in response to exposure to reduced O2 tension requires membrane deformation. If this hypothesis is correct, then decreasing the deformability of the erythrocyte membrane should decrease the release of ATP in response to reduced O2 tension. We report that treating erythrocytes with diamide, a compound that decreases erythrocyte deformability, inhibits low O2 tension induced ATP release. Treating erythrocytes with diamide does not, however, interfere with cAMP accumulation or ATP release in response to a direct activator of Gi (mastoparan 7) or in response to receptor mediated activation of Gs (the prostacyclin analog, iloprost). These results demonstrate that diamide (100 μM) does not directly inhibit the signaling pathways for ATP release from rabbit erythrocytes and support the hypothesis that deformability of the erythrocyte membrane is required for low O2 tension-induced ATP release from these cells.
Keywords: red blood cell, diamide, cAMP, mastoparan 7, iloprost
Introduction
The mammalian erythrocyte, the anucleated cell primarily responsible for transporting oxygen (O2), has also been proposed to regulate local vascular resistance in the microcirculation via its ability to release adenosine triphosphate (ATP) when exposed to reduced O2 tension (1, 2). This erythrocyte-derived ATP can then bind to purinergic receptors found on endothelial cells leading to a vasodilation that is conducted to upstream feed arterioles (3–5). Thus, the release of ATP from erythrocytes in response to local decreases in O2 tension provides a mechanism by which the cell responsible for O2 delivery can increase blood flow to areas requiring increased O2 supply (1).
Release of ATP resulting from exposure of erythrocytes to low O2 tension requires activation of the heterotrimeric G protein Gi (6, 7). In addition to exposure to low O2 tension, subjecting erythrocytes to mechanical deformation also leads to the activation of Gi and subsequent ATP release (6–11). Activation of Gi initiates a signal transduction pathway that includes adenylyl cyclase and requires increases in cAMP for ATP release to occur (7, 10, 12–14). Previously, Gi has been shown to be activated by shear stress applied to both epithelial and endothelial cells. (15, 16). Therefore, the finding that mechanical deformation activates Gi in the erythrocyte membrane is inherently consistent with previous findings. However, the mechanism by which the exposure of the erythrocyte to low O2 tension activates Gi-mediated signaling has not been elucidated.
Hemoglobin, the primary O2 carrier within the erythrocyte, is a tetrameric protein that changes conformation based on its oxygenation status. It was reported previously that in severe hypoxia, O2 content of blood is more important than O2 tension in the maintenance of O2 supply in skeletal muscle (4, 17). Since the O2 saturation of hemoglobin determines the O2 content of blood, this would implicate hemoglobin in the ATP release signal transduction pathway. Indeed, it has been shown that ATP release from erythrocytes is dependent upon the O2 saturation of the hemoglobin molecules rather than the O2 tension to which the cell is exposed (18). What remains to be determined is the mechanism by which alterations in hemoglobin O2 saturation activate a signal transduction pathway for ATP release.
Here we investigate the hypothesis that, in rabbit erythrocytes, ATP release in response to exposure to reduced O2 tension requires deformation of the erythrocyte membrane. Under this hypothesis, decreasing the deformability of the erythrocyte membrane would decrease the release of ATP in response to reduced O2 tension. We used diamide to reduce erythrocyte membrane deformability and evaluated ATP release in response to exposure to lowered O2 tension. In addition, we determined the effect of diamide on ATP release in response to exposure of erythrocytes to direct stimulation of Gi by mastoparan 7 as well as in response to receptor-mediated activation of Gs by the prostacyclin analog, iloprost. Although diamide-treated erythrocytes release ATP in response to both direct activation of Gi and receptor-mediated activation of Gs, ATP release in response to exposure to reduced O2 tension was significantly attenuated following diamide incubation. These results support the hypothesis that deformability of the erythrocyte membrane is required for low O2 tension-induced ATP release.
Methods
Isolation of Rabbit Erythrocytes
Male New Zealand white rabbits were anesthetized with ketamine (12.5 mg/kg) and xylazine (1.5 mg/kg) intramuscularly, followed by pentobarbital sodium (10 mg/kg) administered via a cannula placed in an ear vein. A catheter was placed in a carotid artery, heparin (500 units) was administered, and after 10 min the animals were exsanguinated. Immediately after collection, the whole blood was centrifuged at 500 x g at 4 °C for 10 min and the plasma, buffy coat, and uppermost erythrocytes were removed by aspiration and discarded. The remaining erythrocytes were washed three times in buffer containing 21.0 mM tris(hydroxymethyl)aminomethane, 4.7 mM KCl, 2.0 mM CaCl2, 140.5 mM NaCl, 1.2 mM MgSO4, 5.5 mM glucose, and 0.5% bovine albumin fraction V, final pH 7.4. Erythrocytes isolated in this fashion contain fewer than 1 leukocyte per 50 high power fields (approximately 8–10 leukocytes per mm3) and are devoid of platelets (19). Cells were prepared on the day of use.
Measurement of Erythrocyte Deformability
A 10% suspension of washed rabbit erythrocytes was incubated with diamide (Sigma-Aldrich, 100 μM) or its vehicle (saline) and erythrocyte deformability was measured 30, 45, and 60 min after the addition of diamide. Erythrocyte deformability was assessed using the St. George’s Blood Filtrometer (Carri-Med Ltd., Dorking, U.K.) (11, 20). This device develops a pressure gradient distal to a vertically mounted 13 mm diameter polycarbonate filter (Nucleopore). The filter has a 9.53 mm exposed surface diameter, filter thickness of 11 μm and average pore size of 5 μm. Proximal to the filter, the chamber and an open-ended capillary tube were filled with either buffer or buffer containing erythrocytes (hematocrit 10%). Upon opening of a tap on the outflow tube the buffer or buffer containing erythrocytes was subjected to a negative pressure. The fluid was pulled through the filter with the rate of filtration determined by the time required for the air-fluid meniscus to pass fiberoptic sensors surrounding the capillary tube. The filtration rates for the erythrocyte suspension were determined. Red cell transit time (RCTT), a dimensionless number determined by comparing the filtration rates for the erythrocyte suspension with the filtration rate of buffer alone, is calculated using manufacturer-supplied software and a dedicated computer. An increase in RCTT reflects a decrease in erythrocyte deformability (11, 20).
Determination of ATP release from Erythrocytes in Response to Exposure to Reduced O2 Tension in the Absence and Presence of Diamide
Washed erythrocytes were diluted to a 10% hematocrit in a Ringer’s buffer containing bicarbonate (4.7 KCl, 2.0 mM CaCl2, 140.5 mM NaCl, 1.2 mM MgSO4, 11 mM glucose, 23.8 mM NaHCO3, 0.2% dextrose,0.5% BSA, pH 7.4) at 37 °C. In the presence of 100 μM diamide or its vehicle (saline), erythrocytes were equilibrated for 30 min in a tonometer (model 237, Instrumentation Laboratory) with a gas mixture containing 15% O2, 6% CO2, balance N2 (normoxia) (21). The incubation time of erythrocytes with diamide was chosen based on the preliminary studies demonstrating that diamide significantly increased RCTT after 30 min. The gas mixture was then changed to one containing 4.5% O2, 6% CO2, balance N2. Finally, the gas mixture was changed to one containing 0% O2, 6% CO2, balance N2. The pH, pO2, and pCO2 were determined after a 10 min exposure to each gas mixture (pHOx, Nova Biomedical) (Table 1). The amount of ATP released from erythrocytes was determined during normoxia and following the 10 min exposure to each gas mixture and is reported as a % change from normoxia. Although the tonometer spins the erythrocyte suspension, the shear stress placed on the erythrocytes in this device is minimal and is not sufficient to stimulate ATP release. Baseline ATP levels in suspensions of erythrocytes exposed to normoxia in the tonometer or placed in a test tube at the same hematocrit were 7.4±2.7 (n=8) and 9.6±2.3 (n=15) nanomoles per 4 X 108 erythrocytes, respectively.
Table 1.
pH, pCO2, pO2 (n=8) and SO2 (n=3) of Saline and Diamide (100 uM)-Treated Erythrocytes
| Saline Treated Erythrocytes | Diamide Treated Erythrocytes | ||
|---|---|---|---|
| 15%O2, CO2 | 6% | ||
| pH | 7.43 ± 0.01 | 7.42 ± 0.01 | |
| pCO2 | 36.7 ± 1.0 | 37.3 ± 0.7 | |
| pO2 | 116.1 ± 5.7 | 118.0 ± 6.2 | |
| SO2 | 96.0 ± 0.3 | 97.1 ± 1.7 | |
|
| |||
| 4.5%O2, CO2 | 6% | ||
| pH | 7.42 ± 0.01 | 7.42 ± 0.01 | |
| pCO2 | 38.3 ± 0.6 | 38.3 ± 0.3 | |
| pO2 | 35.4 ± 2.84 | 34.5 ± 2.82 | |
| SO2 | 49.0 ± 1.5 | 50.4 ± 1.6 | |
|
| |||
| 0%O2, CO2 | 6% | ||
| pH | 7.43 ± 0.01 | 7.44 ± 0.01 | |
| pCO2 | 38.0 ± 0.6 | 38.3 ± 0.4 | |
| pO2 | 11.1 ± 1.8 | 10.8 ± 2.0 | |
| SO2 | 13.7 ± 0.6 | 13.7 ± 2.7 | |
In separate experiments, hemoglobin O2 saturations (OSM3, Radiometer) were determined following exposure of the erythrocytes to the three different gas mixtures in the presence and absence of diamide.
Incubation of Erythrocytes with Pharmacological Agents that Stimulate Increases in ATP in the Absence and Presence of Diamide
Isolated erythrocytes were diluted to a hematocrit of 10% and preincubated with diamide (100 μM) or its vehicle (saline) for 30 min. Erythrocytes were then incubated with either the direct activator of Gi, Mastoparan 7 (MAS7, 10 μM, GenScript) or the prostacyclin analog, iloprost (ILO, 1 μM, Cayman), an activator of the Gs-coupled prostacyclin receptor. ATP release was measured after 5, 10, and 15 min.
Measurement of ATP
ATP was measured using the luciferin–luciferase assay as described previously (11, 22). A 200 μL sample of erythrocyte suspension (0.04% hematocrit) was injected into a cuvette containing 100 μL of firefly lantern extract (10 mg/mL, FLE 250; Sigma) and 100 μL of a solution of synthetic D–luciferin (50 mg/100 mL; Sigma). The light emitted was detected using a luminometer (Turner Designs). A standard curve for ATP (Sigma) was obtained for each experiment. Cell counts were obtained from the suspension of erythrocytes and amounts of ATP measured were normalized to 4x108 cells/mL.
Measurement of Total Intracellular ATP of Erythrocytes
A known number of erythrocytes, determined by counting the number of erythrocytes in a known volume by using a hemocytometer, were lysed in distilled water. ATP was measured as described above. Values were normalized to ATP concentration per erythrocyte.
Measurement of Free Hemoglobin
To exclude the presence of significant hemolysis in studies where the release of ATP was measured, samples were centrifuged at 500 x g at 4ºC for 10 min and the presence of free hemoglobin in the supernatant was determined by light absorption at a wavelength of 405 nm. If increases in free hemoglobin were detected, the data was excluded to ensure that levels of ATP reported do not reflect that released as a result of erythrocyte lysis.
Incubation of Erythrocytes with Pharmacological Agents that Stimulate Increases in cAMP in the Absence and Presence of Diamide
Isolated erythrocytes, diluted to a 50% hematocrit, were preincubated with diamide (100 μM) or its vehicle (saline) for 30 min. The erythrocytes were subsequently incubated with either MAS7 (30 μM, 30 min) or ILO (1 μM, 15 min). Reactions were stopped with the addition of 4 mL of ice-cold ethanol containing 1mM HCl. The incubation times and agonist concentrations were chosen based on preliminary studies (6, 21, 23).
Measurement of cAMP
The erythrocyte-ethanol mixture was centrifuged at 14,000 x g for 10 min at 4°C to remove precipitated proteins. The supernatant was removed and stored overnight at -20 °C. Samples were centrifuged a second time at 3,700 x g for 10 min at 4 °C to remove cryoprecipitates. The supernatant was removed and dried under vacuum centrifugation. Concentrations of cAMP were determined by enzyme immunoassay (GE Healthcare) according to the manufacturer’s instructions.
Data Analysis
Statistical significance among groups was determined using an analysis of variance (ANOVA). In the event that the F ratio indicated that a change had occurred, a Fisher’s LSD test was performed to identify individual differences. When appropriate a Student t-test was used. Results are reported as means ± the standard error (SE).
Institutional Approval
The protocol describing the use of rabbits was approved by the Institutional Animal Care and Use Committee of Saint Louis University.
Results
Effect of Diamide on Erythrocyte Deformability
Exposure of erythrocytes to 100 μM diamide for 30 min resulted in a decrease in erythrocyte deformability (increase in RCTT) that persisted for 30 additional min (Figure 1, n=4). Importantly, this concentration of diamide had no effect on basal cAMP concentration or total ATP content of erythrocytes (Table 2)
Figure 1. Effect of diamide on red cell transit time (RCTT).
Rabbit erythrocytes were incubated with 100 μM diamide for 30, 45, and 60 min and RCTT was measured (n=4). Values are the means ± SE. * p < 0.05, different from baseline ; † p<0.01, different from baseline .
Table 2.
Effect of Diamide (100 uM) on Baseline cAMP Levels (n=8) and Intracellular ATP levels (n=21) in Rabbit Erythrocytes
| GROUP | cAMP (pmol per 1010 RBCs) | ATP (mM/ cell) |
|---|---|---|
| Saline Treated | 0.47 ± 0.054 | 2.53 ± 0.225 |
| Diamide Treated | 0.53 ± 0.049 | 2.54 ± 0.235 |
Values are means ± SE
Effect of Diamide on Low-O2 Tension Induced ATP Release from Rabbit Erythrocytes
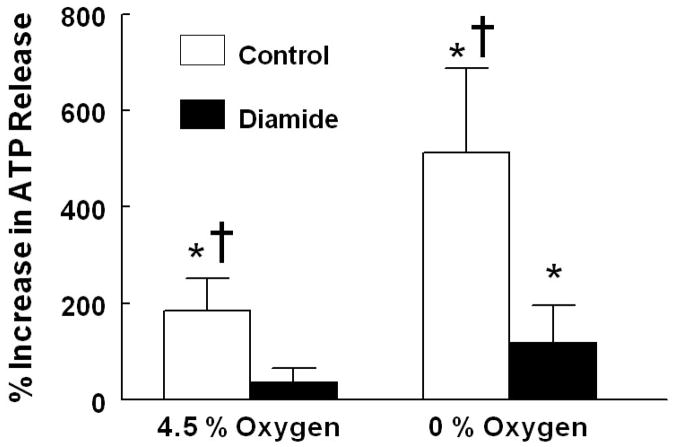
Baseline ATP release (incubation with 15% O2/ 6% CO2) between saline-and diamide- treated cells was not different (7.4 ± 3.6 nmols and 10.1 ± 2.9 nmols per 4 x108erythrocytes, respectively). However, erythrocytes pretreated with diamide released significantly less ATP in response to reduced O2 tension than erythrocytes pretreated with the vehicle for diamide (saline) (Figure 2, n=8). The pCO2, pH, pO2, and SO2 achieved at each gas composition were not significantly different between the diamide and saline treated groups (Table 1).
Figure 2. Effect of diamide on low O2 tension-induced ATP release from rabbit erythrocytes.
Erythrocytes were incubated with 100 μM diamide or its vehicle (saline) for 30 min while exposed to gas with a composition of 15% O2, 6 % CO2, balance N2 in a tonometer. The cells were then exposed sequentially to gases with compositions of 4.5 % O2. 6% CO2, balance N2 and 0% O2, 6% CO2, balance N2. ATP was measured 30 min after exposure to 15% O2 and 10 min after exposure to 4.5% O2 and 0% O2 (n=8). The percent increase in ATP release from baseline (15% O2) is reported. Values are the means ± SE. * p< 0.05, different from respective baseline, † p< 0.05, different from respective diamide treated group
Effect of Diamide on the Ability of Erythrocytes to Accumulate cAMP and Release ATP in Response to Activation of Gi
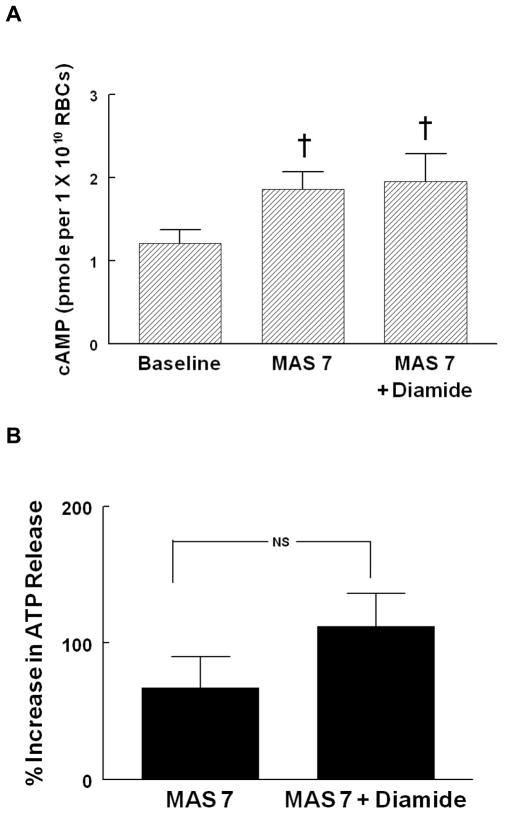
To establish that the Gi-mediated pathway for ATP release from erythrocytes that is activated in response to lowered O2 tension is intact in diamide-treated erythrocytes, cAMP levels and ATP release in response to Mastoparan 7 (MAS 7) were measured in the absence and presence of diamide. Baseline ATP release between diamide-treated and saline treated cells was not different (12.7 ± 3.6 nmols and 14.4 ± 3.7 nmols per 4 x108 erythrocytes, respectively). Diamide (100 μM, 30 min) also did not alter increases in either cAMP (Figure 3A, n=5) or ATP release (Figure 3B, n=8) in response to incubation of erythrocytes with 30 or 10 μM MAS7, respectively. These results indicate that the previously described signal transduction pathway for ATP release in response to reduced O2 tension (6, 7, 24) is intact in diamide-treated erythrocytes.
Figure 3. Effect of diamide on mastoparan 7 (MAS 7)–induced cAMP accumulation (A) and ATP release (B) from rabbit erythrocytes.
Erythrocytes were incubated with 100 μM diamide or its vehicle (saline) 30 min before the addition of MAS 7. cAMP accumulation (A) was measured 30 min after the addition of MAS 7 (30 μM, n=5) and ATP release (B) was measured 5, 10, and 15 min after the addition of MAS 7 (10 μM, n=8). For ATP release, percent increase from baseline for peak values is reported. Values are the means ± SE. † p<0.01, different from baseline; NS, not significantly different.
Effect of 100 μM Diamide on Iloprost Induced cAMP Accumulation and ATP Release
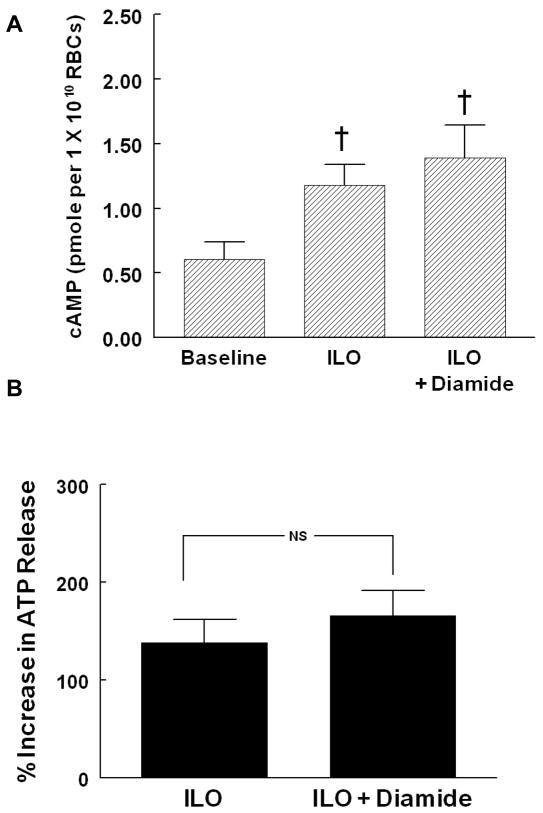
To ensure that erythrocytes treated with diamide remained capable of accumulating cAMP and releasing ATP in response to receptor-mediated activation of Gs, a stimulus for ATP release that is independent of changes in deformability, rabbit erythrocytes were incubated with the prostacyclin analog, ILO (1 μM) in the absence and presence of diamide. Diamide pretreatment (100 μM, 30 min) had no effect on baseline ATP levels (6.0 ± 2.3 and 8.0 ± 3.7 nmols per 4 x108 erythrocytes in the absence and presence of diamide, respectively) and did not alter ILO-induced increases in cAMP (Figure 4A, n=9) or ATP release (Figure 4b, n=7).
Figure 4. Effect of diamide on iloprost (ILO)-induced cAMP accumulation (A) and ATP release (B) from rabbit erythrocytes.
Erythrocytes were incubated with 100 μM diamide or its vehicle (saline) 30 min before the addition of ILO. cAMP accumulation (A) was measured 15 min after the addition of ILO (1 μM, n=9) , and ATP release (B) was measured after 5, 10, and 15 (1 μM, n=7). For ATP release, percent increase from baseline for peak values is reported. Values are the means ± SE. † p<0.01, different from baseline; NS, not significantly different
DISCUSSION
Erythrocytes release ATP in response to exposure to reduced O2 tension as would be encountered as these cells traverse the microcirculation of metabolically active tissue (1, 3, 5, 25). The ability of the erythrocyte to release ATP in response to lowered O2 tension has been suggested to permit this cell to participate in the matching of O2 supply with demand in skeletal muscle (1, 3, 4, 24). It has been demonstrated that ATP release in response to either mechanical deformation or exposure to reduced O2 tension requires activation of the heterotrimeric G protein, Gi (6, 7, 22). In addition to the latter stimuli, human and rabbit erythrocytes release ATP in response to agonist-induced activation of β-adrenergic receptors and prostacyclin receptors (IPR), both coupled to the heterotrimeric G protein, Gs (23, 26).
Regardless of whether Gi or Gs is activated in erythrocytes, signaling pathways are activated that include adenylyl cyclase and protein kinase A (6, 12–14). Although the contribution of the individual components of these signaling pathways to ATP release have been studied, the mechanism by which Gi is activated in the erythrocyte in response to lowered O2 tension was not established.
Here we determined that diamide, at a concentration which reduced erythrocyte deformability (100 μM), selectively attenuates ATP release in response to exposure of erythrocytes to reduced O2 tension. Importantly, the concentration of diamide used had no effect on total intracellular ATP levels or on ATP release in response to either direct activation of Gi or receptor-mediated activation of the IP receptor. These results are consistent with the hypothesis that membrane deformability is required for low O2 tension-induced ATP release from rabbit erythrocytes.
Several studies in endothelial cells and other cell types have demonstrated that G proteins, and in particular, Gi, can be activated through mechanotransduction (shear stress) (15, 16). The erythrocyte membrane-cytoskeleton is a complex network of interconnecting proteins. The major extrinsic proteins of the cytoskeleton are spectrin (bands 1 and 2), ankyrin (band 2.1), band 4.1, and actin (band 5). Of these proteins, spectrin accounts for 75% of the mass of the cytoskeleton and forms a mesh-like network that is attached to the erythrocyte membrane through ankyrin. Ankryin is bound to the membrane through the integral membrane protein band 3. The spectrin-ankyrin-band 3 interaction provides the major association between the cytoskeleton and erythrocyte membrane (27). Although the majority of hemoglobin is present in the cytoplasm of erythrocytes, hemoglobin is also found associated with the cytoplasmic surface of the erythrocyte membrane where it interacts with membrane proteins, including band 3 (28). Studies by Jagger et al. demonstrated that the amount of ATP released from the erythrocyte during exposure to reduced O2 tension is highly correlated with the hemoglobin O2 saturation (18). In the same study, it was demonstrated that the conformational change of hemoglobin from its ‘oxygenated’ to ‘deoxygenated’ state must occur for ATP to be released when erythrocytes are exposed to lowered O2 tension (18) Although this study demonstrated that decreases in hemoglobin saturation serve as the signal for ATP release, the mechanism that relates these decreases with a signaling pathway that regulates ATP release was not established. One mechanism that could couple reductions in hemoglobin saturation with ATP release is if the conformational changes that occur upon hemoglobin desaturation induce local membrane deformations. These local deformations could then activate Gi leading to stimulation of the signaling pathway for ATP release.
The physical properties of the cytoskeletal protein spectrin are essential in maintaining the shape and deformability of the erythrocyte (29). Diamide, a compound that depletes erythrocyte glutathione levels and oxidizes sulfhydryl groups in spectrin, induces cross linking of spectrin producing decreased membrane deformability (29–31). Importantly, when compared to other compounds used to decrease erythrocyte deformability, diamide has fewer adverse effects on the erythrocyte membrane (29, 30). At concentrations below 500 μM, diamide-induced decreases in erythrocyte deformability can be attributed solely to effects on the viscoelastic properties of the erythrocyte membrane. Thus, in our study, diamide-induced decreases in deformability cannot be attributed to effects on intracellular viscosity or cell shape (29, 30, 32).
It has been previously reported that treating erythrocytes with diamide inhibits ATP release in response to external shear force (33, 34). In addition, work by Faris et al. demonstrated that exposure to diamide attenuated ATP release from rabbit erythrocytes in response to reduced O2 tension. However, in the latter study the concentration of diamide used (40 μM for 18 min) was not demonstrated to decrease erythrocyte deformability (33). In addition, this study did not investigate the effects of diamide on Gi or Gs-signaling pathways for ATP release from erythrocytes.
Since diamide is an oxidizing agent, we wished to establish that this agent did not directly inhibit either Gi- or Gs-associated signaling pathways for ATP release from erythrocytes or the ability of these cells to synthesize ATP. In thyroid cells and pancreatic islets, diamide has been demonstrated to either potentiate or inhibit cAMP accumulation depending on the concentration used (35, 36). Therefore, we measured increases in cAMP and ATP release in response to direct activation of Gi with MAS7 and receptor-mediated activation of Gs with the ILO in the absence and presence of diamide. We found that, similar to the effect of diamide on cAMP accumulation in other cell types, high concentrations (5 mM) inhibited ILO-induced ATP release (data not shown). However, as shown in Figures 3 and 4, at 100 μM, a concentration that did not alter either basal cAMP or ATP levels, diamide had no effect on MAS7- or ILO-induced increases in cAMP accumulation and ATP release. These results demonstrate that the concentration of diamide used in our studies does not directly inhibit these signaling pathways for ATP release. This is of importance since it has been shown that ATP release in response exposure of erythrocytes to low oxygen tension requires activation of Gi(7, 9)
Another potential concern with diamide is that treatment of mouse erythrocytes with diamide at concentrations of 2 mM or greater was reported to induce a loss of cooperativity in the binding of O2 by hemoglobin (37). In another study using human erythrocytes it was shown that a decrease in the P50 (O2 tension at which hemoglobin is 50% saturated) when erythrocytes were treated with 5 mM diamide but no change in P50 was observed when erythrocytes were treated with 2 mM diamide (29). To ensure that the far smaller concentration of diamide used in these studies (100 μM) did not alter the ability of hemoglobin to bind or release O2, hemoglobin, O2 saturations in the presence and absence of diamide were determined. In rabbit erythrocytes, we observed no differences in the measured hemoglobin O2 saturations in response to 100 μM diamide (Table 1) demonstrating that the concentration of diamide used in our studies has no effect on the O2 binding characteristics of hemoglobin.
Taken together, these results are consistent with the hypothesis that diamide inhibits low O2 tension-induced ATP release by decreasing membrane deformability resulting in the attenuation of the activation of Gi in response to local membrane deformation. The contribution of deoxygenation to erythrocyte deformability is controversial. Some studies report a decrease in erythrocyte deformability upon deoxygenation (38, 39), although this finding has not been confirmed by other investigators (40). In the studies demonstrating a decrease in deformability of deoxygenated erythrocytes, the measurements were made after the deoxygenation process had been completed (38, 39). Therefore, it would still be possible for local membrane deformation to occur during the transition from the oxygenated to the fully deoxygenated state. We hypothesize that local membrane deformation initiated by conformation changes in membrane-associated hemoglobin could activate the heterotrimeric G protein, Gi, and initiate a signal transduction pathway culminating in ATP release. Therefore, the fact that some studies find a decrease in erythrocyte deformability upon deoxygenation could be taken as support for this hypothesis because it suggests that changes in the erythrocyte membrane/cytoskeletal network are occurring and those changes could activate Gi.
The studies described here do not define how hemoglobin interacts with the membrane to produce local deformation when O2 is released. As noted above, hemoglobin can interact with the membrane by binding to band 3. Previous studies using mouse and human erythrocytes demonstrated that the binding of hemoglobin to band 3, glycolytic enzymes are disassociated from the membrane and glycolysis is stimulated (41–44). In a study using rat erythrocytes it was suggested that the binding of hemoglobin to band 3 also stimulates ATP release (18). Thus, it is possible that band 3 is the protein with which hemoglobin interacts to produce local membrane deformation. However, the resolution of this issue is beyond the scope of the current study. It should be noted that treating erythrocytes with diamide has been shown to tyrosine phosphorylate band 3 at the same site that hemoglobin and glycolytic enzymes are known to bind (45, 46). Phosphorylated band 3 is known to have a decreased affinity for hemoglobin and glycolytic enzymes (45–47). The concentration of diamide used in these studies (100 μM) was chosen based on reports that a concentration of greater than 300 μM is required to stimulate tyrosine phosphorylation of band 3 making it unlikely that inhibition of low O2- induced ATP release is the result of altered phosphorylation of band 3 (46).
In summary, we demonstrate that, treating rabbit erythrocytes with diamide (100 μM) results in reduced erythrocyte deformability and attenuated ATP release in response to exposure of these cells to reduced O2 tension. However, this concentration of diamide does not inhibit adenylyl cyclase activity or ATP release in response to receptor-mediated activation of Gs (ILO) or direct activation of Gi (MAS7). These results are consistent with the hypothesis that low O2 tension-induced ATP release from rabbit erythrocytes is initiated by stress-induced alterations in the cell membrane resulting in the activation of Gi and that this may be a consequence of the conformational change induced by the desaturation of membrane associated hemoglobin.
Acknowledgments
The authors thank J.L. Sprague for inspiration.
This work is supported by NIH grants HL-64180 and HL-89094.
References
- 1.Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS. Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology (Bethesda) 2009;24:107–116. doi: 10.1152/physiol.00038.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res. 1992;26:40–47. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- 3.Ellsworth ML. Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med Sci Sports Exerc. 2004;36:35–41. doi: 10.1249/01.MSS.0000106284.80300.B2. [DOI] [PubMed] [Google Scholar]
- 4.Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol. 1995;269:H2155–2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- 5.Dietrich HH, Ellsworth ML, Sprague RS, Dacey RG., Jr Red blood cell regulation of microvascular tone through adenosine triphosphate. Am J Physiol Heart Circ Physiol. 2000;278:H1294–1298. doi: 10.1152/ajpheart.2000.278.4.H1294. [DOI] [PubMed] [Google Scholar]
- 6.Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. Heterotrimeric G protein Gi is involved in a signal transduction pathway for ATP release from erythrocytes. Am J Physiol Heart Circ Physiol. 2004;286:H940–945. doi: 10.1152/ajpheart.00677.2003. [DOI] [PubMed] [Google Scholar]
- 7.Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. NO inhibits signal transduction pathway for ATP release from erythrocytes via its action on heterotrimeric G protein Gi. Am J Physiol Heart Circ Physiol. 2004;287:H748–754. doi: 10.1152/ajpheart.00161.2004. [DOI] [PubMed] [Google Scholar]
- 8.Sprague RS, Bowles EA, Olearczyk JJ, Stephenson AH, Lonigro AJ. The role of G protein beta subunits in the release of ATP from human erythrocytes. J Physiol Pharmacol. 2002;53:667–674. [PubMed] [Google Scholar]
- 9.Olearczyk JJ, Ellsworth ML, Stephenson AH, Lonigro AJ, Sprague RS. Nitric oxide inhibits ATP release from erythrocytes. J Pharmacol Exp Ther. 2004;309:1079–1084. doi: 10.1124/jpet.103.064709. [DOI] [PubMed] [Google Scholar]
- 10.Liang G, Stephenson AH, Lonigro AJ, Sprague RS. Erythrocytes of humans with cystic fibrosis fail to stimulate nitric oxide synthesis in isolated rabbit lungs. Am J Physiol Heart Circ Physiol. 2005;288:H1580–1585. doi: 10.1152/ajpheart.00807.2004. [DOI] [PubMed] [Google Scholar]
- 11.Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol. 1998;275:H1726–1732. doi: 10.1152/ajpheart.1998.275.5.H1726. [DOI] [PubMed] [Google Scholar]
- 12.Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. Receptor-mediated activation of the heterotrimeric G-protein Gs results in ATP release from erythrocytes. Med Sci Monit. 2001;7:669–674. [PubMed] [Google Scholar]
- 13.Sprague R, Bowles E, Stumpf M, Ricketts G, Freidman A, Hou WH, Stephenson A, Lonigro A. Rabbit erythrocytes possess adenylyl cyclase type II that is activated by the heterotrimeric G proteins Gs and Gi. Pharmacol Rep. 2005;57 (Suppl):222–228. [PubMed] [Google Scholar]
- 14.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol Cell Physiol. 2001;281:C1158–1164. doi: 10.1152/ajpcell.2001.281.4.C1158. [DOI] [PubMed] [Google Scholar]
- 15.Gudi SR, Clark CB, Frangos JA. Fluid flow rapidly activates G proteins in human endothelial cells. Involvement of G proteins in mechanochemical signal transduction. Circ Res. 1996;79:834–839. doi: 10.1161/01.res.79.4.834. [DOI] [PubMed] [Google Scholar]
- 16.Gudi SR, Lee AA, Clark CB, Frangos JA. Equibiaxial strain and strain rate stimulate early activation of G proteins in cardiac fibroblasts. Am J Physiol. 1998;274:C1424–1428. doi: 10.1152/ajpcell.1998.274.5.C1424. [DOI] [PubMed] [Google Scholar]
- 17.Stein JC, Ellsworth ML. Capillary oxygen transport during severe hypoxia: role of hemoglobin oxygen affinity. J Appl Physiol. 1993;75:1601–1607. doi: 10.1152/jappl.1993.75.4.1601. [DOI] [PubMed] [Google Scholar]
- 18.Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circ Physiol. 2001;280:H2833–2839. doi: 10.1152/ajpheart.2001.280.6.H2833. [DOI] [PubMed] [Google Scholar]
- 19.Hanson MS, Stephenson AH, Bowles EA, Sridharan M, Adderley S, Sprague RS. Phosphodiesterase 3 is present in rabbit and human erythrocytes and its inhibition potentiates iloprost-induced increases in cAMP. Am J Physiol Heart Circ Physiol. 2008;295:H786–793. doi: 10.1152/ajpheart.00349.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dormandy J, Flute P, Matrai A, Boger L, Mikita J. The new St. George’s blood filtrometer. Clin Hemorheol Microcirc. 1985;5:975–983. [Google Scholar]
- 21.Hanson MS, Ellsworth ML, Achilleus D, Stephenson AH, Bowles EA, Sridharan M, Adderley S, Sprague RS. Insulin inhibits low oxygen-induced ATP release from human erythrocytes: implication for vascular control. Microcirculation. 2009;16:424–433. doi: 10.1080/10739680902855218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sprague RS, Olearczyk JJ, Spence DM, Stephenson AH, Sprung RW, Lonigro AJ. Extracellular ATP signaling in the rabbit lung: erythrocytes as determinants of vascular resistance. Am J Physiol Heart Circ Physiol. 2003;285:H693–700. doi: 10.1152/ajpheart.01026.2002. [DOI] [PubMed] [Google Scholar]
- 23.Sprague RS, Bowles EA, Hanson MS, Dufaux EA, Sridharan M, Adderley S, Ellsworth ML, Stephenson AH. Prostacyclin analogs stimulate receptor-mediated cAMP synthesis and ATP release from rabbit and human erythrocytes. Microcirculation. 2008;15:461–471. doi: 10.1080/10739680701833804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellsworth ML. The red blood cell as an oxygen sensor: what is the evidence? Acta Physiol Scand. 2000;168:551–559. doi: 10.1046/j.1365-201x.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 25.Sprague RS, Stephenson AH, Ellsworth ML. Red not dead: signaling in and from erythrocytes. Trends Endocrinol Metab. 2007;18:350–355. doi: 10.1016/j.tem.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Adderley SP, Dufaux EA, Sridharan M, Bowles EA, Hanson MS, Stephenson AH, Ellsworth ML, Sprague RS. Iloprost- and isoproterenol-induced increases in cAMP are regulated by different phosphodiesterases in erythrocytes of both rabbits and humans. Am J Physiol Heart Circ Physiol. 2009;296:H1617–1624. doi: 10.1152/ajpheart.01226.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luna EJ, Hitt AL. Cytoskeleton--plasma membrane interactions. Science. 1992;258:955–964. doi: 10.1126/science.1439807. [DOI] [PubMed] [Google Scholar]
- 28.Rauenbuehler PB, Cordes KA, Salhany JM. Identification of the hemoglobin binding sites on the inner surface of the erythrocyte membrane. Biochim Biophys Acta. 1982;692:361–370. doi: 10.1016/0005-2736(82)90385-6. [DOI] [PubMed] [Google Scholar]
- 29.Maeda N, Kon K, Imaizumi K, Sekiya M, Shiga T. Alteration of rheological properties of human erythrocytes by crosslinking of membrane proteins. Biochim Biophys Acta. 1983;735:104–112. doi: 10.1016/0005-2736(83)90265-1. [DOI] [PubMed] [Google Scholar]
- 30.Fischer TM, Haest CW, Stohr M, Kamp D, Deuticke B. Selective alteration of erythrocyte deformabiliby by SH-reagents: evidence for an involvement of spectrin in membrane shear elasticity. Biochim Biophys Acta. 1978;510:270–282. doi: 10.1016/0005-2736(78)90027-5. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki Y, Tateishi N, Soutani M, Maeda N. Deformation of erythrocytes in microvessels and glass capillaries: effects of erythrocyte deformability. Microcirculation. 1996;3:49–57. doi: 10.3109/10739689609146782. [DOI] [PubMed] [Google Scholar]
- 32.Mohandas N, Clark MR, Jacobs MS, Shohet SB. Analysis of factors regulating erythrocyte deformability. J Clin Invest. 1980;66:563–573. doi: 10.1172/JCI109888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faris A, Spence DM. Measuring the simultaneous effects of hypoxia and deformation on ATP release from erythrocytes. Analyst. 2008;133:678–682. doi: 10.1039/b719990b. [DOI] [PubMed] [Google Scholar]
- 34.Moehlenbrock MJ, Price AK, Martin RS. Use of microchip-based hydrodynamic focusing to measure the deformation-induced release of ATP from erythrocytes. Analyst. 2006;131:930–937. doi: 10.1039/b605136g. [DOI] [PubMed] [Google Scholar]
- 35.Anazodo MI, Muller AB, Safayhi H, Ammon HP. Potentiation of forskolin-induced increase of cAMP by diamide and N-ethylmaleimide in rat pancreatic islets. Horm Metab Res. 1990;22:61–64. doi: 10.1055/s-2007-1004852. [DOI] [PubMed] [Google Scholar]
- 36.Smallwood Y, Dekker A, Field JB. Effects of diamide on basal and thyrotropin-stimulated thyroid metabolism. Endocrinology. 1979;104:667–671. doi: 10.1210/endo-104-3-667. [DOI] [PubMed] [Google Scholar]
- 37.Lotero LA, Jordan JA, Lopez RM, Garcia-Perez AI, Diez JC. Influence of oxidation and crosslinking on oxygen binding properties of mouse erythrocytes. Cell Biochem Funct. 2001;19:89–95. doi: 10.1002/cbf.901. [DOI] [PubMed] [Google Scholar]
- 38.Uyuklu M, Meiselman HJ, Baskurt OK. Effect of hemoglobin oxygenation level on red blood cell deformability and aggregation parameters. Clin Hemorheol Microcirc. 2009;41:179–188. doi: 10.3233/CH-2009-1168. [DOI] [PubMed] [Google Scholar]
- 39.Uyuklu M, Meiselman HJ, Baskurt OK. Role of hemoglobin oxygenation in the modulation of red blood cell mechanical properties by nitric oxide. Nitric Oxide. 2009;21:20–26. doi: 10.1016/j.niox.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaniewski WS, Hakim TS, Freedman JC. Cellular deformability of normoxic and hypoxic mammalian red blood cells. Biorheology. 1994;31:91–101. doi: 10.3233/bir-1994-31108. [DOI] [PubMed] [Google Scholar]
- 41.Low PS, Rathinavelu P, Harrison ML. Regulation of glycolysis via reversible enzyme binding to the membrane protein, band 3. J Biol Chem. 1993;268:14627–14631. [PubMed] [Google Scholar]
- 42.Weber RE, Voelter W, Fago A, Echner H, Campanella E, Low PS. Modulation of red cell glycolysis: interactions between vertebrate hemoglobins and cytoplasmic domains of band 3 red cell membrane proteins. Am J Physiol Regul Integr Comp Physiol. 2004;287:R454–464. doi: 10.1152/ajpregu.00060.2004. [DOI] [PubMed] [Google Scholar]
- 43.Campanella ME, Chu H, Low PS. Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane. Proc Natl Acad Sci U S A. 2005;102:2402–2407. doi: 10.1073/pnas.0409741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campanella ME, Chu H, Wandersee NJ, Peters LL, Mohandas N, Gilligan DM, Low PS. Characterization of glycolytic enzyme interactions with murine erythrocyte membranes in wild-type and membrane protein knockout mice. Blood. 2008;112:3900–3906. doi: 10.1182/blood-2008-03-146159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bordin L, Ion-Popa F, Brunati AM, Clari G, Low PS. Effector-induced Syk-mediated phosphorylation in human erythrocytes. Biochim Biophys Acta. 2005;1745:20–28. doi: 10.1016/j.bbamcr.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 46.Bordin L, Zen F, Ion-Popa F, Barbetta M, Baggio B, Clari G. Band 3 tyr-phosphorylation in normal and glucose-6-phospate dehydrogenase-deficient human erythrocytes. Mol Membr Biol. 2005;22:411–420. doi: 10.1080/09687860500233679. [DOI] [PubMed] [Google Scholar]
- 47.Low PS, Allen DP, Zioncheck TF, Chari P, Willardson BM, Geahlen RL, Harrison ML. Tyrosine phosphorylation of band 3 inhibits peripheral protein binding. J Biol Chem. 1987;262:4592–4596. [PubMed] [Google Scholar]