Abstract
Background and Aims
Depletion of interstitial cells of Cajal (ICC) is associated with several gastrointestinal motility disorders. Changes in ICC networks are usually detected by immunolabeling for the receptor tyrosine kinase Kit. Ano1 (DOG1 or TMEM16A) was recently described as a marker of ICC in gastrointestinal tract. Our aim was to determine whether Ano1-immunoreactivity can be used as a reliable marker for ICC in tissues from patients with motility disorders.
Methods
Four tissues from patients with normal ICC numbers and four tissues from patients with slow transit constipation and loss of Kit-positive ICC were studied. ICC were detected by double labeling using antisera to Kit and Ano1.
Results
Both the processes and cell bodies of ICC in tissue from controls and slow transit constipation were immunoreactive for Ano1. There was near complete overlap between Kit and Ano1 immunoreactivity. Tissues from patients with slow transit constipation contained significantly fewer Ano1-positive ICC than control tissues. The numbers of ICC identified by Ano1 and Kit-immunoreactivity were nearly identical across the range of ICC numbers from an average of 1.64 to 7.05 cells per field and correlated with an R2 value of 0.99.
Conclusion
Ano1 is a reliable and sensitive marker for detecting changes in ICC networks in humans. Labeling with antibodies selective for Ano1 reproducibly detects depletion of Kit-positive ICC in tissues from patients with slow transit constipation.
Keywords: ICC, Ano1, slow transit constipation, Kit
Introduction
Interstitial Cells of Cajal (ICC) can be found throughout the gastrointestinal tract and are recognized for their importance in maintaining normal gastrointestinal motility. They serve several important functions including the generation of electrical slow waves, mechano-transduction, participating in cholinergic and nitrergic neurotransmission and setting the smooth muscle membrane potential.1
Loss of Kit-positive ICC has been associated with several gastrointestinal motility disorders.2-10 Until recently Kit was the only reliable antigenic marker for ICC. However, Kit is a ligand for stem cell factor, a growth factor required for survival of ICC,11 hence it can be difficult to differentiate loss of Kit from loss of ICC. Also the antibody to Kit stains mast cells making quantification of ICC harder and less easy to automate. Several other markers such as Na+/K+2Cl− co-transporter (NKCC1),12 neurokinin-1 receptor13 and CD4414 have been investigated given their selective expression on some or all subtypes of ICC but they have not yet been shown to be reliable independent markers of all classes of ICC throughout the gastrointestinal tract. The recently discovered Ano1, a protein first found on gastrointestinal stromal tumors15, 16 holds promise as being such a marker. Ano1 belongs to the anoctamin family of chloride transporters17 and is also known as FLJ1026118 and TMEM16A. The gene product of Ano1 gene contributes to Ca2+ activated Cl- conductances based on expression studies19-21 and Ano1 has been associated with the generation of ICC slow waves.22, 23 Recent work in our laboratory has shown that Ano1 is a selective marker for all classes of ICC in the stomach, small intestine and large intestine of normal humans.24 Ano1 expression is markedly increased in gastrointestinal stromal tumors (GIST) and it is a sensitive marker for detection of GIST.25 However Ano1-immunoreactivity has not been investigated in motility disorders associated with loss of Kit-positive ICC.
The aim of our study was to determine if Ano1-immunoreactivity can be used as a reliable marker for ICC in tissue where Kit-positive ICC are depleted and if it can be used as an independent marker.
Materials and Methhods
Human tissues
All studies were approved by the Institutional Review Board of the Mayo Clinic. Colon tissue was obtained as previously described.24 Four colon tissues from patients with non obstructive colon cancer (2 male, 2 female, ages 36 to 53) and normal ICC numbers were selected as controls and 4 tissues from patients with slow transit constipation (4 female, ages 21 to 47) not responsive to medical therapy with fewer ICC on Kit immunostaining were selected as the experimental group.
Immunostaining for Kit and Ano1 in fresh frozen human tissue
Sections of tissue, 12 μm thick, were stained with the primary antibodies for Kit and Ano1 (Table 1) as previously described.24
Table 1.
| Cat #/Clone | Target | Host Species/Type | Supplier | Final concentration |
| Ab53212 | TMEM16A (DOG-1) | Rabbit polyclonal | Abcam | 0.25 μg/ml |
| Ms-483-P0 | Kit | Mouse monoclonal | Lab Vision | 0.5 μg/ml |
| Donkey-Cy5 | Mouse IgG | Donkey polyclonal | Chemicon | 7.5 μg/ml |
| Donkey-Cy3 | Rabbit IgG | Donkey polyclonal | Chemicon | 1.25 μg/ml |
Image Acquisition
High resolution images were obtained using an Olympus FV300 confocal microscope. A 60× 1.2 NA water immersion objective was used with the pinhole size set to an Airy number of 1. The following lasers and emission filters were used to visualize the labeled structures and collect images: 543nm HeNe laser (for Cy3); emission filter 575-630nm and 633nm HeNe laser (for Cy5); emission filter 660nm (650-700nm).
Low resolution images of the whole thickness of the tissues were obtained using an Olympus BX51WI epifluorescence microscope. Images were collected using a 10× Zeiss water immersion objective and combined in a montage to show the distribution of ICC in the full thickness of the preparations.
For the purposes of quantifying ICC numbers in the tissues, 39 images (13 fields per slide from 3 non-adjacent slides) were obtained using a 40×, 0.75 NA air objective. ICC bodies, defined as Kit or Ano1 positive structures surrounding a DAPI-positive nucleus, were counted in the circular muscle; cells along the submucosal border of the circular muscle were not included. Mast cells, identified by their round or oval shape and lack of processes, were excluded from the counts. Fields were 390 × 314 μm (0.12 mm2). Prior to counting, the images were de-identified and assigned a random number so that the counter was blind to the tissue source of the image.
Materials
Unless indicated, reagents were from Sigma-Aldrich (St Louis, MO).
Results
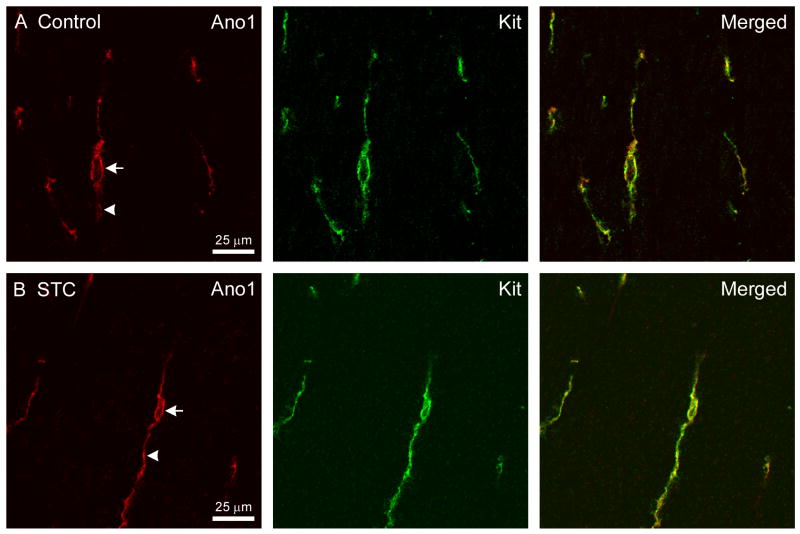
The distribution of Ano1 immunoreactive structures in all specimens examined for this study was consistent with our previous report that Ano1 specifically labels the cell bodies and processes of all types of ICC in the human colon.24 Examples of single ICC in the circular muscle layer of healthy colon tissue with normal ICC numbers obtained from a 53-year-old, male patient with non obstructive colon cancer are shown in Fig 1A. The processes (arrowheads) and cell bodies (arrow) of the cells were immunoreactive for both Ano1 and Kit and the observed signal was similarly distributed within each labeled structure, as shown in the merged image. Ano1 immunoreactivity was also found to colocalize with Kit immunoreactivity in the ICC remaining in the colon tissue from the patients with slow transit constipation. The example shown is of an ICC from the circular muscle layer of the colon tissue from a 41-year-old, female patient with slow transit constipation (Fig 1B). The brightness of fluorescence was similar for most cells labeled with Kit or Ano1 with the exception of 4 cells with ICC morphology that were positive for Ano1 and appeared Kit-negative. Two of these cells were seen in control patients and two were seen in patients with slow transit constipation.
Fig 1.
Ano1-immunoreactivity is seen in both the ICC cell body and in the processes (A) Ano1 and Kit-immunoreactivity in the ICC cell body and the processes in colon tissue obtained from a patient with non obstructive colon cancer. The merged image shows Ano1 and Kit-immunoreactivity were coincident. (B) Ano1 and Kit-immunoreactivity in an ICC cell body and its processes in colon tissue obtained from a patient with slow transit constipation. The merged image shows Ano1 and Kit-immunoreactivity were also coincident in slow transit constipation. These images were collected using a 60×, 1.2NA water immersion objective and represent a single section obtained from a confocal stack collected with 0.3 μm z axis steps. The signal obtained with the rabbit antiserum (Ano1) is shown in red and the signal from mouse antiserum (Kit) is shown in green.
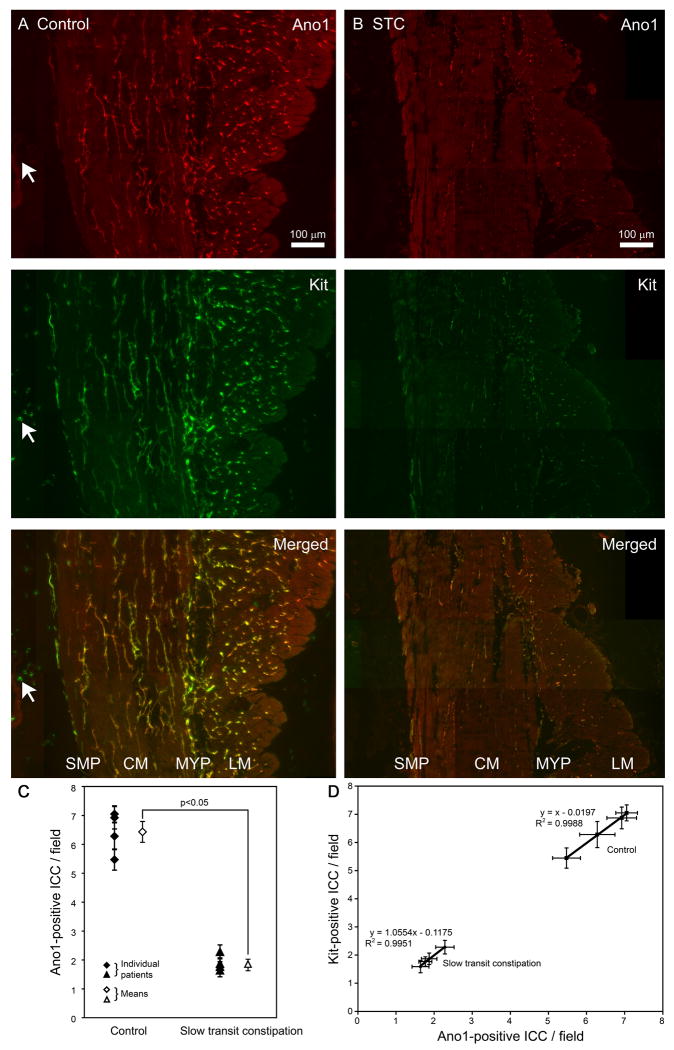
Ano1-immunoreactive structures representing ICC were present in both the longitudinal and circular muscle layers as well as in the myenteric plexus and submucosal border of the circular muscle in colon tissue obtained from a 40-year-old female patient with non obstructive colon cancer (Fig 2A). In contrast Ano1-immunoreactive structures representing ICC were markedly decreased in colon tissue obtained from a 47-year-old female patient with slow transit constipation (Fig 2B). Kit-positive ICC, as expected, were also preserved in colon tissue obtained from the patient with non obstructive colon cancer (Fig 2A) and decreased similar to Ano1-immunoreactive structures in all layers of the wall of the colon in the patient with slow transit constipation (Fig 2B). Ano1-immunoreactivity was found to co-localize with Kit-immunoreactivity in all layers of the colon tissue both in the patient with non-obstructive colon cancer (Fig 2A) and slow transit constipation (Fig 2B) as seen on the merged images. The decrease in Ano1-positive ICC was similar to decrease in Kit-positive ICC in colon tissue from the patient with slow transit constipation (Fig 2B). As previously described, antibody to Ano1 did not label mast cells (arrow, Fig 2A). The decrease in Ano1-positive ICC was further confirmed by counting Ano1 and Kit-positive ICC in the circular muscle layer of the colon tissue. In the circular muscle layer of colon tissue obtained from patients with slow transit constipation there were significantly fewer Ano1-positive ICC than control tissues as seen in Figure 2C (1.89±0.13 vs. 6.43±0.37 cells per field, P < 0.05, n = 4, unpaired t test). Furthermore, the numbers of ICC identified in the circular muscle layer of the colon by Ano1 and Kit-immunoreactivity were nearly identical across the range of ICC numbers from an average of 1.64 to 7.05 cells per field and correlated with an R2 value of 0.99 (Fig 2D).
Fig 2.
Ano1-positive ICC are decreased in all layers of the colon wall in slow transit constipation. (A) In colon tissue from control patients, Ano1 and Kit-immunolabeling showed numerous ICC in the circular muscle, submucosal border of circular muscle, myenteric plexus and the longitudinal muscle. The merged image shows overlap of Ano1 and Kit-mmunolabeling. The arrow points to a mast cell in the mucosa which was Kit-positive but negative for Ano1. (B) In colon tissue from patients with slow transit constipation, Ano1 and Kit-immunolabeling show markedly reduced ICC in the circular muscle, submucosal border of circular muscle, myenteric plexus and the longitudinal muscle as compared to the control tissue. The merged images show overlap of residual Ano1 and Kit- immunolabeling. Both panels A and B are montages of images that were collected using a 10× water immersion objective. The signal obtained with the rabbit antiserum (Ano1) is shown in red and the signal from mouse antiserum (Kit) is shown in green. (C) There were significantly fewer ICC in the circular muscle layer of the colon in patients with slow transit constipation as detected by Ano1-immunolabeling (1.89±0.13 vs. 6.43±0.37 cells per field, P <0.05, n = 4, unpaired t test). Closed symbols represent mean number of ICC in each patient per field with the standard error of mean while the open symbols represent mean number of ICC per field from all 4 patients with standard error of mean. (D) The number of ICC per field detected as Kit and Ano1-positive are identical in control patients and patients with slow transit constipation. The left part of the graph shows the number of ICC per field detected by Ano1 and Kit-immunoreactivity in colon tissue from patients with slow transit constipation (1.8 cells/field) and the right part shows similar data for colon tissue from control patients (6.4 cells/field) showing near complete overlap.
Discussion
This study identifies Ano1 as a sensitive and reliable marker to quantify decreases in ICC numbers in human tissues obtained from patients with motility disorders associated with loss of Kit-positive ICC. Ano1 can therefore be used as an independent marker for ICC with an added advantage of staining only ICC and not mast cells. However Ano1 immunolabeling requires relatively specialized fixation methods24 that currently limit its use and optimal staining was seen only with the polyclonal rabbit antibody (Abcam) with the antibody generated in the chicken (Abcam) giving higher background.
We have previously shown that Ano1-immunoreactivity identifies all subclasses of ICC in the stomach, small intestine and colon in humans and mice.24 Additionally antisera to Ano1 did not label Kit positive mast cells, a finding which was further confirmed in our current study. In this study, we show that antiserum to Ano1 labels both ICC cell body and processes in colon tissue from patients with slow transit constipation with a decrease in number of ICC. There was near complete overlap of Ano1 and Kit-immunoreactivity further confirming the specificity of Ano1 labeling. A small number of cells were Ano1 positive and Kit negative and further studies with larger sample size will be needed to determine if parallel loss of both Kit and Ano1 is a hallmark of slow transit constipation and other motility disorders. As in our previous study we did not see any Ano1 immunoreactivity in the smooth muscle in the colon, though other groups have described Ano1 immunoreactivity in the vascular smooth muscle.26 We and others have previously shown that there is loss of both ICC cell bodies and networks throughout the colon in a subset of patients with slow transit constipation.2-4 We therefore elected to use tissue from these patients to test the reliability of Ano1 as a marker to quantify loss of ICC. The numbers of Ano1-positive ICC were decreased in all layers of the wall of colon in a patient with slow transit constipation and this was similar to loss of Kit-positive ICC in this tissue. The qualitative loss of Ano1-positive ICC in all layers of the colon wall observed at lower magnification was further confirmed by counting ICC cell bodies in the circular muscle layer of colon tissue at a much higher magnification. We decided to count ICC in the circular muscle layer as the circular muscle layer is thicker than longitudinal muscle layer in human colon and allows imaging of sufficient fields to have enough power to detect a change in number of ICC.
Based on studies using neutralizing antibodies that inhibit Kit signaling, we know that Kit is required for the development and maintenance of ICC.27 Ano1 lacks any structural or functional homology to Kit and hence represents an independent marker to quantify changes in ICC. This is important because there has been controversy in the field on whether the decrease in ICC reported in motility disorders such as slow transit constipation is due to loss of the cell or loss of surface Kit immunoreactivity. The near complete overlap between loss of Kit and loss of Ano1-immunoreactivity suggests that both Kit and Ano1 can be used reliably to document loss of ICC. However, even these results cannot fully exclude the possibility that ICC that lost both Kit and Ano1 expression could linger in the diseased tissues. Also needs to be determined is if the loss of Ano1immunoreactivity affects ICC function in patients with motility disorders.
In summary, Ano1 is both a sensitive and specific marker of ICC and can serve as an independent alternative marker of ICC in health and disease states. Depletion of Kit-positive ICC in tissues from patients with slow transit constipation is reproducibly detected by Ano1-immunoreactivity. An advantage of Ano1 is that it does not label Kit-positive mast cells allowing for more accurate quantification of ICC and easier automation of the counting process.
Acknowledgments
The authors thank Peter Strege for technical assistance and Kristy Zodrow for secretarial assistance. Grant Support: This work was supported by NIH grant DK57061 and DK84567. Maria J. Pozo is supported by grant BFU2007-60563 from the Spanish MEC and RETICEF (RD060013/1012). Pedro J. Gomez-Pinilla is funded by the Spanish MEC and FECYT (2007-0637).
Abbreviations not mentioned in Style Guide
- ICC
interstitial cells of Cajal
- GIST
gastrointestinal stromal tumors
- DAPI
4′,6-diamidino-2-phenylindole dilactate
- STC
slow transit constipation
Footnotes
Competing interests: The authors have no competing interests.
References
- 1.Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil. 2008;20 1:54–63. doi: 10.1111/j.1365-2982.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 2.Lyford GL, He CL, Soffer E, et al. Pan-colonic decrease in interstitial cells of Cajal in patients with slow transit constipation. Gut. 2002;51:496–501. doi: 10.1136/gut.51.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He CL, Burgart L, Wang L, et al. Decreased interstitial cell of cajal volume in patients with slow-transit constipation. Gastroenterology. 2000;118:14–21. doi: 10.1016/s0016-5085(00)70409-4. [DOI] [PubMed] [Google Scholar]
- 4.Wedel T, Spiegler J, Soellner S, et al. Enteric nerves and interstitial cells of Cajal are altered in patients with slow-transit constipation and megacolon. Gastroenterology. 2002;123:1459–67. doi: 10.1053/gast.2002.36600. [DOI] [PubMed] [Google Scholar]
- 5.Forster J, Damjanov I, Lin Z, Sarosiek I, Wetzel P, McCallum RW. Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings. J Gastrointest Surg. 2005;9:102–8. doi: 10.1016/j.gassur.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Feldstein AE, Miller SM, El-Youssef M, et al. Chronic intestinal pseudoobstruction associated with altered interstitial cells of cajal networks. J Pediatr Gastroenterol Nutr. 2003;36:492–7. doi: 10.1097/00005176-200304000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Faussone-Pellegrini MS, Cortesini C. The muscle coat of the lower esophageal sphincter in patients with achalasia and hypertensive sphincter. An electron microscopic study. J Submicrosc Cytol. 1985;17:673–85. [PubMed] [Google Scholar]
- 8.Vanderwinden JM, Liu H, De Laet MH, Vanderhaeghen JJ. Study of the interstitial cells of Cajal in infantile hypertrophic pyloric stenosis. Gastroenterology. 1996;111:279–88. doi: 10.1053/gast.1996.v111.pm8690192. [DOI] [PubMed] [Google Scholar]
- 9.Vanderwinden JM, Rumessen JJ, Liu H, Descamps D, De Laet MH, Vanderhaeghen JJ. Interstitial cells of Cajal in human colon and in Hirschsprung's disease. Gastroenterology. 1996;111:901–10. doi: 10.1016/s0016-5085(96)70057-4. [DOI] [PubMed] [Google Scholar]
- 10.Isozaki K, Hirota S, Miyagawa J, Taniguchi M, Shinomura Y, Matsuzawa Y. Deficiency of c-kit+ cells in patients with a myopathic form of chronic idiopathic intestinal pseudo-obstruction. Am J Gastroenterol. 1997;92:332–4. [PubMed] [Google Scholar]
- 11.Wu JJ, Rothman TP, Gershon MD. Development of the interstitial cell of Cajal: origin, kit dependence and neuronal and nonneuronal sources of kit ligand. J Neurosci Res. 2000;59:384–401. doi: 10.1002/(SICI)1097-4547(20000201)59:3<384::AID-JNR13>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Wouters M, De Laet A, Donck LV, et al. Subtractive hybridization unravels a role for the ion cotransporter NKCC1 in the murine intestinal pacemaker. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1219–27. doi: 10.1152/ajpgi.00032.2005. [DOI] [PubMed] [Google Scholar]
- 13.Chen H, Redelman D, Ro S, Ward SM, Ordog T, Sanders KM. Selective labeling and isolation of functional classes of interstitial cells of Cajal of human and murine small intestine. Am J Physiol Cell Physiol. 2007;292:C497–507. doi: 10.1152/ajpcell.00147.2006. [DOI] [PubMed] [Google Scholar]
- 14.Lorincz A, Redelman D, Horvath VJ, Bardsley MR, Chen H, Ordog T. Progenitors of interstitial cells of cajal in the postnatal murine stomach. Gastroenterology. 2008;134:1083–93. doi: 10.1053/j.gastro.2008.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Espinosa I, Lee CH, Kim MK, et al. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am J Surg Pathol. 2008;32:210–8. doi: 10.1097/PAS.0b013e3181238cec. [DOI] [PubMed] [Google Scholar]
- 16.West RB, Corless CL, Chen X, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol. 2004;165:107–13. doi: 10.1016/S0002-9440(10)63279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartzell HC, Yu K, Xiao Q, Chien LT, Qu Z. Anoctamin/TMEM16 family members are Ca2+-activated Cl- channels. J Physiol. 2009;587:2127–39. doi: 10.1113/jphysiol.2008.163709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katoh M, Katoh M. FLJ10261 gene, located within the CCND1-EMS1 locus on human chromosome 11q13, encodes the eight-transmembrane protein homologous to C12orf3, C11orf25 and FLJ34272 gene products. Int J Oncol. 2003;22:1375–81. [PubMed] [Google Scholar]
- 19.Caputo A, Caci E, Ferrera L, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–4. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–29. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang YD, Cho H, Koo JY, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–5. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 22.Hwang SJ, Blair PJ, Britton FC, et al. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol. 2009;587:4887–904. doi: 10.1113/jphysiol.2009.176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu MH, Kim TW, Ro S, et al. A Ca(2+)-activated Cl(-) conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol. 2009;587:4905–18. doi: 10.1113/jphysiol.2009.176206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, et al. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1370–81. doi: 10.1152/ajpgi.00074.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liegl B, Hornick JL, Corless CL, Fletcher CD. Monoclonal antibody DOG1.1 shows higher sensitivity than KIT in the diagnosis of gastrointestinal stromal tumors, including unusual subtypes. Am J Surg Pathol. 2009;33:437–46. doi: 10.1097/PAS.0b013e318186b158. [DOI] [PubMed] [Google Scholar]
- 26.Davis AJ, Forrest AS, Jepps TA, et al. Expression profile and protein translation of TMEM16A in murine smooth muscle. Am J Physiol Cell Physiol. 2010;299:C948–59. doi: 10.1152/ajpcell.00018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torihashi S, Ward SM, Nishikawa S, Nishi K, Kobayashi S, Sanders KM. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 1995;280:97–111. doi: 10.1007/BF00304515. [DOI] [PubMed] [Google Scholar]