Summary
The diseases caused by trypanosomes are medically and economically devastating to the population of sub-Saharan Africa. Parasites of the genus Trypanosoma, infect both humans, causing African sleeping sickness, and livestock, causing Nagana. The development of effective treatment strategies has suffered from the severe side effects of approved drugs, resistance and major difficulties in delivering drugs. Antimicrobial peptides are ubiquitous components of immune defense and are being rigorously pursued as novel sources of new therapeutics for a variety of pathogens. Here we review the role of antimicrobial peptides in the innate immune response of the tsetse fly to African trypanosomes, catalogue trypanocidal antimicrobial peptides from diverse organisms and highlight the susceptibility of bloodstream form African trypanosomes to killing by unconventional toxic peptides.
Keywords: African trypanosomiasis, human sleeping sickness, antimicrobial peptides, tsetse
Introduction
African trypanosomes are the causative agents of human African trypanosomiasis (HAT), also known as sleeping sickness, and Nagana, a wasting disease of livestock (1). The parasites that infect humans are subspecies of Trypanosoma brucei, Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense. The subspecies, Trypanosoma brucei brucei causes livestock disease as well as Trypanosoma vivax, Trypanosoma congolense and Trypanosoma evansi. Trypanosomiasis is a medical and socioeconomic burden primarily to Sub-Saharan Africa, however T. vivax has been introduced into South America (2). Treatment is difficult for many reasons including the logistics of drug delivery and dosage requirements in impoverished rural areas, severe side effects, lack of overlapping drug effectiveness against T. b. gambiense or T. b. rhodesiense and the need to cross the blood-brain barrier in order to treat advanced HAT.
The lifecycle of African trypanosomes involves several morphologically and physiologically distinct stages in both a mammalian and insect host, specifically flies of the genus Glossina, also known as tsetse flies. In order to survive within different hosts, and also within significantly different tissue environments of the same host, the parasite has evolved physiological strategies to acquire nutrients and evade destruction by host immune factors. Perhaps the best understood of these strategies is the successive expression of glycerophosphatidylinositol (GPI)-anchored variant surface glycoproteins (VSG) in the mammalian bloodstream form (BSF) trypanosome (3). The developmental forms of African trypanosomes exhibit multiple physiological differences (4), including non-dividing stages, variation in the acyl- and amino acid identity of GPI-anchored surface proteins (5, 6), differential rates of endocytosis (7) and motility (8) and differences in mitochondrial structure and function (9, 10).
One potential source of new therapeutic agents is the vast and diverse biological repertoire of antimicrobial peptides (AMPs) (11). These small, typically cationic molecules are ubiquitous components of the innate immune system of metazoans and as such have evolved simple biochemical mechanisms of target cell specificity. The mode of action of many AMPs involves increasing the permeability of the cell membrane, often through the formation of transmembrane pores (11). Conventional AMPs with trypanocidal activity have been identified in multiple phyla, including humans (12), and are specifically involved in the insect vector's immune response to African trypanosomes (13-19) (table 1). The unsatisfactory state of pharmacological intervention strategies for HAT has prompted the identification of natural products and synthetic peptides that exhibit trypanocidal activity (20-22) (table 1). Additionally, trypanocidal peptides with unconventional modes of action have been identified from unusual sources, including neuropeptides (23) and secretory signal peptides (24) (table 1). Antimicrobial peptides and synthetic derivatives with activity against the related kinetoplast organisms T. cruzi and Leishmania spp. have been identified and are described in a recent review by McGwire and Kulkarni (25). Here I limit discussion to the African trypanosomes, specifically the role of antimicrobial peptides in the insect vector immune response to African trypanosomes, the characteristics of trypanocidal peptides identified to date and the mechanisms of unconventional trypanocidal peptides from unusual sources.
| Peptide | Source | Mass (dal) | Structure (in non-polar environment) | Net charge (at neutral pH) | Mechanism | Trypanocidal activity1 | |
|---|---|---|---|---|---|---|---|
| PC | BSF | ||||||
| Attacin (15)2 | Tsetse fat body, proventriculus (14), hemolymph(19) | 18,500 | Random (α-helix) (53) | +2 | Plasma membrane permeabilization | 0.075 μM | 0.3 μM |
| Defensin | Tsetse fat body, proventriculus(14), hemolymph(19) | 3601 | β-sheet | +3 | Plasma membrane permeabilization | N.D.3 | N.D. |
| Diptericin (13) | Tsetse midgut, fat body, proventriculus(14) | 8200 | Random (54) | +4 | Plasma membrane permeabilization | 10 μM | N.D. |
| Cecropin (19) | Tsetse hemolymph | 4952 | α-helix (55) | +4 | Plasma membrane permeabilization | N.D. | N.D. |
| Stomoxyn (29) | S. calcitrans midgut | 4415 | Random (α-helix) | +6 | Plasma membrane permeabilization | >>113 μM | 37 μM |
| β-defensin-1 (12) | Human epithelium (33) | 3936 | β-sheet (56) | +5 | Plasma membrane permeabilization | > 50 μM (17.8)4 | > 50 μM (25.3)4 |
| β-defensin-2 (12) | Human epithelium, keratinocytes (57) | 4391 | β-sheet (58) | +6 | Plasma membrane permeabilization | > 50 μM (33.1)4 | > 50 μM (18.6)4 |
| Cryptin-4 (α-defensin) (12) | Mouse Paneth cells (59) | 3762 | β-sheet (60) | +8 | Plasma membrane permeabilization | > 50 μM (29.6)4 | N. A.5 |
| LL-37 (36) | Human neutrophils (61) | 4493 | α-helix (62) | +6 | Plasma membrane permeabilization | 4.5 μM | 1.7 μM |
| SMAP-29 (12) | Ovine myeloids(63) | 3199 | α-helix(64) | +9 | Plasma membrane permeabilization | < 50 μM (95.0)4 | < 50 μM (71.5)4 |
| Novispirin (12) | SMAP-29 derivative (65) | 2207 | α-helix | +7 | Plasma membrane permeabilization | < 50 μM (81.3)4 | ~ 50 μM |
| Ovispirin (12) | SMAP-29 derivative | 2263 | α-helix | +7 | Plasma membrane permeabilization | < 50 μM (86.3)4 | < 50 uM (68.5)4 |
| Protegrin-1 (12) | Porcine leukocytes(66) | 2207 | β-hairpin(67) | +6 | Plasma membrane permeabilization | < 50 μM (95.2)4 | < 50 μM (39.4)4 |
| OaBAC-5-mini (36) | Sheep (36) | 3006 | α-helix | +8 | Plasma membrane permeabilization | 51.6 μM | 44.9 μM |
| Indolicidin (36) | Bovine neutrophils (68) | 1908 | α-helix (69) | +3 | Plasma membrane permeabilization | 15.7 μM | 5.2 μM |
| BAC-CN (36) | Bovine neutrophils (70) | 1486 | γ-turn (70) | +4 | Plasma membrane permeabilization | 11.8 μM | 47.1 μM |
| BMAP-27 (36) | Bovine myeloids (71) | 3283 | α-helix | +10 | Plasma membrane permeabilization | 1.5 μM | < 0.3 μM |
| BMAP-18 (44) | BMAP-27 derivative | 2343 | α-helix | +10 | Plasma membrane permeabilization | 5.1 μM | 3.4 μM |
| TP10 (42) | Shortened transportan analog (43) | 2183 | α-helix | +4 | Plasma membrane permeabilization, GTPase inhibition | N.D. | 2.7 μM |
| Pleurocidin (36) | Winter flounder skin secretions | 2711 | α-helix | +4 | Plasma membrane permeabilization | 27.7 μM | 3.7 μM |
| CP-26 (36) | Cecropin/mellitin hybrid | 2862 | α-helix | +7 | Plasma membrane permeabilization | 17.5 μM | 1.7 μM |
| V681 (36) | Cecropin/mellitin hybrid | 2921 | α-helix | +6 | Plasma membrane permeabilization | 5.1 μM | < 1.7 μM |
| Nonamer peptide A (D-amino acids) (21) | Insect defensin based | 1202 | β-sheet | +4 | Plasma membrane permeabilization | 31.3 μM7 | 4.7 μM, 205 μM6,7 (116 μM) |
| Nonamer peptide B (D-amino acids) (21) | Insect defensin based | 1195 | β-sheet | +5 | Plasma membrane permeabilization | N.D. | 500 μM6,7 (90 μM) |
| Nonamer peptide C (D-amino acids) (21) | Insect defensin based | 1130 | β-sheet | +3 | Plasma membrane permeabilization | N.D. | 125 μM6,7 (95 μM) |
| Nonamer peptide D (D-amino acids) (21) | Insect defensin based | 1151 | β-sheet | +4 | Plasma membrane permeabilization | N.D. | 325 μM6,7 (80 μM) |
| Nonamer peptide E (D-amino acids) (21) | Insect defensin based | 901 | β-sheet | +1 | Plasma membrane permeabilization | N.D. | N.D.6,7 (>500 μM) |
| Leucinostatin A (20) | Soil fungi, Paecilomyces sp. | 1217 | α-helix | Inhibit ATP synthesis, plasma membrane permeabilization | N.D. | 6.4 nM7 | |
| Leucinostatin B (20) | Soil fungi, Paecilomyces sp. | 1203 | α-helix | Inhibit ATP synthesis, plasma membrane permeabilization | N.D. | 6.9 nM7 | |
| Alamethicin I (20) | Trichoderma viride | 1964 | α-helix | Plasma membrane permeabilization | N.D | 86.6 nM7 | |
| Tsushimycin (20) | Streptomyces | 308 | Acylated cyclic peptide | Inhibits dolichol-phosphate-sugar synthesis, membrane interaction | N.D. | 3.5 μM7 | |
| Vasoactive intestinal peptide (23) | Human gut, pancreas and hypothalmus | 3327 | α-helical | +3 | Endosomal/lysosomal membrane disruption | N.A. | 2.8 μM |
| Adrenomedullin (23) | Many human tissues | 5727 | α-helical | +5 | Endosomal/lysosomal membrane disruption | N.A. | 1.8 μM |
| α-Melanocyte-stimulating hormone (23) | Human pituitary | 1624 | α-helical | +1 | Endosomal/lysosomal membrane disruption | N.A. | 8.9 μM |
| Urocortin (23) | Many human tissues | 4708 | α-helical | -2 | Endosomal/lysosomal membrane disruption | N.A. | 1.1 μM |
| Corticotropin-releasing hormone (23) | Human hypothalmus | 4758 | α-helical | -3 | Endosomal/lysosomal membrane disruption | N.A. | 5.3 μM |
| Ghrelin (23) | Human stomach and pancreas | 3173 | α-helical | +4 | Endosomal/lysosomal membrane disruption | N.A. | 3.5 μM |
| Small hydrophobic peptide-1 (24) | N-terminal signal peptide of human haptoglobin-related protein | 1959 | Random (α-helix) (72) | 0 | Plasma membrane rigidification | N.A. | 8 μM |
| Small hydrophobic peptide-2 (24) | N-terminal signal peptide of human paraoxonase-1 | 2268 | Random (α-helix) (72) | +2 | Plasma membrane rigidification | N.A. | 8 μM |
Values are presented as IC50 estimated from published data. Data were acquired from various assays and therefore provide limited comparisons.
Unless indicated otherwise, numbers in parenthesis are references
N.D., not determined
Values in parentheses are percent killed by 50 μM peptide.
N.A., no activity
Values are minimum inhibitory concentrations for 100% killing, no data on IC50 is available.
Values are for strain GUTat 3.1
Trypanocidal peptides in tsetse immunity
A role for AMPs in the immune response of the insect vector has been well established. Perhaps surprisingly, only a small percentage (< 5-17 %) of tsetse are infected in endemic areas (26), only a small number of trypanosomes within a bloodmeal successfully develop into insect stage procyclic forms (PC) (27) and a large portion of tsetse eliminate the parasites entirely at around day 3 post infection (28). Additionally, some tsetse species, i.e. Glossina pallidipes and Glossina palpalis palpalis are more refractory to African trypanosome infection than the main vector Glossina morsitans. The innate immune response has been implicated in preventing or limiting the establishment of gut infections (13, 16). Several AMPs function in tsetse innate immunity and a role for stomoxyn, an insect gut AMP, in preventing the stable fly Stomoxys calcitrans, a blood-sucking insect sympatric with tsetse, from being a successful vector of African trypanosomes has been suggested (29).
Hao et al. (2001) investigated the molecular immune response mounted by tsetse against T. b. rhodesiense (13). Feeding flies a bloodmeal containing PC trypanosomes resulted in increased attacin and defensin mRNA in the fat body, an organ that contributes to the systemic immune response. Bloodstream form trypanosomes also elicited a response but to a lesser degree. Microinjection of trypanosomes did not elicit a transcriptional response of these genes (13). Consistent with the molecular data, Boulanger et al. (2002) identified the defensin and attacin peptides, as well as a cecropin peptide, via mass spectrometry in the hemolymph of G. morsitans fed a bloodmeal containing PC T. b. brucei (19). A diptericin transcript was also identified in the fat body, and synthetic diptericin was shown to kill procyclic T. b. brucei (13). However, time-resolved analysis of mRNA levels indicated that attacin and defensin transcripts, but not diptericin, were specifically upregulated in response to trypanosome challenge and maintained during established infections (13). Priming the immune system with challenge by E. coli results in synthesis of attacin and defensin mRNA and corresponds with a decrease in parasite establishment (13). Spatial analysis of attacin and defensin mRNA synthesis revealed that the fat body and proventriculus, a small organ at the anterior of the midgut, are the major contributors to the AMP pool produced in response to trypanosome infection (14).
A physiological role for the tsetse AMP attacin has been established through in vitro killing assays with recombinant attacin (15), analysis of mRNA synthesis in susceptible and refractory Glossina spp. (17) and RNAi knockdown of attacin and its upstream immune signaling molecule relish (16). Recombinant attacin exhibits killing activity against a range of pathogens including E. coli, but not the Gram-negative tsetse gut symbiont Sodalis (suggesting a paratransgenic strategy for control of trypanosome transmission, see (15, 30-32)). Insect stage T. b. rhodesiense are highly susceptible to killing by attacin (MIC50 = 0.075 μM). Bloodstream form trypanosomes are also killed by attacin, but are less susceptible than PC forms (15). Patterns of attacin mRNA synthesis in newly hatched (teneral) and adult G. morsitans and refractory G. pallidipes and G. palpalis palpalis species suggest a role in limiting the establishment of trypanosome infection. Refractory Glossina show a baseline level of systemic (fat body) and locally synthesized attacin mRNA from the proventriculus and midgut tissue before being fed a bloodmeal. In contrast G. morsitans did not exhibit baseline or bloodmeal-stimulated attacin mRNA synthesis from the fat body (17). Teneral G. morsitans did synthesize attacin mRNA in proventriculus and midgut however transcript levels were significantly lower than in refractory flies. The role of attacin in mediating refractoriness was demonstrated by RNAi knockdown. Refractory G. pallidipes depleted of attacin experienced a 45 % infection rate whereas untreated flies showed 11 % infection rates (17). Similar experiments in G. morsitans gave consistent results. The nature of the signaling pathway controlling AMP expression was probed by RNAi knockdown of the NF-κB-related transcription factor relish. Depletion of relish resulted in no mRNA synthesis of attacin, defensin and cecropin in response to trypanosome challenge. Interestingly, the relative number of successful gut infections leading to infective metacyclic stages appearing in the salivary glands was not significantly different between RNAi-treated and control flies, suggesting that attacin does not function at later time points in the course of a trypanosome infection (16).
Trypanocidal peptides from mammalian hosts
The α- and β-defensins and the cathelicidins are structurally distinct major classes of AMPs and mammalian representatives of each have been shown to be trypanolytic. Both AMP classes are cationic and are generally thought to exert their cytolytic effect via membrane permeabilization (Figure 1). The major differences in these peptides are apparent in their expression profiles and structure. The defensins are expressed in a variety of tissues including neutrophils, Paneth cells and epithelial linings of the gut, lung and skin and are characterized by several anti-parallel β-sheets cross-linked by two or three disulphide bonds (33). The cathelicidins are structurally diverse exhibiting linear, cyclic, α-helical and β-turn structures and are found mainly in neutrophils (34). Cathelicidins can also be induced in keratinocytes by skin barrier disruption (35).
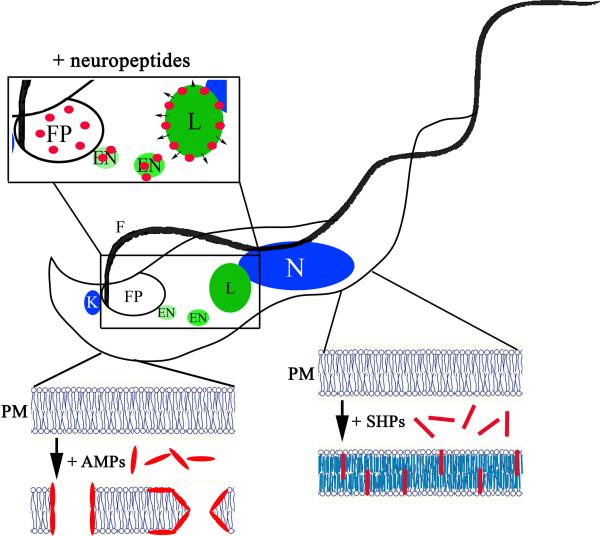
Figure 1.
Schematic representation of the multiple mechanisms of antimicrobial peptide killing of African trypanosomes. Antimicrobial peptides exhibit several modes of action against African trypanosomes. Conventional AMPs most likely act through permeabilization of the plasma membrane (PM) in both BSF and PC developmental forms. Killing of BSF trypanosomes by neuropeptides requires that the peptides be endocytosed through the flagellar pocket (FP) and trafficked from the endosomes (EN) to the acidified lysosome (L) where the peptides disrupt the lysosomal bilayer. Small hydrophobic peptides (SHPs) are specifically toxic to BSF trypanosomes. Intercalation of SHP into the plasma membrane results in an increase in lipid bilayer rigidity and non-lytic cell death. (N, nucleus; K, kinetoplast; F, flagellum.)
Relatively high concentrations of human β-defensins (50 μM) exhibit very weak killing of both PC and BSF T. brucei in vitro. A murine α-defensin, cryptin-4, exhibits similar activity against PC forms but no activity against BSF T. brucei has been demonstrated (12).
The cathelicidins are typically more potent trypanolytic AMPs than the defensins and representative peptides from a variety of mammals have been shown to be trypanolytic. Cathelicidins from human (LL-37), sheep (SMAP-29, OaBAC-5-mini), cattle (BMAP-27, indolicidin, BAC-CN) and pigs (protegrin-1) kill both PC and BSF forms in vitro (12, 36). Electron microscopy of PC trypanosomes treated with cathelicidins reveals a crumpled, rounded morphology with extensive disruption of the plasma membrane and loss of internal structures (12). Two cathelicidin AMPs have been shown to protect mice in vivo. Pretreatment of mice with SMAP-29 or protegrin-1 reduced the parasitaemia and prolonged the survival of mice challenged with BSF 427 T. brucei (12).
Unlike the tsetse, no direct role for AMPs in immunity to African trypanosomes has been demonstrated in mammals. It is unlikely that AMPs such as defensins and cathelicidins contribute to defense against bloodstream parasites in a physiological situation, as they are restricted from freely circulating in plasma to limit cytolytic damage of host tissues. It may be plausible that β-defensins and cathelicidins could contribute to reducing parasite burden from the bite of an infected tsetse due to expression in neutrophils or keratinocytes at the locality of the bite. However no data exists on the killing of metacyclic form trypanosomes by either AMP.
Killing of African trypanosomes by synthetic AMPs and natural products
Motivated by the desire to identify novel agents to treat HAT, several groups have identified synthetic trypanolytic AMPs and AMPs from diverse sources such as insects, fish and soil microorganisms (20-22, 36). With the exception of the fungal derived AMPs and the cell penetrating peptide TP10, these peptides are directly derived from known trypanolytic defensins or cathelicidins.
The peptide antibiotics leucinostatin A and B, alamethicin and tsushimycin are natural products isolated from fungi. These peptides differ from the canonical AMPs by virtue of the presence of unusual amino acids, acylation or both. The leucinostatins, named for their high leucine content, kill trypanosomes in vitro at low nanomolar concentrations (20). The potency of these peptides might be attributable to pleiotropic effects. Studies with model liposomes indicate that leucinostatins increase the permeability of lipid bilayers (37). The leucinostatins have also been shown to inhibit mitochondrial ATP synthesis and uncouple oxidative phosphorylation (38). The relevance of these activities to killing BSF trypanosomes is not clear, due to the lack of a functional electron transport chain in this developmental form, however disrupting the mitochondrial membrane potential may contribute to toxicity. A comparative analysis with the trypanocidal drug suramin indicates greater potency of the leucinostatins in mice. However these mycological metabolites exhibit high oral toxicity (20).
Alamethicin exhibits strong trypanolytic activity in vitro, killing BSF trypanosomes at nanomolar concentrations (20). The membrane permeabilizing activity of alamethicin has been well established. Alamethicin monomers orient perpendicular to the lipid membrane and oligomerize in the bilayer forming cylindrical pores that facilitate the passage of ions and water (39). Studies in mice indicate that alamethicin does not provide greater in vivo activity than suramin (20).
The in vitro trypanolytic activity of tsushimycin may be attributed to its structural similarity to amphomycin, which exhibits activity against T. b. gambiense and T. b. rhodesiense in mice (40). Amphomycin has been shown to inhibit the formation of dolichol-phosphate-sugar complexes, molecules that donate sugar moieties for protein glycosylation and GPI-anchors. This potential mechanism is particularly relevant to African trypanosomes. A relatively large portion of proteins are GPI-anchored including the VSG coat and it has been shown that inhibition of GPI-modification is toxic (41). Intraperitoneal administration of 50 mg/kg tsushimycin, 50-fold greater than the dosage of suramin required, over the course of four days cured mice with established T. b. brucei infections (20).
Several synthetic AMPs have also been shown to be trypanolytic. These peptides are derived from the active sites of known AMPs and presumably operate through the same mechanisms. An exception is the shortened analog of the cell penetrating peptide transportan, TP10 (42), which lyses BSF T. b. brucei at micromolar concentrations. Cell penetrating peptides permeate plasma membranes and are thought to exert their toxic effect through inhibition of GTPases (43). A truncated form of bovine myeloid antimicrobial peptide-27 (BMAP-27), BMAP-18, is active against both developmental forms of African trypanosomes, and shows reduced toxicity towards mammalian cells and the tsetse symbiont Sodalis (again suggesting a paratransgenic control strategy) relative to native BMAP-27 (44). Small synthetic peptides derived from insect defensins have also been shown to exhibit trypanocidal activity against BSF African trypanosomes and to a lesser degree the PC developmental forms (21, 22).
Killing of African trypanosomes by unconventional AMPs
The different developmental forms of African trypanosomes exhibit unique physiologies. These physiological characteristics can contribute to immune evasion, but, as illustrated by the following examples, also sensitize the parasite to killing by AMPs from unusual sources that operate through unconventional mechanisms.
The features of many AMPs (amphipathic helices with regions of cationic residues) are also exhibited by a number of neuropeptides. These similarities led Delgado and colleagues to investigate the potential trypanocidal activity of several neuropeptides (23). A variety of neuropeptides exhibit killing activity against BSF trypanosomes at low micromolar concentrations. Trypanosomes treated with these peptides become swollen, and develop large cytoplasmic vacuoles and detached flagellum. Susceptibility of BSF trypanosomes can be attributed to their robust rate of endocytosis. Fluorescently labeled peptides accumulate in endosomes and colocalize with the lysosomal marker p67 (23) (Figure 1). Procyclic trypanosomes, which exhibit a significantly reduced rate of endocytosis, do not internalize and are thus not killed by neuropeptides (23). Dissection of the endocytic trafficking pathway indicates that neuropeptides exert their cytotoxicity in the acidified lysosome. Inhibiting endocytosis by incubating cells at 4°C or allowing uptake but blocking endosomal trafficking to the lysosome at 17°C spares BSF trypanosomes from killing by neuropeptides. Neutralizing the lysosomal lumen with NH4Cl also inhibits killing, indicating that an acidic environment is necessary (23). Release of fluorescent dextrans from the lysosome indicates that the membrane has been compromised. Subsequent cellular events are characteristic of an autophagic cell death (23).
The trafficking and acidification requirements that result in cell death are not unique to neuropeptides. The trypanosome lytic factor (TLF) that protects many higher primates from veterinary pathogenic trypanosomes is a subset of high-density lipoproteins that is specifically bound and endocytosed by BSF trypanosomes (45-47). Once localized to the acidic lysosome TLF exerts a membrane disrupting activity that results in cell lysis. Acid pH facilitates lytic factor-membrane interaction by neutralizing electrostatic repulsion and allowing TLF to bind the anionic lysosomal membrane (48). This may also be the case for neuropeptides. Alternatively, or in addition to, it may be that protonation of the peptides increases their hydrophobicity thus driving intercalation into the lysosomal bilayer.
Trypanosome lytic factor is also the origin of an unusual AMP that kills trypanosomes through a novel mechanism of membrane rigidification (Figure 1). One unique component of TLF is haptoglobin-related protein (Hpr). This protein is unusual in that it is secreted without cleavage of its N-terminal signal peptide (49). Purified, delipidated Hpr is toxic to BSF trypanosomes (50), however recombinant Hpr that lacks the signal peptide shows no toxicity (51). Recently we have shown that a synthetic small hydrophobic peptide (SHP-1) corresponding in sequence to the Hpr signal peptide specifically kills both veterinary and human pathogenic BSF T. brucei (24). Trypanocidal activity is not limited to SHP-1, the signal peptide of another apolipoprotein (termed SHP-2), paraoxonase-1, which is entirely different in primary structure, but similar in terms of its length, charge and hydrophobicity profile is also toxic to BSF trypanosomes. The SHPs are not toxic to PC T. brucei or mammalian cell lines, nor do they induce hemolysis of human erythrocytes at concentrations orders of magnitude higher than necessary to kill BSF trypanosomes. Studies with model liposomes suggest that the specificity of SHP-1 is due to the high degree of lipid fluidity in the BSF plasma membrane. Procyclic trypanosomes have a more rigid plasma membrane, consistent with the hypothesis that lipid fluidity mediates susceptibility to SHPs (24). The phenotype of death superficially resembles formaldehyde fixed trypanosomes; cells retain their slender, elongated shape but are motionless. Death is preceded by dramatic changes in cell motility, with an initial hyper-activation of the cell followed by decreased motility and subsequent motionlessness (24). The lack of swelling or intracellular vacuolization suggests that membrane permeabilization is not involved in the mechanism of killing. A direct effect of SHP interaction with BSF trypanosomes is rigidification of the plasma membrane (24). It is likely that membrane rigidification is the mechanism of toxicity. The BSF of African trypanosomes offers an attractive target for membrane rigidifying peptides as trypanocidal agents. These cells require a high degree of lateral surface flow of proteins, best exemplified by the directional sorting of antibody-bound VSG by hydrodynamic forces (52), and high rates of membrane remodeling during endocytosis. Membrane rigidification may result in general poisoning of the cell by interfering with these processes.
Conclusion
Antimicrobial peptides are fundamental components of immunity. A role for AMPs in decreasing or eliminating the parasite load in the tsetse fly vector has been established. Despite the identification of mammalian AMPs that show trypanocidal activity, it is not known if these peptides participate in the immune response of the mammalian host. Antimicrobial peptides with variable activity against BSF and PC African trypanosomes may serve as valuable tools for probing the physiology of the different developmental forms. Already work with trypanocidal peptides has highlighted the unusual membrane composition of BSF African trypanosomes and their susceptibility to toxic compounds delivered through robust endocytosis and lysosomal localization. The abundance and diversity of AMPs could offer a vast resource for the development of novel trypanocidal agents.
Acknowledgments
John M. Harrington is supported by a National Research Service Award from the National Institute of Allergy and Infectious Disease and the National Institute of Health Grant AI039033 to Stephen Hajduk. I would like to thank Joseph Russell and Stephen Hajduk for critically reading the manuscript.
Footnotes
Disclosures: None
References
- 1.Barrett MP, Burchmore RJ, Stich A, et al. The trypanosomiases. Lancet. 2003;362(9394):1469–80. doi: 10.1016/S0140-6736(03)14694-6. [DOI] [PubMed] [Google Scholar]
- 2.Jones TW, Davila AM. Trypanosoma vivax--out of Africa. Trends Parasitol. 2001;17(2):99–101. doi: 10.1016/s1471-4922(00)01777-3. [DOI] [PubMed] [Google Scholar]
- 3.Cross GA. Antigenic variation in trypanosomes. Am J Trop Med Hyg. 1977;26(6):240–244. doi: 10.4269/ajtmh.1977.26.240. [DOI] [PubMed] [Google Scholar]
- 4.Fenn K, Matthews KR. The cell biology of Trypanosoma brucei differentiation. Cur Opin Microbiol. 2007;10(6):539–546. doi: 10.1016/j.mib.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson MA, Haldar K, Cross GA. Trypanosoma brucei variant surface glycoprotein has a sn-1,2-dimyristyl glycerol membrane anchor at its COOH terminus. J Biol Chem. 1985;260(8):4963–4938. [PubMed] [Google Scholar]
- 6.Field MC, Menon AK, Cross GA. A glycosylphosphatidylinositol protein anchor from procyclic stage Trypanosoma brucei: lipid structure and biosynthesis. EMBO J. 1991;10(10):2731–2739. doi: 10.1002/j.1460-2075.1991.tb07821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan GW, Allen CL, Jeffries TR, Hollinshead M, Field MC. Developmental and morphological regulation of clathrin-mediated endocytosis in Trypanosoma brucei. J Cell Sci. 2001;114:2605–2615. doi: 10.1242/jcs.114.14.2605. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez JA, Lopez MA, Thayer MC, et al. Propulsion of African trypanosomes is driven by bihelical waves with alternating chirality separated by kinks. Proc Natl Acad Sci U S A. 2009;106(46):19322–19327. doi: 10.1073/pnas.0907001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vickerman K. Polymorphism and mitochondrial activity in sleeping sickness trypanosomes. Nature. 1965;208(5012):762–766. doi: 10.1038/208762a0. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Cui J, Nilsson D, et al. The Trypanosoma brucei MitoCarta and its regulation and splicing pattern during development. Nucleic Acids Res. 2010;38(21):7378–7387. doi: 10.1093/nar/gkq618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michael R, Yeaman NYY. Mechanisms of antimicrobial peptides and resistance. Pharmacol Rev. 2003;55(1):27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- 12.McGwire BS, Olson CL, Tack BF, Engman DM. Killing of African trypanosomes by antimicrobial peptides. J Infect Dis. 2003;188(1):146–52. doi: 10.1086/375747. [DOI] [PubMed] [Google Scholar]
- 13.Hao Z, Kasumba I, Lehane MJ, Gibson WC, Kwon J, Aksoy S. Tsetse immune responses and trypanosome transmission: implications for the development of tsetse-based strategies to reduce trypanosomiasis. Proc Natl Acad Sci U S A. 2001;98(22):12648–12653. doi: 10.1073/pnas.221363798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao Z, Kasumba I, Aksoy S. Proventriculus (cardia) plays a crucial role in immunity in tsetse fly (Diptera: Glossinidiae). Insect Biochem Mol Biol. 2003;33(11):1155–1164. doi: 10.1016/j.ibmb.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Hu Y, Aksoy S. An antimicrobial peptide with trypanocidal activity characterized from Glossina morsitans morsitans. Insect Biochem Mol Biol. 2005;35(2):105–115. doi: 10.1016/j.ibmb.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Hu C, Aksoy S. Innate immune responses regulate trypanosome parasite infection of the tsetse fly Glossina morsitans morsitans. Mol Microbiol. 2006;60(5):1194–1204. doi: 10.1111/j.1365-2958.2006.05180.x. [DOI] [PubMed] [Google Scholar]
- 17.Nayduch D, Aksoy S. Refractoriness in tsetse flies (Diptera: Glossinidae) may be a matter of timing. J Med Entomol. 2007;44(4):660–665. doi: 10.1603/0022-2585(2007)44[660:ritfdg]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Hu C, Wu Y, et al. Characterization of the antimicrobial peptide attacin loci from Glossina morsitans. Insect Mol Biol. 2008;17(3):293–302. doi: 10.1111/j.1365-2583.2008.00805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boulanger N, Brun R, Ehret-Sabatier L, Kunz C, Bulet P. Immunopeptides in the defense reactions of Glossina morsitans to bacterial and Trypanosoma brucei brucei infections. Insect Biochem Mol Biol. 2002;32(4):369–375. doi: 10.1016/s0965-1748(02)00029-2. [DOI] [PubMed] [Google Scholar]
- 20.Ishiyama A, Otoguro K, Iwatsuki M, et al. In vitro and in vivo antitrypanosomal activities of three peptide antibiotics: leucinostatin A and B, alamethicin I and tsushimycin. J Antibiot. 2009;62(6):303–308. doi: 10.1038/ja.2009.32. [DOI] [PubMed] [Google Scholar]
- 21.Yamage M, Yoshiyama M, Grab DJ, et al. Characteristics of novel insect defensin-based membrane-disrupting trypanocidal peptides. Biosci Biotechnol Biochem. 2009;73(7):1520–1526. doi: 10.1271/bbb.90004. [DOI] [PubMed] [Google Scholar]
- 22.Kitani H, Naessens J, Kubo M, et al. Synthetic nonamer peptides derived from insect defensin mediate the killing of African trypanosomes in axenic culture. Parasitol Res. 2009;105(1):217–225. doi: 10.1007/s00436-009-1389-x. [DOI] [PubMed] [Google Scholar]
- 23.Delgado M, Anderson P, Garcia-Salcedo JA, Caro M, Gonzalez-Rey E. Neuropeptides kill African trypanosomes by targeting intracellular compartments and inducing autophagic-like cell death. Cell Death Dif. 2009;16(3):406–416. doi: 10.1038/cdd.2008.161. [DOI] [PubMed] [Google Scholar]
- 24.Harrington JM, Widener J, Stephens N, et al. The plasma membrane of bloodstream-form African trypanosomes confers susceptibility and specificity to killing by hydrophobic peptides. J Biol Chem. 2010;285(37):28659–28666. doi: 10.1074/jbc.M110.151886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGwire BS, Kulkarni MM. Interactions of antimicrobial peptides with Leishmania and trypanosomes and their functional role in host parasitism. Exp Parasitol. 2010;126(3):397–405. doi: 10.1016/j.exppara.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Msangi AR, Whitaker CJ, Lehane MJ. Factors influencing the prevalence of trypanosome infection of Glossina pallidipes on the Ruvu flood plain of Eastern Tanzania. Acta Tropica. 1998;70:143–155. doi: 10.1016/s0001-706x(98)00013-8. [DOI] [PubMed] [Google Scholar]
- 27.Maudlin I, Welburn SC. Maturation of trypanosome infections in tsetse. Exp Parasitol. 1994;79(2):202–205. doi: 10.1006/expr.1994.1081. [DOI] [PubMed] [Google Scholar]
- 28.Gibson W, Bailey M. The development of Trypanosoma brucei within the tsetse fly midgut observed using green fluorescent trypanosomes. Kinetoplastid Biol Dis. 2003;2(1):1. doi: 10.1186/1475-9292-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boulanger N, Munks RJ, Hamilton JV, et al. Epithelial innate immunity. A novel antimicrobial peptide with antiparasitic activity in the blood-sucking insect Stomoxys calcitrans. J Biol Chem. 2002;277(51):49921–49926. doi: 10.1074/jbc.M206296200. [DOI] [PubMed] [Google Scholar]
- 30.Beard CB, O'Neill SL, Mason P, et al. Genetic transformation and phylogeny of bacterial symbionts from tsetse. Insect Mol Biol. 1993;1(3):123–131. doi: 10.1111/j.1365-2583.1993.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 31.Cheng Q, Aksoy S. Tissue tropism, transmission and expression of foreign genes in vivo in midgut symbionts of tsetse flies. Insect Mol Biol. 1999;8(1):125–132. doi: 10.1046/j.1365-2583.1999.810125.x. [DOI] [PubMed] [Google Scholar]
- 32.Aksoy S, Weiss B, Attardo G. Paratransgenesis applied for control of tsetse transmitted sleeping sickness. Adv Exp Med Biol. 2008;627:35–48. doi: 10.1007/978-0-387-78225-6_3. [DOI] [PubMed] [Google Scholar]
- 33.Lehrer RI. Primate defensins. Nature Rev. 2004;2(9):727–738. doi: 10.1038/nrmicro976. [DOI] [PubMed] [Google Scholar]
- 34.Zanetti M. The role of cathelicidins in the innate host defenses of mammals. Curr Issues Mol Biol. 2005;7(2):179–196. [PubMed] [Google Scholar]
- 35.Dorschner RA, Pestonjamasp VK, Tamakuwala S, et al. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Invest Dermatol. 2001;117(1):91–97. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- 36.Haines LR, Hancock RE, Pearson TW. Cationic antimicrobial peptide killing of African trypanosomes and Sodalis glossinidius, a bacterial symbiont of the insect vector of sleeping sickness. Vector Borne Zoonotic Dis. 2003;3(4):175–186. doi: 10.1089/153036603322662165. [DOI] [PubMed] [Google Scholar]
- 37.Ishiguro K, Arai T. Action of the peptide antibiotic leucinostatin. Antimicrob Agents Chemother. 1976;9(6):893–898. doi: 10.1128/aac.9.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shima A, Fukushima K, Arai T, Terada H. Dual inhibitory effects of the peptide antibiotics leucinostatins on oxidative phosphorylation in mitochondria. Cell Struct Funct. 1990;15(1):53–58. doi: 10.1247/csf.15.53. [DOI] [PubMed] [Google Scholar]
- 39.Mihajlovic M, Lazaridis T. Antimicrobial peptides in toroidal and cylindrical pores. Biochim Biophys Acta. 2010;1798(8):1485–1493. doi: 10.1016/j.bbamem.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Packchanian A. Chemotherapy of African sleeping sickness. II. Chemotherapy of experimental Trypanosoma gambiense and Trypanosoma rhodesiense infections in mice (Mus musculus) with a new antibiotic, amphomycin. Antibiot Chemother. 1956;6:684–691. [PubMed] [Google Scholar]
- 41.Morita YS, Paul KS, Englund PT. Specialized fatty acid synthesis in African trypanosomes: myristate for GPI anchors. Science. 2000;288(5463):140–143. doi: 10.1126/science.288.5463.140. [DOI] [PubMed] [Google Scholar]
- 42.Arrighi RB, Ebikeme C, Jiang Y, et al. Cell-penetrating peptide TP10 shows broad-spectrum activity against both Plasmodium falciparum and Trypanosoma brucei brucei. Antimicrob Agents Chemother. 2008;52(9):3414–3417. doi: 10.1128/AAC.01450-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soomets U, Lindgren M, Gallet X, et al. Deletion analogues of transportan. Biochim Biophys Acta. 2000;1467(1):165–176. doi: 10.1016/s0005-2736(00)00216-9. [DOI] [PubMed] [Google Scholar]
- 44.Haines LR, Thomas JM, Jackson AM, et al. Killing of trypanosomatid parasites by a modified bovine host defense peptide, BMAP-18. PLoS Neg Trop Dis. 2009;3(2):e373. doi: 10.1371/journal.pntd.0000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hajduk SL, Moore DR, Vasudevacharya J, et al. Lysis of Trypanosoma brucei by a toxic subspecies of human high density lipoprotein. J Biol Chem. 1989;264(9):5210–5217. [PubMed] [Google Scholar]
- 46.Drain J, Bishop JR, Hajduk SL. Haptoglobin-related protein mediates trypanosome lytic factor binding to trypanosomes. J Biol Chem. 2001;276(32):30254–30260. doi: 10.1074/jbc.M010198200. [DOI] [PubMed] [Google Scholar]
- 47.Vanhollebeke B, De Muylder G, Nielsen MJ, et al. A haptoglobin-hemoglobin receptor conveys innate immunity to Trypanosoma brucei in humans. Science. 2008;320(5876):677–681. doi: 10.1126/science.1156296. [DOI] [PubMed] [Google Scholar]
- 48.Harrington JM, Howell S, Hajduk SL. Membrane permeabilization by trypanosome lytic factor, a cytolytic human high-density lipoprotein. J Biol Chem. 2009;284(20):13505–13512. doi: 10.1074/jbc.M900151200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith AB, Esko JD, Hajduk SL. Killing of trypanosomes by the human haptoglobin-related protein. Science. 1995;268(5208):284–286. doi: 10.1126/science.7716520. [DOI] [PubMed] [Google Scholar]
- 50.Shiflett AM, Bishop JR, Pahwa A, Hajduk SL. Human high density lipoproteins are platforms for the assembly of multi-component innate immune complexes. J Biol Chem. 2005;280(38):32578–32585. doi: 10.1074/jbc.M503510200. [DOI] [PubMed] [Google Scholar]
- 51.Vanhollebeke B, Nielsen MJ, Watanabe Y, et al. Distinct roles of haptoglobin456 related protein and apolipoprotein L-I in trypanolysis by human serum. Proc Natl Acad Sci U S A. 2007;104(10):4118–41123. doi: 10.1073/pnas.0609902104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engstler M, Pfohl T, Herminghaus S, et al. Hydrodynamic flow-mediated protein sorting on the cell surface of trypanosomes. Cell. 2007;131(3):505–515. doi: 10.1016/j.cell.2007.08.046. [DOI] [PubMed] [Google Scholar]
- 53.Gunne H, Hellers M, Steiner H. Structure of preproattacin and its processing in insect cells infected with a recombinant baculovirus. Eur J Biochem/FEBS. 1990;187(3):699–703. doi: 10.1111/j.1432-1033.1990.tb15356.x. [DOI] [PubMed] [Google Scholar]
- 54.Cudic M, Bulet P, Hoffmann R, Craik DJ, Otvos L., Jr. Chemical synthesis, antibacterial activity and conformation of diptericin, an 82-mer peptide originally isolated from insects. Eur J Biochem/FEBS. 1999;266(2):549–558. doi: 10.1046/j.1432-1327.1999.00894.x. [DOI] [PubMed] [Google Scholar]
- 55.Holak TA, Engstrom A, Kraulis PJ, et al. The solution conformation of the antibacterial peptide cecropin A: a nuclear magnetic resonance and dynamical simulated annealing study. Biochemistry. 1988;27(20):7620–7629. doi: 10.1021/bi00420a008. [DOI] [PubMed] [Google Scholar]
- 56.Hoover DM, Chertov O, Lubkowski J. The structure of human beta-defensin-1: new insights into structural properties of beta-defensins. J Biol Chem. 2001;276(42):39021–39026. doi: 10.1074/jbc.M103830200. [DOI] [PubMed] [Google Scholar]
- 57.Harder J, Bartels J, Christophers E, Schroder JM. A peptide antibiotic from human skin. Nature. 1997;387(6636):861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 58.Sawai MV, Jia HP, Liu L, et al. The NMR structure of human beta-defensin-2 reveals a novel alpha-helical segment. Biochemistry. 2001;40(13):3810–3816. doi: 10.1021/bi002519d. [DOI] [PubMed] [Google Scholar]
- 59.Ouellette AJ, Selsted ME. Paneth cell defensins: endogenous peptide components of intestinal host defense. FASEB J. 1996;10(11):1280–1289. doi: 10.1096/fasebj.10.11.8836041. [DOI] [PubMed] [Google Scholar]
- 60.Jing W, Hunter HN, Tanabe H, Ouellette AJ, Vogel HJ. Solution structure of cryptdin-4, a mouse paneth cell alpha-defensin. Biochemistry. 2004;43(50):15759–15766. doi: 10.1021/bi048645p. [DOI] [PubMed] [Google Scholar]
- 61.Larrick JW, Hirata M, Balint RF, Lee J, Zhong J, Wright SC. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63(4):1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oren Z, Lerman JC, Gudmundsson GH, Agerberth B, Shai Y. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem J. 1999;341:501–513. [PMC free article] [PubMed] [Google Scholar]
- 63.Bagella L, Scocchi M, Zanetti M. cDNA sequences of three sheep myeloid cathelicidins. FEBS Lett. 1995;376(3):225–228. doi: 10.1016/0014-5793(95)01285-3. [DOI] [PubMed] [Google Scholar]
- 64.Skerlavaj B, Benincasa M, Risso A, Zanetti M, Gennaro R. SMAP-29: a potent antibacterial and antifungal peptide from sheep leukocytes. FEBS Lett. 1999;463(1-2):58–62. doi: 10.1016/s0014-5793(99)01600-2. [DOI] [PubMed] [Google Scholar]
- 65.Steinstraesser L, Tack BF, Waring AJ, et al. Activity of novispirin G10 against Pseudomonas aeruginosa in vitro and in infected burns. Antimicrob Agents Chemother. 2002;46(6):1837–1844. doi: 10.1128/AAC.46.6.1837-1844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kokryakov VN, Harwig SS, Panyutich EA, et al. Protegrins: leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett. 1993;327(2):231–236. doi: 10.1016/0014-5793(93)80175-t. [DOI] [PubMed] [Google Scholar]
- 67.Fahrner RL, Dieckmann T, Harwig SS, Lehrer RI, Eisenberg D, Feigon J. Solution structure of protegrin-1, a broad-spectrum antimicrobial peptide from porcine leukocytes. Chem Biol. 1996;3(7):543–550. doi: 10.1016/s1074-5521(96)90145-3. [DOI] [PubMed] [Google Scholar]
- 68.Selsted ME, Novotny MJ, Morris WL, Tang YQ, Smith W, Cullor JS. Indolicidin, a novel bactericidal tridecapeptide amide from neutrophils. J Biol Chem. 1992;267(7):4292–4295. [PubMed] [Google Scholar]
- 69.Friedrich CL, Rozek A, Patrzykat A, Hancock RE. Structure and mechanism of action of an indolicidin peptide derivative with improved activity against gram-positive bacteria. J Biol Chem. 2001;276(26):24015–24022. doi: 10.1074/jbc.M009691200. [DOI] [PubMed] [Google Scholar]
- 70.Romeo D, Skerlavaj B, Bolognesi M, Gennaro R. Structure and bactericidal activity of an antibiotic dodecapeptide purified from bovine neutrophils. J Biol Chem. 1988;263(20):9573–9575. [PubMed] [Google Scholar]
- 71.Skerlavaj B, Gennaro R, Bagella L, Merluzzi L, Risso A, Zanetti M. Biological characterization of two novel cathelicidin-derived peptides and identification of structural requirements for their antimicrobial and cell lytic activities. J Biol Chem. 1996;271(45):28375–28381. doi: 10.1074/jbc.271.45.28375. [DOI] [PubMed] [Google Scholar]
- 72.Briggs MS, Cornell DG, Dluhy RA, Gierasch LM. Conformations of signal peptides induced by lipids suggest initial steps in protein export. Science. 1986;233(4760):206–208. doi: 10.1126/science.2941862. [DOI] [PubMed] [Google Scholar]