Summary
Liberation of Ca2+ from the endoplasmic reticulum (ER) through inositol trisphosphate receptors (IP3R) is modulated by the ER Ca2+ content, and overexpression of SERCA2b to accelerate Ca2+ sequestration into the ER has been shown to potentiate the frequency and amplitude of IP3-evoked Ca2+ waves in Xenopus oocytes. Here, we examined the effects of SERCA overexpression on the elementary IP3-evoked puffs to elucidate whether ER [Ca2+] may modulate IP3R function via luminal regulatory sites in addition to simply determining the size of the available store and electrochemical driving force for Ca2+ release. SERCA2b and Ca2+ permeable nicotinic plasmalemmal channels were expressed in oocytes, and hyperpolarizing pulses were delivered to induce Ca2+ influx and thereby load ER stores. Puffs evoked by photoreleased IP3 were significantly potentiated in terms of numbers of responding sites, frequency and amplitude following transient Ca2+ influx in SERCA-overexpressing cells, whereas little change was evident with SERCA overexpression alone or following Ca2+ influx in control cells not overexpressing SERCA. Intriguingly, we observed the appearance of a new population of puffs that arose after long latencies and had prolonged durations supporting the notion of luminal regulation of IP3R gating kinetics.
1. Introduction
Intracellular Ca2+ concentrations are tightly regulated, and local and global elevations of cytosolic free [Ca2+] play pivotal signaling roles in virtually all cells [1]. A major source of Ca2+ derives from the endoplasmic reticulum (ER), which in addition to its crucial roles in protein synthesis sequesters Ca2+ via the ATP-driven sarco/endoplasmic reticulum calcium (SERCA) pump. Ca2+ ions stored in the ER lumen may then be rapidly liberated down their concentration gradient into the cytosol through the opening of inositol 1,4,5,-triphosphate receptor channels (IP3R) and ryanodine receptor channels (RyR) [2]. The gating of both channel types is regulated by cytosolic Ca2+ itself, creating complex feedback loops that orchestrate the activities of these signaling pathways to generate dynamic spatio-temporal patterns of Ca2+ signals that regulate downstream processes as diverse as gene expression and neuronal excitability [1].
The luminal Ca2+ concentration within the ER determines the electrochemical force driving the flux of Ca2+ ions through IP3Rs and RyRs. Moreover, there is strong evidence that RyR function is modulated by luminal [Ca2+] [3, 4]. Elevations of luminal Ca2+ content enhance global Ca2+ release [5, 6] by increasing RyR channel open probability [7-10], possibly by recruiting luminal Ca2+ sensing proteins [11]. It has been suggested that IP3Rs may also be regulated by luminal Ca2+ content, either via a Ca2+ sensor on the luminal face of the receptor or via an accessory luminal Ca2+ sensing protein [12-15]. However, the evidence for luminal Ca2+ regulation of IP3R is less persuasive [16-20]. In part, this may be attributed to technical limitations. Unlike RyRs, activation of IP3Rs requires both IP3 and Ca2+. Thus, experiments in intact cells require a means to manipulate these second messengers as well as luminal Ca2+ content. Furthermore, it may be difficult to discriminate whether changes in ER Ca2+ content are directly modulating IP3R function from the luminal side, or indirectly from the cytosolic side via changes in Ca2+ release flux.
Xenopus oocytes lack RyRs [21] and this feature, together with the large size of the oocyte that facilitates intracellular microinjection, has made the oocyte a favored model cell system to study IP3-mediated Ca2+ signaling processes. Photorelease of IP3 from a caged precursor provides a convenient means to evoke controlled elevations of cytosolic [IP3], which can be adjusted to evoke Ca2+ liberation as either global waves or, with smaller photorelease, as local, transient puffs [22]. The puffs arise through concerted openings of tight clusters containing small numbers of IP3Rs, and thus provide information about channel function that is obscured during global waves [23]. We recently investigated the effects of increasing luminal [Ca2+] on these IP3-mediated signals [24] by capitalizing on our earlier finding that the second messenger cyclic ADP ribose (cADPR) enhances SERCA pump activity, and is thus expected to lead to greater ER Ca2+ store filling [25]. Following transient Ca2+ influx through expressed plasmalemmal nicotinic channels to load ER stores in oocytes injected with a non-metabolizable cADPR analog we observed a potentiation of global wave amplitudes and increased numbers of puffs. Moreover, the appearance of a new population of puffs with longer latencies, prolonged durations and attenuated amplitudes suggested that luminal ER Ca2+ may modulate IP3R function, in addition to simply determining the size of the available store and the electrochemical driving force for release.
Here, we employed a more direct method to promote ER Ca2+ store filling, by overexpressing SERCA2b in Xenopus oocytes [26-28]. Our results confirm and extend those we had obtained using cADPR to enhance SERCA activity, removing possible uncertainty that the actions of cADPR on IP3-mediated signals may have resulted from actions on IP3R independent of store filling, and reinforcing the notion of luminal Ca2+ modulation of IP3R function.
2. Materials and Methods
Procedures for preparation of oocytes, expression of nicotinic acetylcholine receptors (nAChR) and SERCA2b and Ca2+ imaging were as previously described [24, 25, 28]. In brief, oocytes overexpressing nAChR alone or together with SERCA2b were were injected with fluo–4, caged IP3 (D-myo-inositol 1,4,5-trisphosphate P4(5)-[1-(2-nitrophenyl)ethyl]ester (Invitrogen) and EGTA (Invitrogen) to final respective intracellular concentrations of about 40 μM, 8 μM and 300 μM The membrane potential was held at 0 mV during superfusion with non-desensitizing concentration of ACh (100 – 500 nM) in Ringer's solution (mM: NaCl2, 120; KCl, 2; CaCl2, 1.8; HEPES, 5;pH 7.4), and Ca2+ influx through nAChR was induced by transiently stepping the potential to –120 mV to increase the electrical driving force [29]. Cytosolic Ca2+ signals were imaged at room temperature by wide-field fluorescence microscopy using an Olympus inverted microscope (IX 71) equipped with a 40X oil-immersion objective, a 488 nm argon-ion laser for fluorescence excitation and a ccd camera (Cascade 128+: Roper Scientific) for imaging fluorescence emission (510-600 nm) at frame rates of 50 s-1. Fluorescence was imaged within a 40 × 40 μm region within the animal hemisphere of the oocyte, and flashes of UV light were applied to uniformly photorelease IP3 throughout this region. Measurements are expressed as a ratio (ΔF/Fo) of the mean change in fluorescence at a given region of interest (ΔF) relative to the resting fluorescence at that region before stimulation (Fo). Mean values of Fo were obtained by averaging over several frames before stimulation. MetaMorph (Molecular Devices) was used for image processing, and measurements were exported to Microcal Origin version 6.0 (OriginLab, Northamptom, MA, USA) for analysis and graphing. Puff data are from 25 control and 11 SERCA2b-overexpressing oocytes obtained from 3 frogs, and are expressed as mean ± SEM. Significance was assessed by t-tests.
3. Results
3.1 Overexpression of SERCA2b accelerates cytosolic Ca2+ clearance
In order to confirm that oocytes had overexpressed functional SERCA pumps, we monitored the clearance of Ca2+ ions from the cytosol after a transient influx across the plasma membrane [25, 28]. For this purpose, oocytes were induced to express Ca2+-permeable nicotinic acetylcholine receptors (nAChRs) that served as a “Ca2+ switch”, allowing precisely controlled cytosolic Ca2+ transients to be evoked by transiently hyperpolarizing the membrane potential to increase the electrochemical driving force for Ca2+ entry [29]. Oocytes were loaded with the Ca2+-sensitive dye fluo-4 and were voltage-clamped at a holding potential of 0 mV to minimize Ca2+ influx. In the presence of non-desensitizing (100–500 nM) concentrations of acetylcholine, a brief (500 ms) hyperpolarizing pulse to -120 mV was applied to induce Ca2+ influx through opening of nicotinic channels. The decay rate of the fluorescence signals after termination of the voltage pulse was then used to estimate the rate of Ca2+ sequestration from the cytosol. The decay reflects several factors in addition to sequestration into the ER by SERCA pumps, including diffusion of Ca2+ into the interior of the oocyte, mitochondrial uptake and extrusion across the plasma membrane. We had previously shown that the initial decay is dominated by passive diffusion of Ca2+ ions into enormous interior volume of the oocyte, whereas sequestration by thapsigargin-sensitive SERCA pumps is more prominent during the subsequent slow tail of the decay [25]. Fig. 1A plots mean fluorescence profiles obtained from 25 oocytes that expressed only nAChRs (black) and 11 oocytes overexpressing SERCA2b in addition to nAChRs (gray), revealing a marked acceleration of the slow Ca2+ clearance component following overexpression of SERCA2b.
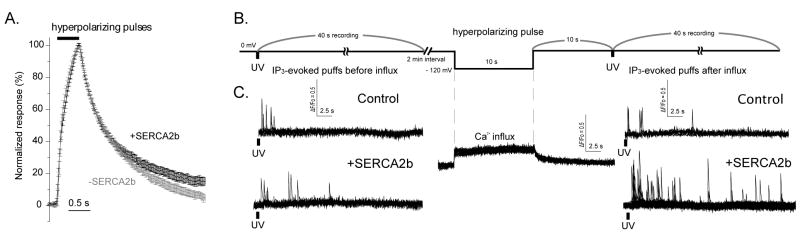
FIGURE 1. Effects of SERCA2b expression on the clearance of Ca2+ from the cytosol and on IP3-evoked Ca2+ puffs.
A. Traces show mean Ca2+-dependent fluorescence changes resulting from 500 ms hyperpolarizing pulses to induce Ca2+ entry through nAChRs expressed in the plasma membrane. Fluorescent profiles were normalized to peak amplitudes as 100 %. Data are from oocytes injected 3 days earlier with α,β,γ,δ nAChR subunits alone (black: 75 records from 25 oocytes) or together with SERCA2b cRNA (gray; 33 recordings from 11 oocytes). B. Experimental protocol to investigate the effects of enhanced ER Ca2+ uptake on IP3-evoked puffs. Oocytes were clamped at a holding potential of 0 mV, and IP3 was photoreleased to evoke puffs (left). After a 2-min interval, a voltage pulse to -120 mV was applied for 10 s to drive Ca2+ influx through nAChR, followed 10 s later by a photolysis flash of the same strength (right). C. Representative examples of fluorescence profiles of IP3-evoked puffs before (left) and after (right) Ca2+ influx (middle), in oocytes expressing nAChR alone (control, top) and oocytes expressing SERA2b together with nAChR (bottom). Traces show representative examples of local fluorescence changes (ΔF/Fo) from a single record under each experimental condition. Puffs were evoked before and after Ca2+ influx, and 1.65 × 1.65 mm regions of interest were centered on puff sites. Superimposed traces show recordings from 5 puff sites before influx and 6 sites after influx in control oocytes (upper traces); and from 10 sites before influx and 26 sites after influx in SERCA2+ overexpressed oocytes (lower traces).
3.2. Effects of ER Ca2+ store filling on IP3-evoked puffs
Fig. 1B shows a schematic of the experimental protocol used to examine the effects of ER Ca2+ store filling on puffs evoked by photoreleased IP3. As described above, nAChR-expressing oocytes were continually exposed to a low concentration of Ach (500 nM) while clamped at 0 mV to minimize Ca2+ entry, and were pulsed for 10 s to -120 mV to promote Ca2+ influx and thereby promote ER store filling. Ca2+ signals evoked by photolysis flashes were compared before and after the hyperpolarizing pulses, with a 10 s delay between the end of the pulse and the second UV flash to allow cytosolic Ca2+ to return to basal levels. The UV flash duration was adjusted (range 50 – 100 ms) to evoke a low frequency of puffs in control oocytes (not overexpressing SERCA2b), and that duration was then fixed for all oocytes within that batch. Figure 1C illustrates representative fluorescent profiles in response to this protocol, showing marked potentiation of puff amplitudes and frequencies in SERCA2b-overexpressing oocytes after Ca2+ influx. Consistent with our previous findings [24], the Ca2+ influx protocol caused little or no change in Ca2+ puffs in control oocytes, and we thus collected quantitative data in control oocytes only in the absence of Ca2+ influx.
3.3 Changes in puff frequency and latency following Ca2+ influx in SERCA2b-overexpressing oocytes
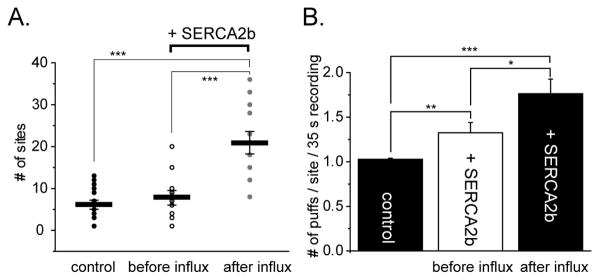
Fig. 2A plots the numbers of discrete sites within the 40 × 40 μm image field where puffs were evoked following photolysis flashes under the three experimental conditions. The strength of the UV flash was fixed so that it evoked puffs at only a few (6.5 ± 0.6) sites in control oocytes. This number was only slightly greater in SERCA2b-overexpressing oocytes before Ca2+ influx (8.2 ± 1.8), but increased almost 3-fold (to 21.7 ± 2.7) following Ca2+ influx. Moreover, the number of puffs observed at each site also increased markedly. Sites almost always gave only a single puff in response to the photolysis flash in control oocytes, but often showed multiple, sequential puffs following Ca2+ influx in SERCA2b-overexpressing oocytes (Fig. 2B).
FIGURE 2. Enhanced ER Ca2+ filling potentiates puff frequencies and the numbers of responding sites.
A. Plots show measurements of the numbers of puff sites within the 40 × 40 μm imaging field that responded to photolysis flashes of fixed duration delivered before and after Ca2+ influx in SERCA2b-overexpressing oocytes (n = 11 oocytes), and in control oocytes (n = 25) without Ca2+ influx. Circles show measurements from individual trials, and bars indicate mean values ± SEM (control oocytes, 6.5 ± 0.6: SERCA2b-overexpressing oocytes before Ca2+ influx, 8.2 ± 1.8: after Ca2+ influx, 21.7 ± 2.7). B. Mean numbers of puffs observed at each site during the 40 s imaging period following photolysis flashes (control oocytes, 1.03 ± 0.012, 159 puffs: SERCA2b-overexpressing oocytes before Ca2+ influx; 1.32 ± 0.11, 66 puffs: after Ca2+ influx, 1.76 ± 0.16, 177 puffs. In this and other figures, asterisks indicate statistically significant differences; * = P< 0.05, ** = P< 0.03, *** = P< 0.01.
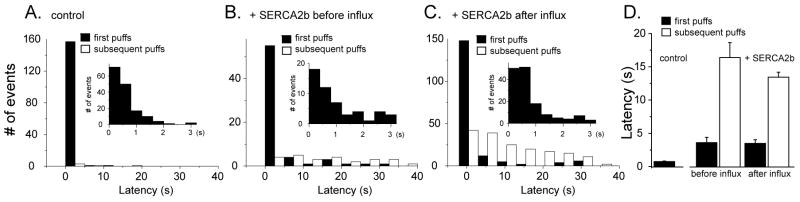
Puff latencies (the time from the end of UV flash to the first puff at a given site) provide a sensitive measure of IP3R activation, and shorten as a steep function of both increasing [IP3] [30] and basal cytosolic [Ca2+] in the presence of [IP3] (M.Y-M. and I.P. unpublished observations). We were thus interested to examine whether puff latencies were further modulated by changes in ER Ca2+ loading induced by Ca2+ influx in SERCA2b-overexpressing oocytes. Figure 3A-C shows distributions of first and subsequent puffs latencies on various timescales for the three experimental conditions, and Fig. 3D presents mean data. In control oocytes, puffs were first initiated within about 1 s of the photolysis flash (mean = 0.75 ± 0.09 s, n = 159), and almost none arose with latencies >2s. In contrast, initial puffs in SERCA2b-expressing oocytes arose with appreciably longer latencies both before and after Ca2+ influx (respective means 3.60 ± 0.80 s, n = 66; 3.48 ± 0.55 s, n = 177, p > 0.05). This difference arose largely from the appearance of a new population of initial puffs arising with latencies of between 2 and 40 s (filled bars, Figs. 3B,C). SERCA2b-overexpression also resulted in the appearance of puffs occurring after the initial event at a given site (Figs. 3B,C, open squares). These ‘subsequent’ puffs were largely absent in control oocytes (Fig. 3A, open square), and arose with similar long mean latencies in SERCA2b-overexpressing oocytes before and after Ca2+ influx (Fig. 3D, open bars, control: 5.80 ± 2.86 s, n = 4, SERCA2b-overexpressing oocytes before: 16.38 ± 2.24 s, n = 25, and after Ca2+ influx: 13.44 ± 0.74 s, n = 178).
FIGURE 3. Effect of ER Ca2+ filling on puff latencies.
A – C. Raw distributions of the numbers of puffs observed within the imaging field during successive 2.5 s time bins following photorelease of IP3 in control oocytes (A), and in SERCA2b-overexpressing oocytes before (B) and after (C) Ca2+ influx. Inset graphs are raw distribution of the puffs responded within 3 s after UV flash during 0.4 s time bins. SERCA2b overexpression prolongs initial puff latencies, and promotes the occurrence of subsequent puffs. D. Graphs show mean values of puff latencies under the three experimental conditions, measured as the time from the photolysis flash to the observation of the first puff at any given site (filled bars) and to all subsequent puffs (open bars) (control oocytes, initial puffs 0.75 ± 0.10 s from 159 puffs, subsequent puffs 5.8 ± 2.86 s from 4 puffs: SERCA2b-overexpressing oocytes before Ca2+ influx, initial puffs 3.60 ± 0.80 s from 66 puffs, subsequent puffs 16.38 ± 2.24 s from 25 puffs: after Ca2+ influx, initial puffs 3.48 ± 0.55 s from 169 puffs, subsequent puffs 13.44 ± 0.74 s from 178 puffs).
3.4. Store filling affects puff amplitudes and durations
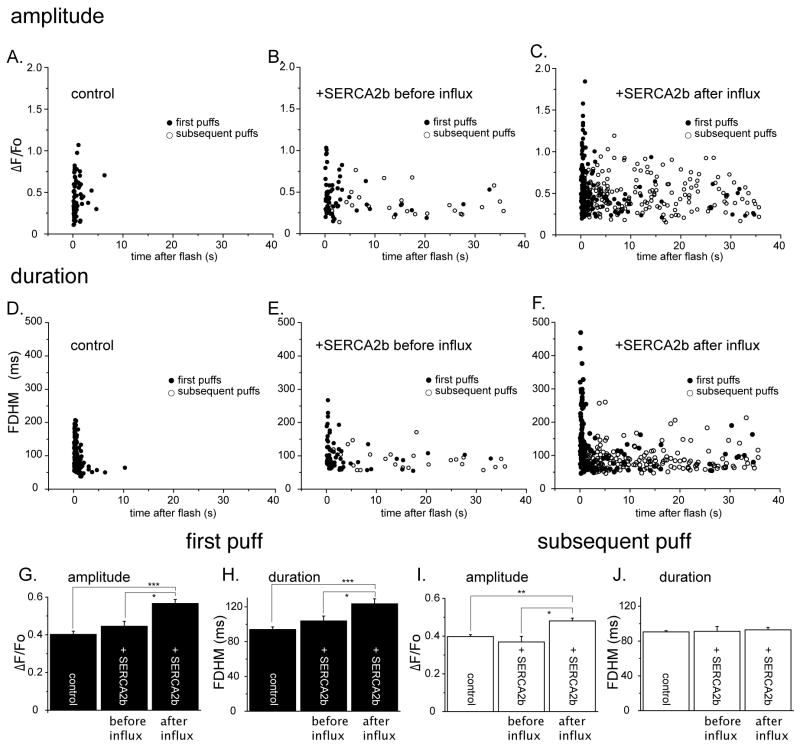
Figs. 4A-C show amplitudes of first and subsequent puffs (ΔF/Fo) in the three experimental groups, plotted against time of occurrence of the puffs following the photolysis flash, and mean data are shown in Figures 4G and I. The amplitudes of initial and subsequent puffs were not significantly different from controls in SERCA2b-overexpressing oocytes before Ca2+ influx (Fig. 4G, control: 0.40 ± 0.02, 66 puffs, SERCA2b-overexpressing before Ca2+ influx: 0.45 ± 0.03, 66 puffs, p < 0.05). On the other hand, Ca2+ influx caused a marked potentiation of mean first-puff amplitude (Fig. 4G, SERCA2b-overexpressing after Ca2+ influx: 0.57 ± 0.02, 169 puffs, p < 0.01, vs. control, p < 0.05, vs. SERCA2b-overexpressing before Ca2+ influx), which was largely attributable to the appearance of a new population of large (ΔF/Fo > 1.0) puffs with short latencies (Fig. 4C). Mean amplitude of subsequent puffs in SERCA2b-overexpressing oocytes before Ca2+ influx was of similar amplitude to control (control: 0.40 ± 0.01, 4 puffs, SERCA2b-overexpressing before Ca2+ influx: 0.37 ± 0.03, 25 puffs, p < 0.05), whereas Ca2+ influx caused significant increase in the mean value from SERCA2b-overexpressing oocytes (SERCA2b-overexpressing after Ca2+ influx: 0.48 ± 0.01, 178 puffs, p < 0.03, vs. SERCA2b-overexpressing after Ca2+ influx).
FIGURE 4. Effects of enhanced ER Ca2+ filling on puff amplitudes and durations.
A - C. Scatter plots show amplitudes (ΔF/Fo) of first (filled circles) and subsequent puffs (open circles) versus their times of occurrence after the photolysis flash for each experimental condition. D - F. Corresponding plots show the durations (full duration at half-maximal amplitude; FDHM) of first (filled circles) and subsequent puffs (open circles) as functions of their times of occurrence. G - J. Mean puff amplitudes and durations derived from the data in (A - F). G. Mean amplitudes (ΔF/Fo) of the initial puff evoked by photoreleased IP3 at each given site in control oocytes (0.40 ± 0.12, 159 puffs), SERCA2b-overexpressing oocytes before Ca2+ influx (0.44 ± 0.06, 66 puffs), and after Ca2+ influx (0.55 ± 0.02, 177 puffs from 11 oocytes). H. Mean durations (full (FDHM) of the initial puffs (control, 92.1 ± 3.0 ms: SERCA2b-overexpressed before influx, 106.0 ± 5.6 ms: SERCA2b-overexpressed after influx, 125 ± 5.5 ms). I and J. are corresponding mean amplitudes and durations after pooling data from all subsequent puffs at each given site (amplitudes: control; ΔF/Fo 0.40 ± 0.01, 4 puffs: SERCA2b-overexpressed before Ca2+ influx, 0.37 ± 0.03, 25 puffs; SERCA2b-overexpressed after Ca2+ influx, 0.48 ± 0.01, 178 puffs).
Figures 4D - F similarly plot puff durations (full duration at half maximum fluorescence; FDHM) against their time of occurrence following the photolysis flash, and mean data are shown in Figures 4H and J. No significant differences were apparent in durations of initial and subsequent puffs between controls and SERCA2b-overexpressing oocytes before Ca2+ influx (Fig. 4H, control: 93.9 ± 3.1 ms, 4 puffs, SERCA2b-overexpressing before Ca2+ influx: 103.7 ± 5.7 ms, 25 puffs, p < 0.05). However, the mean duration of initial puffs in SERCA2b-overexpressing oocytes after Ca2+ influx was significantly prolonged (Fig. 4H, 123.4 ± 5.8 ms, 178 puffs, p < 0.03), largely owing to the appearance of many short-latency puffs with long (>250 ms) durations. The mean durations of subsequent puffs in SERCA2b-overexpressing oocytes were not appreciably different before and after Ca2+ influx, and were similar to the mean duration of initial puffs in control oocytes (Fig. 4J).
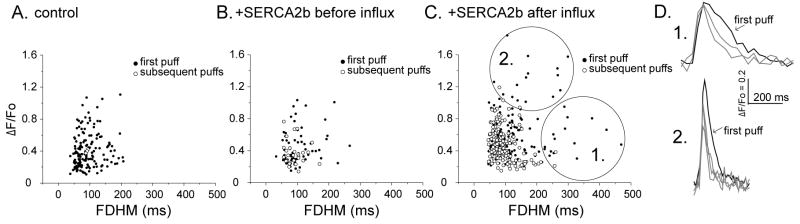
3.5. Relation between puff amplitude and duration
Having found that enhanced ER store filling by Ca2+ influx in SERCA2b-overexpresing oocytes resulted in changes in puff amplitudes and durations, we further examined whether these two effects were interrelated. For both control oocytes and SERCA2b-overexpressing oocytes before Ca2+ influx scatter plots of duration vs. puff amplitude (Fig. 5A and B) revealed little correlation, with amplitudes scattered over a similar range across durations of all puff (first puff in filled circles and subsequent puffs). In contrast, Ca2+ influx in SERCA2b-overexpressing oocytes led to the appearance of new populations of first puffs (Fig. 5 C, filled circles), which were roughly divided into two regions as illustrated in Figure 5C and D: (1) Puffs with normal amplitudes (ΔF/Fo < 1.0) but unusually prolonged durations (>250 ms), and (2) puffs with large amplitudes but durations within the normal range. Intriguingly, subsequent puffs from SERCA2b-overexpressing oocytes after Ca2+ influx (Fig. 5C open circles) showed similar distribution of control and SERCA2b-overexpressing oocytes before Ca2+ influx.
FIGURE 5. Effect of enhanced ER Ca2+ filling on puff kinetics.
A - C. Scatter plots show peak puff amplitudes as a function of puff durations (FDHM) under the three experimental conditions. Measurements from initial puffs are indicated by filled circles, and open circles are from all subsequent puffs observed during the recordings. Following Ca2+ influx in SERCA2b-overexprrssing oocytes two distinct populations of initial puffs (circled as regions 1 and 2 in G) were observed, which were not evident in control oocytes or in SERCA2b-expressing oocytes before Ca2+ influx. D. Representative superimposed traces of initial puffs (black) and subsequent puffs (gray) obtained from two sites in region 1 and 2. First puffs in region 1 displayed prolonged durations but had amplitudes similar to controls, whereas puffs in region 2 showed enhanced amplitudes but with shorter durations.
4. Discussion
Enhanced filling of ER Ca2+ stores by overexpression of SERCA2b has been shown to potentiate the frequency and amplitude of IP3-evoked Ca2+ waves in Xenopus oocytes [26, 27]. We were interested to extend that finding to the level of the elementary IP3-evoked puffs which form the building blocks of global Ca2+ waves [23]. Puffs arise from the transient openings of small numbers of IP3R channels, and might therefore provide clues as to whether the potentiation of Ca2+ signals arose simply as a consequence of the increased driving force and available reservoir for Ca2+ liberation, or whether luminal [Ca2+] within the ER may additionally modulate IP3R function [17].
Overexpression of SERCA2b by itself produced only small changes in IP3-evoked Ca2+ signals, likely because homeostatic cellular mechanisms maintain ER Ca2+ at a relatively constant level despite the increased number of SERCA pumps. We thus loaded the ER stores by evoking transient influx of extracellular Ca2+ through expressed nicotinic receptors before testing IP3-evoked responses. We had previously shown that this procedure has little or no effect on puffs in control oocytes [24], suggesting that the pumping rate of native SERCA is low; a conclusion supported by the finding that thapsigargin had only a slight effect on the rate of cytosolic Ca2+ clearance in control cells [28] [25]. Moreover, the lack of effect of Ca2+ influx in control oocytes indicates that the transient elevation of cytosolic [Ca2+] itself produced no lingering effect on IP3R function when tested after allowing cytosolic [Ca2+] to return to basal levels. Instead, we interpret our results in terms of an overfilling of ER Ca2+ stores, resulting from enhanced uptake by overexpressed SERCA during transient elevation of cytosolic [Ca2+] [24]. It is also important to note that cytosolic Ca2+ transient itself was not sufficient enough to evoke puffs, supporting the idea of sequential cooperative activation of IP3Rs by both IP3 and cytosolic Ca2+ [31].
Store filling induced by transient Ca2+ influx in SERCA-overexpressing oocytes resulted in marked increases in the numbers of sites at which puffs were evoked by photoreleased IP3, as well as in an increase in the frequency of puffs per site, and a modest (∼ 40%) enhancement in mean puff amplitude. These effects may be attributed to the increased driving force for Ca2+ liberation, resulting in an increased single channel Ca2+ flux (current) through IP3Rs. Puffs are initiated by the stochastic opening of an individual IP3R channel [32]. An increase in the amount of Ca2+ released through a trigger channel would be expected to increase the probability that neighboring receptors become activated by CICR, thereby increasing the likelihood of evoking a puff and hence increasing the number of sites at which puffs are observed, as well as the mean frequency of puffs at a given site. Correspondingly, increased puff amplitude may be expected if the Ca2+ flux through each open channel is enhanced, an effect that may be further potentiated if a greater number of channels open due to enhanced CICR. We had previously reported that global IP3-evoked Ca2+ signals were enhanced by using cADPR to accelerate SERCA pump activity but, surprisingly, we did not then observe any appreciable changes in puff amplitude [24]. The reason for the difference with our present results is unclear, but may simply reflect a greater accumulation of ER Ca2+ in SERCA-overexpressing oocytes as compared to those stimulated by cADPR. Mean puff amplitudes are relatively insensitive to IP3 concentration [33], leading us to propose that inhibitory feedback by Ca2+ tends to clamp the mean amount of Ca2+ released during puffs at a roughly constant level despite changes in the numbers of activatable IP3R channels that could potentially contribute to the puff [34]. Such a mechanism might similarly blunt the effect of increased ER Ca2+ store filling on puff amplitudes.
On the other hand, the effects of ER store filling on puff kinetics are not easily explained on the sole basis of increased single-channel Ca2+ flux. Puff latencies (time from photorelease of IP3 to occurrence of a first puff at a given site) shorten as a steep function of increasing [IP3] [30], and we expected that the latency would therefore also shorten with increasing lumenal ER [Ca2+] because of the increased probability that stochastic channel openings would trigger a puff [35]. Instead, we observed a marked (about 5-fold) prolongation of mean first-puff latencies in SERCA-overexpressing oocytes, which was largely attributable to the appearance of a new population of puffs arising after long (>2 s) latencies (Fig. 3). In addition, enhanced ER Ca2+ store filling resulted in the appearance of a new population of puffs with unusually prolonged durations, contrary to the expectation that increased Ca2+ flux and consequent Ca2+ inhibition might lead to more rapid puff termination. The concentration of photoreleased IP3 in oocytes decays with a time constant of about 20 s [36], so it is likely that the delayed puffs are evoked by ‘lingering’ IP3, and do not arise through an IP3-independent mechanism promoted by increased ER Ca2+ store filling
Taken together with other findings using cADPR to accelerate SERCA activity [24, 25], our results support the notion of a modulatory action of ER [Ca2+] on luminal sites of the IP3R [13, 15, 37, 38]. In light of the role of localized Ca2+ signalling to neighbouring organelles, such as mitochondria, changes seen in the kinetics of puffs are intriguing. For example, constitutive release of Ca2+ from IP3R to mitochondria is essential for efficient mitochondrial regulation and for suppression of macroautophagy [39]. In addition to regulation by [IP3], such intra-organelle signalling may be affected by the filling state of the ER Ca2+ store, acting not only on the driving force for Ca2+ liberation but also to modulate IP3R functioning.
Acknowledgments
We thank Dr. Angelo Demuro for technical advice and insightful discussion. We are also grateful to Dr. Stephen F. Heinemann for providing the muscle nAChRs subunits cDNAs. This work was supported by a grant (GM48071) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berridge MJ, Bootman MD, Roderick HL. Calcium: Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ. Calcium microdomains: organization and function. Cell Calcium. 2006;40:405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Jiang D, Wang R, Xiao B, Kong H, Hunt DJ, Choi P, Zhang L, Chen SR. Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circ Res. 2005;97:1173–1181. doi: 10.1161/01.RES.0000192146.85173.4b. [DOI] [PubMed] [Google Scholar]
- 4.Jiang D, Chen W, Xiao J, Wang R, Kong H, Jones PP, Zhang L, Fruen B, Chen SR. Reduced threshold for luminal Ca2+ activation of RyR1 underlies a causal mechanism of porcine malignant hyperthermia. J Biol Chem. 2008;283:20813–20820. doi: 10.1074/jbc.M801944200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fabiato A. Two kinds of calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cardiac cells. Adv Exp Med Biol. 1992;311:245–262. doi: 10.1007/978-1-4615-3362-7_18. [DOI] [PubMed] [Google Scholar]
- 6.Han S, Schiefer A, Isenberg G. Ca2+ load of guinea-pig ventricular myocytes determines efficacy of brief Ca2+ currents as trigger for Ca2+ release. J Physiol. 1994;480(Pt 3):411–421. doi: 10.1113/jphysiol.1994.sp020371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sitsapesan R, Williams AJ. The gating of the sheep skeletal sarcoplasmic reticulum Ca(2+)-release channel is regulated by luminal Ca2+ J Membr Biol. 1995;146:133–144. doi: 10.1007/BF00238004. [DOI] [PubMed] [Google Scholar]
- 8.Sitsapesan R, Williams AJ. Regulation of current flow through ryanodine receptors by luminal Ca2+ J Membr Biol. 1997;159:179–185. doi: 10.1007/s002329900281. [DOI] [PubMed] [Google Scholar]
- 9.Gyorke I, Gyorke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J. 1998;75:2801–2810. doi: 10.1016/S0006-3495(98)77723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ching LL, Williams AJ, Sitsapesan R. Evidence for Ca(2+) activation and inactivation sites on the luminal side of the cardiac ryanodine receptor complex. Circ Res. 2000;87:201–206. doi: 10.1161/01.res.87.3.201. [DOI] [PubMed] [Google Scholar]
- 11.Gyorke I, Hester N, Jones LR, Gyorke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J. 2004;86:2121–2128. doi: 10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irvine RF. ‘Quantal’ Ca2+ release and the control of Ca2+ entry by inositol phosphates--a possible mechanism. FEBS letters. 1990;263:5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- 13.Sienaert I, De Smedt H, Parys JB, Missiaen L, Vanlingen S, Sipma H, Casteels R. Characterization of a cytosolic and a luminal Ca2+ binding site in the type I inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1996;271:27005–27012. doi: 10.1074/jbc.271.43.27005. [DOI] [PubMed] [Google Scholar]
- 14.Sienaert I, Missiaen L, De Smedt H, Parys JB, Sipma H, Casteels R. Molecular and functional evidence for multiple Ca2+-binding domains in the type 1 inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1997;272:25899–25906. doi: 10.1074/jbc.272.41.25899. [DOI] [PubMed] [Google Scholar]
- 15.Higo T, Hattori M, Nakamura T, Natsume T, Michikawa T, Mikoshiba K. Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell. 2005;120:85–98. doi: 10.1016/j.cell.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 16.Nunn DL, Taylor CW. Luminal Ca2+ increases the sensitivity of Ca2+ stores to inositol 1,4,5-trisphosphate. Mol Pharmacol. 1992;41:115–119. [PubMed] [Google Scholar]
- 17.Missiaen L, De Smedt H, Droogmans G, Casteels R. Luminal Ca2+ controls the activation of the inositol 1,4,5-trisphosphate receptor by cytosolic Ca2+ J Biol Chem. 1992;267:22961–22966. [PubMed] [Google Scholar]
- 18.Parys JB, Missiaen L, De Smedt H, Casteels R. Loading dependence of inositol 1,4,5-trisphosphate-induced Ca2+ release in the clonal cell line A7r5. Implications for the mechanism of quantal Ca2+ release. J Biol Chem. 1993;268:25206–25212. [PubMed] [Google Scholar]
- 19.Shuttleworth TJ. Ca2+ release from inositol trisphosphate-sensitive stores is not modulated by intraluminal [Ca2+] J Biol Chem. 1992;267:3573–3576. [PubMed] [Google Scholar]
- 20.Combettes L, Berthon B, Claret M. Taurolithocholate-induced Ca2+ release is inhibited by phorbol esters in isolated hepatocytes. Biochem J. 1992;287(Pt 3):891–896. doi: 10.1042/bj2870891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parys JB, Sernett SW, DeLisle S, Snyder PM, Welsh MJ, Campbell KP. Isolation, characterization, and localization of the inositol 1,4,5-trisphosphate receptor protein in Xenopus laevis oocytes. J Biol Chem. 1992;267:18776–18782. [PubMed] [Google Scholar]
- 22.Callamaras N, Marchant JS, Sun XP, Parker I. Activation and co-ordination of InsP3-mediated elementary Ca2+ events during global Ca2+ signals in Xenopus oocytes. J Physiol. 1998;509(Pt 1):81–91. doi: 10.1111/j.1469-7793.1998.081bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchant JS, Parker I. Role of elementary Ca(2+) puffs in generating repetitive Ca(2+) oscillations. Embo J. 2001;20:65–76. doi: 10.1093/emboj/20.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamasaki-Mann M, Demuro A, Parker I. Modulation of endoplasmic reticulum Ca2+ store filling by cyclic ADP-ribose promotes inositol trisphosphate (IP3)-evoked Ca2+ signals. J Biol Chem. 2010;285:25053–25061. doi: 10.1074/jbc.M109.095257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamasaki-Mann M, Demuro A, Parker I. cADPR stimulates SERCA activity in Xenopus oocytes. Cell Calcium. 2009;45:293–299. doi: 10.1016/j.ceca.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camacho P, Lechleiter JD. Increased frequency of calcium waves in Xenopus laevis oocytes that express a calcium-ATPase. Science. 1993;260:226–229. doi: 10.1126/science.8385800. [DOI] [PubMed] [Google Scholar]
- 27.Lechleiter JD, John LM, Camacho P. Ca2+ wave dispersion and spiral wave entrainment in Xenopus laevis oocytes overexpressing Ca2+ ATPases. Biophys Chem. 1998;72:123–129. doi: 10.1016/s0301-4622(98)00128-8. [DOI] [PubMed] [Google Scholar]
- 28.Green KN, Demuro A, Akbari Y, Hitt BD, Smith IF, Parker I, Laferla FM. SERCA pump activity is physiologically regulated by presenilin and regulates amyloid {beta} production. J Cell Biol. 2008;181:1107–1116. doi: 10.1083/jcb.200706171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demuro A, Parker I. Optical single-channel recording: imaging Ca2+ flux through individual ion channels with high temporal and spatial resolution. J Biomed Opt. 2005;10:11002. doi: 10.1117/1.1846074. [DOI] [PubMed] [Google Scholar]
- 30.Yao Y, Choi J, Parker I. Quantal puffs of intracellular Ca2+ evoked by inositol trisphosphate in Xenopus oocytes. J Physiol. 1995;482(Pt 3):533–553. doi: 10.1113/jphysiol.1995.sp020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marchant JS, Taylor CW. Cooperative activation of IP3 receptors by sequential binding of IP3 and Ca2+ safeguards against spontaneous activity. Curr Biol. 1997;7:510–518. doi: 10.1016/s0960-9822(06)00222-3. [DOI] [PubMed] [Google Scholar]
- 32.Rose HJ, Dargan S, Shuai J, Parker I. ‘Trigger’ events precede calcium puffs in Xenopus oocytes. Biophys J. 2006;91:4024–4032. doi: 10.1529/biophysj.106.088872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith IF, Wiltgen SM, Parker I. Localization of puff sites adjacent to the plasma membrane: functional and spatial characterization of Ca2+ signaling in SH-SY5Y cells utilizing membrane-permeant caged IP3. Cell Calcium. 2009;45:65–76. doi: 10.1016/j.ceca.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith IF, Parker I. Imaging the quantal substructure of single IP3R channel activity during Ca2+ puffs in intact mammalian cells. Proc Natl Acad Sci U S A. 2009;106:6404–6409. doi: 10.1073/pnas.0810799106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shuai J, Pearson JE, Foskett JK, Mak DO, Parker I. A kinetic model of single and clustered IP3 receptors in the absence of Ca2+ feedback. Biophys J. 2007;93:1151–1162. doi: 10.1529/biophysj.107.108795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parker I, Ivorra I. Characteristics of membrane currents evoked by photoreleased inositol trisphosphate in Xenopus oocytes. Am J Physiol. 1992;263:C154–165. doi: 10.1152/ajpcell.1992.263.1.C154. [DOI] [PubMed] [Google Scholar]
- 37.Horne JH, Meyer T. Luminal calcium regulates the inositol trisphosphate receptor of rat basophilic leukemia cells at a cytosolic site. Biochemistry. 1995;34:12738–12746. doi: 10.1021/bi00039a033. [DOI] [PubMed] [Google Scholar]
- 38.Thrower EC, Choe CU, So SH, Jeon SH, Ehrlich BE, Yoo SH. A functional interaction between chromogranin B and the inositol 1,4,5-trisphosphate receptor/Ca2+ channel. J Biol Chem. 2003;278:49699–49706. doi: 10.1074/jbc.M309307200. [DOI] [PubMed] [Google Scholar]
- 39.Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, Vais H, Cheung KH, Yang J, Parker I, Thompson CB, Birnbaum MJ, Hallows KR, Foskett JK. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]