Abstract
The GABAC receptor, a postsynaptic membrane receptor expressed prominently in the retina, is a ligand-gated ion channel that consists of a combination of ρ subunits. We report characterization of a novel guinea pig polyclonal antibody, termed GABAC Ab N-14, directed against a 14-mer peptide (N-14) of the extracellular domain of the human ρ1 subunit. The antibody exhibits high sensitivity for N-14 by ELISA. In Western blots, GABAC Ab N-14 shows reactivity with the ρ1 subunit of preparations obtained from ρ1 GABAC-expressing neuroblastoma cells, Xenopus oocytes, and mammalian retina and brain. Flow cytometry reveals a rightward shift in mean fluorescence intensity of GABAC-expressing neuroblastoma cells probed with GABAC Ab N-14. Immunostaining of neuroblastoma cells and oocytes with GABAC Ab N-14 yields fluorescence only with GABAC-expressing cells. Antibody binding has no effect on GABA-elicited membrane current responses. Immunostaining of human retinal sections shows preferential staining within the inner plexiform layer. GABAC Ab N-14 appears well suited for investigative studies of GABAC ρ1 subunit in retina and other neural tissues.
Keywords: GABA receptor, GABAC, retina, ρ1 subunit, antibody
INTRODUCTION
Gamma-aminobutyric acid (GABA) is the main inhibitory neurotransmitter of the central nervous system. Postsynaptic receptors of the GABAA family are pentameric ligand-gated chloride channels. GABAC receptors, a sub-family of GABAA, consist of ρ subunits and are expressed in brain tissue, with especially high expression in retina (Amin and Weiss, 1996; Boue-Grabot et al., 1998; Cutting et al., 1991; Drew, and Johnston, 1992; Enz et al., 1996; Euler and Wässle, 1998; Feigenspan et al., 1993; Lukasiewicz and Shields, 1998; Polenzani et al., 1991; Qian and Dowling, 1993; Schlicker et al., 2009; Zhang et al., 1995). Three types of GABA ρ subunits (ρ1, ρ2, and ρ3) have been cloned from mammalian retina, with the ρ1 subunit exhibiting much higher expression level than ρ2. It is well documented that ρ1 subunits can assemble to form functional homopentameric receptors (Cutting et al., 1991; Qian et al., 1998; Zhang et al., 1995).
The availability of an antibody to the human GABAC ρ1 subunit would facilitate research on ρ1-containing GABAC receptors, and potentially also clinical studies aimed at selectively targeting tissues rich in ρ1. To serve as an anchor to target GABAC-expressing cells, this antibody should be directed against an extracellularly accessible epitope, and antibody binding should preserve receptor function. Here we describe a novel polyclonal antibody directed against the extracellular domain of human ρ1 GABAC, properties of which encourage its further use in immunolabeling and physiological studies. Preliminary accounts of this work have been reported (Gussin et al., 2008; Pending U.S. Patent Application number US2009/0269786 A1).
MATERIAL AND METHODS
A 14-mer peptide (N-14) consisting of the amino acid sequence RQRREVHEDAHKQV, located within the N-terminal region of the human ρ1 subunit, was chosen as the target for the antibody based on several considerations. First, this sequence lies outside the “core peptide” region, i.e., is not part of the more conserved region believed to be involved in inter-subunit interaction, ligand binding, and channel formation. Second, the 14-mer sequence resides within the “unstructured tail” of the N-terminal region, which is less conserved among species; BLAST/Blastp analysis of this region did not detect a putative conserved domain. Third, a search for the N-14 sequence using the NCBI website (blast.ncbi.nlm.nih.gov) yielded matches to ρ1 GABAC sequences for human and other species, and to Burkholderia phymatum hydrolase (9/14), but not to sequences for ρ2 GABAC or other GABA receptor subunits or to any other proteins. Thus, an antibody raised against this selected sequence conceivably could exhibit little cross-reactivity with other proteins.
Guinea pig was chosen as the species for antibody production. Peptide synthesis, conjugation to keyhole limpet hemocyanin (KLH), animal immunization and serum collection were contracted to an outside source (Covance, Inc., Denver, PA). IgG was purified from guinea pig serum by affinity chromatography (Borg et al., 1993), using protein A-Sepharose CL-4B beads (Sigma, St. Louis, MO). Peptide-specific antibody was further purified by affinity column chromatography (Affi-Gel 10 beads; Bio-Rad Laboratories, Hercules, CA); column-bound N-14 peptide served as ligand (Khasawneh et al., 2006). Following elution with glycine (100 mM, pH 2.5) and neutralization with 1 M Tris-base (pH 8.0), eluted fractions were analyzed for absorbance (280 nm); fractions of highest absorbance were pooled and dialyzed in PBS. The final concentration of the affinity-purified antibody, henceforth termed GABAC Ab N-14, was 0.24 mg/mL.
ELISAs were performed using microtiter wells pre-coated with neutravidin (Pierce – Thermo Fisher Scientific, Rockford, IL). Wells were either untreated (i.e., neutravidin only), or treated with either N-14 biotinylated peptide (100 µL, 10 µg/mL) or an unrelated biotinylated peptide (RSHSYPAKK). GABAC Ab N-14 was tested at 1/5,000 to 1/150,000 dilution. Secondary antibody was horseradish peroxidase (HRP)-conjugated goat anti-guinea pig IgG (Santa Cruz Biotechnologies, Santa Cruz, CA), 1/2,000.
Neuroblastoma cells stably transfected to express human ρ1 GABAC (SHp5-human ρ1) were a gift from Dr. David S. Weiss (University of Texas Health Science Center at San Antonio). Non-expressing neuroblastoma cells (SHSY5Y; ATCC, Manassas, VA) were used as controls. Xenopus laevis oocytes expressing human ρ1 GABAC, human ρ2, or α1β2γ2 GABAA receptors (α1, β2 and γ2 subunit sequences: rat, rat and human. respectively) were prepared as described (Gussin et al., 2006; Qian et al., 1997; Vu et al., 2005). Membrane protein preparations of Xenopus oocytes were obtained using previously described procedures (Wible et al., 1998).
Human retina was obtained from donor eye tissue (Illinois Eye-Bank, Bloomington, IL), in accordance with institutional policies. All procedures involving experimental animals conformed to institutional policies and to the Statement for the Use of Animals in Ophthalmic and Vision Research adopted by the Association for Research in Vision and Ophthalmology. Retina and brain tissues were lysed in RIPA buffer (Sefton, 2005). Human retinas were fixed in 4% paraformaldehyde, cryopreserved in sucrose, then sliced in Optimal Cutting Temperature medium (OCT, Tissue-Tek). The thickness of the retinal cryosections was 16 µm.
Western blots (15–25 µg protein per lane) were probed with GABAC Ab N-14 (1/10,000 dilution) and, as secondary antibody, HRP-conjugated goat anti-guinea pig IgG (1/7,000 dilution; Santa Cruz Biotechnology). Controls involved probing with secondary antibody only, or with GABAC Ab N-14 that had been pre-absorbed with N-14 (3 µg/mL, 30 min, room temperature), followed by secondary antibody. Control rabbit anti-human GABAC ρ2 polyclonal antibody was obtained from Abcam (Cambridge, MA).
Flow cytometry was performed on SHp5-ρ1 neuroblastoma cells that express ρ1 GABAC, and on control SHSY5Y cells. Cells were incubated with GABAC Ab N-14 (1/25 to 1/1,000 dilution) or (as control) normal guinea pig IgG (1/100) as primary antibody, and with FITC-conjugated goat anti-guinea pig IgG (Santa Cruz Biotechnology; 1/50 dilution) as secondary antibody. Single-color analysis employed a FACStar flow cytometer (BD Biosciences, San Jose, CA). A lower-limit threshold was set for data acquisition, thereby eliminating background scatter.
Immunofluorescence labeling of live SHp5-ρ1 and SHSY5Y cells employed GABAC Ab N-14 (1 hr, 1/1,000 – 1/2,000 dilution) as primary antibody and biotinylated goat anti-guinea pig IgG secondary antibody (Santa Cruz Biotechnology; 45 min, 1/400 dilution), followed by 10 nM streptavidin-conjugated quantum dots 605 (SA-qdots; Invitrogen, Carlsbad, CA; 15 min). Oocytes expressing either GABAC ρ1 or α1β2γ2 GABAA receptors, non-expressing control oocytes, and human retinal sections were incubated (1–2 h) with either GABAC Ab N-14 (1/1,000), with GABAC Ab N-14 that had been pre-absorbed with 0.1 mg/mL of N-14 peptide (45 min, room temperature), or without GABAC Ab N-14. The secondary antibody was goat anti-guinea-pig IgG (Abcam) (FITC-conjugated, 1/200 for human retina sections; Cy5-conjugated, 1/400 for oocytes; 1-h incubation). Slides were mounted using Vectashield H-1000 medium (Vector Laboratories, Burlingame, CA), and images obtained on a Leica DM-IRE2 confocal microscope at 20X (oocytes) or 40X (retinal sections) magnification.
GABA-elicited membrane current responses were recorded from GABAC ρ1 expressing oocytes and from SHp5-ρ1 cells using, respectively, two-electrode voltage clamp and whole cell patch-clamp configurations (Wotring et al., 2003; Vu et al., 2005). Prior to electrophysiological testing, cells were subjected to conditions similar to those used for immunofluorescence (untreated; incubated with GABAC Ab N-14 only; or incubated with GABAC Ab N-14 followed by secondary antibody).
RESULTS
Antibody affinity for the N-14 peptide
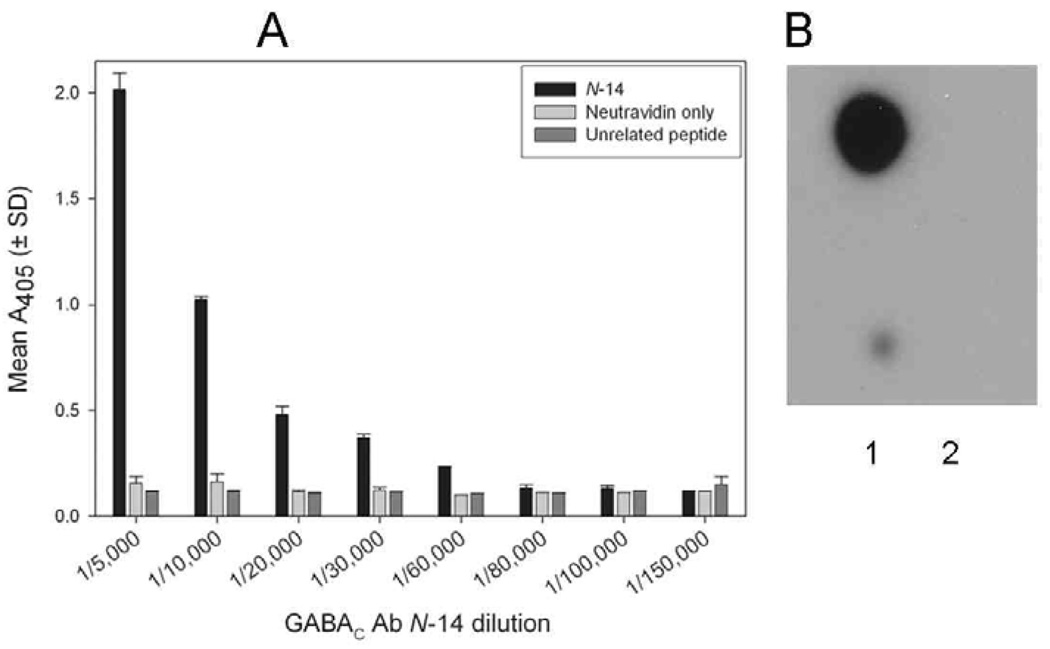
The investigated guinea pig antibody GABAC Ab N-14 was directed against the N-14 region of the human ρ1 subunit of the GABAC receptor. To assess reactivity of the affinity-purified antibody, we conducted both plate-based (ELISA) and membrane-based (dot-blotting) experiments using N-14 as a target. ELISA (antibody dilutions: 1/5,000 – 1/150,000) was used to determine the titer of GABAC Ab N-14 to N-14. Upon reaction with N-14 coated wells, GABAC Ab N-14 dilutions of 1/5,000 to 1/60,000 yielded absorbances above background, i.e., at least twice those determined for control wells (neutravidin only) (Fig. 1A). Higher dilutions (1/80,000 – 1/150,000) exhibited near-background absorbance. At no dilution was there observable binding to wells coated with the unrelated peptide. Antibody affinity was further tested by dot-blotting, using N-14 dotted on a membrane and probed with either GABAC Ab N-14 followed by secondary antibody, or with secondary antibody only. The peptide dots yielded a strong signal when probed with GABAC Ab N-14 (Fig. 1B, lane 1), but not when GABAC Ab N-14 was absent (lane 2).
Fig. 1.
Reactivity of GABAC Ab N-14 with N-14. A: Titration of GABAC Ab N-14 by ELISA. GABAC Ab N-14 was tested at the indicated dilutions for reactivity with biotinylated N-14 (black bars), unrelated biotinylated peptide (dark gray bars), or no peptide (i.e., neutravidin only; light gray bars). Absorbance values (405 nm) are means (± SD) of duplicate measurements. B: Dot-blot assay using 1 ng (top) and 0.1 ng (bottom) of N-14 probed with GABAC Ab N-14 (lane 1) or with secondary antibody only (lane 2).
Antibody affinity for ρ1 GABAC
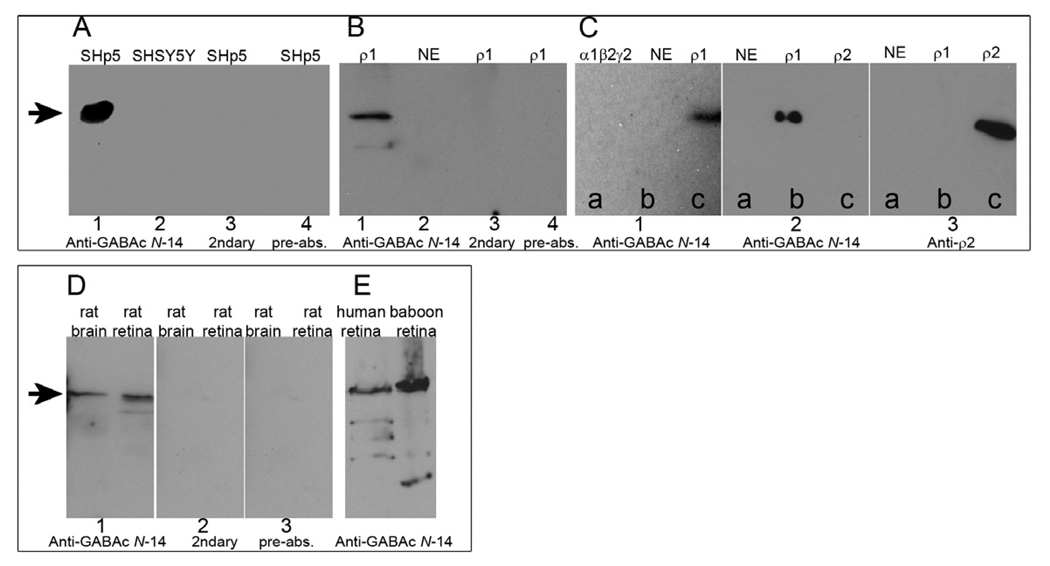
To test GABAC Ab N-14 reactivity with cell line-expressed ρ1 GABAC receptors, we performed Western blots using GABAC-expressing SHp5-ρ1 cells and control SHSY5Y cells (Fig. 2A), as well as oocyte membrane protein preparations (Fig. 2B). Samples from SHp5-ρ1 and from ρ1 GABAC-expressing oocytes probed with GABAC Ab N-14 followed by secondary antibody exhibited a prominent band at ~55 kDa, the expected molecular weight of human GABAC ρ1 subunit (Fig. 2A,B, Lane 1). This band was absent in cells lacking GABAC receptors (Fig. 2A,B, Lane 2). Either omission of GABAC Ab N-14 or pre-absorption of GABAC Ab N-14 led to loss of the ~55 kDa band (Fig. 2A,B, Lanes 3–4).
Fig. 2.
Western blots. A: Whole-cell lysates of GABAC-expressing SHp5-ρ1 cells and non-GABAC expressing SHSY5Y cells. Lane 1: SHp5-ρ1 probed with GABAC Ab N-14. Lane 2: SHSY5Y probed with GABAC Ab N-14. Lane 3: SHp5-ρ1 probed with secondary antibody only. Lane 4: SHp5-ρ1 probed with GABAC Ab N-14 pre-absorbed with N-14. B: Oocyte membrane preparations. Lanes 1, 3 and 4: GABAC ρ1 expressing oocytes. Lane 2: Non-expressing control oocyte. Experimental conditions used for lanes 1–4 are otherwise identical, respectively, to lanes 1–4 in A. C: Oocyte membrane preparations. Section 1: Preparations of α1β2γ2 GABAA-expressing oocytes (lane a), non-expressing oocytes (b), and ρ1 GABAC-expressing oocytes (c) probed with GABAC Ab N-14. Section 2: Preparations of non-expressing oocytes (lane a), ρ1 GABAC-expressing oocytes (b) and human ρ2 GABAC-expressing oocytes (c) probed with GABAC Ab N-14. Section 3: Same preparations as those of section 2, probed with anti-ρ2 antibody. D: Whole cell lysates of rat brain and rat retina probed with: GABAC Ab N-14 (Section 1), secondary antibody only (Section 2), or GABAC Ab N-14 pre-absorbed with N-14 (Section 3). E: Whole cell lysates of human and baboon retina, probed with GABAC Ab N-14. On all blots, the arrows indicate MW ~ 55 kDa.
To examine the selectivity of GABAC Ab N-14 for GABA receptor subtypes, we analyzed, by Western blot, reactivity of the antibody with membrane protein preparations from oocytes expressing ρ1 GABAC, ρ2 GABAC, or α1β2γ2 GABAA receptors. Figure 2C, section 1 shows that the ~55 kDa band, present for ρ1 GABAC (lane c), was absent from GABAA (a) and from the non-expressing control (b). Figure 2C, section 2 shows that membrane preparations from human ρ2 GABAC-expressing oocytes did not react with GABAC Ab N-14, as evidenced by the absence of a band in lane c. However, ρ2 GABAC was detectable by anti-ρ2 antibody (section 3, lane c). Thus, GABAC Ab N-14 immunoreacts with the GABAC ρ1 subunit, but not with either GABAC ρ2 subunit or GABAA receptors.
Western blotting was used to assess the reactivity of GABAC Ab N-14 for ρ1 GABAC expressed in brain and retinal tissue. Samples prepared from rat retina and rat brain, probed with GABAC Ab N-14, exhibited prominent bands at ~55 kDa (Fig. 2D, section 1). Consistent with data obtained in cell line preparations, both exclusion of GABAC Ab N-14 (Fig. 2D, section 2) and pre-absorption of GABAC Ab N-14 with N-14 (Fig. 2D, section 3) resulted in loss of the ~55 kDa band. Furthermore, in preparations of human and baboon retina probed with GABAC Ab N-14, the main observed bands were ~ 55kDa, consistent with GABAC ρ1 (Fig. 2E). Minor bands present in the Western blots can be explained by protein degradation of ρ1, and are consistent with previously-described patterns (Adamian et al., 2009; Wang et al., 2007). Thus, Western blotting with GABAC Ab N-14 specifically detects native GABAC ρ1 receptors from human, baboon and rat.
Interaction of GABAC Ab N-14 with ρ1 GABAC receptors expressed in intact cells
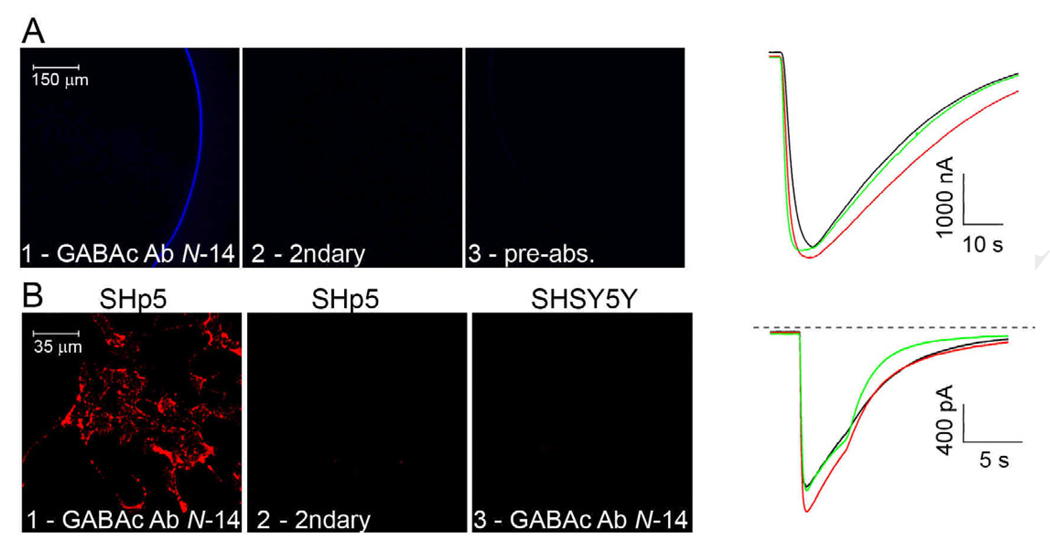
Incubation of ρ1 GABAC-expressing oocytes with GABAC Ab N-14 yielded fluorescence staining of the surface membrane (Fig. 3A, panel 1). No labeling occurred when the oocyte was incubated either without primary antibody (panel 2), or when GABAC Ab N-14 was pre-absorbed with cognate peptide (panel 3). Neither non-expressing oocytes nor oocytes expressing α1β2γ2 GABAA receptors exhibited significant labeling under any of these conditions (not shown). However, oocytes expressing α1β2γ2 GABAA receptors showed surface fluorescence when probed with anti-α1 antibody (not shown). GABAC Ab N-14 labeling is thus specific for oocytes expressing ρ1 GABAC receptors, and cognate peptide competes with labeling.
Fig. 3.
Cell immunostaining and electrophysiology. A: Fluorescence images of ρ1 GABAC-expressing oocytes, following treatment with GABAC Ab N-14 followed by Cy5-conjugated secondary (1), with secondary antibody only (2), or with pre-absorbed GABAC Ab N-14 followed by secondary antibody (3). Right: Membrane current responses of ρ1-expressing oocytes elicited by 3 µM GABA. Untreated oocyte (black trace); oocyte treated with GABAC Ab N-14 only (red); and oocyte treated with GABAC Ab N-14 and secondary antibody (green). B: Fluorescence images of neuroblastoma cells. (1): SHp5-ρ1 cells incubated with GABAC Ab N-14, followed with biotinylated secondary antibody and SA-qdots; (2): SHp5-ρ1 cells incubated without GABAC Ab N-14, but with biotinylated secondary antibody and SA-qdots; (3): SHSY5Y incubated in conditions similar to those of image 1. Right: Membrane current responses of SHp5-ρ1 cells to 10 µM GABA (holding potential = − 60 mV). Untreated cell (black trace); cell treated with GABAC Ab N-14 only (red); and cell treated with GABAC Ab N-14, biotinylated secondary antibody, and SA-qdots (green).
In ρ1 GABAC-expressing oocytes, we assessed the effect of GABAC Ab N-14 binding by electrophysiological recording of GABA-elicited responses. Waveforms in Figure 3A show responses to 3 µM GABA obtained from ρ1 GABAC-expressing oocytes that were untreated, treated with GABAC Ab N-14 only, or treated with GABAC Ab N-14 and secondary antibody. Responses obtained from untreated oocytes (black trace) were similar to those recorded from oocytes treated with GABAC Ab N-14 only (red), or with GABAC Ab N-14 and secondary antibody (green). Peak amplitudes (mean ± SD) of responses elicited by 3 µM GABA were 3770 ± 596 nA (n=5) for untreated oocytes, 3650 ± 212 nA (n=2) for oocytes treated with GABAC Ab N-14 only, and 3892 ± 324 nA (n=4) for oocytes treated with GABAC Ab N-14 and secondary antibody. These data indicate that, with 3 µM GABA, there was no significant difference in peak amplitudes determined for untreated vs. GABAC Ab N-14 only-treated oocytes vs. oocytes treated with GABAC Ab N-14 followed by secondary antibody [ANOVA, F(2, 11)=0.19, p=0.83]. Thus, under the investigated conditions, oocyte labeling with GABAC Ab N-14, alone or followed by secondary antibody, does not significantly affect the GABA-elicited response.
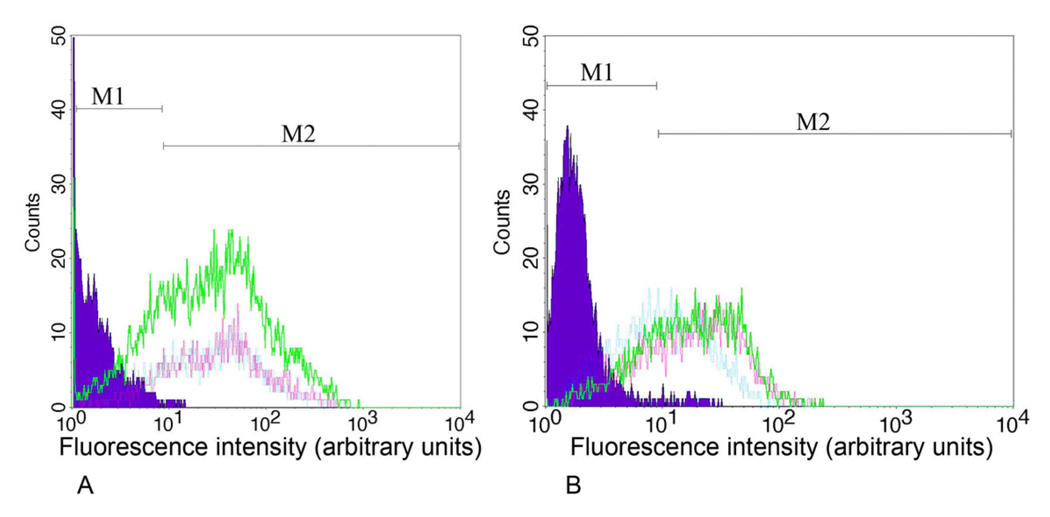
To further examine the interaction of GABAC Ab N-14 with ρ1 GABAC-expressing cells, we employed GABAC Ab N-14 for flow cytometry analysis of SHp5-ρ1 neuroblastoma cells; non-expressing SHSY5Y cells served as controls. Probing the GABAC-expressing cells with GABAC Ab N-14 (1/25, 1/50 and 1/1000 dilution) produced a rightward shift in mean fluorescence intensity (MFI), corresponding with the presence of ~47 to 63% positive cells (Figs. 4A,B, green, pink and blue traces, respectively). Neither SHp5-ρ1 cells probed with non-immune guinea pig IgG as a primary antibody (Fig. 4A, purple) nor non-GABAC expressing SHSY5Y cells probed with GABAC Ab N-14 (Fig. 4B, purple) showed a significant shift in MFI.
Fig. 4.
Flow cytometry profiles for SHp5-ρ1 and SHSY5Y cells, using GABAC Ab N-14 or non-immune guinea pig IgG. A: Purple area: SHp5-ρ1 cells probed with non-immune guinea pig IgG as primary antibody. Green, pink, and blue traces: SHp5-ρ1 cells probed with GABAC Ab N-14 at dilutions of 1/25, 1/50 and 1/1,000, respectively. B: Purple area: SHSY5Y cells probed with GABAC Ab N-14 as a primary antibody. Green, pink and blue traces: SHp5-ρ1 cells probed with GABAC Ab N-14 at dilutions identical to those of A. Regions M1 and M2: background (i.e., unstained) cells and cells with positive staining, respectively.
Enriched populations of GABAC-expressing neuroblastoma cells recovered from flow cytometry were cultured for examination in immunofluorescence and electrophysiological experiments. Fig. 3B, panels 1–3, show results obtained on treatment of these GABAC-expressing cells and non-expressing controls with GABAC Ab N-14 followed by biotinylated secondary antibody and SA-qdots, or with biotinylated secondary and SA-qdots only. These data indicate that labeling depended on both ρ1 GABAC expression and treatment with GABAC Ab N-14. The waveforms show electrophysiological results obtained from SHp5-ρ1 cells that were untreated, treated with GABAC Ab N-14 only, or treated with GABAC Ab N-14 followed by biotinylated secondary antibody and SA-qdots. Presentation of 10 µM GABA under these three conditions elicited responses of similar peak amplitude and kinetics (black, red and green traces, respectively). Peak amplitudes of GABA-elicited responses determined in multiple experiments were 1736 ± 284 pA (n=9) for untreated cells, 1851 ± 278 pA (n=8) for cells treated with GABAC Ab N-14 only, and 1739 ± 319 pA (n=10) for cells treated with GABAC Ab N-14, biotinylated secondary antibody and SA-qdots, and there was no significant difference among the three groups of peak amplitude data [ANOVA, F(2, 27)=0.04, p=0.96]. Thus, as with ρ1 GABAC-expressing oocytes, treatment of ρ1 GABAC-expressing neuroblastoma cells with GABAC Ab N-14 yields robust immunolabeling and no significant perturbation of electrophysiological activity.
Interaction of GABAC Ab N-14 with human retina
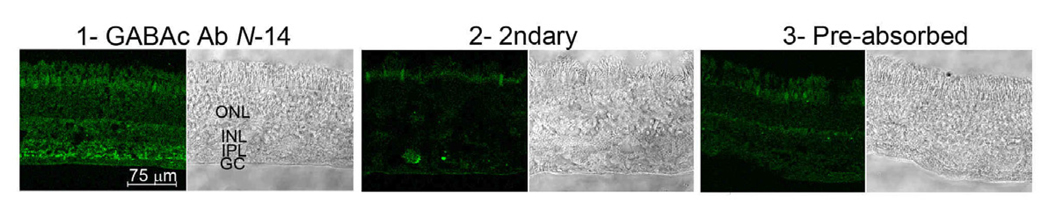
GABAC Ab N-14 treatment of tissue sections prepared from human retina yielded fluorescence staining primarily within the inner plexiform layer (Fig. 5, panel 1). No significant labeling occurred when the slice was incubated either without primary antibody (panel 2), or when GABAC Ab N-14 was pre-absorbed with cognate peptide (panel 3). Labeling by GABAC Ab N-14 is thus relatively specific for the inner plexiform layer of human retina.
Fig. 5.
Fluorescence and brightfield images of human retina cryosections, treated with GABAC Ab N-14 followed by FITC-conjugated secondary (1); with secondary antibody only (2); or with pre-absorbed GABAC Ab N-14 followed by secondary antibody (3). Labels in brightfield image (1) approximate the regions of the ganglion cell layer (GC), inner plexiform layer (IPL), inner nuclear layer (INL), and outer nuclear layer (ONL).
DISCUSSION
The present study reports the characterization of a novel polyclonal antibody, GABAC Ab N-14, that targets the human ρ1 GABAC receptor. This antibody differs in several respects from a polyclonal anti-ρ antibody originally described by Enz et al. (1996) and used in multiple subsequent studies (e.g., Picaud et al., 1998; Pattnaik et al., 2000; McCall et al., 2002; Klooster et al., 2004). Preparation of the previously described antibody employed, as an immunogen, a large recombinant fusion peptide that includes amino acids 1–171 of the mature rat ρ1 sequence. By contrast, the antigen used for the presently investigated antibody was a short peptide that contains amino acids 23–36 of the mature human ρ1 sequence, and that was conjugated to KLH. Furthermore, the polyclonal antibody generated by Enz et al. (1996) recognized ρ2 and ρ3 in addition to the ρ1 subunit, perhaps as a consequence of the large size of the recombinant peptide immunogen used and, thus, a higher likelihood of this immunogen’s sequence homology with multiple subunit types. By contrast, as elaborated below, the present antibody has high affinity and specificity for the ρ1 subunit, and does not cross-react with other GABAC subunits.
Specificity and reactivity of GABAC Ab N-14
The results provide several lines of evidence for a high specificity and high affinity of GABAC Ab N-14 for the human ρ1 GABAC subunit. First, ELISA data indicate robust binding of GABAC Ab N-14 to epitopes displayed by the N-14 peptide (Fig. 1). Second, for ρ1 GABAC-expressing cells, Western blot analyses indicate predominant immunoreactivity of GABAC Ab N-14 with a single protein of MW ~55 kDa, the expected molecular weight of the ρ1 subunit (Fig. 2). Third, the antibody exhibits robust immunostaining of cells expressing ρ1 GABAC receptor (Fig. 3A,B). Fourth, flow cytometry reveals a rightward shift in the MFI of ρ1 GABAC-expressing neuroblastoma cells probed with GABAC Ab N-14 (Fig. 4). Fifth, human retina probed with GABAC Ab N-14 shows immunolabeling primarily within the inner plexiform layer, and this labeling is suppressed by both pre-absorption of GABAC Ab N-14 with the cognate peptide, and the omission of GABAC Ab N-14 (Fig. 5). The inner plexiform layer includes the axonal region of bipolar cells, a known primary locus of GABAC receptors (Feigenspan et al., 1993; Qian and Dowling, 1993; Qian et al., 1997).
The reactivity of GABAC Ab N-14 with GABAC-expressing cells raises the interesting question of whether binding of this antibody preserves the receptor’s physiological activity. The waveforms of Figs. 3 A,B and accompanying text, which indicate the absence of an effect on the GABA-elicited response under conditions of robust immunological staining, are consistent with this possibility. However, a perturbing action by receptor-bound antibody is not ruled out, as the evident immunostaining signal (Figs. 3A,B) could reflect antibody binding to only a minor fraction of the overall receptor population. A previous study describing an antibody raised against the N-terminal half of recombinant ρ1 subunit reported an inhibitory effect on GABA-elicited responses in ρ1 GABAC-expressing oocytes (Ekema et al., 2001).
Based on an antibody concentration of 0.24 mg/ml in the affinity-purified GABAC Ab N-14 preparation (see Material and Methods), dilutions used in the present experiments correspond with antibody concentrations ranging from 0.048 – 0.004 µg/mL in ELISA, 0.24 – 9.6 µg/mL in flow cytometry, 0.12 – 0.24 µg/mL in immunofluorescence labeling, and 0.024 µg/mL in Western blot. Such concentrations compare favorably with typical ranges of polyclonal antibody concentrations used in ELISA (~ 0.1 µg/mL), flow cytometry (~ 1 µg/mL), Western-blot (~ 1 µg/mL) and immunofluorescence labeling of cells (~ 5 µg/mL), indicating a high sensitivity of GABAC Ab N-14 for its target. GABAC Ab N-14 antibody, directed against the unstructured region in the N-terminal extracellular domain of the human GABAC ρ1 subunit, thus joins a group of other polyclonal antibodies with reactivity at the extracellular domains of the cys-loop and other ligand-gated ion channel receptors.
Conclusion
GABAC Ab N-14, a novel polyclonal antibody directed against a peptide in the N-terminal extracellular domain of the human ρ1 subunit of GABAC receptors, exhibits robust binding activity to ρ1 as determined by Western blot, flow cytometry and immunostaining. It furthermore shows affinity for the inner plexiform layer of human retina, a known locus of ρ1-containing GABAC. The present antibody will be valuable for future investigations of ρ1 expression and localization in retina and other neural tissues. It may also have application in fundamental and clinically oriented studies as a ρ1-targeting component for delivering biomolecules to GABAC receptors.
Research highlights.
GABAC Ab N-14 is a new polyclonal antibody directed against the ρ1 GABAC subunit.
The antibody binds specifically to ρ1 expressed in oocytes and neuroblastoma cells.
Antibody binding preserves electrophysiological activity of the target GABAC.
In human retina sections, the antibody preferentially labels inner plexiform layer.
ACKNOWLEDGMENTS
We thank Dr. David S. Weiss for the gift of SHp5-ρ1 neuroblastoma cells; Drs. Nasser M. Qtaishat, Deborah M. Little and Brian K. Kay for helpful discussions; and Mr. Ambarish Pawar, Ms. Hongyu Ying and Dr. Raja Fayad for technical assistance. Supported by NIH grants EY016094, EY001792 and HL24530; by grants from the Daniel F. and Ada L. Rice Foundation (Skokie, IL); by the Macular Degeneration Research Program of the American Health Assistance Foundation (Clarksburg, MD); by Hope for Vision (Washington, DC); and by Research to Prevent Blindness (New York, NY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adamian L, Gussin HA, Tseng YY, Muni NJ, Feng F, Qian H, Pepperberg DR, Liang J. Structural model of ρ1 GABAC receptor based on evolutionary analysis: Testing of predicted protein–protein interactions involved in receptor assembly and function. Protein Sci. 2009;18:2371–2383. doi: 10.1002/pro.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin J, Weiss DS. Insights into the activation mechanism of ρ1 GABA receptors obtained by coexpression of wild type and activation-impaired subunits. Proc. R. Soc. 1996;263:273–282. doi: 10.1098/rspb.1996.0042. [DOI] [PubMed] [Google Scholar]
- Borg C, Lam S, Dieter J, Lim C, Komiotis D, Venton D, Le Breton G. Anti-peptide antibodies against the human blood platelet TXA2/PGH2 receptor: production, purification and characterization. Biochem. Pharmacol. 1993;45:2071–2078. doi: 10.1016/0006-2952(93)90018-r. [DOI] [PubMed] [Google Scholar]
- Boue-Grabot E, Roudbaraki M, Bascles L, Tramu G, Bloch B, Garret M. Expression of GABA receptor ρ subunits in rat brain. J. Neurochem. 1998;70:899–907. doi: 10.1046/j.1471-4159.1998.70030899.x. [DOI] [PubMed] [Google Scholar]
- Cutting GR, Lu L, O’Hara BF, Kasch LM, Montrose-Rafizadeh C, Donovan DM, Shimada S, Antonarakis SE, Guggino WB, Uhl GR, Kazazian HH. Cloning of the γ-aminobutyric acid (GABA) ρ1 cDNA: a GABA receptor subunit highly expressed in the retina. Proc. Natl. Acad. Sci. USA. 1991;88:2673–2677. doi: 10.1073/pnas.88.7.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew CA, Johnston GA. Bicuculline- and baclofen-insensitive gamma-aminobutyric acid binding to rat cerebellar membranes. J. Neurochem. 1992;58:1087–1092. doi: 10.1111/j.1471-4159.1992.tb09366.x. [DOI] [PubMed] [Google Scholar]
- Ekema GM, Zheng W, Wang L, Lu L. Modulation of recombinant GABA receptor/channel subunits by domain-specific antibodies in Xenopus oocytes. J. Membr. Biol. 2001;183:205–213. doi: 10.1007/s00232-001-0068-3. [DOI] [PubMed] [Google Scholar]
- Enz R, Brandstatter JH, Wässle H, Bormann J. Immunocytochemical localization of the GABAC receptor ρ subunits in the mammalian retina. J. Neurosci. 1996;16:4479–4490. doi: 10.1523/JNEUROSCI.16-14-04479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Wässle H. Different contributions of GABAA and GABAC receptors to rod and cone bipolar cells in a rat retinal slice preparation. J. Neurophysiol. 1998;79:1384–1395. doi: 10.1152/jn.1998.79.3.1384. [DOI] [PubMed] [Google Scholar]
- Feigenspan A, Wässle H, Bormann J. Pharmacology of GABA receptor Cl- channels in rat retinal bipolar cells. Nature. 1993;361:159–162. doi: 10.1038/361159a0. [DOI] [PubMed] [Google Scholar]
- Gussin HA, Tomlinson ID, Little DM, Warnement MR, Qian H, Rosenthal SJ, Pepperberg DR. Binding of muscimol-conjugated quantum-dots to GABAC receptors. J. Am. Chem. Soc. 2006;128:15701–15713. doi: 10.1021/ja064324k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussin HA, Khasawneh FT, Xie A, Qian H, Le Breton GC, Pepperberg DR. Characterization of a novel polyclonal anti human ρ1 GABAC antibody. Invest. Ophthalmol. Vis. Sci. 2008;49 E-abstract 1288. [Google Scholar]
- Khasawneh FT, Huang JS, Turek JW, Le Breton GC. Differential mapping of the amino acids mediating agonist and antagonist coordination with the human thromboxane A2 receptor protein. J. Biol. Chem. 2006;281:26951–26965. doi: 10.1074/jbc.M507469200. [DOI] [PubMed] [Google Scholar]
- Klooster J, Nunes Cardozo B, Yazulla S, Kamermans M. Postsynaptic localization of γ-aminobutyric acid transporters and receptors in the outer plexiform layer of the goldfish retina: an ultrastructural study. J. Comp. Neurol. 2004;474:58–74. doi: 10.1002/cne.20114. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz PD, Shields CR. Different combinations of GABAA and GABAC receptors confer distinct temporal properties to retinal synaptic responses. J. Neurophysiol. 1998;79:3157–3167. doi: 10.1152/jn.1998.79.6.3157. [DOI] [PubMed] [Google Scholar]
- McCall MA, Lukasiewicz PD, Gregg RG, Peachy NS. Elimination of the ρ1 subunit abolishes GABAC receptor expression and alters visual processing in the mouse retina. J. Neurosci. 2002;22:4163–4174. doi: 10.1523/JNEUROSCI.22-10-04163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattnaik B, Jellali A, Sahel J, Dreyfus H, Picaud S. GABAC receptors are localized with microtubule-associated protein 1B in mammalian cone photoreceptors. J. Neurosci. 2000;20:6789–6796. doi: 10.1523/JNEUROSCI.20-18-06789.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picaud S, Pattnaik B, Hicks, Forster V, Fontaine V, Sahel J, Dreyfus H. GABAA and GABAC receptors in adult porcine cones: evidence from a photoreceptor-glia co-culture model. J. Physiol. 1998;513.1:33–42. doi: 10.1111/j.1469-7793.1998.033by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polenzani L, Woodward RM, Miledi R. Expression of mammalian gamma-aminobutyric acid receptors with distinct pharmacology in Xenopus oocytes. Proc. Natl. Acad. Sci. USA. 1991;88:4318–4322. doi: 10.1073/pnas.88.10.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Dowling JE. Novel GABA responses from rod-driven retinal horizontal cells. Nature. 1993;361:162–164. doi: 10.1038/361162a0. [DOI] [PubMed] [Google Scholar]
- Qian H, Hyatt G, Schanzer A, Hazra R, Hackam A, Cutting GR, Dowling JE. A comparison of GABAC and ρ subunit receptors from the white perch retina. Vis. Neurosci. 1997;14:843–851. doi: 10.1017/s0952523800011585. [DOI] [PubMed] [Google Scholar]
- Qian H, Dowling JE, Ripps H. Molecular and pharmacological properties of GABA-ρ subunits from white perch retina. J. Neurobiol. 1998;37:305–320. doi: 10.1002/(sici)1097-4695(19981105)37:2<305::aid-neu9>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Schlicker K, McCall MA, Schmidt M. GABAC receptor-mediated inhibition is altered but not eliminated in the superior colliculus of GABAC ρ1 knockout mice. J. Neurophysiol. 2009;101:2974–2983. doi: 10.1152/jn.91001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton BM. Labeling cultured cells with 32Pi and preparing cell lysates for immunoprecipitation. Unit 18.2. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. John Wiley & Sons, Inc.; 2005. [Google Scholar]
- Vu TQ, Chowdhury S, Muni NJ, Qian H, Standaert RF, Pepperberg DR. Activation of membrane receptors by a neurotransmitter conjugate designed for surface attachment. Biomaterials. 2005;26:1895–1903. doi: 10.1016/j.biomaterials.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Wang J, Lester HA, Dougherty DA. Establishing an ion pair interaction in the homomeric ρ1 γ-aminobutyric acid type A receptor that contributes to the gating pathway. J. Biol. Chem. 2007;282:26210–26216. doi: 10.1074/jbc.M702314200. [DOI] [PubMed] [Google Scholar]
- Wible BA, Yang Q, Kuryshev YA, Accili EA, Brown AM. Cloning and expression of a novel K1 channel regulatory protein, KChAP. J. Biol. Chem. 1998;273:11745–11751. doi: 10.1074/jbc.273.19.11745. [DOI] [PubMed] [Google Scholar]
- Wotring VE, Miller TS, Weiss DS. Mutations at the GABA receptor selectivity filter: a possible role for effective charges. J Physiol. 2003;548:527–540. doi: 10.1113/jphysiol.2002.032045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Pan ZH, Zhang X, Brideau AD, Lipton SA. Cloning of a gamma-aminobutyric acid type C receptor subunit in rat retina with a methionine residue critical for picrotoxinin channel block. Proc. Natl. Acad. Sci. USA. 1995;92:11756–11760. doi: 10.1073/pnas.92.25.11756. [DOI] [PMC free article] [PubMed] [Google Scholar]