Abstract
The rotenone-insensitive NADH:hexaammineruthenium III (HAR) oxidoreductase reactions catalyzed by bovine heart and Yarrowia lipolytica submitochondrial particles or purified bovine complex I are stimulated by ATP and other purine nucleotides. The soluble fraction of mammalian complex I (FP) and prokaryotic complex I homolog NDH-1 in Paracoccus denitrificans plasma membrane lack stimulation of their activities by ATP. The stimulation appears as a decrease in apparent Km values for NADH and HAR. Thus, the “accessory” subunits of eukaryotic complex I bear an allosteric ATP-binding site.
Keywords: Complex I, Allosteric nucleotide binding, Mitochondria
1. Introduction
The mitochondrial proton-translocating NADH:ubiquinone oxidoreductase (respiratory complex I) and its prokaryotic homologue (NDH-1) catalyze oxidation of intramitochondrial (or cytoplasmic) NADH, thus maintaining the steady-state NADH/NAD+ ratio required for coordination between ATP-producing respiration and anabolic metabolism [1–4]. The mammalian mitochondrial complex I is an enormously large machine composed of 45 different subunits having total molecular mass of about 1 MDa [5]. The bacterial operons encoding NDH-1 contain 13–14 genes [2], and their transcription products are highly homologous to 14 “core” subunits of the mammalian and fungal enzymes. Up to 10 distinct redox components (FMN, iron-sulfur centers, bound ubiquinone species) [6–9] buried in the hydrophilic arm of the enzyme [10, 11] electronically connect NADH bound at a site located at about 100 Å distant from the coupling membrane plane and the terminal electron acceptor, the membranous quinone/quinol pool.
The catalytic properties of the mammalian [3], fungal [12], and prokaryotic [13, 14] enzymes are similar although not identical. Characteristic L-shaped three-dimensional structures of bovine heart [15], Neurospora crassa [16], Yarrowia lipolytica [11], Escherichia coli [10], and Paracoccus denitrificans [17] complexes have been visualized. It is generally thought that 13-14-subunit bacterial enzymes represent a minimal structural unit required for the catalytic activity. The specific functions of 31 so-called supernumerary or accessory subunits of eukaryotic complex I are not clear. Mammalian SDAP [18] and N. crassa ACP [19] subunits have been identified as acyl-carrier protein of fatty acid synthase complex. The subunit B16.6 (bovine heart nomenclature) is the GRIM-19 apoptosis-inducing factor [20]. A number of the subunits were shown to be susceptible to posttranslational modifications such as phosphorylation [21, 22], glutathionylation [23], and acetylation/de-acetylation [24] although no substantial changes in the enzymatic activity resulting from these modifications have been documented. It seems unlikely that complex I, the enzyme operating as the major entry to the respiratory chain, is not subjected to regulation. One such a mechanism, so-called A/D transition [25] characteristic for a number of eukaryotic species [26] and absent in prokaryotes, has been described for preparations of various degree of resolution (purified enzyme [27], inside-out submitochondrial particles [28], intact mitochondria [29], and perfused hearts [30]). No specific effects of the natural, low molecular mass mitochondrial constituents except for free fatty acids [31] have been described so far.
In this report we will show that ATP in the micromolar concentration range specifically stimulates the NADH:hexaammineruthenium(III) reductase activity catalyzed by eukaryotic complex I when assayed at low NADH and acceptor concentrations. No such stimulatory effect was seen for the three-subunit FP fragment of complex I or for P. denitrificans membrane-bound NDH-1. Although the physiological significance of the ATP-induced effect remains to be established, our data are the first to demonstrate that a functionally significant, high-affinity ATP-specific binding site exists within accessory subunits of mitochondrial complex I.
2. Materials and methods
Bovine heart submitochondrial particles (SMP) were prepared as described [28], and their NADH oxidase was activated by aerobic incubation with NADPH [32]. After activation the SMP were precipitated by centrifugation, suspended in a mixture comprised of 0.25 M sucrose, 50 mM Tris-Cl (pH 8.0), and 0.2 mM EDTA-KOH, 0.1 mM potassium malonate and stored in liquid nitrogen. Complex I [33] and its low molecular mass fragment FP [34] were purified as described. Yarrowia lipolytica (var. alkalitolerance [35]) mitochondria were kindly provided by Dr. Renata Zvyagilskaya (A. N. Bach Institute of Biochemistry, Russian Academy of Sciences). Y-SMP were prepared as follows. Mitochondria (15 mg protein) were suspended in 6 ml of 0.15 M sucrose, and EDTA (1 mM final concentration) was added. The suspension was placed on ice, saturated with argon for 10 min with continuous stirring, the pH was adjusted to 8.6 with NH4OH, and sonicated (MSE Soniprep 50, maximal power output) under argon flow (5 times, 30s each with 1 min intervals). The suspension was centrifuged for 10 min at 20,000 g, the precipitated material was discarded, and the supernatant was centrifuged for 1 h at 120,000 g. The pellet was rinsed with a solution composed of 0.25 M sucrose, 50 mM Tris-Cl buffer (pH 8.0), and 0.2 mM EDTA and suspended in 1 ml of the same solution. The final preparation of Y-SMP (3 mg protein per ml) was stored in liquid nitrogen. About half of the NADH oxidase activity catalyzed by Y-SMP was sensitive to rotenone (due to the presence of alternative NADH dehydrogenase, NDH-2) and deamino-NADH, the specific substrate for NADH-1 was used as the substrate when the Y-SMP were analyzed.
NADH oxidase, the NADH:artificial acceptor reductase, and other activities were assayed at 30°C in the standard reaction mixture composed of 0.25 M sucrose, 50 mM Tris-Cl (pH 8.0), and 0.2 mM K-EDTA. NADH:HAR reductase activities of SMP and purified complex I were assayed in the presence of rotenone (5 μM). Rotenone (5 μM) and KCN (5 mM) were added to the assays when NADH:HAR reductase activities of Y-SMP and P-PMV were measured. Antimycin A (1 μg/ml) was present when the rotenone-sensitive NADH:Q1 reductase activity of SMP was assayed. Other specific additions to the standard reaction mixture and details are indicated in the captions to the figures and footnotes to the Table. All the activities reported in Figs. 1, 3 and 4 and in the Table were determined in at least three independent assays with the experimental errors of less than 10%. Wurster’s Blue (WB), the perchlorate salt of free cation-radical of tetramethyl-p-phenylenediamine was prepared as described [36]. All other fine chemicals were from Sigma-Aldrich (U.S.A.).
Table.
Effect of nucleotides on the NADH:HAR reductase activity of complex I in bovine heart submitochondrial particles
| Nucleotide addeda | Specific activityb, μmol/min per mg | Apparent Kac, μM | Maximal stimulationd, fold |
|---|---|---|---|
| 1. none | 0.10 | ||
| 2. ATP, 100 μM | 0.97 | 20 | 10 |
| 3. as (2) + Mg2+, 1 mM | 0.18 | ||
| 4. as (2) + atractylate, 5 μM | 0.72 | ||
| 5. ADP, 100 μM | 0.26 | 100 | 4.5 |
| 6. AMP-PNP, 50 μM | 0.24 | n.d.e | n.d. |
| 7. GTP, 100 μM | 0.42 | n.d. | n.d. |
| 8. GDP, 100 μM | 0.13 | n.d. | n.d. |
| 9. Adenosine, 100 μM | 0.10 | n.d. | n.d. |
| 10. AMP, 100 μM | 0.10 | n.d. | n.d. |
| 11. cAMP, 100 μM | 0.10 | n.d. | n.d. |
| 12. CTP, 100 μM | 0.10 | n.d. | n.d. |
Nucleotides and other additions were added to the standard assay mixture and the reaction was initiated by the addition of SMP.
Initial rate of 5 μM NADH oxidation in the presence of 50 μM HAR.
Determined from the double reciprocal plot 1/v versus 1/[nucleotide].
Ratio of activity in the presence of saturating nucleotide to activity in the absence of nucleotide.
n.d., not determined.
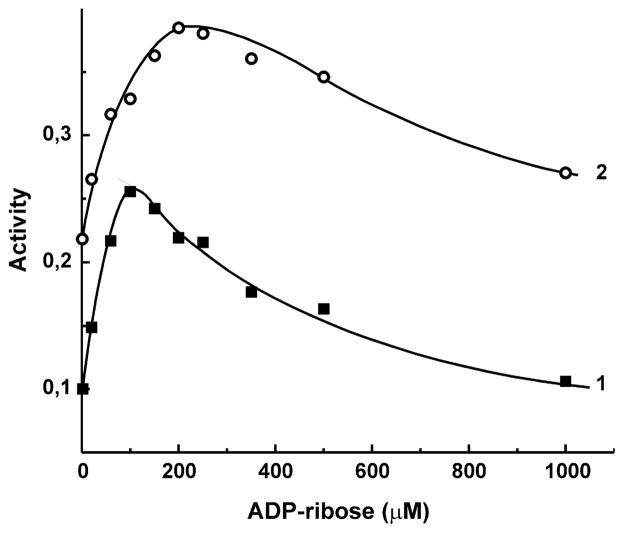
Fig. 1.
Effect of ADP-ribose on the initial rate of NADH:HAR reductase reaction catalyzed by bovine heart SMP. HAR (50 μM) and NADH (5 μM, curve 1, and 20 μM, curve 2) were present in the standard assay mixture. The activity is expressed as μmole of NADH oxidized per minute per mg of protein.
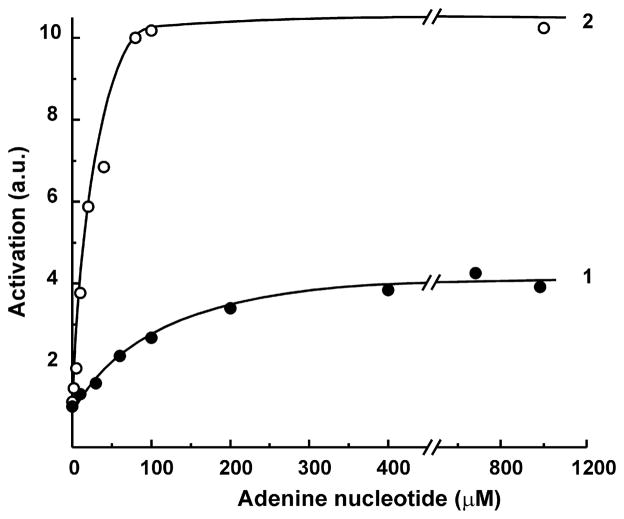
Fig. 3.
Activation of bovine heart SMP NADH:HAR reductase by adenine nucleotides. The concentrations of the reactants were: NADH, 5 μM and HAR, 50 μM. The specific activity in the absence of added nucleotides (0.1 μmol per min/mg) was taken as 1 unit. Curves 1 and 2, ADP or ATP were added to the standard assay mixture, respectively.
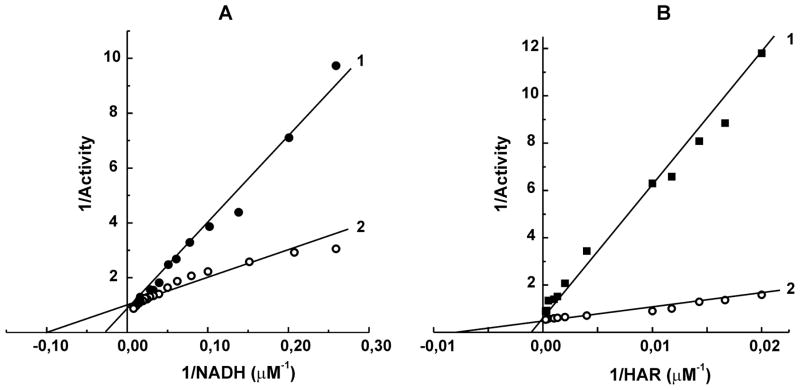
Fig. 4.
Kinetic pattern of ATP-dependent stimulation of NADH:HAR reductase catalyzed by bovine heart SMP. Lines 1, controls; lines 2, 10 μM ATP (A) and 20 μM ATP (B) was added. A, 100 μM HAR was present; B, 5 μM NADH was present.
The protein content was determined by the biuret assay using bovine serum albumin as the standard.
3. Results
The membrane-bound and purified dispersed mitochondrial complex I and their prokaryotic homologue NDH-1 catalyze a number of the NADH:artificial electron acceptor oxidoreductase activities showing particularly high turnover numbers with ferricyanide [37] and hexaammineruthenium(III) [38]. The kinetic patterns of the steady-state NADH oxidation with these two electron acceptors are quite different. When assayed at limiting reactant concentrations, the ferricyanide reductase reaction proceeds according to a ping-pong mechanism with inhibition of the reaction rate by both substrate and acceptor [37], whereas the HAR reductase reaction proceeds according to the ternary complex or Theorell–Chance mechanism [38] and no inhibition by excess of either reactant is seen. These data unequivocally suggest that these two oxidants accept electrons from different sites of the multi-redox component complex I. The kinetic patterns of mammalian complex I in a number of its catalytic activities such as forward and reverse electron transfer [39], transhydrogenase reaction [40. 41], and superoxide generation [42] are far from being trivial, and we feel that a closer look at the reactions with different artificial electron acceptors would be insightful. As the first step of this strategy, the effect of ADP-ribose (a competitive inhibitor of the NADH binding site [43]) on the HAR reductase activity of SMP was evaluated over a wide range of NADH concentrations. Figure 1 demonstrates a nontrivial effect of the competitive inhibitor on HAR reductase, i.e. at low concentration of the substrate (NADH) ADP-ribose significantly stimulated the reaction. The only plausible explanation for biphasic effect of the competitive inhibitor as depicted in Fig. 1 seemed to be the presence of a nucleotide binding site different from that where NADH binds as the substrate, and this hypothesis was further elaborated. To avoid a complexity due to dual activation and inhibition by ADP-ribose, the effects of a simpler nucleotide, ATP, were further investigated.
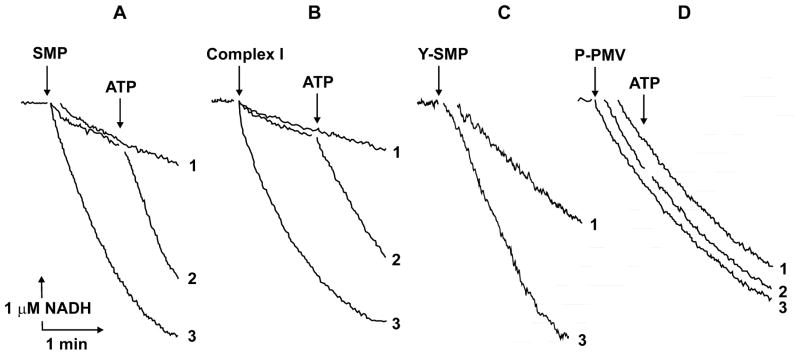
Figure 2 demonstrates the effect of ATP on NADH:HAR reductase as it was seen when different complex I preparations were assayed. ATP either present in the reaction mixture or added during assay caused immediate stimulation of the reactions catalyzed by SMP (A), purified bovine heart complex I (B), and the other eukaryotic complex I, Y-SMP (C). No stimulatory effects were seen when prokaryotic P-PMV (D) or bovine heart FP (not shown) were used as the enzyme preparations. Half-maximal stimulation of the activity by ATP at constant concentration of NADH appeared at 20 μM (Fig. 3). Since the activating effect of ADP-ribose was evident only at low NADH concentration, the kinetic pattern of ATP-dependent stimulatory effect was assessed (Fig. 4), and competitive with NADH (A) and HAR (B) activation was evident, i.e. the presence of ATP decreased apparent Km values for both substrates without affecting the maximal activity.
Fig. 2.
Effect of ATP on the NADH:HAR reductase reaction catalyzed by different complex I preparations. Actual tracings of NADH (5 μM) oxidation by 50 μM HAR are shown. ATP (100 μM) was present in the assay before the reactions were initiated by the enzyme preparations (curves 3) or added where indicated (curves 2). No ATP was present or added (curves 1). A, bovine heart submitochondrial particles (SMP, 5 μg/ml); B, purified bovine heart complex I (1.5 μg/ml); C, Y. lipolytica submitochondrial particles (Y-SMP, 7 μg/ml); D, P. denitrificans plasma membrane vesicles (P-PMV, 10 μg/ml). Deamino-NADH was used as the substrate in panel C.
As depicted in the Table, other purine nucleotides (ADP, GTP, and AMP-PNP) also stimulated the NADH:HAR reductase at low NADH concentration, whereas AMP, adenosine, cAMP, and CTP did not. The activation by ATP was prevented or reversed by Mg+2, thus suggesting that free nucleotide, not ATP-Mg complex, binds to the enzyme.
It was of obvious interest to see if other than HAR reductase activity would be affected by ATP. The following activities of complex I in coupled or uncoupled SMP preparations were tested: (i) NADH oxidase measured in the presence of 100 μM NADH or 1 μM NADH (ethanol and alcohol dehydrogenase were used as the NADH-regenerating system in the latter case); (ii) NADH:ferricyanide reductase, (iii) NADH:WB reductase [44]; (iv) rotenone-sensitive NADH:Q1 reductase; (v) energy-dependent, succinate-supported reverse electron transfer activity, (vi) NADH:acetyl-NAD+ transhydrogenase; (vii) NADH- and succinate-supported superoxide generation. None of these activities was affected by ATP under a variety of experimental conditions (negative results are not shown). ATP also affected neither the rates of thermally induced deactivation nor the turnover-dependent activation of complex I in SMP.
4. Discussion
Significant stimulation of eukaryotic complex I activity by ATP, if assayed with low concentration of NADH and HAR, unequivocally suggests the presence of a nucleotide-binding site different from that where the substrate NADH binds. Obvious questions relevant to this finding arise: (i) where is this site located?; (ii) what is the mechanistic explanation of the competitive (with the substrates) stimulation?; and (iii) is the allosteric nucleotide binding relevant to physiological regulation of complex I? At present we are unable to answer these questions in a straightforward way, and only some possibilities merit brief discussion.
The stimulatory effect of ATP is only seen if eukaryotic enzymes are assayed (Fig. 2AC). This could not exclude that the effects of ATP as seen in SMP or Y-SMP are not direct but mediated by some other membrane-bound ATP-binding protein that interacts with complex I, thus modulating its activity. The data shown in Fig. 2B seem to eliminate this possibility unless the presence of such a “mediating” component in complex I purified by the conventional procedure [33] is to be postulated. The absence of ATP-dependent stimulation in P. denitrificans vesicles (Fig. 2D) excludes the location an ATP-binding site on any “core” subunits of complex I. In light of recent data on the subunit composition of P. denitrificans NDH-1 [17], three homologues of mitochondrial B17.2, AQDQ, and 13 kDa (bovine nomenclature) subunits can also be excluded. Five nucleotide-binding subunits of bovine heart complex I have been identified by UV-induced photolabeling with 32P-nicotinamide adenine dinucleotides and (β-32P)ADP [45]. The 51 and 30 kDa subunits were labeled by ADP, the former was evidently the NADH-binding subunit located in FP fraction, whereas the identity of the latter could not be definitely established [45]. The eliminating effect of Mg2+ on the ATP-dependent effect (Table) shows that free ATP is the activating specie. If a contaminating “mediator protein” proposal is to be accepted, the ATP/ADP translocator, the most abundant protein in the inner mitochondrial membrane, would be the best candidate for this role. However, atractylate, the specific inhibitor of nucleotide binding by ATP/ADP translocator did not abolish the ATP-induced activation (Table), an observation which contradicts the “contaminating mediator” proposal.
Whether the ATP-induced increase in NADH:HAR reductase is direct or mediated, it appears as a substrate- and oxidant-competitive activation, i.e. a decrease in apparent Km values for the reactants (Fig. 4). An apparent Km for the substrate-electron donor is a complex function of the binding term (KS), the kinetic term (kcat), and the terms describing equilibria between the primary and several consequently arranged redox components leading to the electron acceptor reactive site [46]. A decrease in apparent KmNADH would be expected if ATP binding results in a positive shift of the midpoint redox potential of a component which donates electrons to HAR. The structural studies show that most of the multiple redox components in complex I (except for FMN and iron-sulfur clusters N-1a and N-3) are not easily accessible for external oxidants [4]. For kinetic reasons [37, 38], the redox components located in the cavity where NADH binds (FMN, N-1a, N-3) can hardly be assigned as the sites at which HAR interacts with as the electron acceptor. A deep acidic groove is seen in the structure of the hydrophilic part of Thermus thermophilus NDH-1 close to the area where the N-2 iron-sulfur cluster is located [4]. We propose that HAR accepts electrons from the same site (N-2) where the first electron is donated to the natural quinone acceptor. The overall rates of NADH oxidase (coupled or uncoupled) or ubiquinone reductase are evidently limited by the step(s) that follow reoxidation of N-2, and it is not surprising that alteration of the midpoint potential of N-2 by ATP (as we propose) does not affect other than HAR reductase activity. Remarkably, a mutation of Y. lipolytica 49 kDa subunit His226 located in close vicinity (4 Å in T. thermophilus NDH-1) of iron-sulfur cluster N-2 was shown to shift its redox midpoint potential by 80 mV and eliminate its pH-dependency without affecting either the catalytic activity or H+/ē proton pumping stoichiometry [47].
The only activity among several others tested in this study that is strongly affected by ATP is NADH:HAR reductase. We believe that stimulation of this activity should be interpreted as a signature of some other yet unknown functionally significant effect of nucleotide binding. Relatively high affinity of ATP (ADP) for its (their) binding site (Table) suggests that complex I in mitochondria is always present as an allosteric nucleotide-binding site saturated form. At present only hypothetical proposals on functional importance of ATP binding can be offered. One possibility is that it changes an accessibility of the protein for posttranslational modifications such as, for example, phosphorylation/dephosphorylation, which in turn affects the catalytic activity of complex I. Another possibility is that allosteric nucleotide binding influences the interaction of complex I with other respiratory chain components (so-called “supercomplex” formation). Also, the effect of ATP binding on the resistance of complex I to intracellular proteolysis and/or oxidative damage cannot be excluded. It is pertinent to the present discussion to note that allosteric binding of nucleotides ATP/ADP to the respiratory enzyme (complex I as reported here) is not unique: mammalian cytochrome c oxidase was shown to bind ATP and ADP, and this binding affects the enzyme activity [48].
Highlights.
Mitochondrial respiratory complex I binds purine nucleotides at allosteric site.
ATP binding stimulates NADH: hexaammineruthenium(III) reductase activity.
Prokaryotic homologue of complex I lacks of allosteric ATP-binding site.
Acknowledgments
We are grateful to Dr. Tatyana Zharova for P. denitrificans plasma membrane preparations. We thank Dr. Renata Zvyagilskaya who kindly provides us with Y. lipolytica mitochondria. Linguistic help of Dr. Richard Lozier in preparation of the manuscript is gratefully acknowledged. This study was partially supported by the Russian Foundation for Fundamental Research (grants 08-04-00594 to ADV and 09-04-00505 to VGG) and NIH Research Grant #R03 TW07825 funded by the Fogarty International Center.
Abbreviations
- SMP, Y-SMP, and P-PMV
submitochondrial particles derived from bovine heart, Yarrowia lipolytica mitochondria, and Paracoccus denitrificans inside-out plasma membrane vesicles, respectively
- FP
low mol. mass fragment of bovine heart complex I
- AMP-PNP
Adenylylimidodiphosphate, non-hydrolysable analogue of ATP
- Q1
2,3-dimethoxy,5-methyl,6-isoprenyl,1,4-benzoquinone
- WB
free cation radical of tetramethyl-p-phenylenediamine
- HAR
hexaammineruthenium(III)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brandt U. Energy converting NADH:quinone oxidoreductase (complex I) Annu Rev Biochem. 2006;75:69–92. doi: 10.1146/annurev.biochem.75.103004.142539. [DOI] [PubMed] [Google Scholar]
- 2.Yagi T. The bacterial energy-transducing NADH-quinone oxidoreductases. Biochim Biophys Acta. 1993;1141:1–17. doi: 10.1016/0005-2728(93)90182-f. [DOI] [PubMed] [Google Scholar]
- 3.Vinogradov AD. Catalytic properties of the mitochondrial NADH-ubiquinone oxidoreductase (complex I) and the pseudo-reversible active/inactive enzyme transition. Biochim Biophys Acta. 1998;1364:169–185. doi: 10.1016/s0005-2728(98)00026-7. [DOI] [PubMed] [Google Scholar]
- 4.Sazanov LA. Respiratory complex I: mechanistic and structural insights provided by the crystal structure of the hydrophilic domain. Biochemistry. 2007;46:2275–2288. doi: 10.1021/bi602508x. [DOI] [PubMed] [Google Scholar]
- 5.Carroll J, Fearnley IM, Skehel JM, Shannon RJ, Hirst J, Walker JE. Bovine complex I is a complex of 45 different subunits. J Biol Chem. 2006;281:32724–32727. doi: 10.1074/jbc.M607135200. [DOI] [PubMed] [Google Scholar]
- 6.Gostimskaya IS, Grivennikova VG, Cecchini G, Vinogradov AD. Reversible dissociation of flavin mononucleotide from the mammalian membrane-bound NADH: ubiquinone oxidoreductase (complex I) FEBS Lett. 2007;581:5803–5806. doi: 10.1016/j.febslet.2007.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sled VD, Friedrich T, Leif H, Weiss H, Meinhardt SW, Fukumori Y, Calhoun M, Gennis RB, Ohnishi T. Bacterial NADH-quinone oxidoreductases: iron-sulfur clusters and related problems. J Bioenerg Biomembr. 1993;25:347–355. doi: 10.1007/BF00762460. [DOI] [PubMed] [Google Scholar]
- 8.Ohnishi T. Iron-sulfur clusters/semiquinones in Complex I. Biochim Biophys Acta. 1975;387:475–490. doi: 10.1016/0005-2728(75)90087-0. [DOI] [PubMed] [Google Scholar]
- 9.Ohnishi T, Sled VD, Yano T, Yagi T, Burbaev DS, Vinogradov AD. Structure-function studies of iron-sulfur clusters and semiquinones in the NADH:Q oxidoreductase segment of the respiratory chain. Biochim Biophys Acta. 1998;1365:301–308. doi: 10.1016/s0005-2728(98)00082-6. [DOI] [PubMed] [Google Scholar]
- 10.Efremov RG, Baradaran R, Sazanov LA. The architecture of respiratory complex I. Nature. 2010;465:441–445. doi: 10.1038/nature09066. [DOI] [PubMed] [Google Scholar]
- 11.Hunte C, Zickermann V, Brandt U. Functional modules and structural basis of conformational coupling in mitochondrial complex I. Science. 2010;329:448–451. doi: 10.1126/science.1191046. [DOI] [PubMed] [Google Scholar]
- 12.Grivennikova VG, Serebryanaya DV, Isakova EP, Belozerskaya TA, Vinogradov AD. Active/de-active transition of NADH:ubiquinone oxidoreductase (Complex I) in the mitochondrial membrane of Neurospora crassa. Biochemical J. 2003;369:619–626. doi: 10.1042/BJ20021165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grivennikova VG, Roth R, Zakharova NV, Hagerhall C, Vinogradov AD. The mitochondrial and prokaryotic proton-translocating NADH:ubiquinone oxidoreductases: similarities and dissimilarities of the quinone-junction sites. Biochim Biophys Acta. 2003;1607:79–90. doi: 10.1016/j.bbabio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Zickermann V, Kurki S, Kervinen M, Hassinen I, Finel M. The NADH oxidation domain of complex I: do bacterial and mitochondrial enzymes catalyze ferricyanide reduction similarly? Biochim Biophys Acta. 2000;1459:61–68. doi: 10.1016/s0005-2728(00)00113-4. [DOI] [PubMed] [Google Scholar]
- 15.Grigorieff N. Three-dimensional structure of bovine NADH:ubiquinone oxidoreductase (complex I) at 22 Å in ice. J Mol Biol. 1998;277:1033–1046. doi: 10.1006/jmbi.1998.1668. [DOI] [PubMed] [Google Scholar]
- 16.Leonard K, Haiker H, Weiss H. Three-dimensional structure of NADH: ubiquinone reductase (complex I) from Neurospora mitochondria determined by electron microscopy of membrane crystals. J Mol Biol. 1987;194:277–286. doi: 10.1016/0022-2836(87)90375-5. [DOI] [PubMed] [Google Scholar]
- 17.Yip CY, Harbour ME, Jayawardena K, Fearnley IM, Sazanov LA. Evolution of Respiratory Complex I. “Supernumerary” subunits are present in the α-proteobacterial enzyme. J Biol Chem. 2011;286:5023–5033. doi: 10.1074/jbc.M110.194993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Runswick MJ, Fearnley IM, Skehel JM, Walker JE. Presence of an acyl carrier protein in NADH:ubiquinone oxidoreductase from bovine heart mitochondria. FEBS Lett. 1991;286:121–124. doi: 10.1016/0014-5793(91)80955-3. [DOI] [PubMed] [Google Scholar]
- 19.Sackmann U, Zensen R, Rohlen D, Jahnke U, Weiss H. The acyl-carrier protein in Neurospora crassa mitochondria is a subunit of NADH:ubiquinone reductase (complex I) Eur J Biochem. 1991;200:463–469. doi: 10.1111/j.1432-1033.1991.tb16205.x. [DOI] [PubMed] [Google Scholar]
- 20.Fearnley IM, Carroll J, Shannon RJ, Runswick MJ, Walker JE, Hirst J. GRIM-19, a cell death regulatory gene product, is a subunit of bovine mitochondrial NADH:ubiquinone oxidoreductase (complex I) J Biol Chem. 2001;276:38345–38348. doi: 10.1074/jbc.C100444200. [DOI] [PubMed] [Google Scholar]
- 21.Papa S, Sardanelli AM, Cocco T, Speranza F, Scacco SC, Technikova-Dobrova Z. The nuclear-encoded 18 kDa (IP) AQDQ subunit of bovine heart complex I is phosphorylated by the mitochondrial cAMP-dependent protein kinase. FEBS Lett. 1996;379:299–301. doi: 10.1016/0014-5793(95)01532-9. [DOI] [PubMed] [Google Scholar]
- 22.Chen R, Fearnley IM, Peak-Chew SY, Walker JE. The phosphorylation of subunits of complex I from bovine heart mitochondria. J Biol Chem. 279:26036–26045. doi: 10.1074/jbc.M402710200. [DOI] [PubMed] [Google Scholar]
- 23.Chen CL, Zhang L, Yeh A, Chen CA, Green-Church KD, Zweir JL, Chen YR. Site-specific S-glutathiolation of mitochondrial NADH ubiquinone reductase. Biochemistry. 2007;46:5754–5765. doi: 10.1021/bi602580c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vinogradov AD, Grivennikova VG. The mitochondrial complex I: progress in understanding of catalytic properties. IUBMB Life. 2001;52:129–134. doi: 10.1080/15216540152845920. [DOI] [PubMed] [Google Scholar]
- 26.Maklashina E, Kotlyar AB, Cecchini G. Active/de-active transition of respiratory complex I in bacteria, fungi, and animals. Biochim Biophys Acta. 2003;1606:95–103. doi: 10.1016/s0005-2728(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 27.Maklashina EO, Sled’ VD, Vinogradov AD. Hysteresis behavior of complex I from bovine heart mitochondria: kinetic and thermodynamic parameters of retarded reverse transition from the inactive to active state. Biokhimiia (USSR) 1994;59:946–957. [PubMed] [Google Scholar]
- 28.Kotlyar AB, Vinogradov AD. Slow active/inactive transition of the mitochondrial NADH-ubiquinone reductase. Biochim Biophys Acta. 1990;1019:151–158. doi: 10.1016/0005-2728(90)90137-s. [DOI] [PubMed] [Google Scholar]
- 29.Grivennikova VG, Kapustin AN, Vinogradov AD. Catalytic activity of NADH-ubiquinone oxidoreductase (complex I) in intact mitochondria. Evidence for the slow active/inactive transition. J Biol Chem. 2001;276:9038–9044. doi: 10.1074/jbc.M009661200. [DOI] [PubMed] [Google Scholar]
- 30.Maklashina E, Sher Y, Zhou HZ, Gray MO, Karliner JS, Cecchini G. Effect of anoxia/reperfusion on the reversible active/de-active transition of NADH-ubiquinone oxidoreductase (complex I) in rat heart. Biochim Biophys Acta. 2002;1556:6–12. doi: 10.1016/s0005-2728(02)00280-3. [DOI] [PubMed] [Google Scholar]
- 31.Loskovich MV, Grivennikova VG, Cecchini G, Vinogradov AD. Inhibitory effect of palmitate on the mitochondrial NADH:ubiquinone oxidoreductase (complex I) as related to the active-de-active enzyme transition. Biochem J. 2005;387:677–683. doi: 10.1042/BJ20041703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burbaev DSh, Moroz IA, Kotlyar AB, Sled VD, Vinogradov AD. Ubisemiquinone in the NADH-ubiquinone reductase region of the mitochondrial respiratory chain. FEBS Lett. 1989;254:47–51. [Google Scholar]
- 33.Hatefi Y. Preparation and properties of NADH: ubiquinone oxidoreductase (complex I), EC 1.6.5.3. Meth Enzymol. 1978;53:11–14. doi: 10.1016/s0076-6879(78)53006-1. [DOI] [PubMed] [Google Scholar]
- 34.Galante YM, Hatefi Y. Resolution of complex I and isolation of NADH dehydrogenase and an iron-sulfur protein. Meth Enzymol. 1978;53:15–21. doi: 10.1016/s0076-6879(78)53007-3. [DOI] [PubMed] [Google Scholar]
- 35.Zvyagilskaya R, Andreishcheva E, Soares MI, Khozin I, Berhe A, Persson BL. Isolation and characterization of a novel leaf-inhabiting osmo-, salt-, and alkali-tolerant Yarrowia lipolytica yeast strain. J Basic Microbiol. 2001;41:289–303. doi: 10.1002/1521-4028(200110)41:5<289::AID-JOBM289>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 36.Michaelis L, Granick S. The polymerization of the free radicals of the Wurster Dye Type: the dimeric resonance bond. J Amer Chem Soc. 1943;65:1747–1755. [Google Scholar]
- 37.Dooijewaard G, Slater EC. Steady-state kinetics of high molecular weight (type-I) NADH dehydrogenase. Biochim Biophys Acta. 1976;440:1–15. doi: 10.1016/0005-2728(76)90109-2. [DOI] [PubMed] [Google Scholar]
- 38.Sled VD, Vinogradov AD. Kinetics of the mitochondrial NADH-ubiquinone oxidoreductase interaction with hexammineruthenium(III) Biochim Biophys Acta. 1993;1141:262–268. doi: 10.1016/0005-2728(93)90051-g. [DOI] [PubMed] [Google Scholar]
- 39.Grivennikova VG, Kotlyar AB, Karliner JS, Cecchini G, Vinogradov AD. Redox-dependent change of nucleotide affinity to the active site of the mammalian complex I. Biochemistry. 2007;46:10971–10978. doi: 10.1021/bi7009822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zakharova NV. Kinetics of the transhydrogenase reaction catalyzed by mitochondrial NADH:ubiquinone oxidoreductase (complex I) Biochemistry (Mosc) 2002;67:651–661. doi: 10.1023/a:1016194120930. [DOI] [PubMed] [Google Scholar]
- 41.Yakovlev G, Hirst J. Transhydrogenation reactions catalyzed by mitochondrial NADH-ubiquinone oxidoreductase (Complex I) Biochemistry. 2007;46:14250–14258. doi: 10.1021/bi7017915. [DOI] [PubMed] [Google Scholar]
- 42.Grivennikova VG, Vinogradov AD. Generation of superoxide by the mitochondrial Complex I. Biochim Biophys Acta. 2006;1757:553–561. doi: 10.1016/j.bbabio.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 43.Zharova TV, Vinogradov AD. A competitive inhibition of the mitochondrial NADH-ubiquinone oxidoreductase (Complex I) by ADP-ribose. Biochim Biophys Acta. 1997;1320:256–264. doi: 10.1016/s0005-2728(97)00029-7. [DOI] [PubMed] [Google Scholar]
- 44.Avraam R, Kotlyar AB. Inhibition of NADH-dehydrogenase by low concentrations of NAD+ Biokhimiia (USSR) 1991;56:2253–2260. [PubMed] [Google Scholar]
- 45.Yamaguchi M, Belogrudov GI, Matsuno-Yagi A, Hatefi Y. The multiple nicotinamide nucleotide-binding subunits of bovine heart mitochondrial NADH:ubiquinone oxidoreductase (complex I) Eur J Biochem. 2000;267:329–336. doi: 10.1046/j.1432-1327.2000.00999.x. [DOI] [PubMed] [Google Scholar]
- 46.Vinogradov AD. NADH/NAD+ interaction with NADH: ubiquinone oxidoreductase (complex I) Biochim Biophys Acta. 2008;1777:729–734. doi: 10.1016/j.bbabio.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zwicker K, Galkin A, Dröse S, Grgic L, Kerscher S, Brandt U. The Redox-Bohr group associated with iron-sulfur cluster N2 of complex I. J Biol Chem. 2006;281:23013–23017. doi: 10.1074/jbc.M603442200. [DOI] [PubMed] [Google Scholar]
- 48.Arnold S, Kadenbach B. Cell respiration is controlled by ATP, an allosteric inhibitor of cytochrome c oxidase. Eur J Biochem. 1997;249:350–354. doi: 10.1111/j.1432-1033.1997.t01-1-00350.x. [DOI] [PubMed] [Google Scholar]