Abstract
This prospective, randomized, controlled experimental study looks at the effects of the peroxynitrite decomposition catalyst WW-85 on global hemodynamics and regional microvascular blood flow (RMBF) in an established ovine model of septic shock following severe smoke inhalation injury. Twenty-one sheep were randomized into a sham group (no injury), a control group (smoke/sepsis), and a treatment group (smoke/sepsis/WW-85; n=7 each). WW-85 was administered 1h post injury as bolus (0.1 mg/kg), followed by a continuous infusion of 0.02 mg/kg/h RMBF was analyzed using colored microspheres. All control animals developed a hypotensive, hyperdynamic circulation and increased plasma levels of nitrate/-nitrite (NOx). All hemodynamic variables and NOx levels were significantly improved in the treatment group. In visceral organs of controls, blood flow to trachea, ileum, and spleen significantly increased (p<0.05). Blood flow to kidneys and pancreas significantly decreased (p<0.05). Treatment with WW-85 stabilized blood flow to ileum, spleen, and kidneys on baseline levels and was significantly improved compared to controls (p<0.05). Cerebral blood flow deteriorated in controls, but was significantly improved in cerebral cortex, cerebellum, pons, medulla oblongata, and thalamus (p<0.05) by WW-85. These results provide evidence that WW-85 blocks NO production, thereby improving cardiovascular function and microcirculation.
Keywords: Acute lung injury, ARDS, Cerebral autoregulation, Cerebral blood flow, Nitric oxide, Smoke inhalation
1. Introduction
Every year, 5,000 to 10,000 patients die in the United States by smoke inhalation, 23,000 patients get injured, and about 5,000 of these are fire fighters [1]. Acute lung injury (ALI) after smoke inhalation trauma is frequently associated with multiple non-pulmonary organ failure [2], along with pneumonia and sepsis, which in turn is a major cause of morbidity and mortality in thermally injured patients [3, 4]. Yearly, more than 750,000 patients in the U.S. develop sepsis, and ~ 30% of these patients die [5]. More than 75% of these deaths occur within the first 24 hrs after establishing the diagnosis and result mainly from persistent arterial hypotension. The remaining deaths occur after successful treatment of this hypotension and result from the development of multiple organ failure, associated with impairment of regional and micro-regional blood flow, contributing to reperfusion injury and/or irreversible damage sustained during the period of hypotension [6]. In addition, cerebral dysfunction often occurs after smoke inhalation injury, and in septic patients, the pathophysiologic background, however, is still not fully understood.
Systemic inflammatory response syndrome (SIRS) and sepsis are associated with an excessive production of reactive nitrogen species, such as nitric oxide (NO•). Furthermore, it is proposed that in septic shock, the release of reactive oxygen species (ROS) from macrophages, e.g. superoxide (O2−) is increased. Subsequently, peroxynitrite (ONOO−) is formed by a rapid reaction between NO• and O2−. ONOO−, in turn, acts as a terminal mediator of cellular injury under various pathophysiologic conditions of oxidative and nitrosative stress, and is able to induce cell necrosis and apotosis [7].
We hypothesized that blocking ONOO− formation will improve cardiovascular and micro-regional blood flow in septic shock associated with smoke inhalation injury. The purpose of the present study was to asses the effects of the metalloporphyrinic peroxynitrite decomposition catalyst WW-85 on a) cardiovascular functions and b) distribution of blood flow during the development of non-pulmonary organ dysfunction, using an established and clinically relevant model of septic shock in association with smoke inhalation injury in sheep [8].
2. Materials and methods
2.1. Animal care and use
The Institutional Animal Care and Use Committee at the University of Texas Medical Branch (UTMB) at Galveston approved this study. The Investigational Intensive Care Unit (IICU) at UTMB is an Association for Assessment and Accreditation of Laboratory Animal Care International approved facility (AAALAC). The guidelines of the National Institutes of Health (NIH) for the care and use of experimental animals were carefully followed. The animals were individually housed in metabolic cages and were studied in awake state.
2.2. Surgical preparation
Twenty-one female Merino sheep, weighing 34–38 kg, were anesthetized with isoflurane (Vedco Inc., St. Joseph, MO) using an inhalation mixture of 1–2 Vol% in oxygen, and endotracheally intubated. The sheep were ventilated with a mixture of room air and O2 (FiO2 0.5). Under aseptic conditions, the animals were chronically instrumented for hemodynamic monitoring as previously described [9–11]. Briefly, the right femoral artery was cannulated, and a polyvinylchloride catheter (Intracath™, 16-G, 24-inch, Becton Dickinson Vascular Access, Sandy, UT) was positioned in the descending aorta. A 7-French Swan-Ganz™ thermodilution catheter (model 93A-131-7F; Edwards Critical Care Division, Irvine, CA) was inserted into the right jugular vein through an 8.5-French percutaneous introducer sheath (Edwards Lifescience, Irvine, CA) and was advanced into the common pulmonary artery. Through a left thoracotomy at the level of the 5th intercostal space, a Silastic® catheter (0.062 inch inner diameter, 0.125 inch outer diameter, Dow-Corning, Midland, MI) was positioned in the left atrium. The animals were given a seven-day recovery period. During this time, they had free access to food and water.
2.3. Experimental protocol and measurements
One day before the experiment began; catheters were connected to pressure transducers (Model PX3X3, Baxter Edwards Critical Care Division, Irvine, CA) with continuous flushing devices. Electronically calculated mean pressures were recorded on a monitor with graphic and digital displays (model 7830A, Hewlett Packard, Santa Clara, CA). Cardiac output (CO) was determined with the thermal dilution technique and displayed on a cardiac output computer (COM-1; Baxter Edwards Critical Care Division, Irvine, CA). Pressures were measured while the sheep were standing and calm. Zero calibrations were taken at the olecranon joint on the front leg while the animals were standing. Core body temperature was measured with the thermistor of the Swan Ganz™ catheter. Ten mL of 5 % dextrose solution at 1–2 °C served as the thermal indicator. Arterial blood gas samples were analyzed at 37 °C using a conventional blood gas analyzer (Synthesis 15, Instrumentation Laboratories, Lexington, MA) and were corrected for core body temperature. Carboxy-hemoglobin (COHb) saturation was measured using a CO-Oximeter (model IL482, Instrumentation Laboratory, Lexington, MA). (6).
Following a baseline (BL) measurement in the healthy state, sheep were randomly allocated to one of the three groups (each n=7): sham (uninjured, untreated), control (injured, untreated), and WW-85 (injured, treated with WW-85). Thereafter, a tracheotomy was performed under ketamine anesthesia (5 mg/kg). In addition, a Foley® urinary retention catheter was placed in the urinary bladder of all animals to monitor fluid balance. Anesthesia was then maintained using 1.5–2.5% halothane (Vedco Inc., St. Joseph, MO) in O2.
The control and WW-85 animals were subjected to smoke inhalation injury and bacterial challenge according to an established protocol [8, 12]. Each animal in both groups was insufflated with 4 sets of 12 breaths of cotton smoke (< 40 °C). The smoke was generated and delivered by a modified bee smoker that was filled with 40 g of burning cotton toweling and attached to the tracheotomy tube via a modified endotracheal tube. The tube contained an indwelling thermistor from a Swan-Ganz™ catheter to measure the airway temperature during the procedure. The sham group received 4 sets of 12 breaths of room air through the bee smoker. Arterial COHb plasma concentrations were determined after each set of smoke or air inhalation and served as an index of lung injury.
After smoke inhalation, an experimental bacterial solution was instilled into the lung lobes of control and WW-85 animals, using a bronchoscope (Model PF-P40, Olympus America Inc. Melville, NY). Live Pseudomonas aeruginosa (2–5 ×1011cfu), was suspended in 30 mL of saline and instilled into the right lower and middle lobes (10 mL each) as well as the left lower lobe (10 mL). The sham group received only the vehicle (30 mL of NaCl 0.9%) that was instilled in the same way. Anesthesia was then discontinued and the sheep were allowed to awaken [8].
All animals were mechanically ventilated (Servo-Ventilator 900C, Siemens, Elema, Sweden) with a tidal volume of 15 mL/kg and an initial respiration rate of 30/min for the duration of the 24-h study period. In this context it is important to mention that sheep have a higher lung compliance than man, and therefore require higher tidal volumes than humans. Ventilator settings were periodically adjusted to maintain the arterial pCO2 of the blood 10% below of baseline values to allow mechanical ventilation in the awake state. Positive end-expiratory pressure (PEEP) remained on a fixed level of 5 cmH2O to avoid ventilation-related differences in the study groups. These ventilator settings were chosen in accordance with those originally described by Murakami et al. [8].
All animals were fluid resuscitated, initially starting with an infusion rate of 2 mL•kg−1•h−1 lactated Ringer’s solution. The fluid rate was then adjusted, depending on hematocrit (Hct), to prevent hemoconcentration/dilution. Fluid resuscitation was limited to a maximum of 1 L/h, since we have noted in previous studies that fluid administration at a rate of more than 1 L/h does not affect Hct in our sheep model because of vascular leakage associated with septic shock [12]. Since sheep don’t perspire and the inhaled gases were humidified, the fluid balance was calculated as the difference of fluid input and urinary output (mL). Hematocrit was assessed in heparinized microhematocrit capillary tubes (Fisher Brand, Pittsburgh, PA). Values < baseline indicated hemodilution, and values > baseline reflected hemoconcentration. During the experiment, the animals had free access to food, but not to water to accurately determine fluid intake [13].
One hour post injury, WW-85 was dissolved in saline and was intravenously administered with an initial bolus of 0.1 mg/kg followed by a continuous infusion of 0.02 mg•kg−1•h−1 until the end of the 24-h experimental period. The sham and control group received the same amount of the vehicle (NaCl 0.9%) [14].
The determination of regional blood flow was performed using colored microspheres. Approximately five million microspheres (15.0 ± 0.1 µm) were injected into the left atrium at BL, 6, 12, and 24 h, while reference blood was withdrawn from the femoral arterial catheter at a constant rate of 10 mL/min. The color of the microspheres was randomized for each injection [2, 15].
The concentration of NOx (total amount of nitric oxide metabolites) in the plasma was measured intermittently. Plasma samples were subjected to NOx reduction using vanadium (III) as a reducing agent in a commercial instrument (model 745, Antek Instruments, Houston, TX). The resulting NO was measured with a chemiluminescent NO analyzer (model 7020, Antek) and was recorded by dedicated software as the NO content (in µM) [8].
2.4. Postmortem examination
After completion of the experiment, the animals were anesthetized with ketamine (15 mg/kg) and sacrificed by intravenous injection of 60 mL saturated potassium chloride. Immediately after death, representative transmural tissue samples were obtained from the distal trachea, pancreas, spleen, both kidneys (cortex), and ileum. In addition, brain tissue was taken from different cerebral areas, such as cortex cerebri, medulla oblongata, cerebellum, thalamus, and pons. All these tissue samples were analyzed by Interactive Medical Technologies Ltd. (Los Angeles, CA) by determining the weight of each tissue sample, digesting the entire sample in a high concentration of NaOH, and measuring the total number of different colored spheres using flow cytometry. Regional blood flow in mL•min−1•g−1 was then calculated using the following formula: Regional blood flow = (Total tissue spheres) / ((Tissue weight, grams) × (Reference spheres / mL / min)) [15].
2.5. Statistics
For statistical analysis, Sigma Stat 2.03 software (SPSS Inc., Chicago, IL) was used. After confirming normal distribution (Kolmogorov-Smirnov test), a two-way analysis of variance (ANOVA) for repeated measurements with appropriate Student-Newman-Keuls post hoc comparisons was used to detect differences within and between groups. Significance was assumed when p was less than 0.05. Data are presented as means ± standard errors of the mean (SEM).
3. Results
3.1. Injury and survival
The arterial COHb determined immediately after the 4th set of smoke exposure averaged 63 ± 4 % in the control group and 66 ± 2.5 % in the WW-85 group. The sham group, which was not exposed to smoke inhalation, showed a COHb level of 4 ± 1 % after receiving 4 sets of 12 breaths of room air through the bee smoker. The core body temperature in the control and WW-85 group significantly increased over time compared to the sham group and BL (p<0.05, Table 1). The COHb levels as well as the increase in core body temperature in the control and WW-85 groups were not statistically different from one another and reflected the consistency of the injury in each group. With aggressive fluid administration, all animals survived the 24-h study period.
Table 1.
CVP, central venous pressure; MPAP, mean pulmonary artery pressure; PAOP, pulmonary artery occlusion pressure; LAP, left atrial pressure; CI, cardiac index; SVRI, systemic vascular resistance index; HCT, hematocrit; PONC, plasma oncotic pressure; PNOx, plasma nitrate-to-nitrite level; TEMP, temperature; PaO2/FiO2, Horovitz-Index; PaCO2, arterial carbon dioxide tension; a.pH, arterial power of hydrogen; SaO2, arterial oxygen saturation.
| Variables | Group | Values (Mean ± S.E.M.) |
|||||
|---|---|---|---|---|---|---|---|
| Time | BL = 0 hr | 3 hr | 6 hr | 12 hr | 18 hr | 24 hr | |
| CVP | Sham | 7.6 ± 0.9 | 10.5 ± 1.4# | 10.9 ± 1.1# | 10.9 ± 1.2# | 10.4 ± 1.2# | 10.1 ± 1.1# |
| (mm Hg) | Control | 7.0 ± 0.8 | 9.0 ± 1.1# | 11.0 ± 0.9# | 12.0 ± 1.1# | 14.0 ± 1.0# | 14.0 ± 1.0# |
| WW-85 | 6.0 ± 1.0 | 9.7 ± 1.6# | 12.0 ± 1.4# | 12.6 ± 0.6# | 13.4 ± 0.8# | 12.9 ± 1.1# | |
| MPAP | Sham | 22.3 ± 0.8 | 25.6 ± 0.8 | 25.3 ± 1.1 | 25.6 ± 1.1 | 25.7 ± 1.6 | 26.1 ± 1.8 |
| (mm Hg) | Control | 21.1 ± 0.6 | 26.6 ± 1.6# | 29.3 ± 1.9#† | 29.1 ± 0.5# | 32.0 ± 0.9#† | 33.3 ± 1.3#† |
| WW-85 | 19.7 ± 0.5 | 21.4 ± 1.7#*† | 24.3 ± 1.4#* | 27.7 ± 0.9# | 29.1 ± 1.2#† | 32.6 ± 1.4#† | |
| PAOP | Sham | 11.6 ± 1.2 | 14.0 ± 1.1# | 15.9 ± 0.7# | 14.1 ± 1.1# | 15.0 ± 0.8# | 14.4 ± 0.8# |
| (mm Hg) | Control | 10.3 ± 0.6 | 15.0 ± 1.4# | 16.4 ± 0.8# | 17.4 ± 1.0#† | 17.6 ± 0.8# | 19.1 ± 0.6#† |
| WW-85 | 9.1 ± 0.6 | 14.1 ± 1.5# | 15.1 ± 1.3# | 18.4 ± 1.1#† | 18.4 ± 1.5# | 16.3 ± 1.4# | |
| LAP | Sham | 6.9 ± 1.1 | 11.0 ± 0.8# | 10.7 ± 0.9# | 10.9 ± 1.1# | 10.7 ± 1.0# | 11.0 ± 0.9# |
| (mm Hg) | Control | 6.9 ± 0.8 | 10.3 ± 0.8# | 13.0 ± 1.5# | 13.6 ± 1.3# | 14.9 ± 1.7#† | 15.3 ± 1.8#† |
| WW-85 | 6.4 ± 0.5 | 11.1 ± 1.0# | 12.9 ± 1.1# | 15.0 ± 1.5#† | 17.6 ± 2.1#† | 16.0 ± 1.9#† | |
| CI | Sham | 5.6 ± 0.2 | 5.1 ± 0.3 | 5.2 ± 0.2 | 4.9 ± 0.3 | 5.0 ± 0.2 | 4.7 ± 0.3 |
| (L•min−1•m−2) | Control | 5.7 ± 0.4 | 5.3 ± 0.6 | 5.8 ± 0.5 | 6.3 ± 0.4 | 7.0 ± 0.5† | 7.7 ± 0.7#† |
| WW-85 | 5.8 ± 0.2 | 5.8 ± 0.3 | 6.1 ± 0.2 | 6.7 ± 0.3† | 6.7 ± 0.5† | 6.6 ± 0.4† | |
| SVRI | Sham | 1341 ± 97 | 1460 ± 99 | 1399 ± 65 | 1484 ± 77 | 1545 ± 66 | 1616 ± 88 |
| (dynes•m−5•m−2) | Control | 1381 ± 81 | 1388 ± 168 | 1228 ± 113 | 869 ± 66#† | 767 ± 59#† | 694 ± 88#† |
| WW-85 | 1241 ± 50 | 1242 ± 103 | 1162 ± 64 | 988 ± 55† | 1011 ± 116*† | 986 ± 71*† | |
| HCT | Sham | 24.6 ± 0.8 | 24.7 ± 1.8 | 24.7 ± 1.9 | 23.6 ± 1.4 | 23.3 ± 1.1 | 24.6 ± 1.4 |
| (%) | Control | 27.6 ± 2.2 | 27.9 ± 1.9 | 28.6 ± 2.0 | 28.9 ± 2.0 | 28.4 ± 2.6 | 28.4 ± 2.9 |
| WW-85 | 25.3 ± 1.3 | 27.0 ± 2.0 | 27.4 ± 2.5 | 24.9 ± 2.8 | 23.7 ± 1.8 | 24.9 ± 1.9 | |
| PONC | Sham | 22.2 ± 0.7 | 21.3 ± 1.2 | 21.2 ± 1.4 | 21.7 ± 1.1 | 22.7 ± 1.0 | 22.9 ± 1.1 |
| (mm Hg) | Control | 23.3 ± 0.6 | 19.1 ± 0.6# | 17.7 ± 0.8# | 15.8 ± 0.8#† | 14.1 ± 1.1#† | 13.1 ± 1.3#† |
| WW-85 | 22.2 ± 1.3 | 21.0 ± 0.9 | 20.3 ± 0.9 | 18.4 ± 1.1#† | 18.0 ± 1.3#*† | 17.9 ± 1.3#*† | |
| P NOx | Sham | 5.3 ± 0.5 | 6.0 ± 0.5 | 6.1 ± 0.7 | 5.5 ± 0.8 | 6.0 ± 0.6 | 5.7 ± 0.7 |
| (µM) | Control | 5.8 ± 1.2 | 7.2 ± 1.0 | 8.1 ± 0.8 | 8.8 ± 0.5#† | 9.5 ± 1.0#† | 8.1 ± 1.5 |
| WW-85 | 5.0 ± 0.3 | 6.5 ± 0.5 | 7.9 ± 1.0# | 7.5 ± 0.5# | 7.1 ± 1.0* | 5.5 ± 0.6* | |
| TEMP | Sham | 38.9 ± 0.1 | 39.3 ± 0.1 | 39.3 ± 0.1 | 39.1 ± 0.2 | 39.2 ± 0.1 | 39.3 ± 0.2 |
| (°C) | Control | 39.2 ± 0.1 | 40.2 ± 0.3 | 40.5 ± 0.3 | 40.5 ± 0.2 | 40.5 ± 0.3 | 40.2 ± 0.2 |
| WW-85 | 39.3 ± 0.1 | 40.0 ± 0.1 | 40.3 ± 0.2 | 40.7 ± 0.2 | 40.6 ± 0.1 | 40.7 ± 0.1 | |
| PaO2/FiO2 | Sham | 524 ± 8 | 477 ± 12 | 522 ± 29 | 524 ± 34 | 535 ± 34 | 544 ± 31 |
| Control | 503 ± 21 | 154 ± 39#† | 106 ± 26#† | 72 ± 5#† | 70 ± 8#† | 70 ± 4#† | |
| WW-85 | 524 ± 21 | 361 ± 22#*† | 294 ± 12#*† | 218 ± 13#*† | 212 ± 13#*† | 184 ± 18#*† | |
| PaCO2 | Sham | 32.4 ± 1.1 | 27.3 ± 1.4# | 24.4 ± 2.0# | 21.6 ± 1.0# | 20.7 ± 1.4# | 21.0 ± 2.4# |
| (mm Hg) | Control | 35.1 ± 0.5 | 24.9 ± 3.4# | 27.5 ± 1.8# | 28.8 ± 1.7#† | 28.8 ± 1.6#† | 27.0 ± 1.5#† |
| WW-85 | 36.4 ± 0.9 | 26.2 ± 2.1# | 27.6 ± 1.4# | 24.8 ± 1.3# | 23.3 ± 1.3#* | 25.8 ± 1.5#† | |
| a.pH | Sham | 7.48 ± 0.02 | 7.58 ± 0.03 | 7.57 ± 0.03 | 7.56 ± 0.01 | 7.55 ± 0.02 | 7.55 ± 0.03 |
| Control | 7.47 ± 0.01 | 7.60 ± 0.03# | 7.54 ± 0.02 | 7.52 ± 0.02 | 7.50 ± 0.03 | 7.50 ± 0.02 | |
| WW-85 | 7.48 ± 0.01 | 7.62 ± 0.03# | 7.58 ± 0.02# | 7.56 ± 0.01# | 7.55 ± 0.02# | 7.52 ± 0.03# | |
| SaO2 | Sham | 94.4 ± 0.9 | 96.7 ± 0.8 | 96.8 ± 0.6 | 96.5 ± 0.7 | 96.8 ± 0.8 | 96.3 ± 0.8 |
| (%) | Control | 93.1 ± 1.2 | 93.6 ± 1.2 | 88.1 ± 3.2† | 84.7 ± 2.6#† | 81.2 ± 3.3#† | 84.8 ± 1.9#† |
| WW-85 | 92.5 ± 0.4 | 95.8 ± 0.9# | 95.9 ± 0.5# | 93.3 ± 0.9 | 92.2 ± 1.8* | 88.0 ± 5.3 | |
Data are expressed as mean ± S.E.M.; significance was assumed when p was less than 0.05;
vs. BL,
vs. control,
vs. sham.
3.2. Global hemodynamics
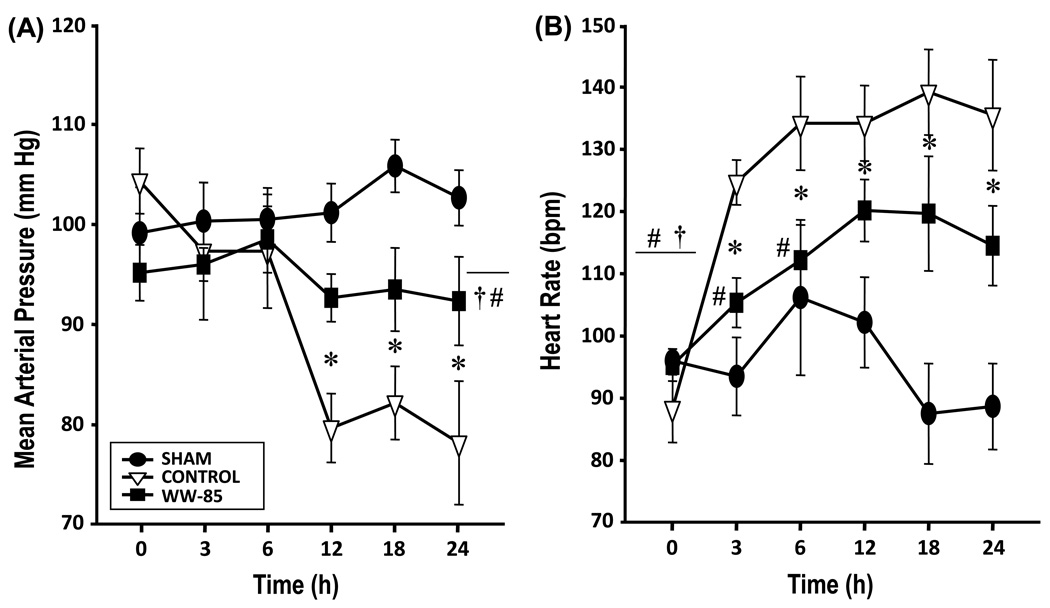
Cardiovascular variables remained stable in sham animals. In the control and WW-85 groups, mean arterial pressure (MAP, Figure 1) and systemic vascular resistance index (SVRI, Table 1) dropped significantly vs. baseline (BL) and vs. the sham group (p<0.05, each). MAP and SVRI in the WW-85 group, however, did not fall to the same extent as in the control group and were significantly improved as compared to controls (p<0.05).
Fig. 1.
Hemodynamic changes after smoke inhalation injury and bacterial challenge (1A) MAP, mean arterial pressure, mmHg; and 1(B) HR, heart rate, beats/min.
Data are expressed as mean ± SEM of 7 animals per group.
Heart rate (HR) significantly increased over time in both injured groups as compared to the sham group and BL (p<0.05). The increase in HR in sheep treated with WW-85 was significantly lower than in control animals (p<0.05, Figure 1).
Cardiac index (CI, Table 1) in sham animals remained stable over time. In the control group, CI increased over time and was significantly higher compared with the sham group. In WW-85-treated sheep, CI slightly increased over time, a statistical difference to BL was not detectable. CI tended to be lower in the WW-85 group as compared to the control group (n.s.).
Total fluid balance in the control (+ 1431±717 mL) and WW-85 (+ 690±870 mL) group was significantly higher as compared with the sham (− 1298±248 mL) group after 24 hrs (p<0.05, each). Fluid balance in the WW-85 group tended to be lower than in the control group.
PONC in the sham group remained on BL level during the 24-h study period. PONC significantly decreased over time in both injured groups. However, PONC in the WW-85 group did not fall to the same extend as in the control group and was significantly higher after 24 hrs (p<0.05, Table 1).
Plasma NOx levels in the sham group remained on BL levels during the entire experiment. In the control group, NOx levels increased significantly over time compared to the sham group, and the WW-85 group (each p<0.05, Table 1).
Central venous pressure (CVP), mean pulmonary artery pressure (MPAP), pulmonary artery occlusion pressure (PAOP), and left atrial pressure (LAP) remained stable in sham animals and showed an increase in both injured groups vs. BL and the sham group over time (Table 1). There was no difference in CVP between controls and sham, or controls and WW-85. MPAP was significantly lower in the WW-85 group as compared to the control group at 3 and 6 hrs post injury. PAOP and LAP were significantly higher in both injured groups than in the sham group (p<0.05). No statistical difference was detectable between the injured groups, indicating adequate fluid resuscitation in both groups.
Whereas the PaO2/FiO2 ratio remained stable in the sham group, it decreased significantly in the control and WW-85 group (p<0.05 vs. BL and vs. sham). The PaO2/FiO2 ratio in the WW-85 group did not fall to the same extent as in the control group and was significantly improved over the entire duration of the experiment (p<0.05, Table 1).
Oxygen saturation (SaO2, Table 1) remained unchanged in sham animals over time. In the control group, SaO2 was significantly lower than in sham animals from 6–24 h, and vs. BL from 12–24 h post injury. SaO2 in the WW-85 group improved and was significantly higher than BL at 6 and 12h post injury, and tended to be higher than controls throughout. However, even though a statistical significant difference could only be seen at 18h post injury, the difference of SaO2 between groups is of clinical importance. Hematocrit (Hct) remained on BL levels in all groups, indicating adequate fluid resuscitation.
3.3. Regional microvascular blood flow
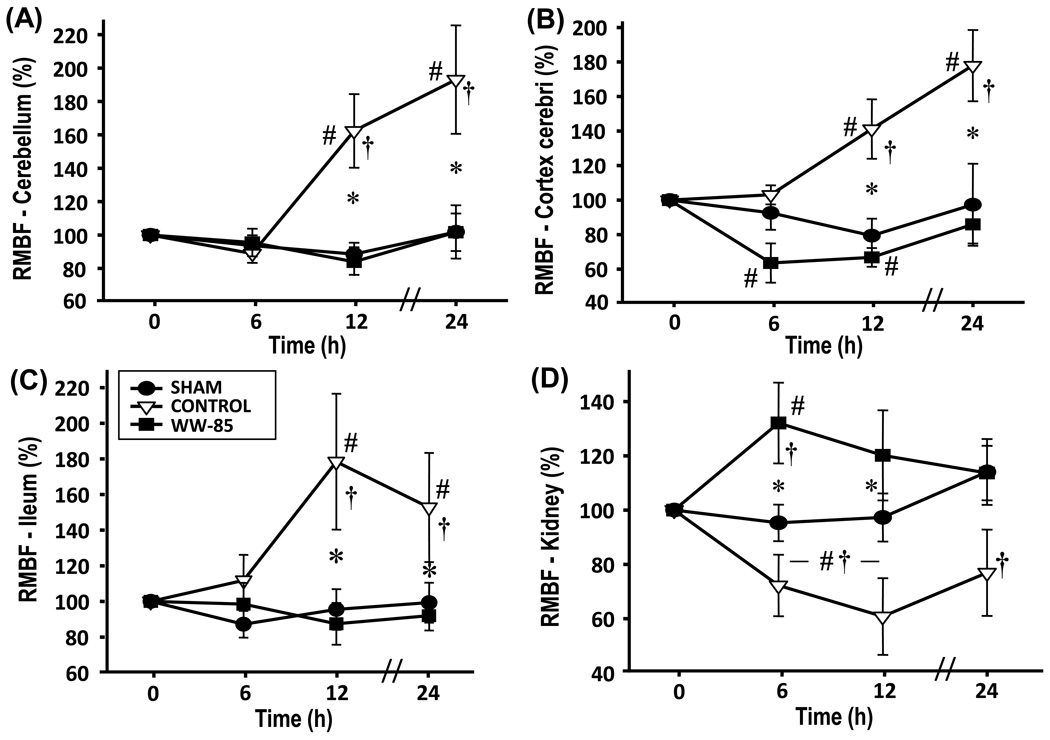
Blood flow in ileum (Figure 2) and spleen (Table 2) significantly increased in the control group compared to the sham and WW-85 group (p<0.05). There was no statistical difference between the sham and the WW-85 groups. The pancreatic blood flow significantly decreased in control and WW-85 animals as compared to sham animals (p<0.05, Table 2), without statistical differences between the injured groups. Renal blood flow significantly decreased in the control group as compared to sham animals and the WW-85 group (p<0.05 each, Figure 2). In WW-85 animals, renal blood flow was initially increased vs. BL and sham animals at 6 hrs and approached sham levels over time. Tracheal blood flow below the tracheotomy tube significantly increased in the control and WW-85 group during the experiment vs. BL and vs. the sham group (Table 2, p<0.001) without statistical differences between the injured groups. Whereas the visceral organs displayed increased and decreased blood flows respectively, all investigated cerebral structures showed a significant increase in RMBF of control animals vs. BL, vs. the sham, and vs. the WW-85 group (p<0.05, Figure 2 and Table 2). There were no statistical differences detectable between the sham and the WW-85 group in all investigated cerebral areas.
Fig. 2.
Regional microvascular blood flow (RMBF) 2(A) Cerebellum; 2(B) Cerebral cortex; 2(C) Ileum; and 2(D) Kidneys.
Baseline blood flow = normal organ blood flow (100%), changes in blood flow as percentage from baseline.
Significance: p<0.05, #vs. baseline, *vs. control, †vs. sham.
Table 2.
RMBF, regional microvascular blood flow
| Variables | Group | Values (Mean ± S.E.M.) |
|||
|---|---|---|---|---|---|
| Time | BL | 6h | 12h | 24h | |
| Trachea | Sham | 100 ± 0 | 114.8 ± 12.6 | 100.6 ± 17.6 | 104.9 ± 15.8 |
| (%) | Control | 100 ± 0 | 992.5 ± 143.9#† | 838.6 ± 144.3#† | 818.8 ± 218.4#† |
| WW-85 | 100 ± 0 | 1173.6 ± 87.3† | 1090.1 ± 86.2*† | 920.1 ± 210.4† | |
| Pancreas | Sham | 100 ± 0 | 98.6 ± 17.2 | 89.2 ± 6.2 | 102.5 ± 16.1 |
| (%) | Control | 100 ± 0 | 54.7 ± 9.3#† | 49.3 ± 5.2#† | 51.4 ± 6.8#† |
| WW-85 | 100 ± 0 | 65.1 ± 4.2#† | 52.9 ± 5.0#† | 65.0 ± 16.1#† | |
| Spleen | Sham | 100 ± 0 | 106.5 ± 17.4 | 94.1 ± 8.3 | 99.5 ± 12.2 |
| (%) | Control | 100 ± 0 | 121.2 ± 14.3 | 163.6 ± 13.5#† | 157.5 ± 20.4#† |
| WW-85 | 100 ± 0 | 78.7 ± 7.7* | 78.4 ± 12.7* | 93.4 ± 4.5* | |
| Medulla oblongata | Sham | 100 ± 0 | 84.3 ± 6.3 | 80.4 ± 9.0 | 93.1 ± 17.1 |
| (%) | Control | 100 ± 0 | 74.6 ± 6.1 | 131.3 ± 17.4#† | 143.5 ± 16.7#† |
| WW-85 | 100 ± 0 | 60.9 ± 8.0# | 74.1 ± 8.5* | 78.9 ± 12.3* | |
| Thalamus | Sham | 100 ± 0 | 82.1 ± 5.8 | 89.4 ± 9.5 | 106.2 ± 19.3 |
| (%) | Control | 100 ± 0 | 90.9 ± 9.3 | 136.6 ± 18.1#† | 156.8 ± 31.6#† |
| WW-85 | 100 ± 0 | 82.5 ± 13.3 | 73.2 ± 5.1* | 96.5 ± 15.8* | |
| Pons | Sham | 100 ± 0 | 80.2 ± 9.8 | 90.8 ± 6.8 | 96.0 ± 18.5 |
| (%) | Control | 100 ± 0 | 81.0 ± 7.5 | 130.9 ± 14.9#† | 134.3 ± 6.3#† |
| WW-85 | 100 ± 0 | 72.1 ± 4.4 | 78.6 ± 4.9* | 87.5 ± 10.7* | |
Data are expressed as mean ± S.E.M.; significance was assumed when p was less than 0.05;
vs. BL,
vs. control,
vs. sham.
4. Discussion
In the present study, the novel peroxynitrite decomposition catalyst WW-85 significantly improved macro- and micro-vascular blood flow to essential organs such as kidneys and several investigated cerebral structures in sheep suffering septic shock associated with smoke inhalation injury.
WW-85 attenuated the pathological changes through catalytically decomposing peroxynitrite, the cytotoxic by-product of NO produced in the presence of superoxide. Peroxynitrite is able to catalyze a variety of oxidative and nitrosative reactions and may exert multiple cytotoxic effects [16]. There are a number of metalloproteinic peroxynitrite decomposition catalysts, including MnTyPyP and FP15. These compounds provide beneficial effects in various models of ischemia, shock and neuro-injury [16, 17]. WW-85 is a new catalytic antioxidant that catalyzes the breakdown of ONOO−. WW-85 has been shown to improve transplant rejection in a rat allograft model [18]. In a mouse model of spinal cord injury, WW-85 also reduced the development of inflammation and tissue injury [19]. Our group has recently shown that WW-85 significantly improved pulmonary function and oxygenation in an ovine model of acute lung injury, induced by IL-2 toxicity [14]. In this study, WW-85 significantly improved lung transvascular fluid flux, decreased lipid peroxidation, and limited iNOS as well as PAR intensity [14]. In an experimental study on pulmonary function in septic shock, our group could show that WW-85-treated animals had significantly improved gas exchange, reductions in airway obstruction, shunt formation, lung myeloperoxidase- (MPO), lung malondialdehyde- (MDA), and lung 3-nitrotyrosine (3-NT) concentrations. Animals treated with WW-85 exhibited less microvascular leakage and significant improvements in pulmonary function [20]. These results provide evidence that WW-85 sufficiently blocks the nitric oxide - peroxynitrite pathway and thereby improves disturbances from septic shock.
Given this mechanistic knowledge on WW-85 from our previous studies, a study on the cardiovascular effects of WW-85 in systemic inflammation was warranted. The current study is the first, investigating the effects of WW-85 on micro-vascular blood flow in a large animal model of septic shock. It is important to point out that WW-85 reduced the formation of plasma nitrite/nitrate in the current experimental model. This is presumably due to the fact that iNOS expression (and subsequent NO production) is a continuous process in the sheep, and by interrupting NF-κB-activation, peroxynitrite decomposition catalysts block this process [22, 23]. We must keep in mind, however, that peroxynitrite is a cytotoxic by-product of NO, and NO, during systemic inflammation, exerts multiple pathophysiological effects, such as vascular and myocardial dysfunction, hepatic, renal, and pulmonary dysfunction, but also intestinal and pancreatic injury, as well as skeletal muscle dysfunction [24]. In addition, peroxynitrite may possibly also contribute to the pathogenesis of cellular metabolic failure. Several studies also demonstrate a correlation between its formation and the severity of the disease in human circulatory shock. Therefore, selective neutralization of peroxynitrite formation seems to represent a preferred approach over non-selective pharmacological inhibition of NO generation [24].
This sheep model is suitable for studying the effects of smoke inhalation and sepsis [8, 10, 12, 13, 15], because it closely mimics the pathophysiology seen in human sepsis, and matches the sepsis criteria as described by Bone et al. [25]. In the present study, all animals were fluid resuscitated to maintain Hct at baseline to achieve similar filling pressures between groups to insure adequate intravascular volume replacement. Therefore, we can exclude that changes in regional blood flow resulted from under-resuscitation or intravascular volume depletion [15]. It is well known that ALI is frequently associated with alterations in non-pulmonary organ function. Non-pulmonary organ failure may in part explain why the respiratory complications and the development of septic shock resulting from inhalation injury have become the major cause of mortality in these patients [3, 4]. There are several lines of evidence supporting the view that overproduction of NO by iNOS contributes significantly to circulatory shock. Not only NO itself, but also its downstream biological effects may play a role in vasodilatory hypotension [26]. Peroxynitrite is capable of inducing endothelial dysfunction and vascular hyporeactivity [7]. Recent data also showed that ONOO− is implicated in the inactivation of alpha-1-adrenoreceptors [27] and norepinephrine [28] and that O2− deactivates catecholamines resulting in loss of their vasopressor activity [29]. These findings are in accordance with the results of our present study, which clearly showed improved global hemodynamics, increased mean arterial pressure, and improved regional microvascular blood flow to the kidneys. In pancreas, blood flow dropped in both, the injured and treated groups, indicating that WW-85 had no effect on the pancreatic endothelium. Blood flow of ileum and spleen significantly increased in septic controls. In treated animals, blood flow to both organs was stabilized and was comparable to sham animals. The dramatic early increase in tracheal blood flow was anticipated. Starting the treatment with WW-85 1hour post injury, was too late for a potential curative effect on the trachea, given the degree and severity of direct inflammatory damage by smoke inhalation at this site. These findings are in accordance with former studies of our group [2, 15]. However, an earlier start of treatment would not have mimicked a clinically relevant scenario.
Septic challenges induce endothelial barrier injury. The endothelial resistance appears to be organ and/or tissue dependent and associated with a redistribution of blood [30]. Perfusion abnormalities are an overall phenomenon in severe sepsis and septic shock, leading to organ and neurological dysfunction. Changes in mental status occur early in sepsis, and are associated with increased rates of morbidity and mortality. Although cerebral dysfunction often occurs, the pathophysiological background is still not fully understood [31]. All animals in our study were mildly hyperventilated to study the sheep in awake state, and to exclude that sedative or narcotic drugs may have impact on cerebral blood flow. The unchanged cerebral blood flow in the sham group proves that the ventilation-related decrease in PaCO2 had no influence on cerebral blood flow in this model, since PaCO2 was similar between groups [15]. The increased blood flows to all investigated cerebral areas in the untreated septic control group was associated with an increase in CI and most likely related to a loss of cerebral autoregulation. These findings are in line with the findings of Smith et al. [32] who reported that cerebral blood flow is proportional to CI in patients with septic shock. However, in the present study, CI was higher in controls than in WW-85 animals, but not statistically different. These results likely suggest that NO and peroxynitrite are involved in cerebral vasoregulation, and that the reduction of plasma NOx stabilized MAP and lead to stabile systemic vascular conditions in WW-85 treated animals. These results are also in accordance with the latest study of our group, investigating the effects of recombinant human activated protein C (rhAPC) in the same model. RhAPC stabilized cardiovascular functions and attenuated the changes in visceral and cerebral microcirculation in sheep suffering from ALI and septic shock by reduction of cardiac MDA and cardiac 3-NT [21], providing more evidence that the breakdown of peroxynitrite improves cardiovascular performance during septic shock.
It may be a limitation of our study that we cannot show mechanistic findings of the NO - ONOO− - PARP pathway in every investigated organ. However, we found evidence on the beneficial effects of WW-85 in our recent studies on IL-2 induced lung injury [14] and sepsis induced lung injury [20]. The descriptive approach in the present study is of importance, to clearly delineate the changes in hemodynamics and microcirculation under certain conditions in certain organs. However, we measured plasma NOx levels, which are utmost important, given the direct effects of NO to the vascular endothelium.
These results proof that the peroxynitrite decomposition catalyst WW-85 is effective in attenuating the microcirculatory deterioration during septic shock associated with smoke inhalation injury, by reducing plasma NOx levels in a clinically relevant experimental model. Further clinical investigations are necessary to transfer these results to human beings.
Acknowledgements
The authors thank the team of the Investigational ICU at the University of Texas Medical Branch for their valuable assistance in conducting these studies. This work was supported in part, by grant GM066312, GM060688 from the National Institutes of Health (NIH), by grants 8450, 8541, and 8954 from the Shriners of North America, and Inotek Pharmaceuticals Corporation, Beverly, MA, U.S.A.; The first (DMM) and second (MOM) author contributed equally to this study and manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Alcorta R. Smoke inhalation & acute cyanide poisoning. Hydrogen cyanide poisoning proves increasingly common in smoke-inhalation victims. JEMS. 2004;29 suppl:6–15. [PubMed] [Google Scholar]
- 2.Schenarts PJ, Bone HG, Traber LD, Traber DL. Effect of severe smoke inhalation injury on systemic microvascular blood flow in sheep. Shock. 1996;6:201–205. [PubMed] [Google Scholar]
- 3.Shirani KZ, Pruitt BA, Jr, Mason AD., Jr The influence of inhalation injury and pneumonia on burn mortality. Ann Surg. 1987;205:82–87. doi: 10.1097/00000658-198701000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maybauer MO, Maybauer DM, Herndon DN. Incidence and outcomes of acute lung injury. N Engl J Med. 2006;354:416–417. author reply 416-7. [PubMed] [Google Scholar]
- 5.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37:1670–1677. doi: 10.1097/CCM.0b013e31819fcf68. [DOI] [PubMed] [Google Scholar]
- 7.Szabo C. The pathophysiological role of peroxynitrite in shock, inflammation, and ischemia-reperfusion injury. Shock. 1996;6:79–88. doi: 10.1097/00024382-199608000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Murakami K, Bjertnaes LJ, Schmalstieg FC, McGuire R, Cox RA, Hawkins HK, et al. A novel animal model of sepsis after acute lung injury in sheep. Crit Care Med. 2002;30:2083–2090. doi: 10.1097/00003246-200209000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Maybauer MO, Kikuchi Y, Westphal M, Maybauer DM, Nishida K, Traber LD, et al. Effects of manganese superoxide dismutase nebulization on pulmonary function in an ovine model of acute lung injury. Shock. 2005;23:138–143. doi: 10.1097/01.shk.0000150777.39484.b0. [DOI] [PubMed] [Google Scholar]
- 10.Maybauer MO, Maybauer DM, Traber LD, Westphal M, Enkhbaatar P, Morita N, et al. Gentamicin improves hemodynamics in ovine septic shock after smoke inhalation injury. Shock. 2005;24:226–231. doi: 10.1097/01.shk.0000174021.95063.f4. [DOI] [PubMed] [Google Scholar]
- 11.Nakano Y, Maybauer MO, Maybauer DM, Enkhbaatar P, Traber DL. A novel antibiotic based long-term model of ovine smoke inhalation injury and septic shock. Burns. 2010;36(7):1050–1058. doi: 10.1016/j.burns.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maybauer MO, Maybauer DM, Fraser JF, Traber LD, Westphal M, Enkhbaatar P, et al. Recombinant human activated protein C improves pulmonary function in ovine acute lung injury resulting from smoke inhalation and sepsis. Crit Care Med. 2006;34:2432–2438. doi: 10.1097/01.CCM.0000230384.61350.FA. [DOI] [PubMed] [Google Scholar]
- 13.Maybauer MO, Maybauer DM, Fraser JF, Traber LD, Westphal M, Cox RA, et al. Ceftazidime improves hemodynamics and oxygenation in ovine smoke inhalation injury and septic shock. Intensive Care Med. 2007;33:1219–1227. doi: 10.1007/s00134-007-0658-3. [DOI] [PubMed] [Google Scholar]
- 14.Maybauer DM, Maybauer MO, Szabo C, Westphal M, Traber LD, Enkhbaatar P, et al. Lung-protective effects of the metalloporphyrinic peroxynitrite decomposition catalyst WW-85 in interleukin-2 induced toxicity. Biochem Biophys Res Commun. 2008;377(3):786–791. doi: 10.1016/j.bbrc.2008.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maybauer DM, Maybauer MO, Traber LD, Westphal M, Nakano YY, Enkhbaatar P, et al. Effects of severe smoke inhalation injury and septic shock on global hemodynamics and microvascular blood flow in sheep. Shock. 2006;26:489–495. doi: 10.1097/01.shk.0000230300.78515.ed. [DOI] [PubMed] [Google Scholar]
- 16.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pacher P, Szabo C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am J Pathol. 2008;173:2–13. doi: 10.2353/ajpath.2008.080019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pieper GM, Nilakantan V, Chen M, Zhou J, Khanna AK, Henderson JD, Jr, et al. Protective mechanisms of a metalloporphyrinic peroxynitrite decomposition catalyst, WW85, in rat cardiac transplants. J Pharmacol Exp Ther. 2005;314:53–60. doi: 10.1124/jpet.105.083493. [DOI] [PubMed] [Google Scholar]
- 19.Genovese T, Mazzon E, Esposito E, Di Paola R, Murthy K, Neville L, et al. Effects of a metalloporphyrinic peroxynitrite decomposition catalyst, ww-85, in a mouse model of spinal cord injury. Free Radic Res. 2009;43:631–645. doi: 10.1080/10715760902954126. [DOI] [PubMed] [Google Scholar]
- 20.Maybauer DM, Maybauer MO, Szabo C, Cox RA, Westphal M, Kiss L, et al. The Peroxynitrite Catalyst WW-85 Improves Pulmonary Function in Ovine Septic Shock. Shock. 2010 doi: 10.1097/SHK.0b013e3181eb4556. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maybauer MO, Maybauer DM, Fraser JF, Szabo C, Westphal M, Kiss L, et al. Recombinant human activated protein C attenuates cardiovascular and microcirculatory dysfunction in acute lung injury and septic shock. Crit Care. 2010;14:R217. doi: 10.1186/cc9342. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naidu BV, Fraga C, Salzman AL, Szabo C, Verrier ED, Mulligan MS. Critical role of reactive nitrogen species in lung ischemia-reperfusion injury. J Heart Lung Transplant. 2003;22:784–793. doi: 10.1016/s1053-2498(02)00556-9. [DOI] [PubMed] [Google Scholar]
- 23.Naidu BV, Farivar AS, Woolley SM, Fraga C, Salzman AL, Szabo C, et al. Enhanced peroxynitrite decomposition protects against experimental obliterative bronchiolitis. Exp Mol Pathol. 2003;75:12–17. doi: 10.1016/s0014-4800(03)00015-7. [DOI] [PubMed] [Google Scholar]
- 24.Szabo C, Modis K. Pathophysiological roles of peroxynitrite in circulatory shock. Shock. 2010;34 Suppl 1:4–14. doi: 10.1097/SHK.0b013e3181e7e9ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992;101:1481–1483. doi: 10.1378/chest.101.6.1481. [DOI] [PubMed] [Google Scholar]
- 26.Asfar P, Hauser B, Radermacher P, Matejovic M. Catecholamines and vasopressin during critical illness. Crit Care Clin. 2006;22:131–149. doi: 10.1016/j.ccc.2005.08.007. vii-viii. [DOI] [PubMed] [Google Scholar]
- 27.Takakura K, Taniguchi T, Muramatsu I, Takeuchi K, Fukuda S. Modification of alpha1 -adrenoceptors by peroxynitrite as a possible mechanism of systemic hypotension in sepsis. Crit Care Med. 2002;30:894–899. doi: 10.1097/00003246-200204000-00030. [DOI] [PubMed] [Google Scholar]
- 28.Takakura K, Xiaohong W, Takeuchi K, Yasuda Y, Fukuda S. Deactivation of norepinephrine by peroxynitrite as a new pathogenesis in the hypotension of septic shock. Anesthesiology. 2003;98:928–934. doi: 10.1097/00000542-200304000-00020. [DOI] [PubMed] [Google Scholar]
- 29.Macarthur H, Couri DM, Wilken GH, Westfall TC, Lechner AJ, Matuschak GM, et al. Modulation of serum cytokine levels by a novel superoxide dismutase mimetic, M40401, in an Escherichia coli model of septic shock: correlation with preserved circulating catecholamines. Crit Care Med. 2003;31:237–245. doi: 10.1097/00003246-200301000-00037. [DOI] [PubMed] [Google Scholar]
- 30.Deng X, Wang X, Andersson R. Endothelial barrier resistance in multiple organs after septic and nonseptic challenges in the rat. J Appl Physiol. 1995;78:2052–2061. doi: 10.1152/jappl.1995.78.6.2052. [DOI] [PubMed] [Google Scholar]
- 31.Sprung CL, Peduzzi PN, Shatney CH, Schein RM, Wilson MF, Sheagren JN, et al. Impact of encephalopathy on mortality in the sepsis syndrome. The Veterans Administration Systemic Sepsis Cooperative Study Group. Crit Care Med. 1990;18:801–806. doi: 10.1097/00003246-199008000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Smith SM, Padayachee S, Modaresi KB, Smithies MN, Bihari DJ. Cerebral blood flow is proportional to cardiac index in patients with septic shock. J Crit Care. 1998;13:104–109. doi: 10.1016/s0883-9441(98)90013-2. [DOI] [PubMed] [Google Scholar]