Abstract
Microinjections are a major tool in modern neuroscience. Microinjection techniques in conscious animals typically involve four steps: 1) animal adapts to experimental setup; 2) injection system is filled and the microinjector is carefully inserted; 3) a drug solution is injected; 4) one to two minutes later the microinjector is carefully removed. Steps 2 and 4 are difficult to perform in rodents without disturbing the animal. This disruption can cause stress and accompanying tachycardia and hyperthermia - unwanted artifacts in physiological research. To reduce these effects, we altered the traditional approach. Our procedure of microinjection consisted of the following steps: 1) We filled the injection setup and fixed the microinjector in its guide cannula; 2) allowed an animal to adapt to the setup; 3) performed an experiment including microinjection(s); 4) removed the microinjector after the experiment was complete. The key change we incorporated was a one meter long piece of tubing with a small internal diameter; it allowed us to inject nanoliter volumes through the injector which had been placed into the guide cannula in advance. This way we avoided the usual manipulations related to microinjection, and minimized extraneous disturbances to the rat. In this report we describe the details of this technique in conscious rats and provide examples of the effects and the reproducibility of a 100 nL drug injection on cardiovascular function.
Keywords: Microinjection, Stress-free, Conscious rat, Animal surgery
1. Introduction
The topical application of drugs to specific brain areas in non-anaesthetized animals is an experimental technique dating back almost a hundred years. Since the initial study in 1915 by Hashimoto (Hashimoto et al., 1915) in which 200 µl volumes were injected intracerebrally in rabbits, this approach has been refined and modified in many ways (Greenshaw, 1998). For instance, smaller animals such as rats, which are less expensive and easier to standardize, are now widely used. In addition, the use of small injectors sizes and volumes has allowed a greater degree of anatomical resolution. In this regard, microinjection with glass pipettes using air-pressure puffs to deliver accurate and reproducible volumes in the nanoliter range (Amaral and Price, 1983) constituted a technical revolution. Despite limited success (Azami et al., 1980), due to the extreme fragility of glass pipettes this approach is impractical in conscious animals. Microinjections using metal or polymer needles which target the brain area of interest through guide cannulas implanted in advance, and using injection volumes in the range of 50 to 100 nL are most common in current rat studies.
The current paradigm in microinjection generally includes four steps with variations. First, a guide cannula is cemented to the rat’s skull. During the healing period, the rat is handled by testers and habituated to the conditions. Second, on the testing day after the animal is acclimated to experimental conditions, a microinjector is carefully inserted into a guide cannula with the intent of creating as little disturbance to the animal as possible. Third, immediately after the placement of the microinjector, the drug or solution of interest is injected, typically over 30 to 60 s. Fourth, approximately 1–2 min after the injections, the microinjector is carefully removed, bothering the animal again.
One of problems with microinjections in rodents is that the placement of the injectors typically arouses the animals and causes stress, shown by an increased heart rate (HR) and activity. These effects can mask changes from the injected drugs. Given this problem, there is a need for a reliable and reproducible method by which small volumes of drugs can be injected, which does not disturb conscious animals, so that physiological parameters can be measured shortly after the injection is made.
In our method we inserted the microinjectors, and then allowed the animals to acclimate for 1–2 h; this permitted injection of the drug or solution of interest without evoking stress. This eliminated often seen artifacts which can mask immediate effects of whatever is injected. In addition, we found that leaving the injector in place until the end of the experiment likewise reduced animal stress.
The purpose of this manuscript is to both describe in detail our microinjection technique, and to provide evidence supporting its reliability and reproducibility.
2. Materials
Hardware
The Dataquest telemetry system (Data Sciences Int., St. Paul, MN) was used for measurements of arterial blood pressure, HR, and locomotor activity. Telemetric probes were sterilized according to manufacturer’s recommendations before implantation.
Guide cannulas for acute experiments (#C315GA; 26 ga; ID=0.24 mm; OD=0.46 mm; length 10 mm below pedestal; Plastics One, Roanoke, VA) were used with corresponding single internal cannulas (#C315A; Plastics One; 33 ga, ID=0.1 mm; OD=0.2 mm). The internal cannula had been made with long enough to allow the tip of the injector to protrude 1 mm below the tip of guide cannula. Dummy cannulas (#C315DC; OD=0.2 mm; Plastics One) were cut to the exact length of the guide cannula. In experiments which involved chronic guide cannulas (#C315G, same specifications as #C315GA), we used the corresponding injectors (#C315I). Guide cannulas were fixed to the skull with metal screws (#0–80, Plastics One), veterinary glue (Vetbond, 3M, St.Paul, MN) and fast-curing cranioplastic cement (Jet Denture Repair, Lang Dental Manufacturing Co, Wheeling, IL).
Arterial and venous lines were prepared from microbore polyvinylchloride (PVC, Tygon) tubing (Y-TGY-020; ID=0.5 mm; OD=1.5 mm; Small Parts Inc., Miami Lakes, FL) and Teflon tubing (Y-SWTT-28; 28 ga; ID=0.38 mm; OD=0.83 mm; Small Parts). One end of a 22 cm piece of Tygon tubing was soaked in acetone for 10 min, and then a segment of Teflon tubing was inserted 5 mm into the temporarily expanded end of the Tygon tubing. This joint was allowed to dry for at least 30–60 min and cut with a sharp blade to leave 5.5 cm long Teflon leader which was later inserted into the blood vessel. No glue was required. Prepared catheters were sterilized for at least 2 h by UV light before being placed in the animal (3.2).
Microinjection setup
Microinjections were performed through commercially available microinjectors (Plastics One). The microinjector was connected to a 10 µl syringe using thick-wall, low-internal volume Teflon tubing (FEB tubing; ID=0.12 mm; OD=0.6 mm; internal volume=12 µl/meter) from CMA Microdialysis (North Chelmsford, MA). Connectors were cut from the same microbore PVC (Tygon) tubing, which was used to manufacture intravascular catheters. Polyetheretherketone (PEEK) tubing (ID=0.12 mm; OD=0.5 mm; Upchurch Scientific, Oak Harbor, WA) could have been used for solution delivery, but due to smaller outer diameter we found it difficult to provide tight connections between components.
To affix the injector to the guide cannula, a custom cap with a 0.5–1 mm hole at the top was fashioned from a dust cap (#C303DC/1) or from a used dummy cannula (#C315DC, wire was removed during drilling). A bigger dust cap (#C303DC) could also be used.
Syringes (10 µl, 1800 series, Hamilton Company, Reno, Nevada) with a 22S needle (point style No.3) were mounted on a syringe pump appropriate for reliable delivery of 100 nL (KD Scientific, Model 200, Holliston, MA).
3. Methods
3.1. Animals
Male Sprague-Dawley rats (280–300 g) (Harlan, Indianapolis, IN) were maintained under standard animal housing conditions, including a 12h-light (lights on 07:00am), 12-h dark illumination cycle. Animals were housed singly. Care and use of rats was in accordance with protocols approved by the Indiana University Animal Care and Use Committee and was carried out under the supervision of veterinarians. Rats were housed in the Indiana University Laboratory Animal Resource Center, an Association for Assessment and Accreditation of Laboratory Animal Care-approved facility, following IACUC guidelines. Animal experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23, revised 1996). All procedures were performed using antiseptic and aseptic techniques. For surgical procedures rats were anesthetized with Nembutal (50 mg/kg, i.p.; Abbott Laboratories, North Chicago, IL) or a Ketamine/Xylazine mixture (80 mg/kg Ketamine, Hospira Inc, Lake Forest, IL and 11.5 mg/kg Xylazine, Lloyd Inc., Shenandoah, IA, i.p.). After surgery, animals received injections of buprenorphine (15 ug/kg s.c., Hospira) and were monitored until they recovered from anesthesia. All animals for which data was reported in this manuscript remained in good health throughout the surgical procedures and experimental protocols as assessed by appearance, behavior, and maintenance of body weight.
3.2. Telemetric probe implantation
Telemetric probe for recording of HR, mean blood pressure (MBP) and locomotor activity were implanted intraabdominally using manufacturer’s instructions.
3.3. Guide cannula implantation
At least seven days after implantation of the telemetric transmitter, the rats underwent cranial guide cannula placement. Rats were anesthetized as above and placed in a stereotaxic apparatus with the incisor bar set 5 mm above the interaural line. Skin overlying the dorsal surface of the skull was infiltrated with Lidocaine HCl/Epinephrine (2%/1:100,000; Hospira), then cut and retracted. Soft tissue was removed to expose the surface of the skull. This area was then washed using cotton-tipped applicators saturated with a 30% hydrogen peroxide solution. In addition to reducing bleeding and aiding in the maintenance of sterility, hydrogen peroxide enhances the visibility of sutures used as stereotaxic landmarks.
Stainless steel guide cannulas were placed unilaterally to allow microinjections targeting the dorsomedial hypothalamus (DMH) as described previously (Bailey and Dimicco, 2001). Disinhibition of the DMH produces dramatic cardiovascular, neurohumoral and behavioral responses (Bailey and Dimicco, 2001; Zaretskaia et al., 2002). As neurons in this region are extremely sensitive to the placement of microinjectors and to injections of solutions, it serves as an excellent model to validate our technique. Stereotaxic coordinates for cannula placement, using bregma as the reference point and a 10° angle from the sagittal plane were 1.2 mm posterior, 2.1 mm lateral and 9.1 mm ventral.
A small hole in the skull to insert a cannula was made with a rotary tool (MiniMite Cordless 4.8V, Dremel, Racine, WI) equipped with a surgical carbide burr (DHP557, Miltex, Plainsboro, NJ). Three jeweler’s screws (size 80) were placed into the skull near the hole to guarantee the reliable attachment of cement cap. After positioning of the guide cannula, the exposed skull and most of the wound surface was covered with veterinary cyanoacrylate glue, followed immediately by cranioplastic cement. The glue affixed the assembly firmly to the skull without the need for the bone to be thoroughly dried. The glue also protected living soft tissues from direct contact with the cranioplastic cement solvent and stopped minor bleeding. With the guide cannula cemented to the rat’s skull a dummy wire cannula was inserted, and the rat was returned to its home cage for recovery for at least five days.
3.4. Intra-arterial catheter implantation
When necessary, the last surgical procedure on the rats, the implantation of intra-arterial catheters, was carried out as previously described (Zaretsky et al., 2006). The catheters were stabilized at the nape of the neck with a small leather harness. Catheters were flushed immediately after surgery with sterile saline without heparin, then every other day with 0.2–0.3 ml of sterile heparinized saline starting the first day after surgery. This maintained patency for up to 3–4 weeks.
3.5. Microinjection system
A schematic of the microinjection setup is shown in Fig.1. Routinely we delivered picomolar amounts of drug in a 100 nL volume. A 10 µl Hamilton air-tight syringe was used both to fill the system and to perform the microinjection(s).
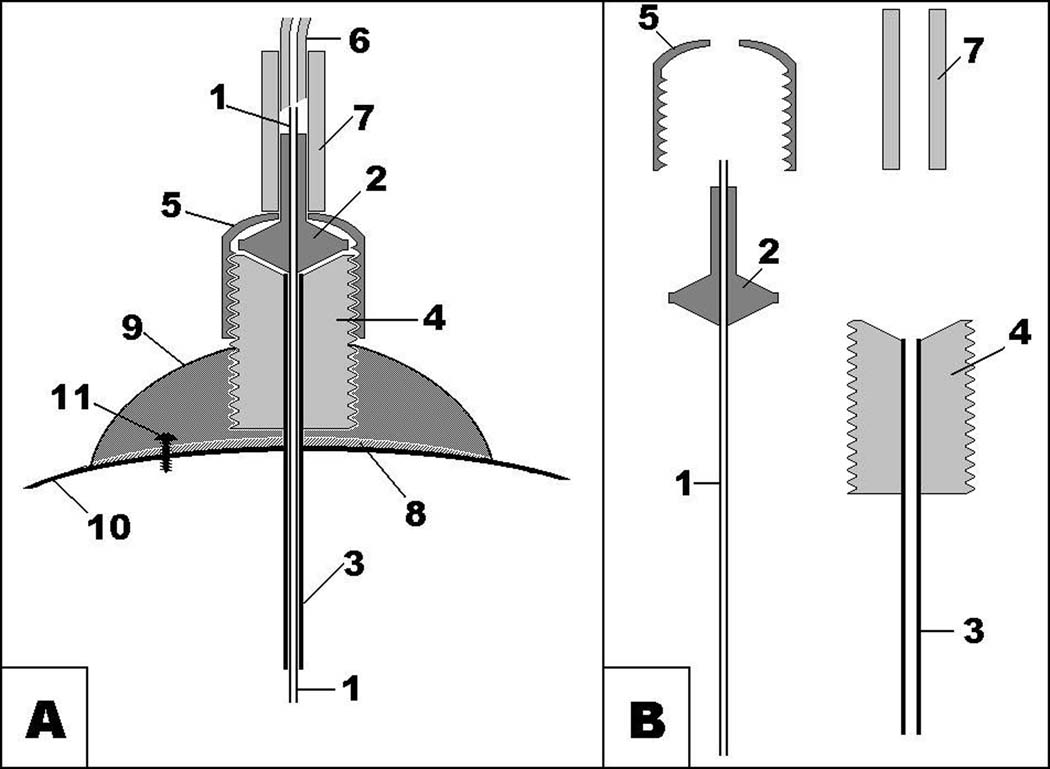
Fig. 1.
- metal tubing of microinjector;
- plastic part of microinjector;
- metal tubing of guide cannula;
- plastic pedestal of guide cannula with thread;
- plastic cup with thread matching thread of guide cannula;
- thick-wall low-volume Teflon tubing;
- PVC connector;
- cyanoacrylate;
- dental cement;
- skull bone;
- jeweler’s screw.
First, to prepare the setup, a microinjection assembly was made: the injector was inserted into a free guide cannula, and affixed to it by the dummy cap with a drilled hole. The guide cannula held the injector protruding through the hole in the cap to allow the connection of the tubing later.
Then, the Hamilton syringe was carefully filled with the injectate excluding any air bubbles. A small drop at needle tip helped to avoid air bubbles at the connection between the tubing connector (10 mm piece of microbore PVC tubing) and needle. Because refilling is required, the syringe needle was inserted into the first connector no more than 1 mm. Then this first connector was filled with liquid until a small drop of solution appeared at the free end.
Next, a long piece of thick-walled Teflon tubing (1m) was beveled slightly to facilitate insertion into the above mentioned PVC connector. The beveled part of the tubing was inserted into a drop of the solution at the tip of connector and was pushed 2–3 mm into connector. This Teflon tube was slowly filled with solution. The internal volume of 1 m of Teflon tubing is 12 µl, so the syringe was refilled once during this process. The small air-filled space remaining after disconnection at the tip of the connector was refilled by squeezing the connector and then releasing pressure while the solution was added from the syringe.
Finally, another 10 mm piece of microbore PVC tubing was connected to the distal, also beveled end side of Teflon tubing. This connector was cut to allow the insertion of the plastic part of the injector into the injector assembly so that the tip is pressed against the Teflon tubing, keeping the dead space minimal. Since the flow of the injectate can be blocked if the opening of the injector gets up against the Teflon wall, it was necessary to check the patency of the connection at this time.
With the tubing connected to the injector with the cap on it, the guide cannula was now safely removed from the microinjector assembly. Then, the needle of the syringe was pushed inside the first PVC connector until the needle touched the Teflon tubing. Usually, there were no sealing problems, because the needle has a wide opening compared to the injector. The syringe was mounted on an infusion pump set at the appropriate flow rate (for 100 nL injections we used the rate of 0.2 µl/min).
We have always prepared the microinjection system just prior to each experiment, with drug stability in mind.
3.6 Connection of microinjector to animal
To ensure that it was functioning properly, the pump was turned ON and OFF a few times. The flow of injectate, which was always checked by a trace on hydrophilic matte surface, should follow the activity of the pump. The disappearance of flow, when the pump was turned off, was equally important as the appearance of flow when the pump was activated. If flow continued with the pump off, before proceeding we replaced the worn Teflon-tipped plunger of the air-tight Hamilton syringe and reassembled the microinjection setup.
To attach the injector to an animal, the dummy cannula was removed from the permanently implanted guide cannula with the pump switched off. The injector was gently advanced until the cap touched the guide cannula, then the cap was rotated until it was fixed to the guide cannula. Since the injector was not affixed to the cap, it did not, typically, rotate together with cap, and did not twist the injection line.
Once the injector was inserted, it was left in place until any microinjections were made. Thus, ample time was allowed for HR, blood pressure, and activity to return to baseline before any microinjection. The injection system (Fig. 1) allowed undisturbed spontaneous activity of the rat (even “wet dog shakes”) without dislodging the injector.
This microinjection setup does not allow for experiments which involve rotations of the animal, such as stereotypy. During the day, nocturnal animals such as rats are not prone to such complications, so in the experiments described here the animals remained in their home cages. However, in experiments in which animal rotation is known to occur, such as in our recent studies of amphetamines, we routinely used Raturn (BASi, West Lafayette, IN) to prevent twisting of lines.
3.7. Microinjection
After the animal has achieved a stable baseline value for all the physiological parameters being monitored, the pump was turned on. In most experiments we injected 100 nL over 30 sec using the rate of 0.2 µl/min.
The microinjector was allowed to stay in place until the end of the experiment. We used two methods to verify delivery. First, after disconnecting the animal at the end of the experiment, the flow was always checked by turning back on the infusion pump and observing the reappearance of flow. With an injection time of 30 s, reappearance of flow in 5 s was considered satisfactory. Clogging of the injector is uncommon if setup is carefully prepared. Second, the microspheres were added to microinjectate, so we could visualize the site of actual microinjection in post-mortem slices of brain (Katz et al., 1984; Samuels et al., 2004). In fact, availability of microspheres of different colors allows us to distinguish sites of multiple microinjections in the same animal (Rusyniak et al., 2007).
3.8. Collection of data in the described experiments
All microinjection experiments were performed between 10 am and 2 pm to avoid circadian variability of physiological parameters. Hemodynamic data were sampled for 10 s every 30 s except during circadian studies in which the data were averaged for 10 s every 60 s. Only the first experiment involved blood sampling. An arterial catheter had been implanted in these nine animals. We slowly withdrew small blood samples (0.35 ml) through PE-50 extension tubing, which had been connected to the arterial line by a metal connector (10 mm piece of 23 ga stainless steel tubing) in advance. The withdrawn blood volume was replaced with sterile saline. When done appropriately, this procedure did not induce any behavioral or autonomic responses.
Blood samples were collected in EDTA/aprotinin-containing syringes, transferred to plastic tubes, and immediately spun down at 4°C at 12,100 g for 30 sec. The collected plasma was stored at −80°C until assayed for adrenocorticotropic hormone (ACTH) using a radioimmunoassay (Li et al., 1993).
3.9. Protocols of the study
In all the experiments described below, the guide cannulas were positioned to target an area of the dorsomedial hypothalamus (DMH) which is known to be sensitive to GABAA antagonists and excitatory aminoacids (DiMicco et al., 2006). The drug we chose for microinjections in these experiments was bicuculline methiodide (BMI) from Tocris Bioscience, Ellisville, MO, a recognized competitive antagonist of GABAA receptors which causes tachycardia immediately when injected directly into the DMH (Bailey and Dimicco, 2001; Zaretskaia et al., 2002).
Experiment 1 [baseline values of HR, MBP, locomotor activity and plasma ACTH obtained before experiments employed microinjection of 10 pmol BMI into the area of DMH]
Seven days after implantation of telemetric probes, the home cages of animals (N=9) were placed on telemetry receivers and animals were left undisturbed overnight. Physiological parameters (HR, MBP and locomotor activity) were recorded and averaged for the period from 10 am to 2 pm. After the recording period, the animals were surgically implanted with guide cannulas targeting the DMH and arterial lines were placed. Seven days later, BMI (10 pmol in 100 nL of phosphate buffered saline (PBS)) was injected through the microinjection system. Baseline values for these experiments were calculated as an average of the 15 min immediately preceding the injection; blood samples were collected 10 min before the microinjection through the extension without handling the animal as described in the previous section.
Experiment 2 (manipulation effect)
Four animals were implanted with telemetric probes and guide cannulas targeting the hypothalamus. Each animal was microinjected with 100 nL of PBS twice on sequential days: one day microinjector was inserted immediately before and removed 60 sec after the microinjection, and on the other day the microinjector was inserted at least 1h before the microinjection and not removed before the end of experiment. Injections were performed in random order.
Experiment 3 (reproducibility)
Three animals were implanted with telemetric probes and guide cannulas targeting the hypothalamus. Two microinjections of 10 pmol of BMI in 100 nL of PBS, using our improved procedure, were performed in these rats to show the reproducibility of the described techniques. The rats designated A and B were injected on two occasions separated by 4 days, rat C received two microinjections separated by 3 h.
Experiment 4 (minimal volume)
To demonstrate the ability to reproducibly microinject volumes as low as 20 nL, we performed microinjections, using our improved procedure, of BMI (10 pmol in 20 nL) in animals with telemetric transmitters and guide cannula targeting the DMH.
3.10. Statistical analysis
The results are presented in the text as the Mean ± S.D. Results were compared using a one way ANOVA with repeated measures. A value of P<0.05 was considered to indicate a significant difference in all comparisons.
4. Results
4.1. Baseline values of HR, MBP, and locomotor activity in the microinjection experiments (Experiment 1)
The baseline values of HR, MBP and locomotor activity for animals acclimated to the placement of microinjectors were similar to those of undisturbed animals in their home cages (Table 1). ACTH levels in blood samples withdrawn before microinjection were typical for baseline values and considerably less than levels observed after microinjection of BMI into the DMH (Zaretskaia et al., 2002).
Table 1.
Cardiovascular, neurohumoral and behavioral parameters of rats in undisturbed conditions or prepared for microinjection (baseline)
| Undisturbed control |
Microinjection Baseline |
|
|---|---|---|
| Heart rate, beats/min | 367 ± 6 | 364 ± 10 |
| Locomotor activity, units | 9 ± 2 | 16 ± 12 |
| Mean blood pressure, mm Hg | 109 ± 2 | 115 ± 4 |
| Blood plasma ACTH, pg/ml | – | 69 ± 12 |
Data in columns:
Undisturbed control –telemetric data from undisturbed animals with only telemetric probes were collected between 10am and 2pm and averaged for entire interval.
Microinjection Baseline – telemetric data from same animals, which had telemetric probe, unilateral guide cannula targeting the DMH and arterial catheter, were collected and averaged for 15 min immediately preceding microinjection of 10 pmol of BMI in 100 nL of PBS into the DMH using described technique. Blood sample was collected 10 min before microinjection.
4.2. Comparison of responses to microinjection of PBS performed by “traditional” and by new described method (Experiment 2)
There was no difference in baseline values of HR, MBP, and locomotor activity between the animals which had an installed injector, and the animals which had been acclimated, but did not have any connections. These data confirm the results of Experiment 1: our technique allows rats to reach physiological baselines typical for undisturbed animals.
When microinjection of PBS was performed traditional way (injector was inserted into guide cannula immediately before microinjection), typical signs of stress response were observed: tachycardia, mild hypertension, and locomotion. In contrast, there was no discernible response to the delivery of PBS using our newly described technique which does not involve handling of the rat (Fig. 2)
Fig. 2.
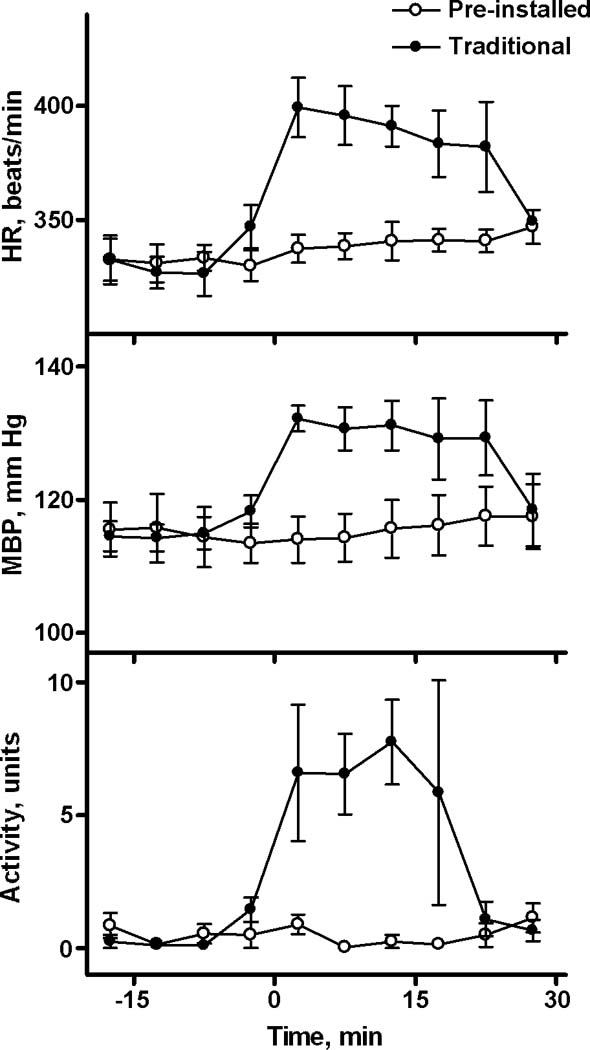
A comparison of responses to the manipulation of microinjection performed in traditional and new described way. Two microinjections of 100 nL of PBS were performed in a group of rats (N=4) using an injector which was inserted immediately before microinjections and removed 60 sec after microinjection (traditional way) or was pre-installed (described in this manuscript) at least 1h before microinjection. The effect of Technique was statistically significant for HR (F(1,3)=12.0, p<0.05) and Locomotor Activity (F(1,3)=19.3, p<0.05). Interaction of Technique and Time was also significant for both HR and Activity. For MBP only interaction between Technique and Time reached the level of significance (F(9,27)=5.7, p<0.001).
4.3. Reproducibility of microinjections (Experiment 3)
Fig. 3 contains the one-minute means for HR, locomotor activity and MBP in three separate animals (presented by A, B, C) before and after microinjection of 10 pmol BMI in 100 nL of PBS. Figs. 3A and 3B show the results of experiments performed on rats on different days (with 4 days between). The animal in Fig. 3C underwent two experiments the same day, 3 h apart. In all three recordings shown in Fig 3 we observed similar baselines of HR, MBP; locomotor activity was minimal before injections. Responses to microinjections of 10 pmol BMI into the targeted area of the DMH were reproducible.
Fig. 3.
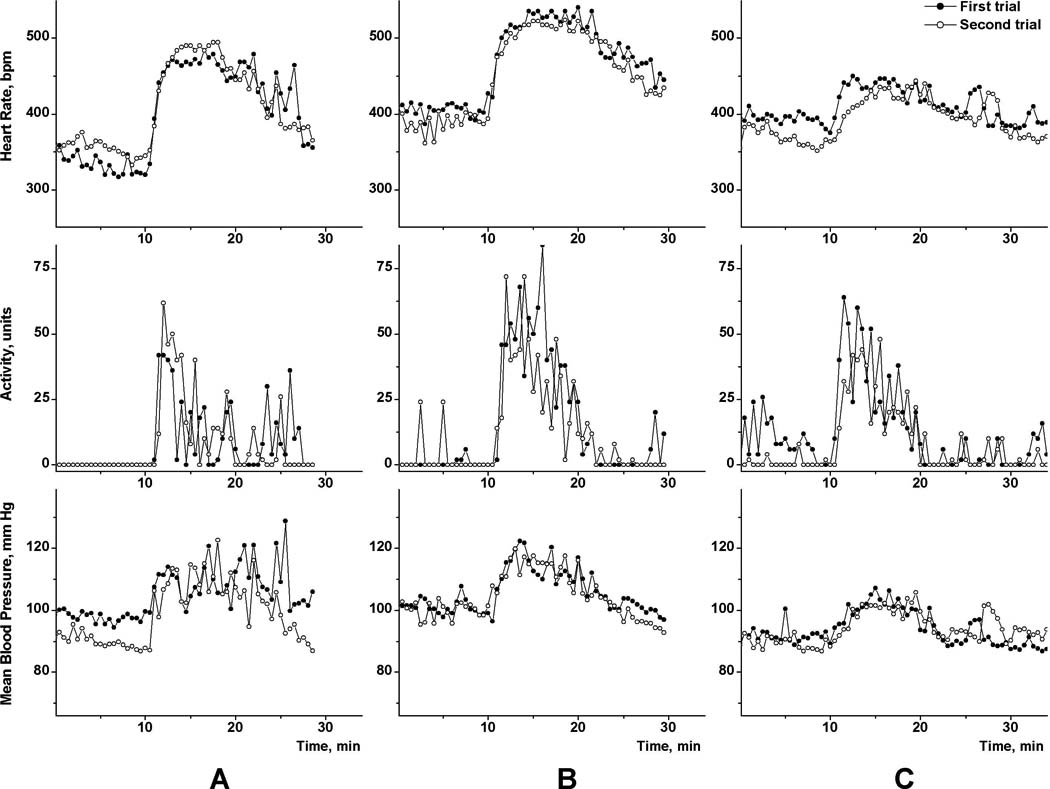
The reproducibility of physiological responses (HR, locomotor activity, and MBP) to microinjections of BMI into the DMH. Responses to two consecutive injections of 10 pmol BMI in 100 nL of PBS, which were performed at 10 min, are shown. Rats A, B received two microinjections separated by four days, rat C - separated by 3 h.
4.4. Microinjection of 20 nL volume (Experiment 4)
To confirm that 100 nL is not the lower limit of this microinjection technique, we performed microinjection of 10 pmol of BMI in 20 nL of PBS. The telemetric recording of HR is shown in Fig.4. Fixation of the injector in this animal produced locomotor activation accompanied by tachycardia. It took about 10 min for HR to return to baseline values. The BMI injection targeted the DMH and evoked the tachycardia which is typical for this site and dose (DiMicco et al., 2006; DiMicco and Zaretsky, 2007). A second injection 35 min later resulted in a very similar response in both magnitude and length (Fig. 4).
Fig. 4.
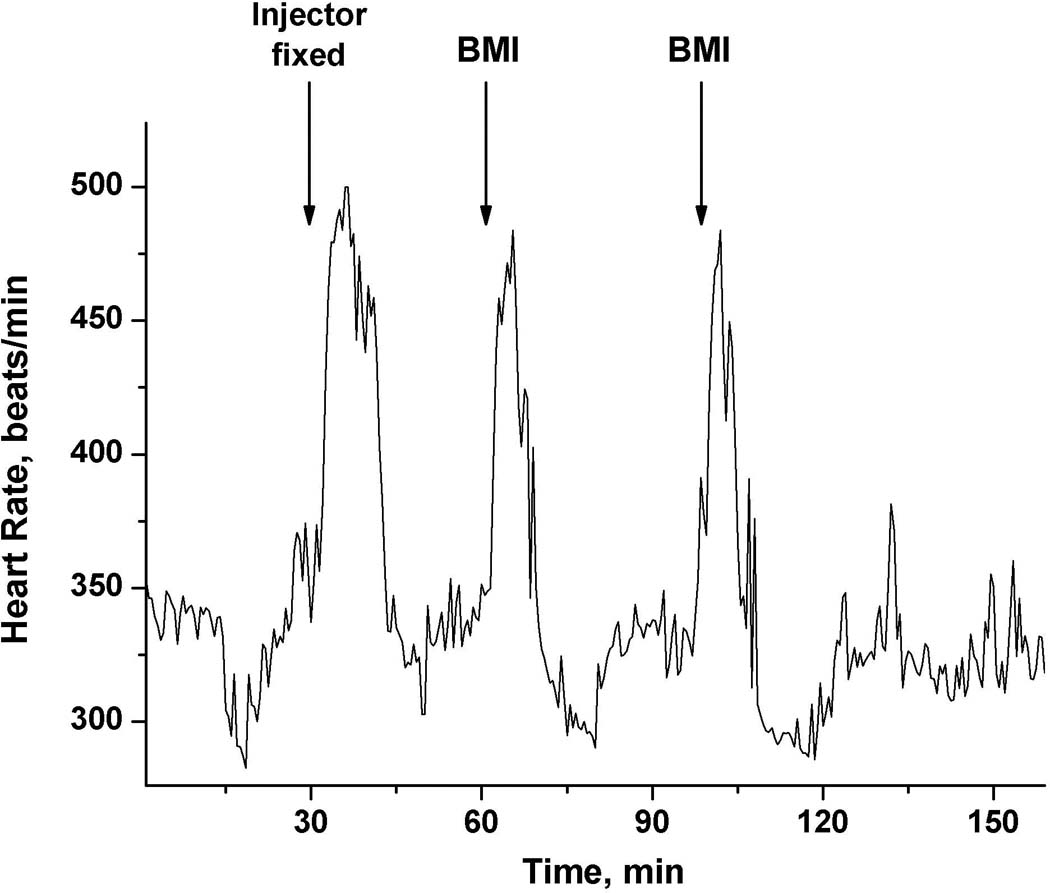
Tachycardic response to a manipulation required to fix the microinjector in the guide cannula and to two consecutive microinjections into the DMH of 10 pmol of BMI in 20 nL of PBS.
5. Discussion
In most current microinjection techniques in conscious rats, the microinjection is performed immediately after insertion of the injector into a guide cannula, and the injector is removed as soon as possible after the microinjection is finished, typically not more than 1 min after injection. This manipulation alone can evoke a response like the one shown in Fig.4. Unfortunately, this could mask effects of the microinjected drug. In this study we proposed a new paradigm of the microinjection technique. The key advantage of our described technique is minimizing any manipulations related to microinjections, with no need to acclimate rats to the microinjection procedures and extra handling.
Our technique consists of four steps: 1) The microinjection setup was prepared, and the microinjector was fixed to the guide cannula; 2) an animal was allowed to adapt to experimental setup, with no extra human interaction required; 3) a complete experiment was performed according to a protocol which included microinjection(s); 4) the microinjector was removed after the end of experiment, and the patency of microinjection setup was verified. The success of the microinjection was confirmed by presence of the co-injected marker, such as fluorescent microspheres in post-mortem brain sections.
This technique has been successfully used in several studies published in peer-reviewed journals (Zaretskaia et al., 2008; Zaretskaia et al., 2002; Zaretsky et al., 2006; Zaretsky et al., 2003a; Zaretsky et al., 2003b), but we have not previously been able to describe each step in a finely detailed manner. The major reason for developing this new technique was to decrease the artifacts of manipulation. However, there is a variety of applications of our technique, which opens new avenues for research. For example, it allows researchers to perform microinjections in “free moving” rats while the animal is sleeping. Since most of our experiments were performed during the light phase of circadian cycle, we frequently microinjected while a rat was asleep. When saline was injected, usually rats continued to sleep, which is virtually impossible when injector is inserted immediately before and removed just after microinjection.
5.1. Animal preparation
To monitor and record MBP and HR we have used telemetry, which provided a noninvasive approach during the experiment. Moreover, it even permitted us to continue recording BP during the withdrawal of arterial blood samples. In most experimental designs an arterial catheter can be used for intravascular administration of substances. In our described experiments, we implanted a telemetric probe, guide cannula(s) and an arterial catheter in three sequential surgeries divided by at least 5 days for recovery. Rats quickly recovered from each surgery and continued to stay healthy in appearance and behavior, and demonstrated weight gain. The total time required for animal preparation is about 2 weeks including recovery periods.
The length of a study is usually limited by the number of microinjections performed through each guide cannula. To avoid excessive damage around injector tip, we limited the number of injections to four per cannula. The experimental preparation was reliable for at least 1–2 weeks, which allowed 2–3 days between trials.
Long-term experiments required very reliable fixation of catheters and cannulas. A free end of the catheter was affixed to a leather harness or saddle to prevent it from being chewed by the animal. If flushed regularly, this Teflon-tipped Tygon catheter guarantees a few weeks of service without clogging. In turn, a combination of metal or nylon screws, biological glue, and the cement cap made external fixation of the guide cannula virtually permanent.
5.2. Microinjection setup
Most of the problems with performing microinjections arise from quick movements of the head and from the unidirectional circling of a freely moving animal in the limited space of experimental cage. Therefore, the optimal setup must accommodate both of these movements. To accomplish this we employed a flexible tubing with a very low internal volume, but with the strength to resist the intense locomotor activity of an animal. Also, our design has no flexible high-diameter junctions, which can “push-pull” injectate when bent.
We switched from polyethylene (PE) and polyvinyl chloride (PVC) tubing to thick-wall low-volume Teflon tubing. This tubing was originally designed for microdialysis applications, but we found it highly useful for microinjections. The internal diameter of this tubing (ID=0.12 mm) is three times less than the traditionally used PE-20 (ID=0.38 mm) and less than half that of PE-10 (ID=0.28 mm). Therefore, the dead volume of Teflon tubing is approximately 1/10 that of PE-20 of the same length and 1/5 that of PE-10.
The other possible tubing material is PEEK which is even more resistant to twisting or tangling. However, the smaller outer diameter of commercially available PEEK tubing does not match the diameter of microbore PVC connectors, so we used Teflon.
The total volume required to fill our microinjection setup with 1m-long connection is less than 30ul. We found that slow filling of the system with a 10 µl Hamilton syringe is essential, because it minimizes the possibility of introducing air bubbles. The low internal volume of the tubing which connected the syringe with the injector was also significant because it allowed us to fill the complete system with the same solution, which avoided the use of mineral oil or backfilling. Degassing of microinjectate was not needed.
The fixation of the injector on the guide cannula was a procedure which required insertion of 33 ga (OD=0.2 mm) tubing into 26 ga tubing (ID=0.24 mm). When an animal sees an approaching experimenter’s hand, it frequently makes very quick avoidance movements. The injector is most vulnerable when it has already been inserted, but not yet fixed to guide cannula. The cap helps to protect the injector because as soon as the cap was threaded onto the guide cannula, the injector can not be bent or broken. Also, the use of the cap allowed us to use the injector for acute experiments. Thanks to the design of the guide cannula for acute experiments, the injector can be inserted quicker and easier.
The microinjection setup can be made with an injector matching standard guide cannula (Fig.5). Commercially available microinjectors are designed for a single use, because without the cap, the tight fixation of the microinjector is only guaranteed for one use. The cap allowed us to reuse microinjectors at least 10–12 times (this is also evidence of the low breakdown rate during experiments). We recommend connecting/disconnecting the injector to/from the guide cannula a few times before assembling the microinjection setup to loosen the tight fit of the plastic parts so that the insertion of the injector into the guide cannula can be easily made.
Fig. 5.
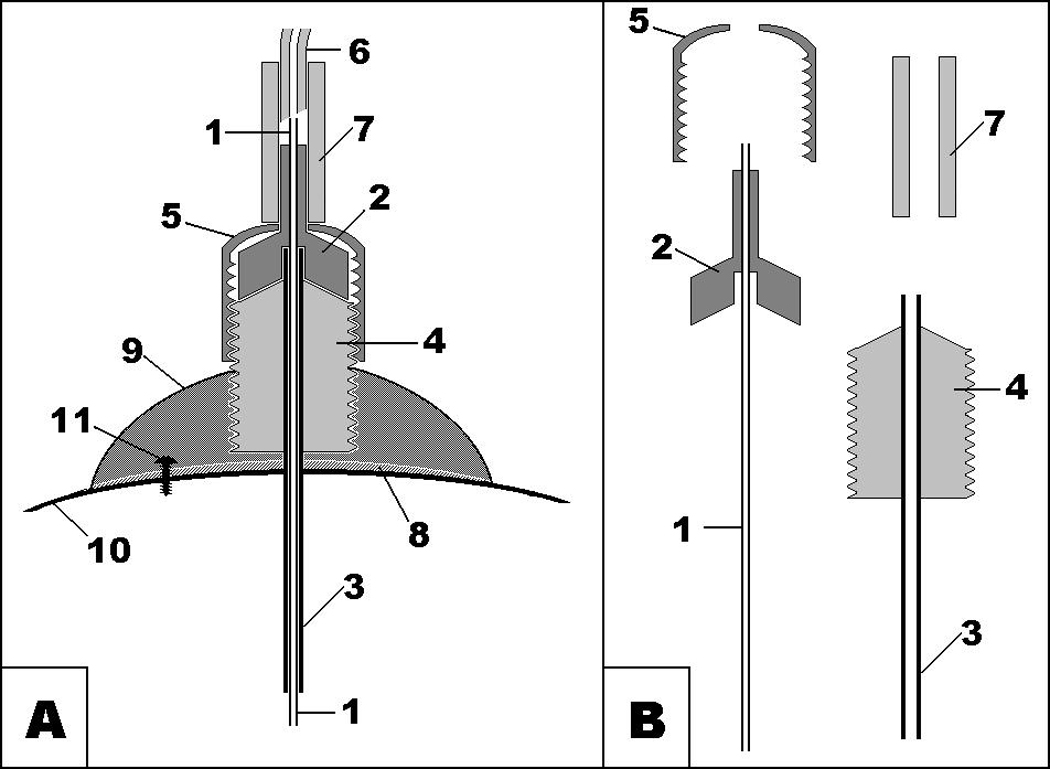
- metal tubing of microinjector;
- plastic part of microinjector;
- metal tubing of guide cannula;
- plastic pedestal of guide cannula with thread;
- plastic cup with thread matching thread of guide cannula;
- thick-wall low-volume Teflon tubing;
- PVC connector;
- cyanoacrylate;
- dental cement;
- skull bone;
- jeweler’s screw.
5.3. Baseline before microinjection
With our technique, baseline values before the microinjection were affected in two ways: presence of additional external connections, and diffusion of an active substance from the tip of injector.
Results of Experiment 1 (Table 1) demonstrated that, if sufficient time was allowed after connection of the injector, the physiological parameters of the connected rats returned to values typical for the same rats in their home cages. This showed that the animals quickly adapted to external connections.
BMI causes tachycardia immediately when injected directly into the DMH. The heart rate in animals with pre-installed microinjectors was just as undisturbed as in the animals without cannulas which clearly showed that diffusion of active substance is negligible when the pump is off. In contrast, activation of the pump evokes a degree of tachycardia typically seen with microinjections of BMI in the DMH. When the pump is turned off, the rat’s heart rate returns to baseline, and there is no further significant diffusion into the brain. Obviously, this may not be the case with all concentrations of active drugs.
5.4. Avoiding the artifact of manipulation in microinjections
Simply connecting a microinjector to the guide cannula evoked a measurable reaction in the rats (Fig.4). Tachycardia, the usual response of a non-adapted animal to this procedure, lasted almost 20 min, at which time the HR returned to baseline. Similarly, the microinjection of PBS, which involved a handling the rat, also induced tachycardia, mild hypertension, and locomotor activation (Fig.2), which are the hallmarks of a stress response. The increase in heart rate was more than 50 beats/min, which is similar to data previously published by our lab (for example, Bailey & DiMicco, 2001). This rise constituted approximately one third of the response of interest in this cited study (Bailey and Dimicco, 2001).
In contrast, microinjection of the vehicle using our described technique does not disturb rats [Fig. 2 and (Zaretskaia et al., 2008; Zaretskaia et al., 2002; Zaretsky et al., 2006)]. This clearly demonstrates the main advantage of our technique: the ability to circumvent the artifact of manipulation and to maintain the baseline conditions seen immediately before a microinjection as judged by HR, blood pressure, and locomotor activity. Our animals, wearing a leather harness to secure the arterial line while connected for microinjections and blood sampling, had similar HR, blood pressure, and locomotor activity as undisturbed animals in their home cages with closed lids (see Table 1).
5.5. Reproducibility of microinjection technique
Physiological responses to microinjections of BMI into the DMH which were performed using the new technique (Fig.3 in this manuscript and shown in data published by us earlier (Zaretskaia et al., 2008; Zaretskaia et al., 2002) appear very similar to published data from experiments which used a “traditional” technique (Bailey and Dimicco, 2001; De Novellis et al., 1995). Duplicate BMI microinjections demonstrated the close similarity in physiologic responses recorded on different days (Figs. 3A & 3B) or divided only by 3 h (Fig.3C). Reproducibility of experiments in biological systems, especially in whole animal experiments largely depends on the ability to reproduce baseline status. Our described technique provides a tool to reach reliable baseline physiologic parameters virtually in any experiment. It is not surprising that responses to the microinjection of 100 nL of BMI were reproducible (Fig. 3), because responses to injections of BMI as small as 20 nL appeared reproducible (Fig. 4).
5.6. Comparison with existing methods of “stress-free” microinjections
Few previously described microinjection techniques in conscious rats are effective at avoiding the artifact of manipulations. One such approach was a remote insertion technique (Parada et al., 1993). Here a long microinjector (202 mm) made of fused silica (the flexible inert tubing which was introduced into neurophysiological studies more than twenty years ago) (Berger et al., 1989), is inserted into a guide cannula with an extension (total length of 200 mm). Our experience showed that 20 cm is not long enough to exclude the influence of an experimenter’s manipulations on a conscious animal. We did a few successful microinjections of 20 nL using a 1-meter-long injector with a corresponding extension of the guide cannula. Although it was possible to insert a microinjector without waking up sleeping rat (data not shown),this procedure was laborious and prone to failure due to the fragility of long pieces of fused silica tubing, so we chose not to use this technique
Another approach called electrolytic microinfusion was developed to the point that commercial versions were available (Bozarth and Wise, 1980; Goeders et al., 1984). We have no personal experience with that approach, but practical problems have been reported by others (Hesse et al., 1997).
5.7. Difficulties and potential improvements for this technique
The problem of stability is inherent to this technique. Filling the system and waiting until the animal adapts to an installed injector usually takes at least 1 h. The active substance needs to be stable enough for this long. Many typical substances of neuroscience research (such as muscimol, bicuculline, kynurenate etc.) are stable in solution in this time frame, but, keeping drug stability in mind, we have always prepared the injection system just prior to each experiment.
The total volume which is required to fill the delivery system is less than 30 µl: 12 µl for 1m of Teflon tubing, 5–7 µl to fill connectors and a few microliters to be in the syringe mounted on the pump. Although we have not encountered a drug so precious that we could not use 30 µl, we can foresee this could be a problem. One possibility to address this is to continue efforts toward miniaturization – smaller internal diameter tubing (ID 0.0025”, volume is about 3 µl per meter) is available, although it turned out to be very difficult to work with it. Another alternative would be to backfill the microinjector with a solution of drug while the rest of the system is filled with another liquid. We did trials with this type of backfilling, but have not used it routinely.
Our described method was developed using commercially available injectors and guide cannulas. At first, we used injectors for chronic experiments using the design shown in Fig.5. A major difficulty of this design was inserting the injector into the guide cannula: 33 ga (OD=0.2 mm) stainless steel tubing of the injector into the 26 ga (ID=0.24 mm) stainless steel tubing. To ease that process we found it possible to use injectors and guide cannulas for acute experiments using the design shown in Fig.1. However, we faced an unexpected problem. While sliding down the “funnel” of the guide cannula, the tip of injector sometimes caught the edge of the metal component of guide cannula as shown in Fig.6a. This problem can be easily solved in anesthetized animals by slightly rotating the injector, but in conscious animals it often resulted in broken injectors when the animal moved sharply. As a precaution, we tested each guide cannula to see if it had a “catchy edge.” If one was found, the metal tubing was pushed down the plastic part with appropriate adjustment of the implantation coordinates. That bothersome edge could be eliminated by a change of the guide cannula design as shown on Fig.6b.
Fig. 6.
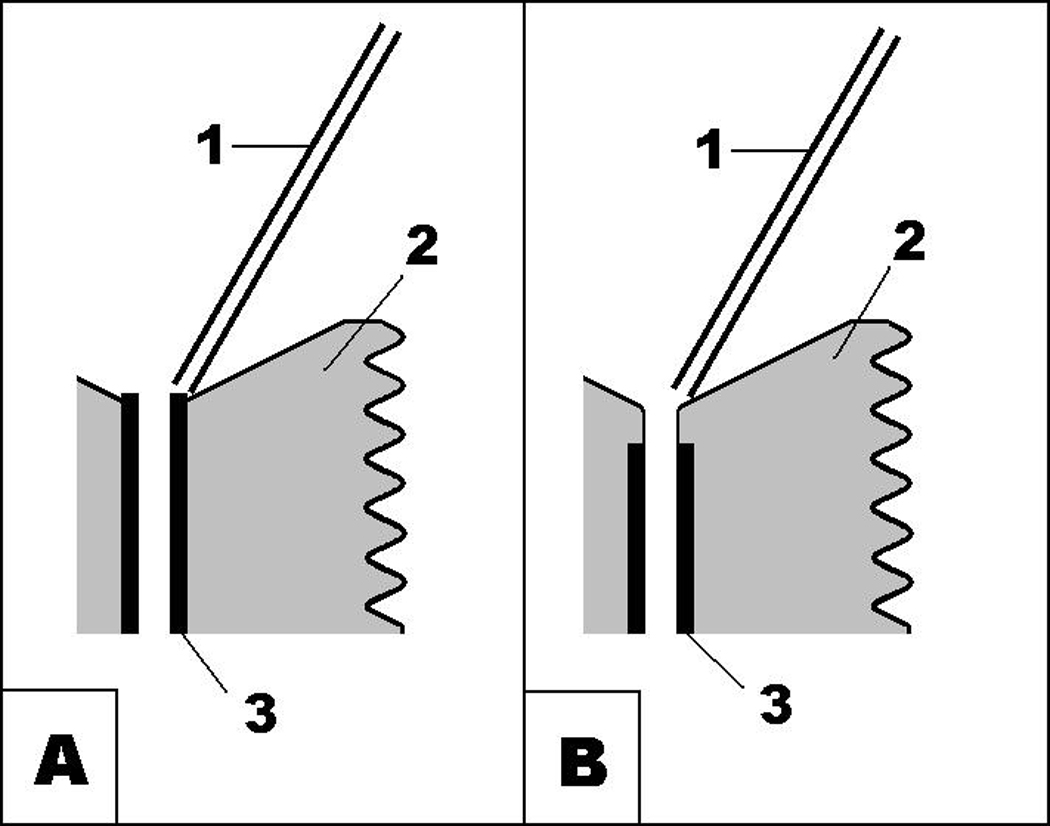
A schematic illustrating the frequent obstacle encountered when microinjectors are inserted into guide cannulas for acute experiments, and (b) the design of guide cannulas which avoid this problem.
Another difficulty is to avoid twisting of the microinjection tubing. When locomotor response is intense and unidirectional (very typical for unilateral stimulation of hypothalamus), it is often impossible to protect lines from excessive twisting, and sometimes it is difficult to disconnect the injector from an aroused rat. Fortunately, there is a commercially available system (Raturn, BASi, Lafayette IN), which rotates the cage in the opposite direction from animal movement. An easily overcome obstacle to this system is the need to adapt rats to this environment, which is very different from their home cage. We observed that it takes at least 8 h for the body temperature of a rat to return to typical baseline values after placing the animal into a Raturn. So, we obtained appropriate authorizations for experimental protocols that allow us to connect rats the day before, allowing them to acclimate overnight when they are most active. In our routine experiments, the hook of the Raturn was connected to a small metal loop which we fixed into the cement cap during implantation of guide cannula.
A major requirement of any microinjection technique is using an appropriate pump. Almost all current high-precision pumps can be computer-controlled or remote-controlled, which helps to not disturb the animal at the moment of injection. Alternatively, some pumps have a control unit separated from pump unit (Baby Bee, BASi). However, any available pumps are large pieces of equipment which are located outside the cage, so an injector must always be connected to a syringe by long tubing.
A mini-pump, which can be mounted on the animal, is a long-waited improvement. A variety of attempts using these for microinjections have been made (Hesse et al., 1997; Ikemoto and Sharpe, 2001). Recent advances in commercial manufacturing of implantable pumps for animals includes a micro-infusion pump called iPrecio (Primtech, Tokyo, Japan) (Abe et al., 2009) which uses a mechanism resembling that described by Iwamoto et al. (Iwamoto et al., 1984). While presently iPrecio is not able to reproducibly deliver volumes of 100 nL and below, it offers a promise of dramatic changes of the microinjection paradigm. If a high-precision pump is fixed on the animal, all tubing can be reduced to a few centimeters of fused silica and no additional connections. Nanoliter volumes of injection will someday become possible, as well as experiments run in home cages without any hardware connections, finally reaching the aim of “performing stress-free experiments on freely behaving animals”
6. Conclusion
Our preparation technique used healthy animals and allowed the collection of data over several weeks. Our experimental data show that baseline physiological parameters achieved before microinjection were not different from those recorded by telemetry in undisturbed animals without implanted guide cannulas. New technique allowed us to look at changes induced by the microinjections without the spectrum of artifacts leaning into the picture.
Research Highlights.
Microinjections are a major tool in modern neuroscience
We propose a change in design which reduces manipulation effects in conscious rats
The key innovation is placement of microinjector into a guide cannula in advance
Manuscript contains a detailed technique with examples of experimental data
Acknowledgements
The authors would like to thank Pamela Durant for her editorial help. This study was funded by NIH grants DA020484, NS19883, MH65697.
Abbreviations
- ACTH
Adrenocorticotropic hormone
- BMI
bicuculline methiodide
- DMH
dorsomedial hypothalamus
- HR
heart rate
- MBP
mean blood pressure
- PBS
phosphate buffered saline
- PVC
polyvinylchloride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dmitry V. Zaretsky, Email: dzaretsk@iupui.edu.
Maria V. Zaretskaia, Email: mazarets@iupui.edu.
Daniel E. Rusyniak, Email: drusynia@iupui.edu.
Joseph A. DiMicco, Email: jdimicco@iupui.edu.
References
- Abe C, Tashiro T, Tanaka K, Ogihara R, Morita H. A novel type of implantable and programmable infusion pump for small laboratory animals. J Pharmacol Toxicol Methods. 2009;59:7–12. doi: 10.1016/j.vascn.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL. An air pressure system for the injection of tracer substances into the brain. J Neurosci Methods. 1983;9:35–43. doi: 10.1016/0165-0270(83)90107-3. [DOI] [PubMed] [Google Scholar]
- Azami J, Llewelyn MB, Roberts MH. An extra-fine assembly for intracerebral microinjection. J Physiol (Lond) 1980;305:18P–19P. [Google Scholar]
- Bailey TW, Dimicco JA. Chemical stimulation of the dorsomedial hypothalamus elevates plasma ACTH in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R8–R15. doi: 10.1152/ajpregu.2001.280.1.R8. [DOI] [PubMed] [Google Scholar]
- Berger ML, Reither H, Schmid RW, Lassmann H. Manufacture and use of fused silica cannulas for intracerebral injections in freely moving rats. J Neurosci Methods. 1989;27:225–234. doi: 10.1016/0165-0270(89)90084-8. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Electrolytic microinfusion transducer system: an alternative method of intracranial drug application. J Neurosci Methods. 1980;2:273–275. doi: 10.1016/0165-0270(80)90016-3. [DOI] [PubMed] [Google Scholar]
- De Novellis V, Stotz-Potter EH, Morin SM, Rossi F, DiMicco JA. Hypothalamic sites mediating cardiovascular effects of microinjected bicuculline and EAAs in rats. Am J Physiol Regul Integr Comp Physiol. 1995;269:R131–R140. doi: 10.1152/ajpregu.1995.269.1.R131. [DOI] [PubMed] [Google Scholar]
- DiMicco JA, Sarkar S, Zaretskaia MV, Zaretsky DV. Stress-induced cardiac stimulation and fever: common hypothalamic origins and brainstem mechanisms. Auton Neurosci. 2006;126–127:106–119. doi: 10.1016/j.autneu.2006.02.010. [DOI] [PubMed] [Google Scholar]
- DiMicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R47–R63. doi: 10.1152/ajpregu.00498.2006. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Lane JD, Smith JE. Self-administration of methionine enkephalin into the nucleus accumbens. Pharmacol Biochem Behav. 1984;20:451–455. doi: 10.1016/0091-3057(84)90284-3. [DOI] [PubMed] [Google Scholar]
- Greenshaw AJ. Electrical and chemical stimulation of brain tissue in vivo. In: Boulton AA, Baker GB, Bateson AN, editors. In Vivo Neuromethods. New Jersey: Humana Press; 1998. [Google Scholar]
- Hashimoto M, Fieberstudien X, Mitteilung Uber die spezifische Uberempfindlichkeit des Warmzentrums an sensibilisierten Tieren. Naunyn-Schmiedeber's Arch Pharmacol. 1915;78:370–393. [Google Scholar]
- Hesse GW, Stellar JR, Chevrette J. A simple and reliable method for delivering small fluid volumes to the brain of a freely moving rat. J Neurosci Methods. 1997;72:35–38. doi: 10.1016/s0165-0270(96)00152-5. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Sharpe LG. A head-attachable device for injecting nanoliter volumes of drug solutions into brain sites of freely moving rats. J Neurosci Methods. 2001;110:135–140. doi: 10.1016/s0165-0270(01)00428-9. [DOI] [PubMed] [Google Scholar]
- Iwamoto ET, Williamson EC, Wash C, Hancock R. An improved drug infusion pump for injecting nanoliter volumes subcortically in awake rats. Pharmacol Biochem Behav. 1984;20:959–963. doi: 10.1016/0091-3057(84)90023-6. [DOI] [PubMed] [Google Scholar]
- Katz LC, Burkhalter A, Dreyer WJ. Fluorescent latex microspheres as a retrograde neuronal marker for in vivo and in vitro studies of visual cortex. Nature. 1984;310:498–500. doi: 10.1038/310498a0. [DOI] [PubMed] [Google Scholar]
- Li M, Wong KS, Whitworth JA. Influence of somatostatin analogue (SMS 201–995, octreotide) on blood pressure in adrenocorticotrophin (ACTH) treated rats: role of hyperinsulinaemia in ACTH hypertension. Clin Exp Pharmacol Physiol. 1993;20:647–653. doi: 10.1111/j.1440-1681.1993.tb01647.x. [DOI] [PubMed] [Google Scholar]
- Parada MA, Puig de Parada M, Hoebel BG. A remote insertion technique for intracerebral microinjections in freely moving animals. J Neurosci Methods. 1993;50:237–241. doi: 10.1016/0165-0270(93)90012-g. [DOI] [PubMed] [Google Scholar]
- Rusyniak DE, Zaretskaia MV, Zaretsky DV, DiMicco JA. 3,4-Methylenedioxymethamphetamine- and 8-hydroxy-2-di-n-propylamino-tetralin-induced hypothermia: role and location of 5-hydroxytryptamine 1A receptors. J Pharmacol Exp Ther. 2007;323:477–487. doi: 10.1124/jpet.107.126169. [DOI] [PubMed] [Google Scholar]
- Samuels BC, Zaretsky DV, DiMicco JA. Dorsomedial hypothalamic sites where disinhibition evokes tachycardia correlate with location of raphe-projecting neurons. Am J Physiol Regul Integr Comp Physiol. 2004;287:R472–R478. doi: 10.1152/ajpregu.00667.2003. [DOI] [PubMed] [Google Scholar]
- Zaretskaia MV, Zaretsky DV, Sarkar S, Shekhar A, DiMicco JA. Induction of Fos-immunoreactivity in the rat brain following disinhibition of the dorsomedial hypothalamus. Brain Res. 2008;1200:39–50. doi: 10.1016/j.brainres.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretskaia MV, Zaretsky DV, Shekhar A, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res. 2002;928:113–125. doi: 10.1016/s0006-8993(01)03369-8. [DOI] [PubMed] [Google Scholar]
- Zaretsky DV, Hunt JL, Zaretskaia MV, DiMicco JA. Microinjection of prostaglandin E2 and muscimol into the preoptic area in conscious rats: comparison of effects on plasma adrenocorticotrophic hormone (ACTH), body temperature, locomotor activity, and cardiovascular function. Neurosci Lett. 2006;397:291–296. doi: 10.1016/j.neulet.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Zaretsky DV, Zaretskaia MV, DiMicco JA. Stimulation and blockade of GABA(A) receptors in the raphe pallidus: effects on body temperature, heart rate, and blood pressure in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2003a;285:R110–R116. doi: 10.1152/ajpregu.00016.2003. [DOI] [PubMed] [Google Scholar]
- Zaretsky DV, Zaretskaia MV, Samuels BC, Cluxton LK, DiMicco JA. Microinjection of muscimol into raphe pallidus suppresses tachycardia associated with air stress in conscious rats. J Physiol. 2003b;546:243–250. doi: 10.1113/jphysiol.2002.032201. [DOI] [PMC free article] [PubMed] [Google Scholar]