Abstract
Interest in single-cell analysis has increased because it allows to understand cell metabolism and characterize disease states, cellular adaptation to environmental changes, cell cycles, etc. Here, the authors propose a device to electrically trap and lyse single-bacterial cells in an array format for high-throughput single-cell analysis. The applied electric field is highly deformed and concentrated toward the inside of the microwell structures patterned on the planar electrode. This configuration effectively generates dielectrophoretic force to attract a single cell per well. The microwell has a comparable size to the target bacterial cell making it possible to trap single cells by physically excluding additional cells. Inducing highly concentrated electric potential on the cell membrane can also effectively lyse the trapped single-bacterial cells. The feasibility of the authors’ approach was demonstrated by trapping and lysing Escherichia coli cells at the single-cell level. The present microwell array can be used as a basic tool for individual bacterial cell analysis.
INTRODUCTION
Single-cell manipulation and analysis hold great promise for studying diverse biological functions.1 For instance, single-cell analyses of gene expression, transcriptome, and proteome are of great importance. However, cell-based biological assays traditionally probe cell ensembles thereby completely averaging over relevant individual cell characteristics and even sometimes misleading the interpretation of cell properties.1, 2 For single-cell analysis, several cell-handling methods have been developed such as microwell arrays, chemical patterning, optical tweezers, and microfluidics.2, 3, 4, 5 Especially, microwell arrays enable large scale arraying and high-throughput single-cell measurements of cellular responses.6 Previous microwell array methods used gravity as a simple mechanism to trap single cells;6, 7, 8, 9 however, this method remains in the applications to mammalian cells for which typical volume is in the range of picoliters. To the best of our knowledge, no single-cell arraying method for bacteria such as Escherichia coli (E. coli) has been presented presumably because gravity cannot be used for trapping small-sized cells that swim.
Additional force is required to overcome the difficulties in trapping single-bacterial cells. Difficulties are mainly of two types. First, bacterial cells are much smaller in size compared to mammalian cells, typically in the range of femtoliters in volume. Second, the locomotion with flagella makes handling of bacterial cells difficult. Electrostatic force, electrophoresis, or dielectrophoresis (DEP) is one of the powerful tools to handle cells with specificity and stability. Especially, DEP, the force on a polarized particle in a spatially nonuniform electric field, has been widely used to manipulate mammalian cells10 or bacterial cells.11 Introducing additional force such as DEP into the microwell arrays would allow handling of bacterial cells followed by lysis for single-cell analysis.
Meanwhile, analysis of intracellular constituents such as nucleic acids and proteins is of great importance to study diverse biological functions. To get intracellular materials for direct analysis, it is necessary to open the cellular membrane. This can be achieved efficiently using electroporation (EP) by inducing an electric potential on the cell membrane.12, 13 When the applied electric field strength is strong enough, it causes an irreversible mechanical breakdown of the membrane leading to cell lysis. Microfluidic devices have advantageous features to perform EP for cell lysis, which enables to achieve high electric field strength with relatively low electric potential by reducing the distance between the electrodes. Several microfluidic devices of this type have been introduced so far.14, 15, 16, 17, 18 It has been reported that bacterial cells such as E. coli require a threshold field strength for lysis significantly higher than the one required for typical mammalian cells.14 This is due to the presence of a peptidoglycan cell wall and their small size. Applying a strong electric field to E. coli lysis leads to generation of bubbles by electrolysis of water or Joule heating. To minimize bubble generation, target cells should be positioned where electric field strength is maximized.
Here, we describe an array of microwells where electrostatic functions are integrated to perform DEP and EP-based single-bacterial cell trapping with subsequent lysis. It contains a large number of arrayed microwells that can lead to parallel manipulation and analysis of populations of bacterial cells. The through-hole photoresist structure is patterned on an ITO-coated glass slide to induce a highly localized electric field. This setup efficiently induces DEP force for pulling down bacterial cells into the microwell. The key to trapping single-bacterial cells is a physical restriction of available space in each microwell having comparable size to the target cell. If a microwell is occupied by one trapped cell, this physically prevents a second cell from entering the microwell. Our microwell array platform also effectively lyses trapped cells with EP since electric fields are highly concentrated inside the microwell without bubble generation. Such a platform represents a first step toward the development of highly integrated devices that will enable trapping and analysis of a population of bacteria at the single-cell level.
THEORY AND DESIGN OF THE DEVICE
Dielectrophoresis and electroporation of the cell
DEP is a phenomenon in which a force is exerted on a dielectric particle when it is subjected to a nonuniform electric field. The time averaged DEP force, , for the case of a spherical cell of radius, a, is approximated by
where εe, f, and E are the permittivity of the external medium, the frequency, and the strength of the applied electric field, respectively. The polarization factor, K, is
where is the complex electrical permittivity of the cell and is the complex electrical permittivity of the external medium (σ is electrical conductivity and ). When the real part of polarization factor, Re[K], is bigger than 0, the particle will be attracted toward the strong field, positive DEP (pDEP). Meanwhile, when Re[K] is smaller than 0, the particle will be directed away from the strong field, negative DEP (nDEP). Re[K] is controlled by adjusting the conductivity of the medium and the frequency of the applied electric fields. Typical cells show strong pDEP around 1 MHz of the applied electric fields.19
When a cell is exposed to an external electric field, a transmembrane potential, Δϕ, is induced. This transmembrane potential for a spherical cell can be approximated13 as
If the transmembrane potential exceeds a critical value (0.2–1.5 V), permeable pores are formed on the cell membrane.
Design of the device
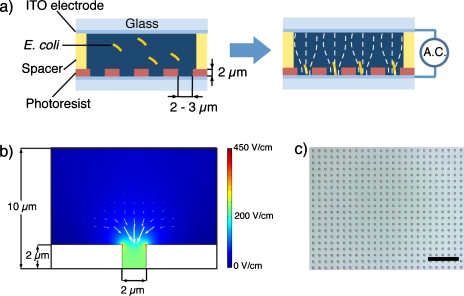
Figure 1a shows the schematic diagram of the experimental concept for cell trapping and lysis. The target cells are loaded on the microwell array and trapped with pDEP into each microwell and lysed after positioning single cells. Since the photoresist is a good insulation material, the electric fields are well blocked except for the area where the microwells are patterned. In this geometry, an applied electric field is highly deformed and concentrated around the microwell, which effectively generates DEP to attract a single cell to a nearby microwell. The depth of the well structure was 2 μm and the diameter was 2–3 μm, which were designed accordingly to E. coli cell dimensions and the size limit of the patterning with conventional photolithography. The distance between the top and the bottom electrodes is kept about 100 μm with the spacer. If a microwell is occupied by one cell, no more cells will be trapped since the previously trapped E. coli cell physically excludes the second one. For evaluation of the DEP force of our device, we performed 2D simulation of the electric fields by using commercially available code (COMSOL MULTIPHYSICS, COMSOL Group). Figure 1b shows simulated E contours, where the electric potential is assigned at the boundaries of the top and bottom lines. The electric field is concentrated inside the microwell due to the different permittivity of microwell structure and the medium, which induces DEP force for cell trapping.
Figure 1.
(a) Schematic image of the single-cell trapping and lysing device. The solution containing target cells is introduced through the gap between the electrodes by capillary flow. The distance between the top and the bottom electrodes is kept about 100 μm with the spacer. A highly localized electric field, caused by the through-hole photoresist structure, efficiently induces DEP force to trap cells. Microwell structure having comparable size to the target cell allows single-cell trapping. (b) Simulated electric field with finite element method, where the electric potential, 1 V, is applied between top and bottom lines, and the permittivity of medium and microwell structure (white area) are 80.4 ε0 and 3.0 ε0 (ε0: vacuum permittivity), respectively. Color scheme shows the electric field strength from blue (lowest) to red (highest). White arrows represent ∇E2 indicating the direction of pDEP force. (c) Arrayed microwells patterned on the plane ITO electrode. The distance between center to center of each microwell is 10 μm. Large array of microwells will allow high-throughput single-cell analysis. Scale bar is 50 μm.
EXPERIMENT
The microwell array was fabricated by conventional photolithography. A 500 nm thick layer of indium tin oxide (ITO) was sputtered on a glass substrate. A positive-type photoresist (S-1813, Shipley Far Ltd.) is spin coated on the electrode substrate and prebaked. The photoresist was exposed to ultraviolet light through a patterned chromium photomask, followed by developing. The substrate was cleaned and rinsed with de-ionized water. Figure 1c shows the large array of electroactive microwells fabricated on the ITO electrodes. The fabricated microwell array was exposed to O2 plasma to activate the surfaces using a reactive ion etching machine (RIE-10NR, Samco Co.). O2 plasma treatment makes the microwell structure hydrophilic, which ensures easy injection of reagent to the microwell. The microwell array was soaked into water and taken out just before the experiment.
E. coli K-12 cells are grown in LB (Luria-Bertani) broth to the early exponential phase and diluted to a concentration of 108 cells ml−1. Prior to DEP and EP experiments, the culture medium was exchanged to a DEP buffer (10 mM HEPES, 0.1 mM CaCl2, 59 mM D-glucose and 236 mM sucrose; pH 7.35) to adjust the conductivity of the cell suspension medium (21.4 mS m−1) for pDEP. Cells in the culture medium were centrifuged at 1000×g for 5 min. We gently removed the culture medium and added DEP buffer. The solution containing E. coli cells labeled by SYBR green (Takara Bio Inc.) was introduced through the gap between the electrodes by capillary flow. The device was mounted on the x−y translational stage located on a microscope (IX 71, Olympus). Cell trapping and lysing were monitored using a charged coupled device (CCD) camera that was installed on the microscope. The electric potential for DEP and EP was applied to the ITO electrodes with a function generator (WF1974; NF Corp.) after amplifying the amplitude with an amplifier (HSA4101; NF Corp.).
RESULTS AND DISCUSSION
Here single-bacterial cell trapping is demonstrated with an electroactive microwell array designed for E. coli cells. Bacterial cells were introduced into the device and the flow subsequently was stopped in order to test if upon simple gravity, cells would enter the microwells. However and not surprisingly, we could not observe such a movement (see supplementary movie). Therefore, it is necessary to assist cell trapping by additional force such as DEP. For the demonstration of our concept, the DEP buffer containing stained E. coli cells was loaded through the gap between the electrodes with capillary flow and an electric potential was applied to the electrodes for pDEP to attract cells into the microwells. E. coli cells started to move for an applied voltage of about 1 Vp-p AC at 1 MHz. Figure 2a (enhanced) displays time-lapse images of typical experiment of E. coli cell trapping. Microwells are gradually occupied by E. coli cells. Figure 2b shows a portion of the array with trapped E. coli cells in 2 μm diameter microwells. Trapped E. coli cells, which have a cylindrical shape, are detected as fluorescent circles due to their orientation with their long axis parallel to the electric field lines, as illustrated in Fig. 1a. The image shows that 61% of microwells are occupied by single E. coli cells.
Figure 2.
(a) Time-lapse images of E. coli cell trapping with 3 μm diameter microwells. SYBR green stained E. coli cells were trapped by pDEP by applying a voltage ac 5 Vp-p at 1 MHz (enhanced online). Scale bar is 30 μm. (b) Trapped E. coli cell array in 2 μm diameter microwells. The image shows that 61 percent of microwells are occupied by single E. coli cells. Scale bar is 30 μm. (c) The ratio of occupied microwells is plotted vs the number of trapped cells. The number of microwells containing zero, one, two, three, or four cells is counted from the randomly selected 10×10 microwell array after trapping. All plotted bars are mean values of five data series (enhanced online).
By modulating the diameter of the microwells, we can control the degree of cell occupancy. The effect of the microwell diameter was investigated by testing with 2 and 3 μm diameter microwells, as shown in Fig. 2c. We did not test smaller microwells because it is practically difficult to fabricate. Larger microwells are not relevant here, as too many cells would be trapped into 4 or 5 μm diameter microwells. The electric potential for DEP and the concentration of E. coli cells were kept constant in order to avoid additional complexities due to the variation of DEP force. The electric fields (10 Vp-p at 1 MHz ac voltage) were applied for 1 min to trap cells. We then counted the number of microwells containing zero, one, two, three, or four cells in a randomly selected 10×10 microwell area. In Fig. 2c, the different percentage values of microwell are plotted versus the number of trapped cells. All plotted bars are the mean values of five data series. As shown in Fig. 2c, the number of microwells containing 2 or 3 cells increased with the diameter of the microwells. 2 μm microwell array shows good trapping efficiency for single cells. Statistically, the density of E. coli cells in the device (104 cells mm−2) is equal to the density of microwells in the array (104 microwells mm−2). Trapping data in Fig. 2c shows that about 60% of introduced cells are trapped into each microwell for 2 μm microwell array. There are other parameters that control trapping efficiency such as the applied electric field strength and cell concentration. Increasing the cell concentration led to an increase, in the total number of trapped cells (data not shown). Hence, further adjustment of experimental parameters will lead to more efficient single-cell trapping.
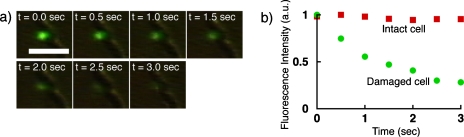
We increased the applied electric field strength in order to trigger E. coli cell lysis and monitored, after the electric pulse, the intracellular level of fluorescence. We expected that disruption of the cell wall would lead to rapid diffusion of the intracellular material resulting in a rapid decrease of fluorescence. Figure 3a shows time-lapse images of single-cell lysis, where 30 Vp-p at 1 MHz ac voltage was applied at t=0 sec. In this case, the applied electric field strength (1060 V cm−1) corresponds to the electric field strength threshold for E. coli cell lysis (1000–1500 V cm−1).20 The fluorescence intensity of cells decreased rapidly and diffused out with time. After 3 s, no fluorescence light was detected from the cell. It likely represents leakage of the cytoplasmic material due to cell lysis. In order to rule out the contribution of photobleaching in the fluorescence decay, the time course of fluorescence intensity of the damaged cell by the electric field is compared with that of an intact cell measured before applying electric field in Fig. 3b. Decrease of fluorescence intensity in intact cells is negligible for 3 s. However, rapid decrease of the fluorescence intensity is observed for the damaged cell.
Figure 3.
(a) Time-lapse images during single E. coli cell lysis, where 30 Vp-p at 1 MHz ac voltage was applied at t=0 s. Following lysis, fluorescence signal from an individual cell decreased rapidly with time. Scale bar is 5 μm. (b) Normalized fluorescence intensity for intact and lysed cells. The rapid decrease of fluorescence is due to the leakage of intracellular materials by EP.
CONCLUSION
Trap and lysis of single-bacterial cells were realized with a simple electroactive microwell array. Integration of electrostatic functions into the microwell enables attraction of E. coli cells into the microwell array and their subsequent lysis. Microwell structures patterned on the planar electrode induces highly localized electric field for pulling down E. coli cells with pDEP. A comparable size of the well to the target cell strongly favors single-bacterial cells trapping by physically excluding additional cells. Moreover, highly concentrated electric fields strength is sufficient to lyse E. coli cell. The device can potentially be used as a basic tool for individual bacterial cell analysis in high-throughput manners. For instance, it will facilitate interpretation of dielectric properties of different bacteria by investigating DEP or EP characteristics if we trap and lyse several kinds of bacterial cells simultaneously. We can also investigate the response of individual bacterial cells upon chemical stimuli by applying target chemicals onto the trapped cells and monitor the cellular response. Moreover, the new device could be used for the analysis of individual cell contents or the utilization of intracellular materials of individual bacteria (for instance, to perform protein synthesis directly by the translational machinery of the trapped bacteria). To realize this operation, development of new functions for confinement or manipulation of intracellular materials of individual bacterial cells is underway.
ACKNOWLEDGMENTS
S.H.K. was supported through the Global COE Program, “Global Center of Excellence for Mechanical Systems Innovation,” by the Ministry of Education, Culture, Sports, Science and Technology, Japan. This work was supported in part by the CNRS Program “Interface Physique Chimie Biologie: aide à la prise de risque” to D. Fourmy.
References
- Lidstrom M. E. and Meldrum D. R., Nat. Rev. Microbiol. 1, 158 (2003). 10.1038/nrmicro755 [DOI] [PubMed] [Google Scholar]
- Di Carlo D. and Lee L. P., Anal. Chem. 78, 7918 (2006). 10.1021/ac069490p [DOI] [PubMed] [Google Scholar]
- Bennett M. R. and Hasty J., Nat. Rev. Genet. 10, 628 (2009). 10.1038/nrg2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims C. E. and Allbritton N. L., Lab Chip 7, 423 (2007). 10.1039/b615235j [DOI] [PubMed] [Google Scholar]
- El-Ali J., Sorger P. K., and Jensen K. F., Nature (London) 442, 403 (2006). 10.1038/nature05063 [DOI] [PubMed] [Google Scholar]
- Rettig J. R. and Folch A., Anal. Chem. 77, 5628 (2005). 10.1021/ac0505977 [DOI] [PubMed] [Google Scholar]
- Lee W. C., Rigante S., Pisano A. P., and Kuypers F. A., Lab Chip 10, 2952 (2010). 10.1039/c0lc00139b [DOI] [PubMed] [Google Scholar]
- Figueroa X. A., Cooksey G. A., Votaw S. V., Horowitz L. F., and Folch A., Lab Chip 10, 1120 (2010). 10.1039/b920585c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokimitsu Y. et al. , Cytometry, Part A 71A, 1003 (2007). 10.1002/cyto.a.20478 [DOI] [Google Scholar]
- Voldman J., Annu. Rev. Biomed. Eng. 8, 425 (2006). 10.1146/annurev.bioeng.8.061505.095739 [DOI] [PubMed] [Google Scholar]
- Castellarnau M., Errachid A., Madrid C., Juarez A., and Samitier J., Biophys. J. 91, 3937 (2006). 10.1529/biophysj.106.088534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann E., Sowers A. E. and Jordan C. A. (Plenum Press, New York, 1989). [Google Scholar]
- Tsong T. Y., Biophys. J. 60, 297 (1991). 10.1016/S0006-3495(91)82054-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. W. and Tai Y. C., Sens. Actuators, A 73, 74 (1999). 10.1016/S0924-4247(98)00257-X [DOI] [Google Scholar]
- Lu H., Schmidt M. A., and Jensen K. F., Lab Chip 5, 23 (2005). 10.1039/b406205a [DOI] [PubMed] [Google Scholar]
- Wang H. Y. and Lu C., Anal. Chem. 78, 5158 (2006). 10.1021/ac060733n [DOI] [PubMed] [Google Scholar]
- Ramadan Q., Samper V., Poenar D., Liang Z., Yu C., and Lim T. M., Sens. Actuators B 113, 944 (2006). 10.1016/j.snb.2005.04.018 [DOI] [Google Scholar]
- Khine M., Lau A., Ionescu-Zanetti C., Seo J., and Lee L. P., Lab Chip 5, 38 (2005). 10.1039/b408352k [DOI] [PubMed] [Google Scholar]
- MacQueen L. A., Buschmann M. D., and Wertheimer M. R., Bioelectrochemistry 72, 141 (2008). 10.1016/j.bioelechem.2008.01.006 [DOI] [PubMed] [Google Scholar]
- Wang H. Y., Bhunia A. K., and Lu C., Biosens. Bioelectron. 22, 582 (2006). 10.1016/j.bios.2006.01.032 [DOI] [PubMed] [Google Scholar]