Abstract
Secreted Protein Acidic and Rich in Cysteine (SPARC) participate in the regulation of morphogenesis and cellular differentiation through its modulation of cell-matrix interactions. We previously reported that SPARC expression significantly impairs medulloblastoma tumor growth in vivo. In this study, we show that Adenoviral-mediated overexpression of SPARC cDNA (Ad-DsRed-SP) elevated the expression of the neuronal markers NeuN, Nestin, Neurofilament (NF) and MAP-2 in medulloblastoma cells and induced neuron like differentiation. SPARC overexpression decreased Signal Transducer and Activator of Transcription 3 (STAT3) phosphorylation; constitutive expression of STAT3 reversed SPARC-mediated expression of neuronal markers. We also show that Notch signaling is suppressed in the presence of SPARC, as well as the Notch effector basic helix-loop-helix (bHLH) transcription factor hairy and enhancer of split 1 (HES1). Notch signaling was found to be responsible for the decreased STAT3 phosphorylation in response to SPARC expression. Furthermore, expression of SPARC decreased the production of IL-6 and supplemented IL-6-abrogated, SPARC-mediated suppression of Notch signaling and expression of neuronal markers. Immunohistochemical analysis of tumor sections from mice treated with Ad-DsRed-SP showed increased immunoreactivity for the neuronal markers and a decrease in Notch1 expression and phosphorylation of STAT3. Taken together, our results suggest that SPARC induces expression of neuronal markers in medulloblastoma cells through its inhibitory effect on IL-6 regulated suppression of Notch pathway-mediated STAT3 signaling, thus giving further support to the potential use of SPARC as a therapeutic candidate for medulloblastoma treatment. Findings show that SPARC-induced neuronal differentiation can sensitize medulloblastoma cells for therapy.
Keywords: SPARC, Notch1, STAT3, neuronal differentiation, NeuN
INTRODUCTION
Medulloblastoma is a malignant tumor of the cerebellum. The median age at diagnosis is 5 years, with the age range extending into young adulthood. Primary management consists of surgical resection followed by radiation therapy and chemotherapy. Current therapies have serious short-term and long-term adverse effects, including neurocognitive deficits, endocrinopathies, sterility, and the risk of secondary high-grade glioma or meningioma (1). Patients with recurrent disease after primary therapy have a particularly poor prognosis, with a median survival of less than 6 months; the 2-year survival rate among these patients is approximately 9% (2). Medulloblastomas are functionally heterogeneous tumors with a subset of cells that have a stem-like phenotype that drives tumor growth. These cells contain two karyotypically similar populations-one comprised of rapidly proliferating, undifferentiated cells and a second (the nodules) composed of largely non-proliferative cells that have differentiated into neurons (3). Tumors with more cells of the second type are known to be less aggressive clinically (4). This suggests that modulating the number of primitive cells in a medulloblastoma can affect the clinical outcome.
The Notch signaling pathway regulates cell differentiation by means of intercellular communication between adjacent cells (5). A role for Notch in medulloblastoma stem cell maintenance has been described. Notch suppression induced differentiation in medulloblastoma cells and viable populations of better-differentiated cells continued to grow when Notch activation was inhibited. However, they were unable to efficiently form soft-agar colonies or tumor xenografts, suggesting that a cell fraction required for tumor propagation had been depleted. Further, Notch blockade reduced the CD133-positive cell fraction suggesting that the loss of tumor-forming capacity could be due to the depletion of stem-like cells. Stem-like cells in medulloblastoma tumors thus seem to be selectively vulnerable to agents inhibiting the Notch pathway (6).
Secreted Protein Acidic and Rich in Cysteine (SPARC/osteonectin/BM40) is a 32kDa calcium-binding glycoprotein that affects a wide variety of cellular processes, including counter adhesion, ECM remodeling (7), cell migration (8), and angiogenesis (7, 9). In adult tissues, expression of SPARC is highest in tissues undergoing active matrix remodeling, where it is hypothesized to function as a negative regulator of growth factor activity and has been shown to possess anti-adhesive and anti-proliferative properties (10–12). SPARC is also thought to play a critical role in epithelial differentiation (13), parietal endoderm differentiation, cardiomyogenesis in embryoid bodies (14), and differentiation of myoblasts (15). In addition, SPARC was also shown to play a role in neural and/or glia differentiation (16). Further, previous studies have shown that Daoy and D283 medulloblastoma cells are arrested along the neuronal differentiation pathway (17).
In a recent study, we established that SPARC expression induced apoptosis and regressed pre-established tumor growth in medulloblastoma cells (18). Previous studies have shown that Daoy and D283 medulloblastoma cells are arrested along the neuronal differentiation pathway (17). In the present study, we first investigated the effect of endogenous expression of SPARC using adenoviral-mediated delivery of SPARC full length cDNA on the expression of neuronal markers in medulloblastoma cells in vitro and in vivo. Further we demonstrate the role of Notch1/Signal Transducer and Activator of Transcription 3 (STAT3) in SPARC-induced neuronal marker expression in human medulloblastoma cell lines. These data define a new role for SPARC as an inducer of neuronal differentiation and could lead to a favorable course of treatment for medulloblastoma patients and it may sensitize tumors for therapy.
MATERIALS AND METHODS
Cell cultures
We used the Daoy (HTB-186), D283 Med (HTB-185), UW228, D425, cell lines and primary medulloblastoma cells (H2405 and H2411) for this study. Daoy and D283 cells were authenticated by DNA profile using the short tandem repeat (STR), cytogenetic analysis and isoenzymes, and obtained from ATCC, (Manassas, VA). D425, H2405 and H2411 cells were kindly provided by Dr. Darell D. Bigner (Duke University Medical Center); and UW228 cells were kindly provided by Dr Ali-Osman (Duke University Medical Center) in June, 2010. These cells were authenticated on the basis of c-myc amplification, chromosomal aberrations by the provider (19,20). At the 3rd or 4th passage of cells were frozen and these frozen stocks were used for further experimental studies up to the 10th passage to obtain consistent results. Daoy cells were cultured in Advanced-MEM and D283, D425, H2405 and H2411 were cultured in Improved-MEM (Zn Option) and UW228 cells were cultured in RPMI-1640. All of the above media were supplemented with 10% fetal bovine serum, 2mM L-glutamine, 2mM sodiumpyruvate, 100 units/mL penicillin, and 100 μg/mLstreptomycin. Cells were maintained in a humidified atmosphere containing 5% CO2 at 37°C.
Antibodies and reagents
Antibodies against SPARC, STAT3, pSTAT3 (Tyr705), pSTAT3 (Ser727), Notch1, Notch2, Notch3, HES1, GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA), Nestin, MAP-2, Neurofilament (NF), NF (Novus Biologicals, Littleton, CO), NeuN (Millipore, Billerica, MA), NeuN (Invitrogen Corporation, Carlsbad, CA), MAP-2 (Bethyl Laboratories, Inc, Montgomery, TX), neutralizing IL-6 antibody (Biovision, Mountain View, CA), Calcium Green-2-AM (Invitrogen, Carlsbad, CA) and N-[(3,5-Difluorophenyl)acetyl]-L-alanyl-2-phenyl]glycin-e-1,1-dimethylethyl ester (DAPT) (Sigma, St. Louis, MO), paraffin embedded Human medulloblastoma tumor sections (a kind gift from Dr. Dan Fults, Comprehensive Care for Brain and Spine Disorders, UT) were used in this study. All other reagents were of analytical reagent grade or better.
Adenovirus construction and Adenoviral infection
We constructed adenoviral vectors carrying full length human SPARC cDNA (Ad-DsRed-SP) and an empty vector (Ad-DsRed) using Adeno-X ViraTrak Expression System-2 (Clontech Laboratories, Mountain View, CA) as described previously (21). The generation, amplification, and titer of the adenovirus were performed according to previously described procedures (21). Infection with recombinant viruses was accomplished by exposing cells to adenovirus in serum-free cell culture medium for 1hr followed by addition of serum-containing medium. Cells were then incubated for various time periods as detailed in the following experiments.
Immunoblotting
Immunoblot analysis was performed as described previously (22). Briefly, cells were infected with mock, 100MOI of Ad-DsRed, or various MOI of Ad-DsRed-SP and incubated for 36hrs at 37°C. Cell lysates were prepared and equal amounts of protein was resolved on SDS-PAGE gel and transferred on to PVDF membrane. Next, the blot was blocked and probed overnight with primary antibody at 4°C followed by HRP-conjugated secondary antibody for 1hr, and signals were detected using ECL reagent.
Transfection with plasmids
Plasmid expressing constitutively active STAT3 (pSTAT3-C) was obtained from Addgene Inc. (Cambridge, MA; plasmid 8722). Plasmids expressing IL-6 and HES1 were obtained from Origene Technologies (Rockville, MD). All transfection experiments were performed with FuGeneHD (Roche, Indianapolis, IN) transfection reagent according to the manufacturer’s protocol.
Intracellular Ca+2 monitoring
Daoy/D283 cell were plated in 96-well plates and infected with Mock, 100MOI of Ad-DsRed or 100MOI of Ad-DsRed-SP for 36hrs and measured intracellular Ca+2 concentration as described previously (23). The Calcium Green-2-AM fluorescence was expressed as (Fmax − Fmin)/Fmin where Fmax was the maximum, Fmin the minimum fluorescence measured in each well.
Immunofluorescence and immunohistochemical analyses
We used a previously described protocol with minor changes (24). Briefly, the cells were infected with Adenovirus as above for 36hrs. Cells were fixed, permeabilized and blocked, and incubated with primary antibody overnight at 4°C, followed by FITC-conjugated secondary antibody for 1hr. Finally, cells were mounted with Vectashield mounting medium (Vector Labs, Burlingame, CA). The results were documented using fluorescence microscope. For immunohistochemical analysis, tissue sections (4–5μm-thick) were deparaffinized, rehydrated, washed with PBS, permeabilized, and incubated overnight with primary antibodies. Tissue sections were then incubated with HRP-conjugated secondary antibodies followed by DAB peroxidase substrate; Sigma, St. Louis, MO) solution, counterstained with hematoxylin and mounted. The images were processed as described previously (24).
Intracranial tumor model
The animal experiments were carried out as described previously by us (24). D425 (1×105 cells/10μl PBS) cells were stereotactically implanted. After 14 days of tumor cell implantation, the animals were randomized into 3 groups (10/group). Each mouse received three intratumoral injections on days 15, 17 and 19 with PBS, Ad-DsRed (5×107 PFU) or Ad-DsRed-SP (5×107 PFU) in 10μl of volume. Animals were monitored for up to 90 days, which is when we arbitrarily terminated the experiment. Mice brains were fixed in 10% buffered formalin and embedded in paraffin. Tissue sections (5μ thick) were obtained from the paraffin blocks and stained with H&E using standard histological techniques. Tissue sections were also subjected to immunostaining as described above.
Statistical analysis
All data are expressed as mean ± SD. Statistical analysis was performed using the student’s t-test or a one-way ANOVA. p <0.05 was considered significant.
RESULTS
SPARC induces neuronal differentiation of medulloblastoma cells
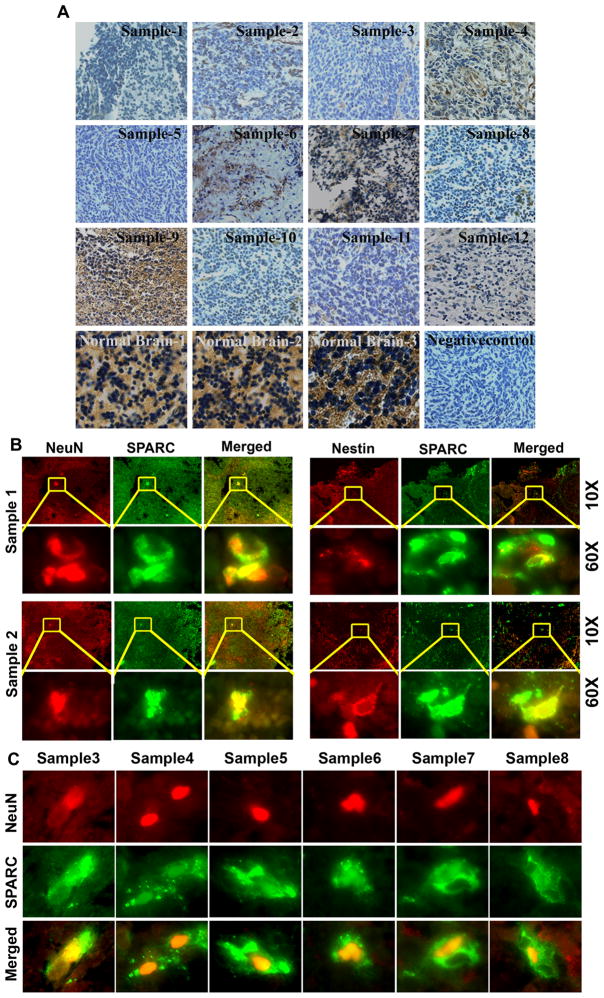
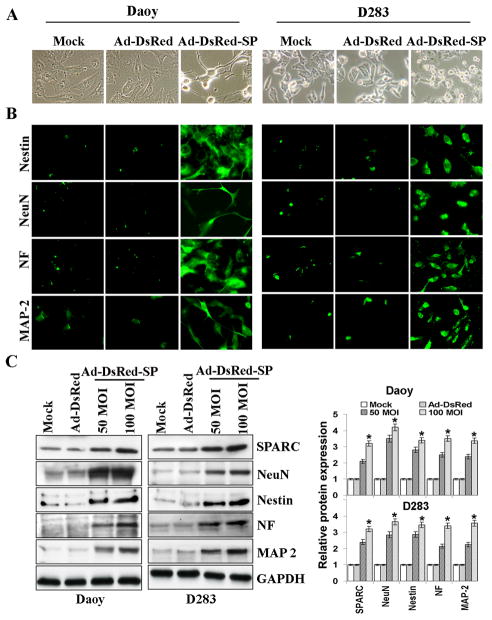
We observed very low or minimal staining for SPARC in Human Medulloblastoma tissue samples compared to normal cerebellum (Fig. 1A). Dual immunoassaying of these tissue samples for neuronal markers and SPARC indicated that very few cell stained positive for neuronal makers and that SPARC expressing tumor cells stained positive for NeuN and Nestin neuronal markers (Fig. 1B&C). Further, previous studies have shown that Daoy and D283 medulloblastoma cells are arrested along the neuronal differentiation pathway (17). We therefore determined weather SPARC induced the expression of neuronal markers in Daoy, D283, UW228, D425 medulloblastoma cell lines and H2405, H2411 primary medulloblastoma cells in vitro and in vivo. To examine the effect of SPARC expression on neuronal differentiation, we used an adenoviral vector expressing SPARC cDNA (Ad-DsRed-SP) in above cell lines. Medulloblastoma cells infected with Ad-DsRed-SP showed obvious neuriteextensions and classic neuronal features, such as shrinkage of the cell body, and these cells were distinguished by highly refractive cell bodies with neuron-like processes terminating in structures that resembled growth cones (Fig. 2A & Suppl. Fig. 1). In contrast, few if any empty vector (Ad-DsRed)-infected cells differentiated into neurons under the same conditions (Fig. 2A). These findings suggest that the medulloblastoma cells treated with Ad-DsRed-SP might have differentiated into neuron-like cells. To further characterize this neuronal induction phenomenon, we examined neuronal marker expression by immunoblot analysis and immunocytochemical analysis. The cells that exhibited contracted cell bodies and processes showed clear staining for the neuronal-specific markers NeuN, Nestin, MAP-2, and NF. In contrast, the flat, unresponsive medulloblastoma cells remained unstained (Fig. 2B). The percentage of cells positively reactive for NeuN, Nestin, MAP-2, and NF was 52% ± 2.5%, 55% ± 2.3%, 56% ± 38%, and 53% ± 2.4%, respectively. Immunoblotting was performed to confirm these results, and the protein analysis showed a marked correlation with the results of the immunocytochemical analysis (Fig. 2C & Suppl. Fig. 2). SPARC expression induced a statistically significant increase (p<0.05) of up to four-fold in the protein expression levels of all the markers as compared to empty vector-treated cells in medulloblastoma cell lines. Similar to adenoviral mediated expression of SPARC cDNA, recombinant human SPARC protein (rhSPARC) induced neuron like cell morphology and expression of neuronal markers in medulloblastoma cell lines. (Suppl. Fig. 3).
Figure 1.
(A) SPARC expression in human medulloblastoma tissue. Human medulloblastoma tissue sections (samples 1–12) were subjected to immunohistochemical analysis using SPARC specific antibody. SPARC expression is high in normal brain tissue (20X). In contrast, the SPARC expression is very weak in most of the medulloblastoma samples (20X). Also shown is a negative control using non specific IgG. (B&C) Association of SPARC expression with neuronal differentiation in human medulloblastoma tumor sections. Paraffin-embedded tissue sections from human medulloblastoma tumors were analyzed by duel immunoflorescence for SPARC (green) and neuronal makers NeuN or Nestin (red) expression using specific antibodies (10X). Enlarged portions of the sections indicated with yellow square boxes (60X). Very few cells express neuronal markers which also stain positive for SPARC expression (yellow).
Figure 2. Overexpression of SPARC in medulloblastoma cells induces expression of neuronal markers.
DAOY and D283 cells were infected with mock, 100MOI Ad-DsRed, or the indicated MOI of Ad-DsRed-SP for 36hrs. (A) Morphology of medulloblastoma tumor cells treated with 100MOI of Ad-DsRed-SP using phase contrast microscopy. (B) Immunocytochemical analysis for neuronal markers in medulloblastoma cells treated with 100MOI of Ad-DsRed-SP. (C) Cell lysates were assessed for SPARC and neuronal markers by immunoblot analysis using anti-SPARC, anti-NeuN, anti-Nestin, anti-NF, and anti-MAP-2 antibodies. GAPDH served as a control. Results are representative of three independent experiments. Protein band intensities were quantified by using ImageJ software (NIH). Columns, mean of three experiments; bars, SD; *, significant difference from Ad-DsRed (p<0.01).
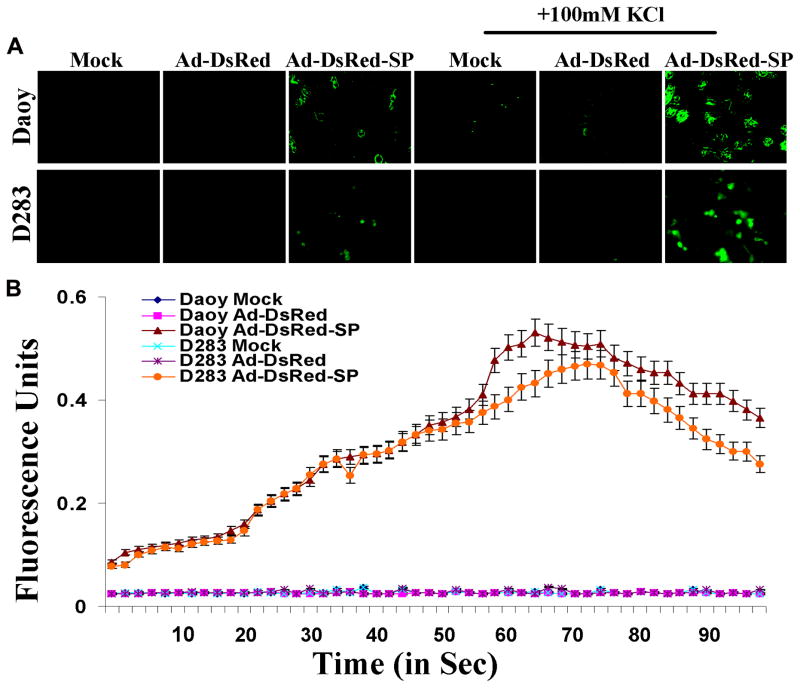
Activity-dependent changes in neuronal processes such as synaptic plasticity and neuronal survival are mediated in large part through elevations in intracellular calcium levels. In many cases, this involves stimulation of calcium influx, particularly via voltage-operated L-type channels or receptor-operated N-methyl-D-aspartate channels (25). To test the neuronal nature of the differentiated cells from Daoy/D283 treated with Ad-DsRed-SP, we examined the changes in intracellular free Ca2+ concentration ([Ca2+]i) in response to depolarization of membrane potential with superfusion of high KCl (100mM) using Calcium Green-AM. Figure 3A shows that intense fluorescence in Ad-DsRed-SP-infected cells compared to mock and Ad-DsRed infected Daoy/D283 cells. We next performed a dual-wavelength excitation fluorimetry using in Calcium Green-AM -loaded cells. High KCl bathing solution for 60 sec increased [Ca2+]i in differentiated neuronal cells (Fig 3B). In contrast, cells treated with mock and Ad-DsRed had no changes in [Ca2+]i by membrane depolarization with high KCl.
Figure 3. KCl-induced Ca+2 imaging in live differentiated medulloblastoma cells.
(A) Medulloblastoma cells were plated in 8-well chamber slides and infected with mock, Ad-DsRed, or Ad-DsRed-SP for 36hrs. The Calcium Green-AM and KCl was added and photographed using fluorescence microscope. (B) Medulloblastoma cells were plated in 96-well plates and infected with mock, Ad-DsRed, or Ad-DsRed-SP for 36hrs, and assessed the changes in intracellular free Ca2+ concentration. Representative time–response data for KCl excitation using differentiated neuron like cells. Cells were treated with Calcium Green-AM in the presence and absence of KCl and a kinetic fluorometric reading was measured using Flex Station and the representative graph is shown. Results are representative of three independent experiments. Symbols, mean of three experiments; bars, SD.
SPARC expression decreases Signal transducer and activator of transcription-3 (STAT3) signaling
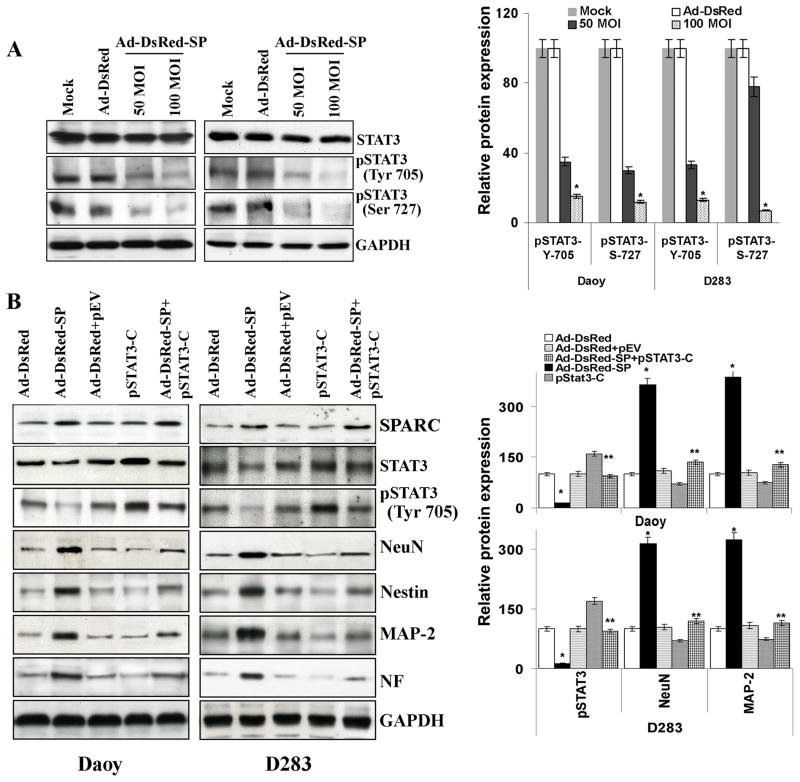
STAT3 signaling has been shown to regulate cell fate determination, renewal, and differentiation in various cells (26). We evaluated whether suppression of STAT3 cell signaling machinery could modulate the neuronal fate of SPARC-expressing medulloblastoma cells. To elucidate the effects of SPARC overexpression on STAT3 signaling, cells were infected with Ad-DsRed-SP for 36hrs and homogenized in lysis buffer. Then, lysates were immunoblotted with antibodies against pSTAT3. Densitometric analysis revealed that the STAT3 phosphorylation was reduced significantly (70.5% in Daoy cells and 67% in D283 cells) as compared to the mock or empty vector-infected cells (Fig. 4A). Further, SPARC expression in primary medulloblastoma cells (H2405, H2411), D425 and UW228 cells also suppressed STAT3 phosphorylation (Suppl. Fig. 2). We therefore speculated that suppression of STAT3 influenced the differentiation fate of neural stem cells. To confirm this notion, we transfected cells with plasmid expressing constitutively active STAT3 (pSTAT3-C) along with Ad-DsRed-SP-infection and total cell lysates. After inoculation, cells were homogenized in lysis buffer and immunoblotted with antibodies against MAP-2, NF, NeuN and Nestin. As illustrated in Figure 4B, transfection with plasmid expressing constitutively active STAT3 induced STAT3 phosphorylation in Ad-DsRed-SP-treated cells comparable to that of the mock and empty vector-transfected cells. Densitometry analysis indicated that pSTAT3-C transfection reversed SPARC-induced MAP-2, NF, NeuN and Nestin neuronal markers by up to 50% (p<0.05) in Ad-DsRed-SP-infected cells. Further, STAT3 siRNA-transfection decreased STAT3 levels in Daoy/D283 cells and induced neuronal markers (Suppl. Fig. 4). These results clearly indicate the role of STAT3 inhibition in the differentiation of medulloblastoma cells. Taken together, these data suggested that STAT3 regulates the expression of neuronal markers.
Figure 4. SPARC expression decreased STAT signaling.
(A) Medulloblastoma cells were infected with mock, 100 MOI Ad-DsRed, or the indicated MOI of Ad-DsRed-SP for 36hrs, and cell lysates were assessed for STAT3 and phosphorylation of STAT3 using specific antibodies. GAPDH served as a control. Protein band intensities were quantified by using ImageJ software (NIH). Columns, mean of three experiments; bars, SD; *, significant difference from Ad-DsRed (p<0.01). (B) Cells were transfected with plasmid constitutively expressing active-STAT3 (pSTAT3-C) for 16hrs and infected with mock, 100MOI Ad-DsRed or Ad-DsRed-SP for an additional 24hrs. Cell lysates were assessed for SPARC, activation of STAT3 and neuronal markers by using their specific antibodies. Results are representative of three independent experiments. Columns, mean of three experiments; bars, SD; *, significant difference from Ad-DsRed (p<0.01); **, significant difference from Ad-DsRed-SP (p<0.05).
SPARC induces expression of neuronal markers by blocking Notch signaling
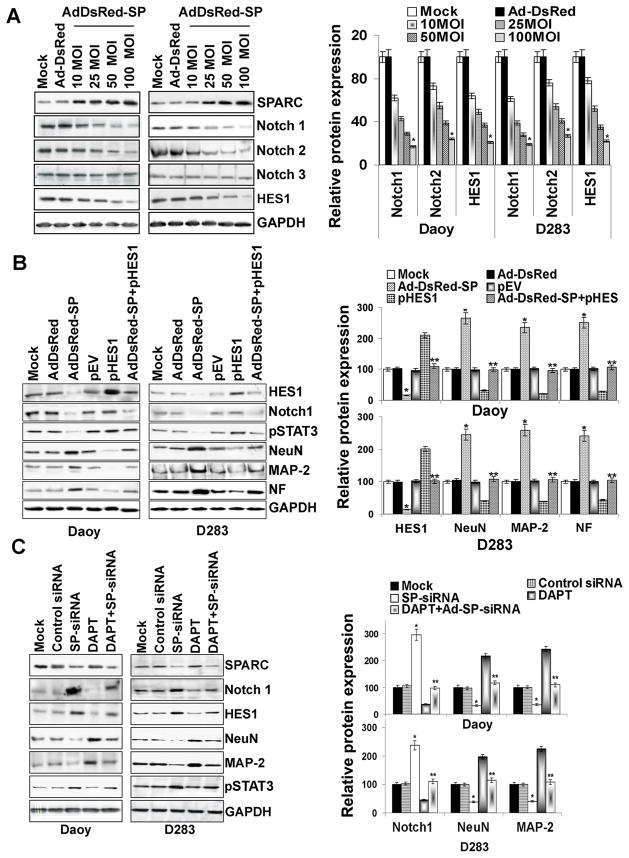
Blockade of Notch signaling, a condition known to induce neuronal differentiation, represses inhibitory basic helix-loop-helix transcription factor-1, HES1 expression (27). It was shown that STAT3 is activated in the presence of active Notch, as well as the Notch effectors HES1 and HES5 (28). We therefore examined the effects of Ad-DsRed-SP-infection on the expression of Notch family members (Notch1 and 2) and HES1 in medulloblastoma cells. Immunoblot analysis revealed that Notch1 and Notch2 were suppressed in a dose-dependent manner reaching about 75% inhibition in Daoy and 70% inhibition in D283 cells at 100 MOI Ad-DsRed-SP-infection when compared to mock or Ad-DsRed-infected cells (Fig. 5A). Similarly, HES1 was inhibited by 68% in Daoy and 65% in D283 (p<0.05) cells infected with Ad-DsRed-SP as compared to mock or Ad-DsRed controls (Fig. 5A). Similarly, SPARC overexpression in the primary medulloblastoma cells (H2405 and H2411), D425 and UW228 cell lines lead to decreased Notch1 and HES1 expression (Suppl. Fig. 2). Since we observed that STAT3 inhibition contributes to the expression of neuronal markers in SPARC-overexpressed cells, we therefore used HES1 cDNA to determine whether HES1 expression is necessary for STAT3-mediated induction of neuronal markers in SPARC-overexpressed cells. Transfection of medulloblastoma cells with a vector specific for HES1 cDNA in SPARC-overexpressed cells resulted in an increase in the abundance of HES1 protein comparable to mock or Ad-DsRed-treated cells (Fig. 5B). Concomitantly, densitometry analysis revealed that STAT3 phosphorylation was increased significantly by 70% and 68% (p<0.05 vs. Ad-DsRed-SP) in Daoy/D283 cells (Fig. 5B). Furthermore, neuron like morphological changes and the induction of neuronal markers as determined by immunocytochemical analysis and immunoblotting, respectively, were suppressed by HES1 overexpression in SPARC-overexpressed cells. (Fig. 5B & Suppl. Fig. 1). Together, these results suggest that HES1 is an essential mediator of the action of STAT3 in SPARC-induced neuronal differentiation in medulloblastoma cells.
Figure 5. SPARC inhibits Notch signaling and induces expression of neuronal markers.
(A) Medulloblastoma cells were infected with mock, 100MOI Ad-DsRed, or the indicated MOI of Ad-DsRed-SP for 36hrs, and cell lysates were assessed for Notch family molecules (Notch1, Notch2 and Notch3) and HES1 using molecule specific antibodies. GAPDH served as a control. Protein band intensities were quantified by using ImageJ software (NIH). Columns, mean of three experiments; bars, SD; *, significant difference from Ad-DsRed (p<0.01). (B) Cells were transfected with plasmid expressing HES1 for 16hrs and infected with mock, 100MOI Ad-DsRed or Ad-DsRed-SP for an additional 24hrs. Cell lysates were assessed for neuronal markers by immunoblotting using their specific antibodies. Results are representative of three independent experiments. GAPDH served as a control. Columns, mean of three experiments; bars, SD; *, significant difference from Ad-DsRed (p<0.01); **, significant difference from Ad-DsRed-SP (p<0.05). (C) Cells were infected with mock, 100MOI of control siRNA or SPARC siRNA (SP-siRNA) for 24hrs. Cells were then treated with 10μM DAPT and cells were allowed to grow for an additional 12hrs. Cell lysates were assessed for Notch signaling molecules and neuronal markers by immunoblotting using their specific antibodies. Results are representative of three independent experiments. GAPDH served as a control. Columns, mean of three experiments; bars, SD; *, significant difference from control-siRNA (p<0.01); **, significant difference from SP-siRNA (p<0.01).
Effects of SPARC siRNA (SP-siRNA) on Notch expression
To confirm that SPARC can induce neurogenesis in medulloblastoma cells via Notch1-mediated HES1 signaling, we examined the effects of SP-siRNA on the expression of Notch family members and neuronal markers in medulloblastoma cells. Figure 5C indicates that infection with an adenoviral vector encoding SP-siRNA decreased SPARC levels as compared to mock or control siRNA-treated cells. Along with SPARC reduction, there was induction of Notch1, HES1 expression and STAT3 phosphorylation, and suppression of the expression of NeuN and MAP-2 neuronal markers in SP-siRNA-treated cells (Fig. 5C). Blocking Notch1 using a known gamma scecretase inhibitor DAPT (29) in SP-siRNA treated cells suppressed HES1 and STAT3 phosphorylation and induced the expression of neuronal markers (Fig. 5C). Taken together, these results suggest that SPARC-induced neuronal differentiation by blocking Notch-mediated STAT3 phosphorylation.
IL-6 regulates Notch-mediated modulation of neuronal markers in SPARC-overexpressed medulloblastoma cells
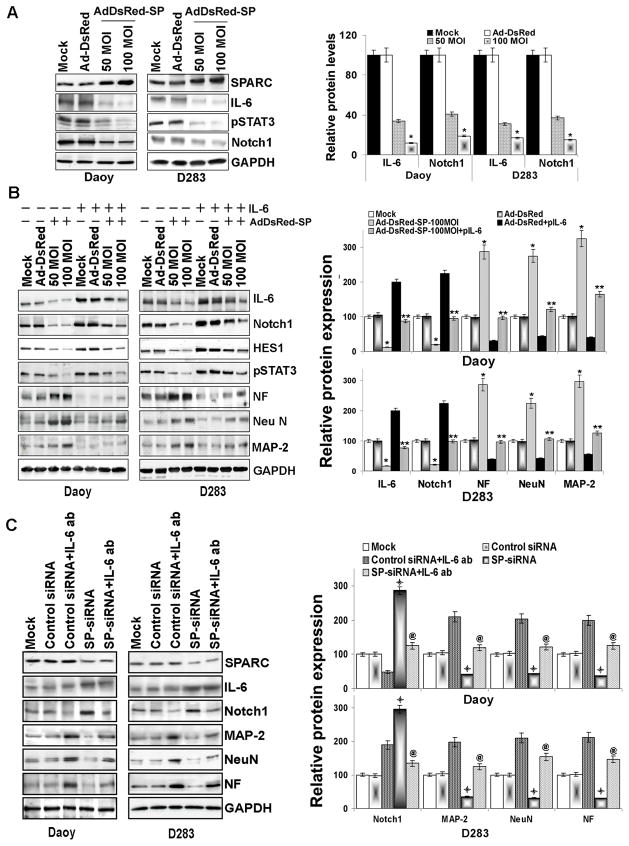
Previous studies demonstrate SPARC expression attenuated IL-6 secretion (30) and that IL-6 up regulates Notch signaling (31). Therefore, we examined the role of IL-6 in SPARC-induced Notch signaling and expression of neuronal markers. Immunoblot analysis for IL-6 expression indicates that SPARC overexpression decreased IL-6 in a dose-dependent manner in Daoy, D283, D425 and UW228 cell lines and primary medulloblastoma cells (H2405, H2411), (Fig. 6A & Suppl. Fig. 2). To better understand the role of IL-6-mediated effects on neuronal markers in SPARC-expressed cells, we overexpressed IL-6 in SPARC-overexpressed medulloblastoma cells. Figure 6B indicated that Notch1 expression increased by 65% and 60% in IL-6 and SPARC-overexpressed cells as compared to only SPARC-overexpressed medulloblastoma cells. Consequently the expression of neuronal markers, MAP-2, NF, and NeuN was also decreased by about 65%, 63% and 60% respectively in cells overexpressed with IL-6 and SPARC compared to only SPARC-overexpressed cells (Fig. 6B; p<0.05), suggesting that SPARC-mediated suppression of IL-6 leads to suppression of Notch1 expression, which leads to induction of neuronal markers.
Figure 6. SPARC overexpression in medulloblastoma cells inhibits IL-6 signaling and induces expression of neuronal markers.
(A) Medulloblastoma cells were infected with mock, 100MOI Ad-DsRed or the indicated MOI of Ad-DsRed-SP for 36hrs. Cell lysates were assessed for SPARC, IL-6, pSTAT3, and Notch1 by immunoblot analysis using specific antibodies. GAPDH served as a control. Protein band intensities were quantified by using ImageJ software (NIH). Columns, mean of three experiments; bars, SD; *, significant difference from Ad-DsRed (p<0.01). (B) Cells were transfected with a plasmid expressing IL-6 for 16hrs and infected with mock, 100MOI Ad-DsRed or the indicated MOI of Ad-DsRed-SP for an additional 24hrs. Cell lysates were assessed for neuronal markers by using their specific antibodies. Results are representative of three independent experiments. Protein band intensities were quantified by using ImageJ software (NIH). Columns, mean of three experiments; bars, SD; *, significant difference from Ad-DsRed (p<0.01); **, significant difference from Ad-DsRed-SP (p<0.05), (C) Cells were infected with mock, 100MOI control siRNA or SP-siRNA for 24hrs and treated with IL-6 blocking antibody for further 12hrs. Cell lysates were assessed for IL-6, Notch1 and neuronal markers by using their specific antibodies. Results are representative of three independent experiments. Protein band intensities were quantified by using ImageJ software (NIH). Columns, mean of three experiments; bars, SD;
 , significant difference from control-siRNA (p<0.01); @, significant difference from SP-siRNA (p<0.01)].
, significant difference from control-siRNA (p<0.01); @, significant difference from SP-siRNA (p<0.01)].
To confirm the role of IL-6 in Notch signaling-mediated expression of neuronal markers in SPARC-expressed cells, we performed parallel experiments using SP-siRNA. Figure 6C indicates that SP-siRNA suppressed SPARC levels when compared to mock or control siRNA-treated cells. Suppression of SPARC using SP-siRNA-induced IL-6 and Notch1 expression by 2–3 fold compared to control siRNA treated cells. Consequently the expression of neuronal markers MAP-2, NeuN and NF were decreased by 60–70% in SP-siRNA treated cells. To further confirm that IL-6 mediates Notch1 suppression which in turn regulates the expression of Neuronal markers in SPARC-modulated cells, we blocked IL-6 activity by using neutralizing antibodies and determined the levels of Notch1 and neuronal markers. IL-6 neutralizing antibody suppressed Notch1 and induced the expression of neuronal markers in SP-siRNA treated cells (Fig. 6C) suggesting that SPARC-mediated effects on Notch1 regulation are mediated by IL-6.
In summary, these results suggest that the inhibitory effect of SPARC on IL-6 leads to Notch-mediated expression of neuronal markers in SPARC-overexpressed cells.
SPARC-mediated expression of neuronal markers in vivo
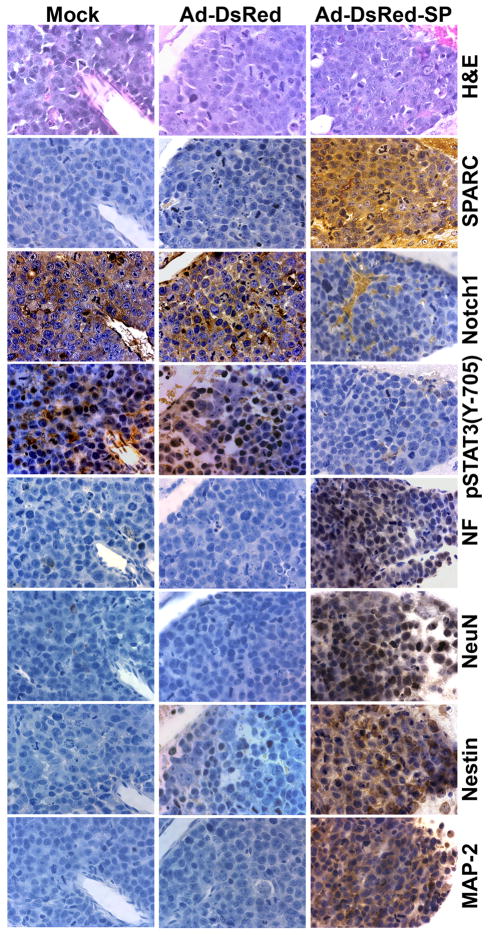
We have previously shown that SPARC expression inhibits medulloblastoma tumor growth in vivo in an intracranial model (18). The present findings raise the question of whether the effects of SPARC on tumor growth inhibition are related to the effect of SPARC on neuronal differentiation in vivo. Immunohistochemical analysis was performed on established tumors from mice implanted with D425 medulloblastoma cells and treated with mock, Ad-DsRed, or Ad-DsRed-SP with antibodies specific to detect neuronal markers of human origin. The results show a clear increase in the expression of neuronal markers MAP-2, NeuN, Nestin and NF in tumor sections from Ad-DsRed-SP-treated mice as compared to sections from mock and Ad-DsRed-treated animals, thereby suggesting that SPARC expression induced the expression of neuronal markers in vivo (Fig. 7). To determine whether SPARC regulates Notch and STAT3 phosphorylation in vivo, phosphorylation of STAT3 and Notch1 expression was measured by immunohistochemical analysis. Consistent with the in vitro results, a decrease in cleaved Notch1 and phosphorylation of STAT3 was found in Ad-DsRed-SP-treated tumors (Fig. 7).
Figure 7. SPARC overexpression induces expression of neuronal markers in vivo.
Orthotopic medulloblastoma tumor sections from mice that received mock, Ad-DsRed and Ad-DsRed-SP treatments were analyzed. Tissue sections were stained for H&E. Immunohistochemical analysis was performed with antibodies specific to SPARC, Notch1, pSTAT3, NF, NeuN, Nestin and MAP-2.
DISCUSSION
Medulloblastoma show a tremendous clinical heterogeneity and the degree of neuronal tumor cell differentiation influences patient outcome (4). Several studies demonstrate that SPARC induces differentiation; however, no studies have shown the functional mechanism by which SPARC induces neuronal differentiation in tumor cells. In the present study, we demonstrate that SPARC expression, using an adenoviral vector expressing SPARC cDNA (Ad-DsRed-SP), induced neuronal differentiation in medulloblastoma tumor cells. Further, we demonstrate the molecular mechanisms that govern SPARC-induced neuronal markers and discuss the potential clinical impact of SPARC on medulloblastoma tumor genesis.
SPARC expression induced the expression of neuronal markers in medulloblastoma in vitro as demonstrated by immunoblotting and immunocytochemical analysis. Moreover, Ca2+ response by high K+ proved functionally mature neuron activity in neuronal cells, differentiated from Ad-DSRed-SP-infected medulloblastoma cells. We also demonstrated that the neuronal differentiation ability of Ad-DsRed-SP depends on STAT3 regulation. The function of STAT3 in differentiation has been investigated extensively. Extracellular stimulus and the cellular context activate the phosphorylation of STAT3. Increasing evidence indicates that STAT3 can maintain the propagation and pluripotency of embryonic stem cells (32). Suppression of STAT3 directly induced neurogenesis in neural stem cells (33). STAT3 activation has also been reported to be sufficient to maintain the undifferentiated state of mouse embryonic stem cells (28, 34).
Our results further emphasize the potential clinical importance of Notch signaling in medulloblastoma. A recent series of experiments demonstrated an oncogenic role for Notch signaling in medulloblastoma and the maintenance of medulloblastoma stem cells. The Notch pathway also plays an important role in inhibition of differentiation in many systems, including the hematopoietic system (35). Notch blockade suppressed expression of the pathway target HES1 and caused cell cycle exit, apoptosis, and differentiation in medulloblastoma cell lines (6). In addition, expression of the Notch pathway target gene HES1 in medulloblastomas was associated with significantly shorter patient survival (36). It was shown that STAT3 is activated in the presence of active Notch, as well as the Notch effectors HES1 and HES5 (28). Furthermore, suppression of endogenous HES1 expression reduces growth factor induction of STAT3 phosphorylation (28). We therefore tested the effect of SPARC on Notch/HES1 mediated regulation of STAT3 phosphorylation. HES1 overexpression induced STAT3 phosphorylation and suppressed expression of neuronal markers suggestion that HES1/STAT3 axis plays a role in SPARC-induced neuronal markers. As consistent with our immunoblot analysis, morphological and immunocytochemical analysis also suggest that HES1 overexpression suppressed neuron like morphological changes and neuronal marker expression. Our study clearly demonstrates that HES1 overexpression induced STAT3 phosphorylation SPARC-overexpressed cells.
We next investigated how SPARC modulates IL-6 signaling in medulloblastoma cells. SPARC had a negative regulatory role on levels of IL-6sR, an IL-6 agonist implicated in IL-6 trans-signaling (37). IL-6 is a multifunctional cytokine that has also been implicated in tumorigenesis (38). Cytokines of the IL-6 family were suggested to block neuronal markers expression of cerebral cortical precursor (39). Our study demonstrates that SPARC expression decreased IL-6 expression. In addition, we also demonstrate that overexpressing IL-6 blocked SPARC-mediated inhibition of Notch1 expression and neuronal markers expression. Conversely, we also demonstrate that SP-siRNA induced IL-6 and Notch expression. Furthermore, we show that blocking IL-6 signaling in SPARC-suppressed cells induced Notch1 expression and neuronal differentiation. These findings suggest that SPARC negatively regulates IL-6 signaling leading to suppression of Notch1 signaling, resulting in neuronal differentiation of medulloblastoma cells.
Immunohistochemistry showed that cells expressing SPARC express high levels of neural markers in the tumor sections of mice treated with Ad-DsRed-SP. This change in histological appearance was also associated with a change in the size of xenograft tumors that formed in the immunodeficient mice (18), suggesting that SPARC-enhanced the expression of neuronal markers in medulloblastoma cells. Our observation can be placed within the larger context of recent progress in cancer treatment involving differentiation. In many cell lines and primary cultures derived from hematologic malignancies, the malignant phenotype can be abrogated by inducing differentiation (40). Cyclopamine, a plant-derived teratogen that targets the SHH pathway, inhibits SHH-dependent gene expression in medulloblastoma in vitro and is able to cause cell cycle arrest consistent with the initiation of neuronal differentiation and loss of neuronal stem cell-like character (41).
In summary, we have previously shown that SPARC expression causes tumor growth inhibition (41). Further we show that SPARC-induced neuronal differentiation which could render these tumors to be more susceptible to chemo and radiotherapy. Previous studies demonstrate that SPARC enhances apoptosis in therapy-refractory MIP101 colon cancer cells exposed to chemotherapy by activating the extrinsic pathway of apoptosis while further enhancing the effect of chemotherapy through the intrinsic pathway (42). The effect of radiotherapy in combination with SPARC is the focus of the ongoing research in our laboratory. There are some medically relevant implications from our in vitro and in vivo data. First, SPARC expression can play a role in the clinical outcome of medulloblastoma patients by increasing the number of non-proliferative cells that have differentiated into neurons. Second, pharmacological interventions aimed at the mechanisms of medulloblastoma differentiation outlined in this study might be therapeutically relevant. Third, our results raise the question whether SPARC also plays a role in the differentiation of other tumor types like colorectal cancer or ovarian cancer in which SPARC has been identified as a potential therapeutic target. Fourth, neuronal differentiation of these cells can sensitize tumors for therapy.
Supplementary Material
Acknowledgments
This research was supported by National Cancer Institute Grant CA132853 (to S.S.L.). Contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
We thankful to Shellee Abraham for manuscript preparation; to Diana Meister and Sushma Jasti for manuscript review. We also thankful to Prof. J.E.Darnell Jr for providing constitutively active STAT3 (Addgene plasmid:8722); to Dr. Darell D.Bigner, Duke University Medical Center for providing D425, H2405 and H2411 medulloblastoma cells; to Dr. Ali-Osman, Duke University Medical Center for providing UW228 cells, and to Dr. Dan Fults, Comprehensive Care for Brain and Spine Disorders, UT for providing paraffin embedded Human medulloblastoma tumor sections.
Reference List
- 1.Crawford JR, MacDonald TJ, Packer RJ. Medulloblastoma in childhood: new biological advances. Lancet Neurol. 2007;6:1073–85. doi: 10.1016/S1474-4422(07)70289-2. [DOI] [PubMed] [Google Scholar]
- 2.Zeltzer PM, Boyett JM, Finlay JL, Albright AL, Rorke LB, Milstein JM, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children's Cancer Group 921 randomized phase III study. J Clin Oncol. 1999;17:832–45. doi: 10.1200/JCO.1999.17.3.832. [DOI] [PubMed] [Google Scholar]
- 3.Ehrbrecht A, Muller U, Wolter M, Hoischen A, Koch A, Radlwimmer B, et al. Comprehensive genomic analysis of desmoplastic medulloblastomas: identification of novel amplified genes and separate evaluation of the different histological components. J Pathol. 2006;208:554–63. doi: 10.1002/path.1925. [DOI] [PubMed] [Google Scholar]
- 4.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Artavanis-Tsakonas K, Riley EM. Innate immune response to malaria: rapid induction of IFN-gamma from human NK cells by live Plasmodium falciparum-infected erythrocytes. J Immunol. 2002;169:2956–63. doi: 10.4049/jimmunol.169.6.2956. [DOI] [PubMed] [Google Scholar]
- 6.Fan X, Matsui W, Khaki L, Stearns D, Chun J, Li YM, et al. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66:7445–52. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- 7.Yan Q, Sage EH. SPARC, a matricellular glycoprotein with important biological functions. J Histochem Cytochem. 1999;47:1495–506. doi: 10.1177/002215549904701201. [DOI] [PubMed] [Google Scholar]
- 8.Huynh MH, Sage EH, Ringuette M. A calcium-binding motif in SPARC/osteonectin inhibits chordomesoderm cell migration during Xenopus laevis gastrulation: evidence of counter-adhesive activity in vivo. Dev Growth Differ. 1999;41:407–18. doi: 10.1046/j.1440-169x.1999.00443.x. [DOI] [PubMed] [Google Scholar]
- 9.Chlenski A, Liu S, Guerrero LJ, Yang Q, Tian Y, Salwen HR, et al. SPARC expression is associated with impaired tumor growth, inhibited angiogenesis and changes in the extracellular matrix. Int J Cancer. 2006;118:310–6. doi: 10.1002/ijc.21357. [DOI] [PubMed] [Google Scholar]
- 10.Brown AL, Ringuette MJ, Prestwich GD, Bagli DJ, Woodhouse KA. Effects of hyaluronan and SPARC on fibroproliferative events assessed in an in vitro bladder acellular matrix model. Biomaterials. 2006;27:3825–35. doi: 10.1016/j.biomaterials.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 11.Lane TF, Sage EH. The biology of SPARC, a protein that modulates cell-matrix interactions. FASEB J. 1994;8:163–73. [PubMed] [Google Scholar]
- 12.Martinek N, Shahab J, Sodek J, Ringuette M. Is SPARC an evolutionarily conserved collagen chaperone? J Dent Res. 2007;86:296–305. doi: 10.1177/154405910708600402. [DOI] [PubMed] [Google Scholar]
- 13.Ford R, Wang G, Jannati P, Adler D, Racanelli P, Higgins PJ, et al. Modulation of SPARC expression during butyrate-induced terminal differentiation of cultured human keratinocytes: regulation via a TGF-beta-dependent pathway. Exp Cell Res. 1993;206:261–75. doi: 10.1006/excr.1993.1146. [DOI] [PubMed] [Google Scholar]
- 14.Hrabchak C, Ringuette M, Woodhouse K. Recombinant mouse SPARC promotes parietal endoderm differentiation and cardiomyogenesis in embryoid bodies. Biochem Cell Biol. 2008;86:487–99. doi: 10.1139/O08-141. [DOI] [PubMed] [Google Scholar]
- 15.Cho WJ, Kim EJ, Lee SJ, Kim HD, Shin HJ, Lim WK. Involvement of SPARC in in vitro differentiation of skeletal myoblasts. Biochem Biophys Res Commun. 2000;271:630–4. doi: 10.1006/bbrc.2000.2682. [DOI] [PubMed] [Google Scholar]
- 16.Martinek N, Shahab J, Saathoff M, Ringuette M. Haemocyte-derived SPARC is required for collagen-IV-dependent stability of basal laminae in Drosophila embryos. J Cell Sci. 2008;121:1671–80. doi: 10.1242/jcs.021931. [DOI] [PubMed] [Google Scholar]
- 17.Lawinger P, Venugopal R, Guo ZS, Immaneni A, Sengupta D, Lu W, et al. The neuronal repressor REST/NRSF is an essential regulator in medulloblastoma cells. Nat Med. 2000;6:826–31. doi: 10.1038/77565. [DOI] [PubMed] [Google Scholar]
- 18.Bhoopathi P, Chetty C, Gujrati M, Dinh DH, Rao JS, Lakka SS. Cathepsin B facilitates autophagy mediated apoptosis in SPARC overexpressed primitive neuroectodermal tumor cells. Cell Death Differ. 2010;17:1529–39. doi: 10.1038/cdd.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bigner SH, Friedman HS, Vogelstein B, Oakes WJ, Bigner DD. Amplification of the c-myc gene in human medulloblastoma cell lines and xenografts. Cancer Res. 1990;50:2347–50. [PubMed] [Google Scholar]
- 20.Ali-Osman F, Stein DE, Renwick A. Glutathione content and glutathione-S-transferase expression in 1,3-bis(2-chloroethyl)-1-nitrosourea-resistant human malignant astrocytoma cell lines. Cancer Res. 1990;50:6976–80. [PubMed] [Google Scholar]
- 21.Mohan PM, Barve M, Chatterjee A, Hosur RV. pH driven conformational dynamics and dimer-to-monomer transition in DLC8. Protein Sci. 2006;15:335–42. doi: 10.1110/ps.051854906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhoopathi P, Chetty C, Kunigal S, Vanamala SK, Rao JS, Lakka SS. Blockade of tumor growth due to matrix metalloproteinase-9 inhibition is mediated by sequential activation of beta1-integrin, ERK, and NF-kappaB. J Biol Chem. 2008;283:1545–52. doi: 10.1074/jbc.M707931200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.George J, Dravid SM, Prakash A, Xie J, Peterson J, Jabba SV, et al. Sodium channel activation augments NMDA receptor function and promotes neurite outgrowth in immature cerebrocortical neurons. J Neurosci. 2009;29:3288–301. doi: 10.1523/JNEUROSCI.6104-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chetty C, Bhoopathi P, Lakka SS, Rao JS. MMP-2 siRNA induced Fas/CD95-mediated extrinsic II apoptotic pathway in the A549 lung adenocarcinoma cell line. Oncogene. 2007;26:7675–83. doi: 10.1038/sj.onc.1210584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicoll RA, Malenka RC. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995;377:115–8. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- 26.Yu LJ, Wu ML, Li H, Chen XY, Wang Q, Sun Y, et al. Inhibition of STAT3 expression and signaling in resveratrol-differentiated medulloblastoma cells. Neoplasia. 2008;10:736–44. doi: 10.1593/neo.08304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimojo H, Ohtsuka T, Kageyama R. Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron. 2008;58:52–64. doi: 10.1016/j.neuron.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Kamakura S, Oishi K, Yoshimatsu T, Nakafuku M, Masuyama N, Gotoh Y. Hes binding to STAT3 mediates crosstalk between Notch and JAK-STAT signalling. Nat Cell Biol. 2004;6:547–54. doi: 10.1038/ncb1138. [DOI] [PubMed] [Google Scholar]
- 29.Wang M, Wu L, Wang L, Xin X. Down-regulation of Notch1 by gamma-secretase inhibition contributes to cell growth inhibition and apoptosis in ovarian cancer cells A2780. Biochem Biophys Res Commun. 2010;393:144–9. doi: 10.1016/j.bbrc.2010.01.103. [DOI] [PubMed] [Google Scholar]
- 30.Said NA, Najwer I, Socha MJ, Fulton DJ, Mok SC, Motamed K. SPARC inhibits LPA- mediated mesothelial-ovarian cancer cell crosstalk. Neoplasia. 2007;9:23–35. doi: 10.1593/neo.06658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117:3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T, et al. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J. 1999;18:4261–9. doi: 10.1093/emboj/18.15.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu F, Hata R, Ma YJ, Tanaka J, Mitsuda N, Kumon Y, et al. Suppression of Stat3 promotes neurogenesis in cultured neural stem cells. J Neurosci Res. 2005;81:163–71. doi: 10.1002/jnr.20561. [DOI] [PubMed] [Google Scholar]
- 34.Yoshimatsu T, Kawaguchi D, Oishi K, Takeda K, Akira S, Masuyama N, et al. Non-cell-autonomous action of STAT3 in maintenance of neural precursor cells in the mouse neocortex. Development. 2006;133:2553–63. doi: 10.1242/dev.02419. [DOI] [PubMed] [Google Scholar]
- 35.Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annu Rev Immunol. 2005;23:945–74. doi: 10.1146/annurev.immunol.23.021704.115747. [DOI] [PubMed] [Google Scholar]
- 36.Fan X, Mikolaenko I, Elhassan I, Ni X, Wang Y, Ball D, et al. Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res. 2004;64:7787–93. doi: 10.1158/0008-5472.CAN-04-1446. [DOI] [PubMed] [Google Scholar]
- 37.Kallen KJ. The role of transsignalling via the agonistic soluble IL-6 receptor in human diseases. Biochim Biophys Acta. 2002;1592:323–43. doi: 10.1016/s0167-4889(02)00325-7. [DOI] [PubMed] [Google Scholar]
- 38.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–12. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, et al. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science. 1997;278:477–83. doi: 10.1126/science.278.5337.477. [DOI] [PubMed] [Google Scholar]
- 40.Ferrari AC, Waxman S. Differentiation agents in cancer therapy. Cancer Chemother Biol Response Modif. 1994;15:337–66. [PubMed] [Google Scholar]
- 41.Romer J, Curran T. Targeting medulloblastoma: small-molecule inhibitors of the Sonic Hedgehog pathway as potential cancer therapeutics. Cancer Res. 2005;65:4975–8. doi: 10.1158/0008-5472.CAN-05-0481. [DOI] [PubMed] [Google Scholar]
- 42.Tai IT, Dai M, Owen DA, Chen LB. Genome-wide expression analysis of therapy-resistant tumors reveals SPARC as a novel target for cancer therapy. J Clin Invest. 2005;115:1492–502. doi: 10.1172/JCI23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.