Abstract
We report for the first time the impact of neoadjuvant oral low-dose chemotherapy consisting of oral trofosfamide, idarubicin, and etoposide (O-TIE) in the case of alveolar Rhabdomyosarcoma (RMS) in the lower jaw of an 18-year-old woman at 27-weeks of gestation, without fetal complications and a highly efficient anti-tumor response. Our study suggests the possible application of O-TIE treatment in a neoadjuvant setting during pregnancy and recommends a schedule that can be considered for the treatment of patients with high-risk sarcomas who cannot be treated with intensive chemotherapy for various reasons.
Keywords: oral chemotherapy, pregnancy, rhabdomyosarcoma
INTRODUCTION
The incidence of cancer during pregnancy is relatively low and has been found in approximately 1:1,000 gestations [1]. Rhabdomyosarcoma (RMS) occurs most frequently in pediatric and adolescent patients, accounting for more than 50% of soft tissue sarcomas in these age groups, but is extremely rare during pregnancy. The multimodal treatment of RMS includes an intense intravenous (i.v.) multiagent chemotherapy, radiotherapy and surgery [2]. However, depending on gestational age, dose and exposure time of the different agents this treatment can cause severe damage to the fetus, including spontaneous abortion, fetal abnormalities, growth retardation, or cardiac and hematologic toxicity [3-5].
We report for the first time the successful and well tolerable neoadjuvant application of oral low-dose chemotherapy in a pregnant woman in the 27th week of gestation who presented with an alveolar RMS infiltrating the mandible. Our patient was treated with oral low-dose chemotherapy consisting of trofosfamide, idarubicin, and etoposide (O-TIE). This regimen was used as induction therapy followed by intense i.v. chemotherapy according to the trial CWS-96-IV for soft tissue sarcomas of the German Pediatric Oncology and Hematology Society (GPOH).
CASE REPORT
An 18-year-old woman at 27-weeks of gestation, gravida 2, para 0, was referred to our hospital with a painless mass of her left lower jaw which had rapidly progressed over approximately four weeks (Fig. 1). Facial magnetic resonance imaging (MRI) disclosed a tumor measuring 4.4 × 4.1 × 3.0cm (volume: 28ml) with infiltration of the left mandibular bone. Grossly, the tumor was well circumscribed with an oral/buccal gelatinous nodular outside portion (Fig. 1). No distant metastases were found in staging studies performed by ultrasonography. The family history was inconspicuous for any malignant diseases.
Fig. 1.
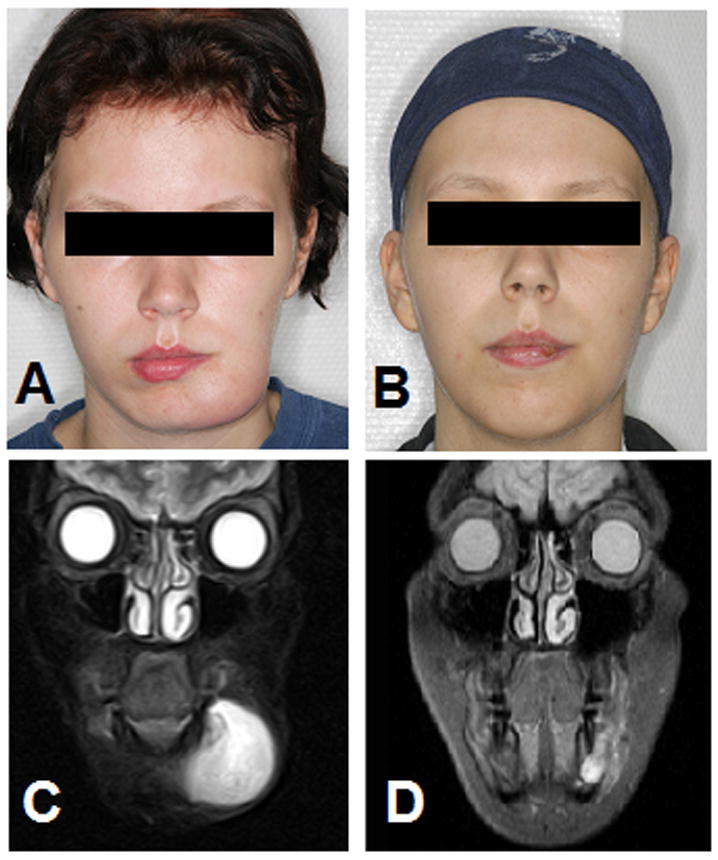
O-TIE treatment of 18-year old pregnant women with facial alveolar RMS. (A) Photograph of swelling of the left lower jaw. (B) Follow-up photograph demonstrating resolution of the facial alveolar RMS after oral low-dose chemotherapy with O-TIE. (C) Cranial MRI scan (STIR sequence, coronar, 0.3 TESLA). Tumor mass in the left inferior mandible that extends into the mandibular bone and the soft tissue of the jaw maximum (tumor size: 4.4 × 4.1 × 3.0cm, volume 28ml). (D) Follow-up cranial MRI scan after O-TIE (STIR sequence, coronar, 1.5 TESLA). Clearly marked regression of the extended alveolar RMS in the lower jaw 13 days after last O-TIE chemotherapy (tumor size: 1.5 × 1.2 × 2.1cm, volume 2ml).
Histopathologic analysis disclosed a mesenchymal neoplasm with musculoskeletal differentiation including alveolar and embryonal rhabdomyoblasts. Immunohistochemical staining was positive for desmin, actin, myogenin and S-100, and negative for pan cytokeratin. The proliferation index (measured as Ki-67 positive cells) was approximately 30%. An independent panel of pathologists gave a final diagnosis of an alveolar RMS, clinical grade III according to the Intergroup Rhabdomyosarcoma Study Group System (IRS), tumor, node, metastases system (TNM) stage T1b N0 M0.
To protect the fetus from severe side effects of intensive intravenous chemotherapy, oral low-dose chemotherapy (O-TIE) was administered to the mother according to the maintenance therapy of the GPOH-CWS-96-IV-protocol. The schedule included alternating courses of (A) 2 × 75mg/m2 trofosfamide for days 1–10 combined with 1 × 5mg/m2 idarubicin on days 1, 4, 7, and 10 and (B) 2 × 75mg/m2 trofosfamide on days 1–10 combined with 2 × 25mg/m2 etoposide on days 1–10. Both courses A and B were given twice, without interruption, for 40 consecutive days. Written consent was obtained from the patient before starting this experimental therapy.
O-TIE chemotherapy began at 28+1 weeks of gestation. With the exception of mild hair loss, the chemotherapy was not accompanied by clinically relevant side effects or complications. The lack of myelosuppression meant that no antibiotics were required for severe infections. Neither platelet nor erythrocyte transfusions were necessary. Abdominal ultrasounds were performed weekly to evaluate the development of the fetus. No pathological features or signs of abnormalities in the fetus were detected.
Five days after the last course of O-TIE with trofosfamide and etoposide, at 34+1 weeks of gestation, a healthy male preterm infant was born by Cesarean section with a birth weight of 1,790g (10th–25th percentile), length 42cm (3rd–10th percentile), head circumference 29.8cm (10th–25th percentile), APGAR score of 9/9/9 at 1/5/10 minutes, umbilical cord blood pH7.39. Echocardiography and ultrasound screening of the newborn revealed no abnormalities. The blood cell count of the newborn was unremarkable with hemoglobin 14.5g/dl, leukocytes 8.000/μl and platelets 465.000/μl. Lactate dehydrogenase, C-reactive protein, bilirubin, and electrolyte values were normal. Cord blood and serum levels of the mother were negative for residual etoposide (as measured by HPLC). The mother’s postpartum facial MRI showed tumor reduction from the initial volume of 28ml (tumor size: 4.4 × 4.1 × 3.0cm) to 2ml (tumor size: 1.5 × 1.2 × 2.1cm) six weeks after start of O-TIE (Fig. 1). Whole-body skeletal scintigraphy and additional computed tomography scan of the chest showed no metastases.
After recovery from Cesarean section and implantation of a Hickman catheter, intravenous chemotherapy was started according to the GPOH-CWS2002-P-VAIA high-risk group protocol. Two months after irradiation and the last intravenous chemotherapy cycle, local surgery with resection of the left mandible and parts of the bottom of the oral cavity with reconstruction followed. Histology of the resected tissue showed rarely disseminated tumor cells (desmin+, MIB-1+), and resection margins and bony parts that were free of tumor. Further, O-TIE maintenance chemotherapy according to the CWS-96-IV protocol was pursued one month after local surgery for about 6 months (8 × TI, 8 × TE; every three weeks). The patient exhibited no evidence of recurrence of RMS 2 years after the end of therapy. The infant had no evidence of any malformations, and exhibited normal neurological development during the 23/12 years of follow-up.
DISCUSSION
Malignancy diagnosed during pregnancy is perceived as a situation where the life of the mother is in conflict with that of the developing fetus [4,6]. Nonetheless, cautious administration of chemotherapy during the second or third trimester of pregnancy may be associated with severe adverse effects, including cardiac toxicity, growth retardation, septicemia, and/or bleeding due to the chemotherapy-induced transient fetal myelosuppression [7-9]. We demonstrated that oral low-dose chemotherapy with O-TIE produced a highly efficient anti-tumor response without associated side effects in the mother or fetus. To our knowledge, to date there are no published case reports on the use of oral low-dose chemotherapy in a pregnant woman with alveolar RMS. Meazza et al. reported customized treatment with mild doses of intravenous chemotherapy and alternating courses of cyclophosphamide with doxorubicin or actinomycin D administered weekly for treatment of a pregnant woman until birth. Unfortunately, despite an initially good tumor response the disease progressed and the patient died after delivery of a healthy child [10]. In contrast, Martin et al. reported the case of an 18-year-old woman with facial alveolar RMS diagnosed during the third trimester, which showed a complete remission after three intravenous courses of intensive multiagent chemotherapy (cyclophosphamide, actinomycin D, vincristine), without fetal complications. However, severe pancytopenia and febrile temperatures developed in the mother, which were associated with life-threatening adverse effects on the fetus [11]. Beside the early teratogenic effects after fetal exposure to intensive intravenous chemotherapy administered to the mothers, the infectious complications caused by transient neonatal myelosuppression play a pivotal role in the third trimester [8].
Our O-TIE treatment schedule considered three essential pharmacological aspects. First, the drug combination in the O-TIE schedule was based on chemotherapy regimens reported to be relatively safe for use in the second and third trimester of pregnancy including primarily oxazaphosphorines and anthracyclines [12-14]. The successful use of etoposide alone or in combination with other antineoplastic agents during pregnancy has been previously described [15-17]. Second, a prospective study of patients with stage IV RMS or “RMS-like” tumors showed that the efficacy of O-TIE, in terms of survival, was significantly better than intravenous high-dose chemotherapy after standard chemotherapy with carboplatin, etoposide, vincristine, actinomycin D, ifosfamide, and epirubicin (CEVAIE) [18]. Third, the reported toxicity of the O-TIE arm was low, even though all patients were pretreated with intensive chemotherapy [18,19]. Although the O-TIE concept was attractive as mild therapy in our patient during the 27th week of gestation, a favourable outcome was not necessarily a matter of course. In contrast to other maintenance chemotherapy concepts, we used O-TIE chemotherapy in the present study as an experimental front-line treatment. These data must be taken into consideration in view of the harmful prognostic features of our patient, i.e. age >10 years, local invasiveness, alveolar subtype, and facial tumor site [20]. However, in accordance with data from the literature, the results demonstrate that the application of O-TIE seems to be an attractive alternative to provide long-lasting, low-dose chemotherapy [18,19], and may act through different mechanisms [13,20], without an increase in side effects and extension of the patients’ hospitalization time. Since different experiences demonstrate that chemotherapy given in the third trimesters of pregnancy is usually not dangerous for the fetal development, even higher doses of the O-TIE concept than those used in the presented case can be discussed in the future. Additional studies on treatment with oral low-dose therapies in RMS are clearly needed to further clarify the role and potential benefit of these anti-cancer strategies.
CONCLUSIONS
In our case report of a pregnant woman with alveolar RMS neoadjuvant oral administration of chemotherapy in an outpatient setting appears feasible during pregnancy, is well tolerable, and seems to provide a good outcome for the mother and the child. This case report is not a proof of principle, but an interesting observation showing that oral low-dose chemotherapy can be highly effective in the neoadjuvant setting in unusual cases like pregnancy, and does not negate the clear benefit of intensive i.v. chemotherapy and surgery (with or without irradiation) for the treatment of standard cases of RMS.
Acknowledgments
This work was supported in part by the US National Institute of Health (RO1CA91889), Scott & White Memorial Hospital and Clinic, the Texas A&M Health Science Center College of Medicine, the Central Texas Veterans Health Administration and an Endowment from the Cain Foundation (to A.A.).
References
- 1.Pereg D, Koren G, Lishner M. Cancer in pregnancy: gaps, challenges and solutions. Cancer Treat Rev. 2008;34:302–312. doi: 10.1016/j.ctrv.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Sultan I, Qaddoumi I, Yaser S, et al. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: an analysis of 2,600 patients. J Clin Oncol. 2009;27:3391–3397. doi: 10.1200/JCO.2008.19.7483. [DOI] [PubMed] [Google Scholar]
- 3.Cardonick E, Iacobucci A. Use of chemotherapy during human pregnancy. Lancet Oncol. 2004;5:283–291. doi: 10.1016/S1470-2045(04)01466-4. [DOI] [PubMed] [Google Scholar]
- 4.Gwyn K. Children exposed to chemotherapy in utero. J Natl Cancer Inst Monogr. 2005:69–71. doi: 10.1093/jncimonographs/lgi009. [DOI] [PubMed] [Google Scholar]
- 5.Zemlickis D, Lishner M, Degendorfer P, et al. Maternal and fetal outcome after invasive cervical cancer in pregnancy. J Clin Oncol. 1991;9:1956–1961. doi: 10.1200/JCO.1991.9.11.1956. [DOI] [PubMed] [Google Scholar]
- 6.Van Calsteren K, Heyns L, De Smet F, et al. Cancer during pregnancy: an analysis of 215 patients emphasizing the obstetrical and the neonatal outcomes. J Clin Oncol. 2010;28:683–689. doi: 10.1200/JCO.2009.23.2801. [DOI] [PubMed] [Google Scholar]
- 7.Sadural E, Smith LG., Jr Hematologic malignancies during pregnancy. Clin Obstet Gynecol. 1995;38:535–546. doi: 10.1097/00003081-199509000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Udink ten Cate FE, ten Hove CH, Nix WM, et al. Transient neonatal myelosuppression after fetal exposure to maternal chemotherapy. Case report and review of the literature. Neonatology. 2009;95:80–85. doi: 10.1159/000151759. [DOI] [PubMed] [Google Scholar]
- 9.Zemlickis D, Lishner M, Degendorfer P, et al. Fetal outcome after in utero exposure to cancer chemotherapy. Arch Intern Med. 1992;152:573–576. [PubMed] [Google Scholar]
- 10.Meazza C, Casanova M, Zaffignani E, et al. An adolescent with rhabdomyosarcoma during pregnancy. Tumori. 2008;94:431–433. doi: 10.1177/030089160809400324. [DOI] [PubMed] [Google Scholar]
- 11.Martin D, Winter SS, Gardner MO, et al. Rhabdomyosarcoma treated with chemotherapy during the third trimester. Obstet Gynecol. 1997;89:828–831. doi: 10.1016/s0029-7844(97)00034-3. [DOI] [PubMed] [Google Scholar]
- 12.Germann N, Goffinet F, Goldwasser F. Anthracyclines during pregnancy: embryo-fetal outcome in 160 patients. Ann Oncol. 2004;15:146–150. doi: 10.1093/annonc/mdh009. [DOI] [PubMed] [Google Scholar]
- 13.Wagner A, Hempel G, Boos J. Trofosfamide: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in the oral treatment of cancer. Anticancer Drugs. 1997;8:419–431. [PubMed] [Google Scholar]
- 14.Tillmann B, Krumpelmann S, Wurthwein G, et al. Pharmacokinetic aspects of oral administration of etoposide. Klin Padiatr. 1998;210:159–164. doi: 10.1055/s-2008-1043872. [DOI] [PubMed] [Google Scholar]
- 15.Kushner BH, Kramer K, Cheung NK. Oral etoposide for refractory and relapsed neuroblastoma. J Clin Oncol. 1999;17:3221–3225. doi: 10.1200/JCO.1999.17.10.3221. [DOI] [PubMed] [Google Scholar]
- 16.Karimi Zarchi M, Behtash N, Modares Gilani M. Good pregnancy outcome after prenatal exposure to bleomycin, etoposide and cisplatin for ovarian immature teratoma: a case report and literature review. Arch Gynecol Obstet. 2008;277:75–78. doi: 10.1007/s00404-007-0416-3. [DOI] [PubMed] [Google Scholar]
- 17.Han JY, Nava-Ocampo AA, Kim TJ, et al. Pregnancy outcome after prenatal exposure to bleomycin, etoposide and cisplatin for malignant ovarian germ cell tumors: report of 2 cases. Reprod Toxicol. 2005;19:557–561. doi: 10.1016/j.reprotox.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Klingebiel T, Boos J, Beske F, et al. Treatment of children with metastatic soft tissue sarcoma with oral maintenance compared to high dose chemotherapy: report of the HD CWS-96 trial. Pediatr Blood Cancer. 2008;50:739–745. doi: 10.1002/pbc.21494. [DOI] [PubMed] [Google Scholar]
- 19.Kamen BA, Rubin E, Aisner J, et al. High-time chemotherapy or high time for low dose. J Clin Oncol. 2000;18:2935–2937. doi: 10.1200/JCO.2000.18.16.2935. [DOI] [PubMed] [Google Scholar]
- 20.Koscielniak E, Morgan M, Treuner J. Soft tissue sarcoma in children: prognosis and management. Paediatr Drugs. 2002;4:21–28. doi: 10.2165/00128072-200204010-00003. [DOI] [PubMed] [Google Scholar]


