Abstract
In this paper, we present a movement-based assay to observe adaptability in Caenorhabditis elegans locomotion behavior. The assay comprises a series of sinusoidal microchannels with a fixed wavelength and modulating (increasing or decreasing) amplitude. The channel width is comparable to the body diameter of the organism. Worms are allowed to enter the channel from the input port and migrate toward the output port. Within channel sections that closely match the worm’s natural undulations, the worm movement is relatively quick and steady. As the channel amplitude increases or decreases along the device, the worm faces difficulty in generating the propulsive thrust, begins to slow down and eventually fails to move forward. A set of locomotion parameters (i.e., average forward velocity, number and duration of stops, range of contact angle, and cut-off region) is defined for worm locomotion in modulated sinusoidal channels and extracted from the recorded videos. The device is tested on wild-type C. elegans (N2) and two mutants (lev-8 and unc-38). We anticipate this passive, movement-based assay can be used to screen nematodes showing difference in locomotion phenotype.
INTRODUCTION
The nematode Caenorhabditis elegans (C. elegans) is a powerful model organism for behavioral studies primarily because of its fully-mapped genome, relatively simple anatomy, short lifespan and ease of genetic manipulation.1, 2, 3, 4, 5 Among the varied behavioral facets of C. elegans, locomotion is considered the most fundamental and is closely related to the neuromuscular functioning of this limbless animal.6, 7, 8, 9, 10, 11 The forward movement of nematodes results from rhythmic undulatory waves propagating from the head to the tail.6, 7 These periodic undulations, caused by contraction and relaxation of body muscles, have helped quantify C. elegans locomotion on various substrates (e.g., agar plates,7 gelatin,11 saturated particulates8, 9 and buffer11). The shape and speed of undulations depend on the response of the C. elegans to the physical environment—they crawl on agarose surface with undulations of low frequency and smaller wavelength; they swim in M9 buffer with undulations of higher frequency and longer wavelength.7, 11
With advances in microfluidic technology,12 a new class of worm assays (T-mazes for memory and learning,13 culture and detection chambers,3, 5 micro-clamps for olfactory sensing,14 integrated microscopy system for rapid phenotyping,15 piezoresistive displacement clamps for force measurement,16 compact disks for geotaxis studies,17 micro-traps for nanosurgery,18 and screening and sorting devices19, 20, 21) have emerged to study the mechanics and neuromuscular functioning of C. elegans. Particularly relevant to this work are microfluidic devices8, 9, 22, 23 that allow the observation of C. elegans locomotion in soil-like environments (compared to that on planar surfaces7, 11). One such example is the waveform generator22 consisting of sinusoidal channels (each of a fixed amplitude and wavelength) to experimentally control the waveform and trajectory of crawling C. elegans. It was shown that, in the waveform generator, wild-type C. elegans were able to pass through several different channels, suggesting that the mechanisms for generating and propagating undulations were largely independent of the channel amplitude and wavelength.22 The worms, however, faced difficulty in passing through sinusoidal channels whose amplitude and wavelength were larger (two times or more) than the dimensions of its natural undulatory movement.22 In a different work, we used straight channels to show significant differences in the morphological and locomotion parameters between parasitic nematodes (Esophagostomum dentatum), reflecting a difference in propulsive thrust between mutants.24, 25
In this study, we build on the above concepts of a waveform generator and differences in morphological∕locomotion parameters among mutants to realize a simple tool for screening C. elegans by differentiating its ability to adapt to sinusoidal channels with controlled waveform. Here, the entire locomotion experiment is conducted in two modulated sinusoidal channels; one with gradually increasing amplitude and the other with gradually decreasing amplitude. We observed that worms moved steadily and vigorously through channel sections whose amplitude and wavelength closely matched that of the worm’s natural undulations. Beyond this adaptable range, the worms found it difficult to move forward and eventually stopped. This enabled us to quantify locomotion in modulated sinusoidal channels by a set of parameters (average forward velocity, number and duration of stops, and cut-off region). We tested the device on wild-type (N2) and two mutants (lev-8 and unc-38) and observed differences in their crawling behavior. A custom worm tracking software was used to visually observe the tracks of worms with the possibility of automated data extraction from recorded videos.
MATERIALS AND METHODS
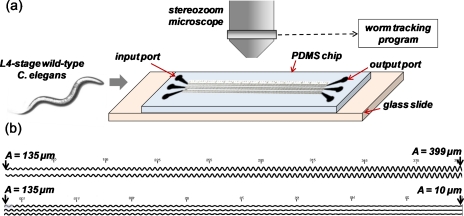
The experimental setup and the modulated sinusoidal channels are shown in Fig. 1. Two microchannels were designed: the first comprises an amplitude A that increases from 135 to 399 μm in steps of 6 μm; the second has A decreasing from 135 μm to 10 μm in steps of 3 μm For both microchannels, an input port is placed at A=135 μm and an output port is placed at A=10 μm or at A=399 μm. The channel wavelength (λ) is fixed at 360 μm. The channel width (60 μm) and height (80 μm) are chosen to allow sinusoidal movement of crawling worms but with limited latitudinal and longitudinal freedom, as discussed for the waveform generator.22 In every chip, multiple (8–12) modulated sinusoidal channels are placed in parallel to record several worms at the same field of view. On-chip vertical markers are included for calibration purpose.
Figure 1.
(a) Experimental setup comprising a microfluidic chip with sinusoidal channels, high-resolution microscope, and worm tracking program. (b) Magnified images of the two (i.e., with increasing and decreasing amplitude) modulated sinusoidal channels with vertical markers.
The mask layout is drawn in AutoCAD and sent to an outside vendor (Fineline Imaging, Colorado Springs, CO) for generation of the physical masks. The microfluidic devices are fabricated using standard soft lithography procedure. A SU-8 photoresist (Microchem Corporation, Newton, MA) is cast on a bare silicon wafer by a double spin-coating technique (spin at 2800 rpm.; soft bake at 65 °C for 5 min; repeat the process) to achieve a 80 μm thick SU-8. The resist is exposed to near UV (375 nm) light at 10 mJ∕cm2 for 50 s. The PDMS prepolymer (Sylgard 184 Silicone Elastomer Kit, Dow Corning Corporation, Midland, MI) is cast over the SU-8 mold and cured on a hot plate at 70 °C for 2 h. Subsequently, the PDMS microfluidic devices are peeled off the SU-8 mold and input∕output ports (2 mm diameter) are punched into the device. Air plasma is exposed to the PDMS devices, which are then bonded to individual glass slides.
Wild-type (N2) and two mutant (CB904 unc-38 and ZZ15 lev-8) C. elegans are obtained from the Caenorhabditis Genetics Center (CGC) at University of Minnesota (St. Paul, USA). The C. elegans are cultivated at 25 °C on Nematode Growth Medium (NGM) plates seeded with Escherichia coli OP50 bacteria. For the experiments, L4 stage C. elegans are picked using a sterilized platinum wire. A polyethylene tubing (OD=1 mm, ID=0.58 mm) is connected to a 1-mL syringe and the sinusoidal channels are filled with standard M9 buffer. Care is taken to ensure that the input ports and channels do not trap any air bubbles. During our initial trials, we noticed that air bubbles would block the path of worms which end up clogging the channels. Thereafter, L4-stage C. elegans (body diameter ∼45 μm) are dropped onto the input ports.
In our experiments, the worms were allowed to enter the sinusoidal channels without applying any attractants (e.g., food source1, 13), deterrents (e.g., citric acid) or mechanical forces.15, 18 Previous work used a syringe to apply suction to push the worms into the sinusoidal channels.22 In our devices, we observed that suction sometimes caused temporary immobilization of the worm and the organism needed time to resume with its forward movement. As an alternate method of inserting the worms, we found that electrotaxis can help direct the worms into the modulated channel (anode at input port, cathode at output port). This is consistent with the current literature that shows nematodes with developed electrosensory neurons respond to applied electric fields.26, 27 However, for our experiments presented here, we preferred not to use any external electrical, chemical or mechanical factors which may affect the measured locomotion parameters.
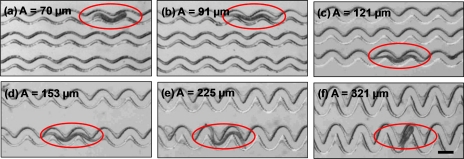
After a brief scouting time (∼3–5 min), worms enter the channels and move toward the output port. A Leica MZ16 transmission stereomicroscope is used to record the worms’ locomotion along the different channel sections (Fig. 2). The microscope has a 1x and 2x objective lens with a magnification range of 7.1x to 230x. It is coupled with a QICam 12-bit Mono Fast 1394 cooled digital camera interfacing with QCapture PRO software. This allows us to capture digital images (1392×1040 pixels) at a specified time interval (1 s in this case). The images are sequenced and compressed into the Audio Video Interleave (.avi) video format. The .avi video is post-processed by a custom worm tracking program that extracts track signatures and locomotion parameters (i.e., number and duration of stops, cut-off region) of individual and∕or multiple worms. The details of the worm tracking program is mentioned elsewhere.24 Briefly, the program analyzes a large number of images (typically ∼1200) to recognize moving objects (i.e., worms) and extract motility parameters such as amplitude, wavelength, body postures and path (i.e., track) traversed by the worm. GraphPad Prism (GraphPad, San Diego, USA) is later used to statistically analyze and fit the generated data.
Figure 2.
Wild-type C. elegans (encircled) crawling in different sections of a modulated sinusoidal channel. The worm shows relatively smooth movement in the adaptable range of channel amplitudes (b-e). In sections beyond this adaptable range (a, f), the worm is unable to move forward.
A set of locomotion parameters are defined for C. elegans movement in the modulated sinusoidal channels: (i) Average forward velocity: This is defined as the ratio of the net displacement of the worm’s head to the total time traversed in a given channel section. (ii) Stops: As the worm passes through the channel, its forward movement is interrupted by frequent stops. We define a stop as a halt (net body displacement less than one channel wavelength) for time duration more than or equal to 3 s. (iii) Range of contact angle: The worm makes contact with and pushes against the channel sidewall to move forward. During a given undulatory cycle, the worm’s body makes contact with different points along the channel. A number of contact angles can be defined with respect to the body or head positions. Here, we define the range of contact angle as the angle within which the middle section of the body touches the crest of the sinusoidal channel. (iv) Cut-off region: The worms face a gradually increasing challenge along the channel before they eventually fail to move forward. During their futile struggle to continue forward locomotion, worms occasionally (>90%) appear to fold and unfold their body utilizing the extra vertical space available in the channel. The cut-off region is defined as the region where a worm appears virtually immobilized (net body displacement less than one wavelength) for time duration greater than 300 s.
RESULTS
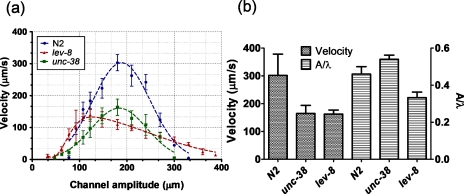
Figure 3a plots the average forward velocity of C. elegans (N2, lev-8 and unc-38) as a function of the channel’s amplitude. For each strain, the velocity curve has a peak (303±25.3 μm∕s for N2, 139.60±19.8 μm∕s for lev-8, and 163.42±25.7 μm∕s for unc-38, mean±S.E.) at a certain amplitude (180 μm for N2, 122.5 μm for lev-8, and 180 μm for unc-38, n=15–20, N=3) and decays on either side of this amplitude. The peak average velocity of N2 is 116.42 and 85.4% greater than that of lev-8 and unc-38 mutants, respectively. A single Gaussian distribution fits the velocity data for N2 and unc-38 mutant, while a double Gaussian distribution fits the lev-8 velocity data. On either side of the peak amplitude, the worm has to acclimatize its body posture along the channel geometry to push forward. This increases the time needed to traverse a certain displacement, which in turn lowers the average forward velocity. Further, the velocity curves for N2 and unc-38 are symmetrical on either side of their peak values. The lev-8 mutant, however, shows a prolonged and gradual decrease in velocity at higher channel amplitudes while having a sharp drop at lower channel amplitudes. This suggests that, at amplitudes above 100 μm, the lev-8 mutant is able to adapt itself better than N2 and unc-38 mutant with a finite forward velocity up to channel amplitudes of 390 μm.
Figure 3.
(a) Average forward velocity versus channel amplitude is plotted for the three L4-stage C. elegans strains (N2, lev-8 and unc-38). (b) Average forward velocity and ratio of amplitude to wavelength (A∕λ) for the three C. elegans strains on 2.5% agarose plates are shown.
In an attempt to correlate the location of peak forward velocity in the modulated sinusoidal channel with the natural undulations of the worms, we measured the amplitude, wavelength and velocity of C. elegans crawling on 2.5% agarose plates without any food (Fig. 3b). The A∕λ ratio for the three strains (n=12–15) on agarose plates are: A∕λ=0.46 for N2, A∕λ=0.33 for lev-8, and A∕λ=0.54 for unc-38. From Fig. 3a, the peak velocities occur at channel regions where A∕λ=0.5 for N2, A∕λ=0.34 for lev-8, and A∕λ=0.5 for unc-38. It thus seems likely that the peak velocity in modulated sinusoidal channel occurs at an amplitude where the channel A∕λ ratio closely matches the A∕λ ratio of worm’s natural undulations on agarose plates. Furthermore, the peak velocities for individual strains in the sinusoidal channels (Fig. 3a) are in close agreement with the forward velocities measured on agarose plates (Fig. 3b). This suggests that, at these channel regions, the worms crawl as comfortably as they would on agarose culture plates.
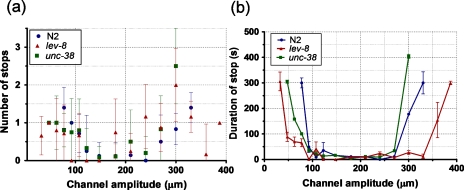
Figure 4 shows the number and duration of stops exhibited by N2, lev-8 and unc-38 C. elegans during their forward movement in modulated sinusoidal channels. In the adaptable range (100 μm–250 μm), all three strains stop less frequently (<1.4 times) and for a short duration (t<35 sec.). As the channel amplitude increases or decreases beyond this adaptable range, the duration and average number of stops increase significantly (∼300 sec. and >1.4 times, respectively). Unlike the Gaussian decay observed in average velocity (Fig. 3a), the duration of stops increases sharply as the worm encounters the channel section close to its cut-off region. A small stop duration (t<25 s) is shown by lev-8 over a wider amplitude range (50 μm–345 μm) when compared to N2 (100 μm–300 μm) and unc-38 (140 μm–285 μm). This again shows that lev-8 is better than N2 and unc-38 at showing steady movement over a wider range of channel amplitudes.
Figure 4.
Average number (a) and duration (b) of stops versus channel amplitude are plotted for the three C. elegans strains (N2, lev-8 and unc-38).
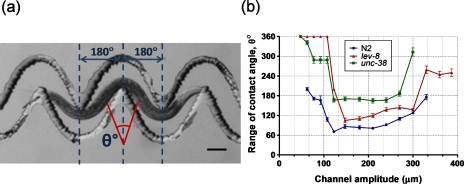
During its forward movement, the worm makes contact with the channel sidewall to overcome friction and generate the propulsive thrust. We analyzed the recorded videos and selected images where the worm’s body had a specific posture (i.e., where body’s middle section is centered at the channel’s crest and the head and tail regions are outside the two troughs on either side of the crest (Fig. 5a)). Then we identified the two extreme contact points of the body’s middle section and extrapolated them individually to a vertical line corresponding to the channel’s crest. The angle between these two lines (intersecting at the vertical line) is denoted as the range of contact angle and is measured for individual worms at different sections of the sinusoidal channel. We observed that the worms made less contact (θ<170°) with the channel sidewalls in the adaptable range of amplitudes (100 μm–250 μm) while increased their range of contact angle in regions of difficulty. Compared to N2 and lev-8, the unc-38 mutant has a larger range of contact angle (θ>170°) throughout the amplitude range which is probably due to its uncoordinated movement pattern (Fig. 5b). The N2 has the smallest range of contact angle among the three strains for the entire distance of the channel. For example, at amplitudes outside the adaptable range, most of the body for unc-38 and lev-8 mutants touches the channel sidewalls (θ>300°) compared to N2 C. elegans that has a maximum range of contact angle of ∼200°±5.15° (mean±S.E.).
Figure 5.
(a) Illustration of the range of contact angle for a L4-stage N2 C. elegans in two sections of the modulated sinusoidal channel. (b) Range of contact angle versus channel amplitude is plotted for the L4-stage C. elegans (N2, lev-8 and unc-38).
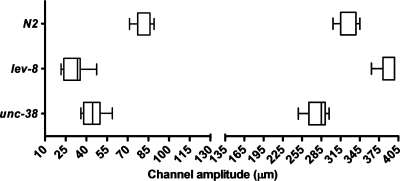
The cut-off region in the lower amplitude range and higher amplitude range (Fig. 6) are shown for the N2, lev-8 and unc-38 C. elegans. In the boxplot, the left and right whiskers denote the minimum and maximum of a data set, respectively. The left and right edges of each box and the vertical line within denote the first quartile, second quartile and median of a data set, respectively. The lower cut-off for the individual strains are 78.73±6.6 μm for N2, 31.41±8.0 μm for lev-8, and 44.43±8.1 μm for unc-38 (mean±S.E., n=15–20, N>3). Unlike lev-8 or unc-38 mutants, the N2 is unable to move beyond a channel amplitude less than 70 μm. It is easier for lev-8 and unc-38 to adapt their body posture and continue moving to sections of lower amplitudes (<40 μm). The upper cut-off for the individual strains are 322.7±14.1 μm for N2, 390.7±14.3 μm for lev-8, and 278.9±15.6 μm for unc-38 (mean±S.E., n=15–20, N>3). In accordance with earlier results (Figs. 345), lev-8 has the farthest cut-off region among the three strains.
Figure 6.
The lower and upper cut-off regions in the modulated sinusoidal channels are shown for the N2, lev-8 and unc-38 C. elegans.
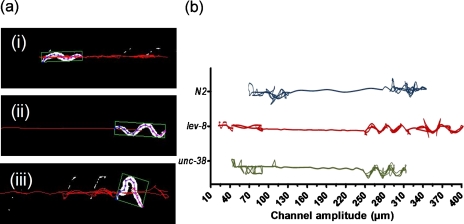
The recorded videos are processed by a custom worm tracking program to obtain locations of the body centroid as a function of time. This is used to plot the path traversed (i.e., tracks) by individual worms in the modulated sinusoidal channels. As shown in Fig. 7a, the worm tracking program converts the recorded grayscale video to black and white, identifies the worm body, measures its centroid location, and plots the tracks over a period of time. Figure 7b shows representative tracks of N2, lev-8 and unc-38 C. elegans along sections of the sinusoidal channel. Each channel section denotes a different level of difficulty for the worms. A somewhat linear track indicates smooth movement of the worm, while a zigzag track denotes increasing difficulty for the worm to continue its forward movement. This visual observation of the worm movement provides a quick means of observing the worm difficulty and complements the previous quantitative analysis (Fig. 3456).
Figure 7.
(a) Snapshots of a L4-stage N2 C. elegans whose body positions and centroid are tracked by a worm tracking program. The tracks show the relative levels of difficulty faced by the worm in the different sections of the channel [(i): lower cut-off region, (ii): adaptable region, and (iii): higher cut-off region (see Ref. 29)] (b) Representative tracks of the body centroid for C. elegans (N2, lev-8 and unc-38) along the modulated sinusoidal channel.
DISCUSSION
For quite some time, it is known that the form and frequency of the waves passing down a nematode’s body depends, among other factors, on the relative resistance exerted by the medium on the normal and tangential components of displacement.6, 11 While tangential forces exerted by the medium retard its motion, the normal forces (or internally generated bending couples) generated by the nematode propels the body forward.6, 7 When observed in straight microfluidic channels with relatively no physical constraints (besides the channel sidewalls separated by 300 μm), body and locomotion parameters (amplitude, wavelength, frequency, and average forward velocity) can be measured along the channel.24, 25 The locomotion parameters were used to calculate the propulsive thrust, which is relatively distinct for a nematode species or isolate under the same set of experimental conditions.24
From this, we hypothesized that the propulsive thrust and adaptability of C. elegans could be tested in modulated sinusoidal channels of varying amplitude. This allowed us to manipulate the natural undulations by forcing the worm to conform its body to the shape of the sinusoidal channel during its forward movement. As the channel width was comparable to the width of its body, the worm pushed against regions of the channel sidewalls to propel itself forward. The gradual change in channel amplitude necessitated the worm to adjust its locomotory behavior. Both UNC-38 and LEV-8 are essential subunits of the body wall muscle acetylcholine receptors.27 This explains why both mutants (unc-38 and lev-8) have a lower peak forward velocity compared to N2 in the sinusoidal channels (Fig. 3a). The unc-38 mutant, known to display uncoordinated movement, shows a larger range of contact angle (Fig. 5b) and a smaller cut-off region at the high A-limit (Fig. 6b) compared to the N2 strain. It is interesting to observe that lev-8 is able to cover the widest range of channel amplitudes, even though with significant difficulty close to its cut-off regions in the device. Previously, it was demonstrated that lev-8 gene is also expressed in the GABAergic neurons innervating the body wall muscles and helps control the relaxation of dorsal muscles during wave oscillations.28 This explains the smaller A∕λ ratio of lev-8 compared to N2 in both sinusoidal channels and 2.5% agarose plates (Fig. 3). The greater flexibility in body posture for lev-8 demonstrated in our experiments suggests the possible role of other biological factors in regulating worm adaptation for crawling in constrained microenvironments.
The proposed study builds upon the previous work on waveform generator22 in three main ways: (i) Increased throughput: The waveform generator tested the crawling behavior of C. elegans in six individual fixed-wave channels (18 different A∕λ ratio). Here, two modulated sinusoidal channels allowed us to observe crawling behavior of worms through several (more than 80 different A∕λ ratio) changing waveforms. For example, the modulated sinusoidal channel with increasing amplitude accommodates (399−135)∕6=44 distinct waveforms. This helps to significantly increase the throughput of the locomotion experiments. (ii) Quantifying locomotion: The waveform generator was separated in three domains (i.e., green, blue, red) depending on the relative ease with which the worm can crawl through. In the modulated sinusoidal channels, we defined a set of locomotion parameters to better quantify the relative ease of worm crawling. (iii) Testing mutants: While the waveform generator was tested with N2 C. elegans to show the feasibility of controlling the crawling behavior, the modulated sinusoidal channels was studied with three C. elegans strains to show differences in crawling behavior between mutants.
The observed differences in locomotion parameters in the modulated sinusoidal channels can be used to differentiate mutant phenotypes. Compared to existing C. elegans screening methods, the movement-based assay presented here has some potential benefits. First, the method does not require any on-chip immobilization techniques (such as suction or cooling)14, 15, 18 which makes the device simple to use and requires a single mask fabrication process. Second, the worm is not subjected to external factors (such as mechanical or chemical stress)1, 14 that may physically harm or produce unknown behavioral changes in the organism. The worm is allowed to move freely through the sinusoidal channel and use its innate internal forces for the forward locomotion. Third, unlike observing single worms one at a time,14 parallel observation of individual worms in multiple modulated sinusoidal channels is possible. Since the input∕output ports are accessible to the user, it is possible to add chemical compounds (e.g., anthelmintics) in individual channels and test their effects on worm locomotion at real-time.24, 25 Furthermore, the existing modulated sinusoidal channels could be used to test other C. elegans mutants (such as cat-2, cat-4, sqt-1, lon-1, unc-54 and goa-1)7 without changing the device dimensions. This is because most mutants, studied on agarose plates, have similar body postures (e.g., A∕λ=90∕540 for goa-1 to 130∕480 for unc-54)7 and their forward velocities (220±50 μm∕s for goa-1 to 40±10 μm∕s for unc-54)7 are in the range that can be observed in our movement-based assay.
This treadmill test in modulated sinusoidal channels provides an advantage of limiting the area of experimental observation within the microscope’s field of view, while measuring the locomotion adaptability in C. elegans. Behavioral intelligence has been suggested in previous studies where the worms adapted to range of physical8, 11, 12, 13, 14 and biochemical1, 2, 3, 4, 5 environments. For example, these animals can migrate over a long distance on agarose plates in search of food or other attractants. This makes it difficult to predict or measure worm locomotion, characterized by frequent forward crawling, reverse crawling and omega turns, on agarose plates across a wide spatial area.22, 23 Additionally, it is challenging to design constrained microenvironments on agarose plates to observe locomotion adaptability. The modulated sinusoidal channel design provides directed path for the worm to move and makes relatively easier to measure changing locomotion parameters. The measured average velocity accounted for the forward∕reverse crawling and the stops during forward movement. The cut-off region indicated the upper limit of propulsive force generated by the worm.
CONCLUSION
In this study, we inserted the C. elegans into sinusoidal channels of a width comparable to the nematode’s body width. While the wavelength of the sinusoidal channel was fixed, its amplitude kept gradually increasing (or decreasing). The restricted channel geometry forced the C. elegans to constantly adapt its locomotory behavior during its forward movement. The modulated sinusoidal channel design thus manipulated the propulsive thrust exerted by the worm as reflected by a set of locomotion parameters. The N2 C. elegans had a significantly higher peak forward velocity compared to lev-8 and unc-38 mutants. The lev-8 mutant was able to cover a wider range of channel amplitudes. The unc-38 mutant traverses the smallest range of increasing channel amplitudes. This passive device does not require any movable parts and allows real-time imaging of locomotion adaptability. The modulated sinusoidal channels are expected to provide a simple platform to automatically identify phenotypic differences in C. elegans locomotion.
Supplementary Materials
Worms moving through various sections of the modulated sinusoidal channels.
Movie 1: A L4-stage N2 C. elegans moves smoothly and steadily in channel sections within its adaptable range of amplitude.
Movie 2: A L4-stage N2 C. elegans eventually reaches cut-off region in the high-A limit.
Movie 3: A L4-stage unc-38 C. elegans reaches cut-off region in the low-A limit.
ACKNOWLEDGMENT
The research was supported by National Science Foundation (Grant No. CMMI-1000808). We are thankful to R. J. Martin and A. P. Robertson from Iowa State University for providing the worms.
References
- Miller A. C., Thiele T. R., Faumont S., Moravec M. L., and Lockery S. R., J. Neurosci. 25, 3369 (2005). 10.1523/JNEUROSCI.5133-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. M., Hill J. J., and Bargmann C. I., Proc. Natl. Acad. Sci. U.S.A. 102, 3184 (2005). 10.1073/pnas.0409009101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronis N., Zimmer M., and Bargmann C. I., Nat. Methods 4, 727 (2007). 10.1038/nmeth1075 [DOI] [PubMed] [Google Scholar]
- Chalasani S. H., Chronis N., Tsunozaki M., Gray J. M., Ramot D., Goodman M. B., and Bargmann C. I., Nature (London) 450, 63 (2007). 10.1038/nature06292 [DOI] [PubMed] [Google Scholar]
- Gray J. M., Karow D. S., Lu H., Chang A. J., Chang J. S., Ellis R. E., Marletta M. A., and Bargmann C. I., Nature (London) 430, 317 (2004). 10.1038/nature02714 [DOI] [PubMed] [Google Scholar]
- Gray J. and Lissmann H. S., J. Exp. Biol. 41, 135 (1964). [DOI] [PubMed] [Google Scholar]
- Karbowski J., Cronin C., Seah A., Mendel J., Cleary D., and Sternberg P., J. Theor. Biol. 242, 652 (2006). 10.1016/j.jtbi.2006.04.012 [DOI] [PubMed] [Google Scholar]
- Jung S., Phys. Fluids 22, 031903 (2010). 10.1063/1.3359611 [DOI] [Google Scholar]
- Juarez G., Lu K., Sznitman J., and Arratia P. E., Europhys. Lett. 92, 44002 (2010). 10.1209/0295-5075/92/44002 [DOI] [Google Scholar]
- Sznitman J., Purohit P., Krajacic P., Lamitina T., and Arratia P. E., Biophys. J. 98, 617 (2010). 10.1016/j.bpj.2009.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang-Yen C., Wyart M., Xie J., Kawai R., Kodger T., Chen S., Wen Q., and Samuel A., Proc. Natl. Acad. Sci. U.S.A. 107, 20323 (2010). 10.1073/pnas.1003016107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesides G. M., Nature (London) 442, 368 (2006). 10.1038/nature05058 [DOI] [PubMed] [Google Scholar]
- Qin J. and Wheeler A. R., Lab Chip 7, 186 (2007). 10.1039/b613414a [DOI] [PubMed] [Google Scholar]
- Zhang Y., Lu H., and Bargmann C. I., Nature (London) 438, 179 (2005). 10.1038/nature04216 [DOI] [PubMed] [Google Scholar]
- Chung K., Crane M. M., and Lu H., Nat. Methods 5, 637 (2008). 10.1038/nmeth.1227 [DOI] [PubMed] [Google Scholar]
- Park S. J., Goodman M. B., and Pruitt B. L., Proc. Natl. Acad. Sci. U.S.A. 104, 17376 (2007). 10.1073/pnas.0702138104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahui K., Dempsey C. M., Zoval J. V., Sze J., and Madou M. J., Sensors Actuators B 122, 511 (2007). 10.1016/j.snb.2006.06.026 [DOI] [Google Scholar]
- Rohde C. B., Zeng F., Gonzalez-Rubio R., Angel M., and Yanik M. F., Proc. Natl. Acad. Sci. U.S.A. 104, 13891 (2007). 10.1073/pnas.0706513104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane M., Chung K., Stirman J., and Lu H., Lab Chip 10, 1509 (2010). 10.1039/b927258e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronis N., Lab Chip 10, 432 (2010). 10.1039/b919983g [DOI] [PubMed] [Google Scholar]
- Chokshi T. V., Ben-Yakar A., and Chronis N., Lab Chip 9, 151 (2009). 10.1039/b807345g [DOI] [PubMed] [Google Scholar]
- Lockery S. R., Lawton K. J., Doll J. C., Faumont S., and Coulthard S. M., J. Neurophysio. 99, 3136 (2008). 10.1152/jn.91327.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Hwang H., Martinez F., Austin R., and Ryu W., PLoS ONE 3, e2550 (2008). 10.1371/journal.pone.0002550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr J., Parashar A., Gibson R., Robertson A. P., Martin R. J., and Pandey S., “A microfluidic platform for high-sensitivity, real-time drug screening on C. elegans and parasitic nematodes,” Lab Chip, 2011, Advance Article . [DOI] [PMC free article] [PubMed]
- Chen B., Deutmeyer A., Carr J., Robertson A. P., Martin R. J., and Pandey S., Parasitology 138, 80 (2011). 10.1017/S0031182010001010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel C. V., Gabel H., Pavlichin D., Kao A., Clark D., and Samuel A., J. Neurosci. 27, 7586 (2007). 10.1523/JNEUROSCI.0775-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezai P., Siddiqui A., Selvaganapathy P., and Gupta B., Lab Chip 10, 220 (2010). 10.1039/b917486a [DOI] [PubMed] [Google Scholar]
- Towers P. R., Edwards B., Richmond J. E., and Sattelle D. B., J. Neurochem. 93, 1 (2005). 10.1111/j.1471-4159.2004.02951.x [DOI] [PubMed] [Google Scholar]
- See supplementary material at http://dx.doi.org/10.1063/1.3604391E-BIOMGB-5-017102 for worm locomotion in different sections of the modulated sinusoidal channel.