Abstract
The development of a vaccine to prevent norovirus infections has been focused on immunization at a mucosal surface, but has been limited by the low immunogenicity of self-assembling Norwalk virus-like particles (NV VLPs) delivered enterically or at nasal surfaces. Nasal immunization, which offers the advantage of ease of immunization, faces obstacles imposed by the normal process of mucociliary clearance, which limits residence time of applied antigens. Herein, we describe the use of a dry powder formulation (GelVac) of an inert in-situ gelling polysaccharide (GelSite) extracted from Aloe vera for nasal delivery of NV VLP antigen. Powder formulations, with or without NV VLP antigen, were similar in structure in dry form or when rehydrated in simulated nasal fluids. Immunogenicity of the dry powder VLP formulation was compared to equivalent antigen/adjuvant liquid formulations in animals. For the GelVac powder, we observed superior NV-specific serum and mucosal (aerodigestive and reproductive tracts) antibody responses relative to liquid formulations. Incorporation of TLR7 agonist gardiquimod in dry powder formulations did not enhance antibody responses, although its inclusion in liquid formulations did enhance VLP immunogenicity irrespective of the presence or absence of GelSite. We interpret these data as showing that GelSite-based dry powder formulations 1.) stabilize the immunogenic structural properties of VLPs and 2.) induce systemic and mucosal antibody titers which are equal or greater than those achieved by VLPs plus adjuvant in a liquid formulation. We conclude that in-situ gelation of the GelVac dry powder formulation at nasal mucosal surfaces delays mucociliary clearance and thereby prolongs VLP antigen exposure to immune effector sites.
Keywords: norovirus vaccine, spray dry powder formulation, mucoadhesive, intranasal delivery, virus-like particles (VLP), TLR agonist
1. INTRODUCTION
Viruses belonging to the genera Norovirus are responsible for over 90% of all non-bacterial gastroenteritis epidemics [1] and a leading cause of global diarrhea [2]. The high prevalence of norovirus infections has led investigators to develop vaccine candidates to prevent disease [3]. Norwalk virus (NV) is the prototype virus of the genera Norovirus and extensive preclinical studies in mice have shown that NV virus-like particles (VLPs) administered parenterally, orally, or intranasally are immunogenic [3-9]. In clinical trials, NV VLPs administered orally or intranasally have been shown to be well tolerated and modestly immunogenic [10-12]. Despite promising results, many challenges to developing a norovirus vaccine remain. A key obstacle has been the incomplete understanding of the immune correlates of protection [3, 9, 13], although a recent publication by Reeck at al. showed that antibodies that block histoblood group antigen binding to NV VLPs correlate with protection against clinical NV gastroenteritis [14].
The most effective means to prevent infectious diseases like norovirus is through vaccination strategies that initiate immune responses at the natural site of infection, the mucosa [15]. The majority of currently licensed vaccines are administered parenterally, even though these vaccines have the disadvantages of patient reluctance to tolerate needle sticks and lack of mucosal immune induction [16]. Previous studies have evaluated the immunogenic potential of oral, nasal, rectal, and vaginal routes of vaccine administration [17-28]. The nasal cavity is a promising site for vaccine delivery because it is easy to access, is highly vascularized, has a relatively large surface area, has low proteolytic activity, and is able to induce systemic immunity as well as both local and distal mucosal immunity via the Common Mucosal Immune System (CMIS) [16, 29-32]. An intranasal influenza vaccine has been approved for clinical use by the U.S. Food and Drug Administration (FDA) [33-35] and intranasal vaccines for hepatitis B virus (HBV), measles, anthrax, bacterial meningitis, and others are being evaluated [18, 36]. Additional VLP-based, nasal vaccines have been shown to induce distal mucosal and systemic immunity in mice [37, 38]. The nasal route has also been shown to be superior to parenteral administration for VLP-based vaccines at eliciting IgA at distal mucosal sites [39].
Nasally administered vaccines initiate an immune response through the nasal-associated lymphoid tissue (NALT) [32, 40]. The NALT is composed of an assembly of antigen-reactive cells including B cells, T cells, and antigen presenting cells (APCs). Upon nasal vaccine administration, antigens can be taken up by specialized epithelial cells called microfold cells (M cells), or by macrophages and dendritic cells, which in turn leads to the activation of T and B cells [40, 41]. A drawback to nasal immunization is the limited time available for antigen absorption due to the rapid mucociliary clearance of foreign particles from the nasal cavity. Beginning in the 1980s the concept of mucosal adhesives, or mucoadhesives, has been explored to improve nasal drug delivery [42]. Various synthetic or natural polymers have been studied for their ability to interact with the mucus layer covering the epithelial surface. Mucoadhesives are thought to improve drug bioavailability by increasing contact time and localization at nasal surfaces and possibly modifying epithelial permeability. These properties increase antigen uptake by M cells and other APCs, and enhance the immune response [32, 43, 44]. In addition, dry powder formulations offer chemical and physical stability for antigens and other vaccine components, in comparison to liquid formulations [32].
GelSite® is an Aloe vera L.-derived polysaccharide (polygalacturonic acid) polymer with mucoadhesive properties. The GelSite polymer, which exists in liquid form or a dry powder formulation called GelVac™, is uniquely capable of in-situ gelation, turning into a gel whether in liquid or powder form upon contact with body fluids at the site of administration [45]. This in-situ gelation property thereby extends the mucosal residence time. An inactivated H5N1 influenza vaccine based on the GelVac nasal powder formulation has been approved for human testing by the FDA, and a phase I clinical study is currently underway (http://clinicaltrials.gov/ct2/show/NCT01258062?term=GelVac&rank=1).
Previously, our research group showed that liquid formulations of plant-derived NV VLPs elicit humoral and mucosal immune responses when delivered via the enteric or intranasal route of immunization in mice [4, 6, 46, 47]. The intranasal route may be preferable for a commercial vaccine because it is easy to access, is highly vascularized, has a large surface area, has low proteolytic activity, and can induce both systemic and distal mucosal immune responses [29, 31, 32, 44]. In addition, NV VLP immunogenicity was shown to be enhanced by codelivery with the imidazoquinoline-based, TLR7 agonist, gardiquimod (GARD) [4]. While the GARD-containing, NV VLP liquid vaccine was effective, we hypothesized that a mucoadhesive-containing, dry powder vaccine might prolong the residence time on the mucosa, thereby increasing antigen uptake and enhancing the immune response [32, 43]. A dry powder formulation may also be preferable for a commercial vaccine because it offers higher sterility and stability, thus facilitating mass production and vaccination in both developed and developing countries [32].
2. MATERIALS AND METHODS
2.1 Preparation of vaccine formulations
Recombinant NV VLPs were expressed in Nicotiana benthamiana by Kentucky Bioprocessing (Owensboro, KY) following previously described protocols [47]. Clarified leaf extracts were filtered through a 0.2 micron capsule filter and concentrated using a 100 Kd polyethersulfone (PES) tangential flow filtration (TFF) membrane (Pall Corporation, Port Washington, NY). A diethylaminoethyl (DEAE) Sepharose column was used to collect a colorless fraction that allowed recovery of the VLPs in >98% protein purity. Endotoxins and remaining small molecules were removed by Q Column fractionation. The resulting concentrated VLPs as a liquid solution in PBS were diluted to 10 or 25 μg NV VLPs in sterile PBS with or without 10 μg GARD (InvivoGen, San Diego, CA).
GelSite liquid formulations were prepared by mixing sterile stock solutions of 0.4% GelSite (DelSite Biotechnologies, Inc., Irving, TX) with PBS liquid formulations containing NV VLP with or without GARD at a 1:1 dilution in a biological hood. GelVac powder formulations (DelSite Biotechnologies, Inc.) were prepared by spray drying the liquid formulations using a Buchi B-290 Mini spray dryer (Buchi laboratories, Switzerland) in a temperature and moisture-controlled class 1000 clean room. The following formulations were prepared: GelVac alone powder, GelVac NV VLP powder, GelVac GARD powder, or GelVac NV VLP + GARD powder. GelVac powder formulations had a GelSite polymer content of 0.25% (w/w). The particle size of the powder formulations was measured using a laser diffraction particle size analyzer (Beckman Coulter LS 230, Brea, CA) and the mean particle size was ~20 μm. The powders were transferred to tight-sealed tubes and packaged in moisture and light resistant aluminum foil bags (3M™, Minneapolis, MN) with a desiccant pack and stored at room temperature until use.
2.2 GelVac NV VLP structural characterization
2.2.1 Light microscopy
Micrographs of GelVac alone powder particles were collected using a Nikon epifluorescent microscope (Nikon, Melville, NY). Powder particles were examined either as dry samples, or when rehydrated in simulated nasal fluid as previously described without the addition of bovine serum albumin [48]. Rehydrated particles were stained with 0.01 mg/ml of toluidine blue dye (EMS, Hatfield, PA).
2.2.2. Scanning electron microscopy
GelVac alone or GelVac NV VLP dry powder formulations were prepared by dispersing each powder on a metal disk and the particles were held in place using double sided sticky carbon tape. Each powder was sputter coated with gold/palladium for 5 min using a Technis Hummer II sputtering device (Technis, Alexandria, VA). Micrographs of each powder were collected using a Philips XL30 environmental scanning electron microscope (ESEM).
2.3 GelVac NV VLP quantification
NV VLP stability and concentration in the GelVac powder formulations was determined by sucrose gradient sedimentation and ELISA, as described previously [47]. Briefly, a 6-layer gradient was created in Beckman SW55 Ti tubes (Beckman Coulter, Fullerton, CA) by layering equal volumes of 60, 50, 40, 30, 20 and 10% sucrose dissolved in modified phosphate buffer (25 mM sodium phosphate, 100 mM NaCl). Following incubation at 4°C for 2 h, GelVac NV VLP or insect cell-derived NV VLP standard (Invitrogen, Carlsbad, CA) were loaded onto the gradient and centrifuged at 90,000 × g for 3 h at 4°C. Fractions were removed from the top to the bottom of the gradient and analyzed by ELISA. Enzyme immunoassay/radioimmunoassay (EIA/RIA) 96-well polystyrene high-binding plates (Corning Inc. Life Sciences, Lowell, MA) were precoated with rabbit anti-NV VLP serum for 4 h at room temperature then loaded with the sucrose fractions serially diluted in 1% (wt/vol) dry milk in PBS containing 0.05% Tween-20 (PBS-T) overnight at 4°C. A standard curve was generated with 2-fold dilutions of insect-cell derived NV VLPs at concentrations ranging from 100 to 0.7 ng/ml. The wells were reacted in succession with guinea pig anti-NV VLP serum and goat anti-guinea pig IgG – horseradish peroxidase (HRP) conjugate (Southern Biotech, Birmingham, AL), each diluted 1:10,000 in 1% dry milk in PBS-T for 2 h at 37°C. Plates were developed with 4% tetramethylbenzidine (TMB) peroxidase liquid substrate system (KPL Inc., Gaithersburg, MD) for 2 min then stopped with 1 M phosphoric acid. Absorbance measurements were made at 450 nm using a MRX automatic plate reader (Dynex Technologies, Chantilly, VA).
2.4 Animal Studies
All animals were housed in accordance with United States Department of Agriculture (USDA) and American Association for Laboratory Animal Care (AALAC) standards, provided unlimited access to food and water, and handled in accordance with the Animal Welfare Act and Institutional Animal Care and Use Committee (IACUC) regulations. The in-situ gelling of GelVac powder alone was evaluated in rats due to the ease of delivery of dry powders as a particulate aerosol to the nasal cavity. Animals were sacrificed at 1 or 3 hours after aerosol powder delivery, and the tissues of the nasal cavity were fixed for histological examination. Prior to nasal delivery, female (175-200 g) Sprague-Dawley rats (Harlan Laboratories, Inc., Indianapolis, IN) were distributed into four groups (n = 3 per group; except control group n = 1). For immunization studies, the use of mice allowed correlation to extensive prior studies with NV VLP liquid vaccine formulations, but the small nare size in mice precluded their use for dry powder delivery. Guinea pigs were therefore used to evaluate dry powder vaccine formulations. Prior to immunization, animals were randomly distributed into vaccination groups and allowed to acclimate for at least one week. Female (250 g) Hartley guinea pigs (Charles River Laboratories International, Inc., Wilmington, MA) were distributed into six immunization groups (n = 5 per group) and female, 6-week old, BALB/c mice (Charles River Laboratories International, Inc.) were distributed into five immunization groups (n = 7 per group; except PBS control group, n = 5).
2.4.1 Histological analysis of nasal epithelium in rats
GelVac alone powder with 1% (w/w) GelSite polymer content was delivered intranasally to rats as described below for guinea pigs in section 2.4.2. Negative control rats received mock immunizations without the GelVac powder. At 1 h and 3 h following administration, rats were euthanized with CO2. Tissues (heads without lower jaws) were fixed in formalin. Cross sections were made of the nasal cavity starting anterior to the orbit of the eye. The tissue sections were stained with toluidine blue dye. This dye revealed the in-situ gel as a pinkish/purplish substance. This staining characteristic has been previously established by examining the gel formed following subcutaneous injection of GelSite polymer solutions.
2.4.2 Guinea pig immunization
Guinea pigs were anaesthetized with ketamine (35 mg/kg; Bioniche Pharma USA LLC) and xylazine (5 mg/kg; Akorn, Inc.) administered intraperitoneally prior to immunization. Dry powder vaccines were administered intranasally on days 0 and 21 with 10-12 mg/naris of GelVac alone, GelVac NV VLP (10 μg), or GelVac NV VLP (10 μg) + GARD (10 μg). Comparable liquid formulations of NV VLP (10 μg), NV VLP (10 μg) + GARD (10 μg), or NV VLP (25 μg) + GARD (10 μg) were delivered at a maximum of 5 μl/naris. An intranasal powder delivery device was prepared by fitting a p200 pipette tip with 2 cm of the end removed to 6 cm of rubber tubing attached to a 5 ml syringe (BD Biosciences, Franklin Lakes, NJ). The p200 pipette tip was used as a connector piece between the syringe and vaccine cartridge. The vaccine cartridge was prepared by cutting a 2 cm piece from the end of a p1000 pipette tip wrapped at the end with parafilm. The narrowest tip of the vaccine cartridge was removed to create wider aperture. One dose of each GelVac powder formulation (10-12 mg/naris) was weighed, loaded into the vaccine cartridge, and subsequently slid into the modified p200 connector tip. A 1.5 cm piece removed from the end of a p1000 pipette tip was fitted onto the vaccine cartridge and used as the point of insertion into the nasal cavity. The modified p1000 nasal tip was coated with KY® jelly lubricant (McNeil-PCC, Inc., Fort Washington, PA) and 5 mm of the tip was inserted into the nostril. The GelVac powder vaccine was delivered by administering 2 ml of air from the 5 ml syringe into the nostril and subsequently repeated on the opposite naris. The modified p1000 vaccine cartridge and nasal tips were discarded after each use and the modified p200 connector tip was changed between experimental groups to prevent cross-contamination of vaccine materials.
2.4.3 Mouse immunization
Mice were intranasally immunized on days 0 and 21 with NV VLP (25 μg) or NV VLP (25 μg) + GARD (10 μg) in a GelSite liquid or PBS liquid formulation. The liquid formulations were administered to conscious mice by gently distributing 5-10 μl of the vaccine dropwise in each naris using a p20 pipette tip. Negative control mice received 10 μl PBS alone.
2.5 Sample collection
2.5.1 Guinea pig sample collection
Guinea pig serum and vaginal lavage samples were collected prior to the first immunization on day 0 (preimmune) and on days 13, 21, 42, and 60. Serum was isolated by centrifugation of whole blood (150 μl) collected from the lateral saphenous vein of each guinea pig and transferred into heparinized microtubes. Vaginal lavages were collected by lavaging 250 μl of PBS intravaginally with an oral feeding needle (Braintree Scientific Inc., Braintree, MA). Fecal pellets were not collected as guinea pigs were group housed. On day 60, guinea pigs were given a pre-anesthetic injection of ketamine (35 mg/kg) and xylazine (5 mg/kg) administered intraperitoneally and then maintained at a surgical plane using isoflurane (2%, Phoenix Pharmaceutical, Inc.) and exsanguinated via cardiocentesis. Distal mucosal samples including salivary, intestinal, nasal, and bronchoalveolar were collected following euthanasia as previously described [4] for mice with some modifications: nasal lavage samples were collected by flushing each naris with 500 μl PBS and bronchoalveolar lavage samples were collected by flushing the lungs with 1 ml PBS. Uterine lavages were collected post-mortem by opening the abdominal cavity, extracting each uterine horn, and flushing each horn with 500 μl PBS. Each horn was excised caudal to the ovary and at the branch where it meets the vagina. All samples were clarified by centrifugation and stored at −80°C prior to analysis.
2.5.2 Mouse sample collection
Mouse serum, fecal pellets, and vaginal lavage samples were collected on days 0, 12, 21, 42, 56, 84, and 112 as previously described [4]. All mice were humanely euthanized on day 112 in accordance with the Animal Welfare Act and ASU IACUC. Distal mucosal samples including salivary, nasal, and bronchoalveolar were collected following euthanasia as previously described [4]. Uterine lavages were collected post-mortem as described above with 200 μl per uterine horn. All samples were clarified by centrifugation and stored at −80°C prior to analysis.
2.6 NV-specific ELISAs
EIA/RIA 96-well polystyrene high-binding plates were coated with 0.5 μg/ml insect cell-derived NV VLPs for 4 h at room temperature then blocked overnight at 4°C with 10% (fecal and intestinal samples) or 5% (all other samples) dry milk in PBS. Samples were prepared in 2.5% (serum samples) or 5% (mucosal samples) dry milk in PBS-T, serially diluted 2-fold down the microtiter plate, and incubated for 2 h at 37°C to permit antibody binding as previously described [4, 5, 47]. Briefly, HRP-conjugated anti-guinea pig or anti-mouse antibodies diluted in 2.5% dry milk in PBS-T were loaded onto the wells and incubated for 1 h at 37°C (see Table 1). Plates were developed with 4% TMB peroxidase liquid substrate system for 5-15 min (depending on the sample). Color development was stopped by the addition of an equal volume of 1 M phosphoric acid and absorbance measurements were made at 450 nm using a MRX automatic plate reader. Endpoint titers are reported as the reciprocal of the highest dilution that had an absorbance value greater than or equal to 0.065 to 0.1 above the background (0.065 for serum and 0.1 for all mucosal samples).
Table 1.
List of antibodies used in this study
| Antibody | Dilution | Vendor |
|---|---|---|
| Goat anti-guinea pig IgG | 1:5,000 | Southern Biotech |
| Goat anti-guinea pig IgG1 | 1:2,500 | Bethyl Laboratories, Inc. (Montgomery, TX) |
| Goat anti-guinea pig IgG2a | 1:50,000 | Bethyl Laboratories, Inc. |
| Rabbit anti-guinea pig IgA | 1:100 | Bethyl Laboratories, Inc. |
| Goat anti-rabbit IgGa | 1:5,000 | Sigma-Aldrich (St. Louis, MO) |
| Goat anti-mouse IgG | 1:5,000 | Southern Biotech |
| Goat anti-mouse IgG1 | 1:2,500 | Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) |
| Goat anti-mouse IgG2a | 1:2,500 | Santa Cruz Biotechnology, Inc |
| Goat anti-mouse IgA | 1:1,000 | Sigma-Aldrich |
Secondary antibody to rabbit anti-guinea pig IgA incubated 1.5 h at 37°C.
2.7 Cell culture
Human embryonic kidney (HEK)-293XL cells constitutively expressing human TLR7 (InvivoGen), were cultured in Dulbecco’s modified eagle medium (DMEM) (Invitrogen) supplemented with 20% fetal bovine serum (FBS) (Invitrogen), 0.01 mg/ml blasticidin (InvivoGen), and 0.1 mg/ml primocin (InvivoGen) as recommended by the vendor. For stimulation experiments, HEK-293XL cells were cultured in 24-well plates (BD Biosciences) to a density of 1.2×106 cells/well. GelVac powder formulations were resuspended in 200 μl H2O and 100 μl of the suspension was added to the HEK-293XL cells in duplicate wells at a concentration of 25.0 mg/ml (GelVac NV VLP) or 16.5 mg/ml (GelVac GARD). PBS liquid formulations containing GARD were added in duplicate at 1, 2.5, 5, 10, and 25 μg/ml. Following 24 h of stimulation, cell culture supernatants were collected and assayed for IL-8 production by ELISA using the Quantikine Human CXCL8/IL-8 Immunoassay (R&D Systems, Inc., Minneapolis, MN). Absorbance measurements made at 450 nm were corrected at 540 nm using a MRX automatic plate reader.
2.8 Statistics
Prism software (GraphPad Inc., San Diego, CA) was used to graph and evaluate statistical comparisons of all data. NV VLP-specific IgA and IgG antibody titers are expressed as geometric mean titers (GMT) for each immunization group at each time point. All responders and nonresponders were included in the computation of the GMT. Negative samples were assigned a value of 1.0 for the purpose of calculating the GMT. The differences in NV-specific IgA and IgG antibody production were evaluated using the Kruskal-Wallis one-way analysis of variance (ANOVA) followed by a Dunns post-test at each time point. The differences in IL-8 secretion were evaluated using an unpaired one tailed t test with Welch’s correction. All statistical comparisons displayed graphically were made between individual treatment groups versus the GelVac alone group (Fig. 5, 6, 7), the H20 group (Fig. 8), or the PBS alone group (Fig. 9, S1, S2). Statistical significance was considered to be a P value of < 0.05.
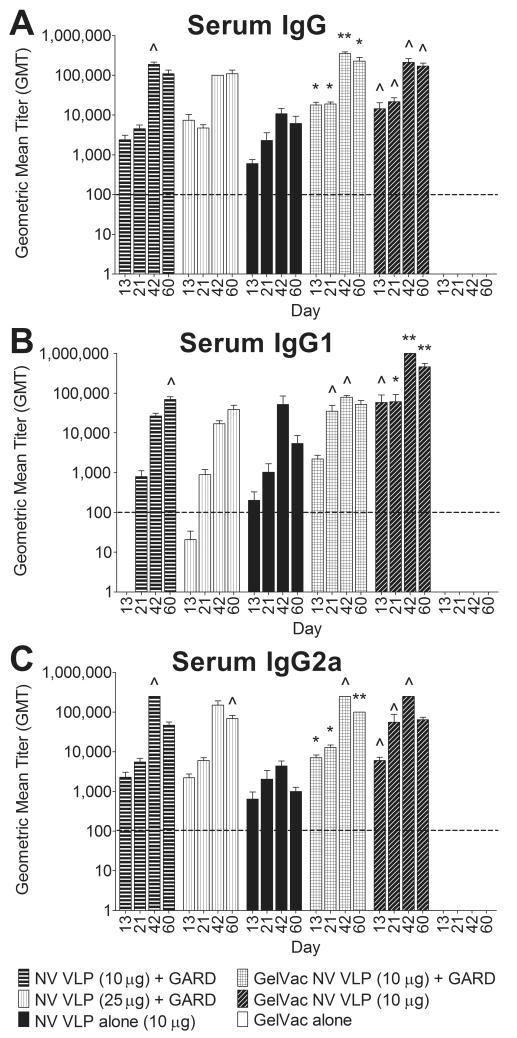
Figure 5. Serum NV-specific IgG and IgG isotype production following intranasal immunization with VLPs in GelVac powder or PBS liquid.
Female Hartley guinea pigs were immunized intranasally with a GelVac dry powder or a PBS liquid formulation of NV VLPs (10 or 25μg) on days 0 and 21 with or without GARD (10μg). Serum samples were collected on days 0, 13, 42, and 60 and analyzed for NV VLP-specific IgG (A), IgG1 (B), and IgG2a (C) by ELISA. Antigen-specific IgG, IgG1, and IgG2a were not detected (GMT < 100) in all pre-immune samples (data not shown). Error bars represent the standard errors of the mean. Horizontal dashed line indicates the limit of detection for the assay. ^P<0.05; *P<0.01; **P<0.001; ***P<0.0001 compared to the GelVac alone control group.
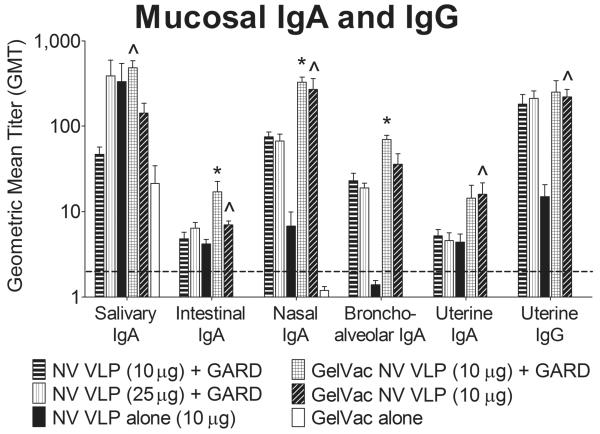
Figure 6. Mucosal NV-specific IgA and IgG production following intranasal immunization with VLPs in GelVac powder or PBS liquid.
Guinea pigs were euthanized on day 60. Salivary, intestinal, nasal, bronchoalveolar, and uterine lavages were collected and analyzed for NV VLP-specific IgA and IgG by ELISA. Error bars represent the standard errors of the mean. Horizontal dashed line indicates the limit of detection for the assay. ^P<0.05; *P<0.01 compared to the GelVac alone control group.
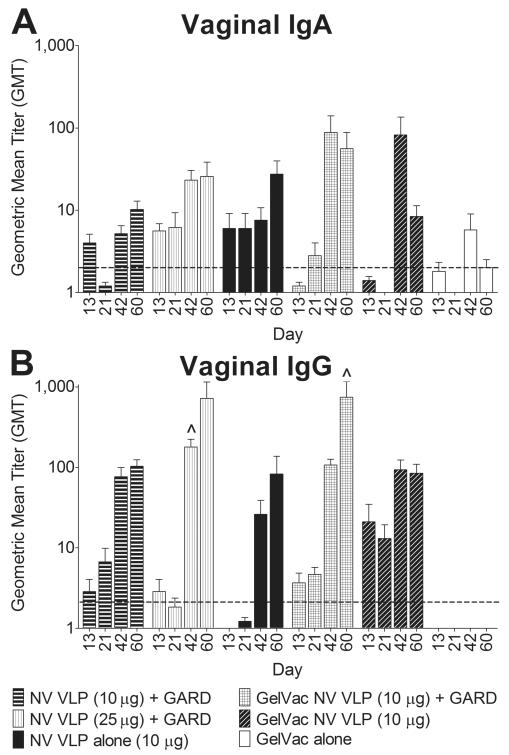
Figure 7. Vaginal NV-specific IgA and IgG production following intranasal immunization with VLPs in GelVac powder or PBS liquid.
Guinea pig vaginal lavages were collected on days 0, 13, 42, and 60 and analyzed for NV VLP-specific IgA (A) and IgG (B) by ELISA. Background levels of vaginal antigen-specific IgA were detected in most pre-immune samples (GMT < 2); antigen-specific IgG was not evaluated in pre-immune samples (data not shown). Error bars represent the standard errors of the mean. Horizontal dashed line indicates the limit of detection for the assay. ^P<0.05; *P<0.01 compared to the GelVac alone control group.
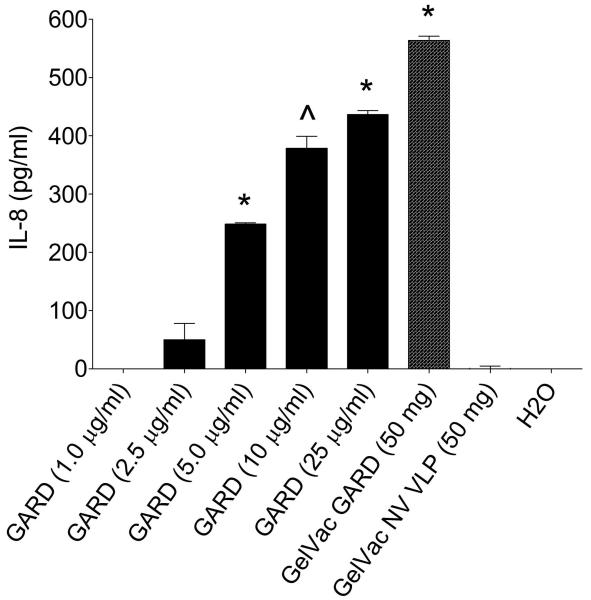
Figure 8. IL-8 secretion by HEK-293XL cells expressing TLR7 following stimulation with GARD in GelVac powder.
HEK-293XL cells expressing human TLR7 were cultured in 24-well plates and stimulated with 15.6 mg/ml GelVac GARD powder formulation or 1.0, 2.5, 5.0, 10, or 25μg/ml of GARD in a PBS liquid formulation. GelVac NV VLP and H2O were used as negative controls. Cell culture supernatants were collected 24 h after stimulation and analyzed in duplicate for IL-8 content by ELISA. GelVac results are expressed as the amount of IL-8 per 50mg of powder. Error bars represent the standard errors of the mean. ^P<0.05; *P<0.01 compared to the H2O control group.
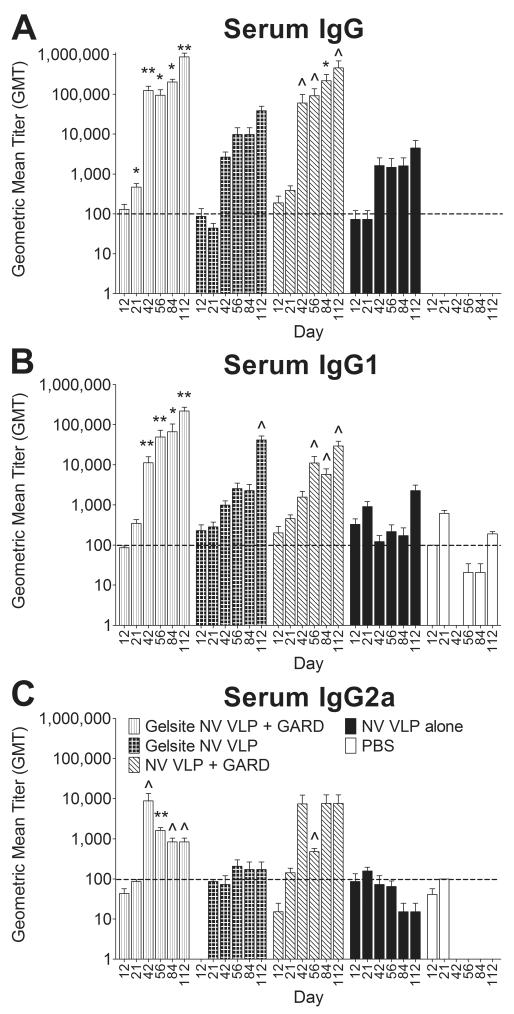
Figure 9. Serum NV-specific IgG and IgG isotype production following intranasal immunization with VLPs in a GelSite or PBS liquid.
Female BALB/c mice were immunized intranasally with a GelSite or PBS liquid formulation of NV VLPs (10μg) on days 0 and 21 with or without GARD (10μg). Serum samples were collected on days 0, 12, 21, 42, 56, 84, and 112 and analyzed for NV VLP-specific IgG (A), IgG1 (B), and IgG2a (C) by ELISA. Antigen-specific IgG was not detected (GMT < 100) in all pre-immune samples; however background levels of antigen-specific IgG1 and IgG2a were detected (GMT ≥ 100) in most pre-immune samples (data not shown). Error bars represent the standard errors of the mean. Horizontal dashed line indicates the limit of detection for the assay. ^P<0.05; *P<0.01 compared to the PBS control group.
3. RESULTS AND DISCUSSION
3.1 Structural characterization of GelVac dry powder with or without NV VLP incorporation
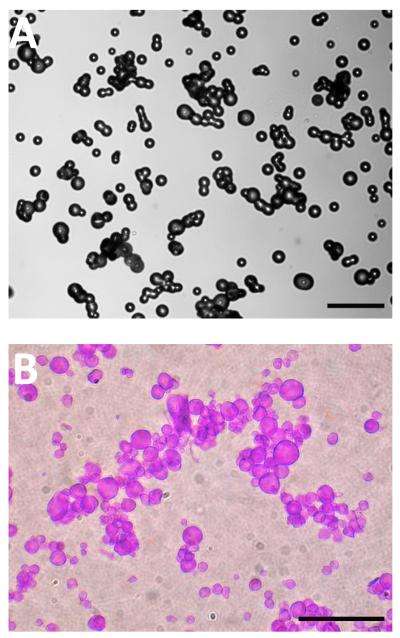
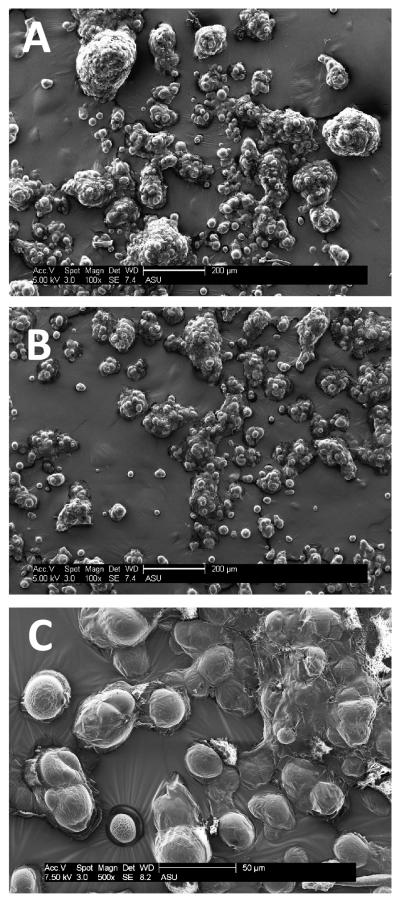
GelVac dry powder formulations were prepared by spray drying and resulted in a white, fine powder that appeared as spherical-shaped particulates (Fig. 1A). The mean particle size was 20 μm as measured by a laser diffraction particle size analyzer. When rehydrated in simulated nasal fluid and stained with toluidine blue dye, particulates formed wet gel particles that were enlarged in size (Fig. 1B). In vivo, this characteristic is important as mucoadhesive polymers act by swelling upon contact with the mucosa, then penetrating into the tissue crevices to increase the residence time of the antigen in the nasal cavity [44]. GelVac dry powder formulations were analyzed by scanning electron microscopy for ultrastructural characterization. The dry powders all had a similar dispersed, particulate profile with individual spherical particles ranging in size from 20-30 μm in diameter (Fig. 2). Some aggregation was evident in the micrographs and is likely due to hydration during sample preparation. The particle surface was non-porous in appearance (Fig. 2), consistent with other reports of spray dried subunit antigen formulations [49]. When we compared GelVac powders with or without NV antigen included in the formulation, no detectable size or other structural differences were observed (Fig. 2).
Figure 1. Visualization of GelVac polymer-containing powder.
A. Spray dried GelVac alone powder contains spherical-shaped particles ranging in size from approximately 20-30 μm in diameter. Aggregation of particles occurs during sample preparation for microscopy, likely due to hydration. B. GelVac alone powder reconstituted and hydrated with simulated nasal fluid and stained with toluidine blue dye. Aggregation of particles, coincident with hydration, is evident. Scale bars are 100 μm.
Figure 2. Ultrastructural characterization of GelVac polymer-containing powders by scanning electron microscopy.
GelVac alone powder (A) or formulated with NV VLPs (B, C). GelVac powder particles were imaged at 100X (A, B; scale bar 200 μm) and 500X (C; scale bar 50 μm) with a scanning electron microscope. Non-aggregated particles range in size from 15-30 μm in diameter, and are smooth in appearance.
3.2 In-situ gelation of GelVac dry powder
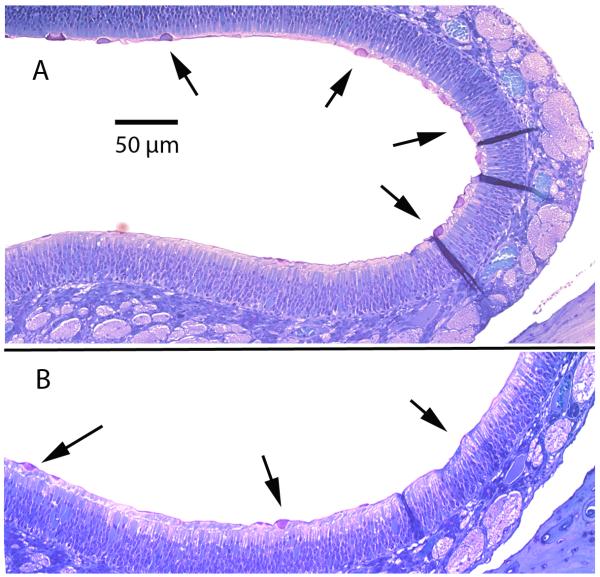
A potential limitation to intranasal immunization is the rapid mucociliary clearance of vaccine components from the nasal cavity. Nasal mucociliary clearance in healthy humans is known to occur in times as short as 10 minutes [50]. In nasal clearance studies using rabbits, greater than 90% clearance of control solutions from the nasal cavity was observed in 1 hr, with a half time of 24 minutes [51]. In the latter study, it was found that formulation of immune stimulating complexes (ISCOMs) could extend the clearance time to half times over 1 hr, due to mucoadhesive characteristics. These types of observations lead us to examine the mucoadhesive and in-situ gelling properties of GelVac powder that contains GelSite (an Aloe vera L.-derived, inert polysaccharide polymer) and has been proposed for use with mucosally-delivered vaccines [45, 52]. Following aerosol delivery of GelVac alone powder to the nasal cavity of rats, microscopic examination of the nasal epithelium was conducted to find evidence of in-situ gelation. At 1 h and 3 h after dry powder delivery, direct evidence of the particulate gel material was observed in close association with the mucus at the surface of the epithelium (Fig. 3, see arrows). Although this experimental approach is not quantitative and does not provide data on clearance time, the fact that particles can still be detected after 3 hrs, indicates that normal mucociliary clearance is delayed. Because our specific goal in this study was to determine if the apparent association enhanced immune responses, we did not expand the clearance studies but instead proceeded to characterize the formulated antigen by nasal immunization and immune induction analysis.
Figure 3. Histologic examination of the nasal epithelium after intranasal delivery of GelVac dry powder.
Female Sprague-Dawley rats were euthanized at 1 h (A) or 3 h (B) following intranasal delivery of GelVac alone powder (scale bar 50 μm). Gelation of the pink/purple particles at the moist nasal epithelium surface are shown by arrows. No pink/purple particles were observed in negative controls (data not shown). While these images are not quantitative for the amount of GelVac powder remaining in the epithelium, particles can still be detected at 3 h indicates that normal mucociliary clearance is delayed.
3.3 NV VLPs are stable in GelVac dry powder
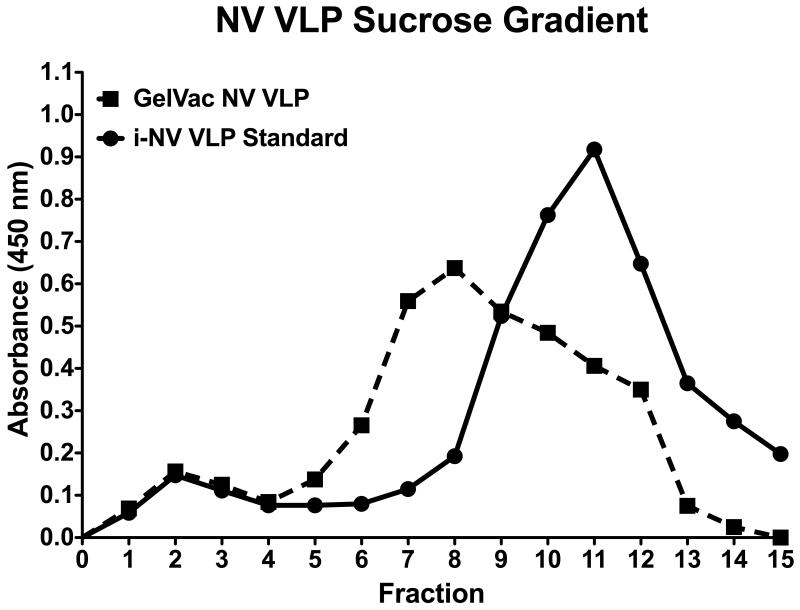
The spray drying processes used to transform the NV VLP preparation from a liquid state to a dry powder includes a hot drying process that might denature vaccine components [32]. To determine if such denaturation occurred, GelVac NV VLP powder (1 mg) was subjected to sucrose gradient sedimentation and the antigen was localized on the gradient by indirect ELISA. It is well known that particulate antigens, such as assembled VLPs, migrate more rapidly into more dense sucrose solutions as compared to unassociated or partially associated capsid protein antigens [47]. The gradient distribution of antigen in the dry powder, which was applied directly to the surface of the sucrose gradient, was compared to a liquid formulation of NV VLP derived from an insect cell expression system (i-NV VLP). As expected, the i-NV VLP standard antigen migrated deeply into the gradient, indicating assembled VLPs (fractions 9-13) (Fig. 4). GelVac NV VLP dry powder also showed that the majority of antigen had migrated deeply into the gradient (fractions 7-12) (Fig. 4). This is strong evidence that the NV VLP antigen was not denatured during the spray drying processes (which would have resulted in detection at the top of the gradient), but was still particulate. We do note that the antigen peak “leading shoulder” of the expected peak corresponding to VLPs is somewhat wider in the GelVac dry powder sample than in the insect cell –NV VLP standard samples. We believe this may be the result of directly applying the powder to the sucrose solution surface, such that subsequent hydration/solubilization of the powder contents was occurring as the gradient was being fractionated, and could have delayed VLP migration into the sucrose as compared to the insect cell-derived control, resulting in a peak broadening. In total, these data support the previously published concept that spray dry vaccine preparation is an effective strategy to preserve antigen stability [53].
Figure 4. Evaluation of VLP stability in GelVac powder by sucrose gradient sedimentation.
GelVac NV VLP dry powder and insect-cell derived NV VLP liquid standard (i-NV VLP) were loaded onto a 6 layer sucrose density gradient and centrifuged. Fractions were removed from the gradient from top (1) to bottom (15) and analyzed by indirect ELISA for NV VLP integrity. Peaks in the absorbance between fractions 7 and 12 correspond to whole NV VLP, indicating that NV VLPs are stable in the GelVac dry powder formulation.
3.4 Intranasal delivery of NV VLPs in a GelVac powder formulation elicits a superior systemic immune response compared to a PBS liquid formulation
Once it was determined that the spray drying process maintained NV VLP structural integrity (Fig. 4) and that the GelVac powders were appropriately rehydrated with simulated nasal fluid (Fig. 1B) and could delay mucociliary clearance (Fig. 3), the immunogenicity of the GelVac dry powder vaccines were evaluated in a guinea pig model. In comparison to conventional murine models, guinea pigs are preferred for delivery and evaluation of dry powder formulations because they provide a larger nasal mucosal surface area for immune induction. In addition, guinea pigs have been used previously to evaluate TLR7 agonist activity in vivo [54, 55]. The immunogenicity of the GelVac powder vaccines were evaluated relative to complementary PBS liquid formulations of NV VLPs (10 or 25 μg) with or without GARD (10 μg). To measure systemic immune induction, serum was assayed for NV VLP-specific IgG, IgG1, and IgG2a levels by ELISA.
Immunization with NV VLPs, whether in a GelVac powder or PBS liquid formulation, induced higher antigen-specific IgG1 production than IgG2a, indicative of a predominant Th2 response (Fig. 5B, 5C). This result is consistent with previous studies and suggests that the Th2 shift is due to the NV VLP antigen [4, 7]. As hypothesized, the GelVac powder formulations were more immunogenic than their liquid counterparts (Fig. 5). In comparison to guinea pigs immunized with GelVac alone powder (mock-immunized), guinea pigs immunized with NV VLP powder with or without GARD produced significantly higher antigen-specific IgG, IgG1, and IgG2a antibody titers on most days throughout the study (days 13-60) (P < 0.05); whereas guinea pigs immunized with comparable liquid formulations rarely induced significant titers (Fig. 5). On average, the magnitude of enhancement by immunization with NV VLP powder formulated without GARD, relative to NV VLP liquid was 20-, 114-, and 40-fold for IgG, IgG1, and IgG2a, respectively (Fig. 5). These levels were statistically different on days 13, 42, and 60 for IgG1 (P < 0.05) (Fig. 5B). The magnitude of immune response enhancement achieved by including GARD in the dry powder formulation, relative to both liquid counterparts was 4-, 300- and 2-fold for IgG, IgG1, and IgG2a, respectively (P ≥ 0.05) (Fig. 5). Unexpectedly, GARD did not significantly enhance serum IgG and IgG isotype production (P ≥ 0.05) when included in the dry powder formulation. The level of IgG and IgG2a production enhancement achieved by the powder vaccine without the TLR7 agonist was higher than that achieved with GARD (Fig. 5).
3.5 Intranasal delivery of NV VLPs in a GelVac powder formulation elicits robust mucosal immune responses
3.5.1 Gastrointestinal Tract (Salivary, Intestinal)
To gain further information related to the mucosal immunogenicity of dry powder formulations, and their ability to stimulate responses at distal sites in the CMIS, we evaluated antibody titers at multiple mucosal surfaces. Because NV initiates disease via the enteric route, IgA titers were evaluated in the enteric pathway. NV-specific IgA production was found in both saliva and intestinal lavages, with higher concentrations per volume recovered in the saliva (however higher levels of non-specific background IgA were detected in salivary samples) (Fig. 6). Powder formulations and their liquid counterparts elicited nearly equivalent IgA production in the saliva, although the NV VLP and GARD powder was the only vaccine regimen to induce statistically significant NV VLP-specific IgA production relative to mock-immunized guinea pigs (P < 0.05) (Fig. 6). In the intestine, both powder formulations induced IgA responses that were higher than their liquid counterparts and significantly higher than mock-immunized guinea pigs (P < 0.05) (Fig. 6). In contrast to serum IgG and IgG isotype results (Fig. 5), the addition of GARD to the powder-formulated VLPs increased salivary and intestinal IgA production by 3- and 2-fold, respectively. These levels however, were not statistically different in the sample size of this experiment (P ≥ 0.05) (Fig. 6).
3.5.2 Respiratory tract (Nasal, Bronchoalveolar)
GelVac powder vaccines were administered intranasally, therefore we evaluated their ability to induce strong NV-specific IgA production at the primary site of immunization, the respiratory tract. Significant IgA titers were observed in nasal and bronchoalveolar lavages collected from guinea pigs immunized with the powder formulations (P < 0.05); whereas no such response was observed for the liquid formulations (P ≥ 0.05) (Fig. 6). The apparent association between the GelVac powder and the nasal epithelium (Fig. 3) may have prolonged residence time on the mucosa, thereby increasing antigen uptake by APCs, and resulting in the enhanced local immune response by the mucoadhesive-containing powder in comparison to the liquid formulation. The NV VLP powder without GARD elicited 40- and 26-fold higher nasal and bronchoalveolar IgA production, respectively, relative to its liquid counterpart (Fig. 6). Similarly, the NV VLP and GARD powder elicited 4- and 3-fold higher nasal and bronchoalveolar IgA production relative to both liquid counterparts (P < 0.05) (Fig. 6). Interestingly, nasal and bronchoalveolar IgA titers were comparable in guinea pigs immunized with NV VLP powder or NV VLP and GARD powder (Fig. 6), a result similar to that observed for serum IgG and IgG2a (Fig. 5A, 5C).
3.5.3 Reproductive tract (Vaginal, Uterine)
To gain insights into the extent of CMIS stimulation by GelVac-formulated VLPs, we evaluated NV-specific IgA and IgG production in the female reproductive tract. Vaginal and uterine IgG production was higher than IgA production (Fig. 6, 7). As was seen in serum (Fig. 5) and the respiratory tract (Fig. 6), the NV VLP powder without GARD induced higher vaginal IgA (day 42; 11-fold) and IgG (days 13 and 21; 11-fold) as well as uterine IgA (4-fold) and IgG (15-fold) production relative to its liquid counterpart (Fig. 6, 7). Similarly, the NV VLP and GARD powder induced higher IgA production in the vagina (days 42 and 60; 2-fold) and uterus (3-fold) relative to both liquid counterparts (Fig. 6, 7). The addition of GARD to the powder-formulated VLPs did not consistently enhance vaginal or uterine IgA and IgG production as compared to the NV VLP powder (Fig. 6, 7).
Collectively, these results indicate that our intranasal NV dry powder vaccine containing the mucoadhesive polymer GelSite elicits robust systemic and mucosal immune responses that in most cases are superior to those induced by liquid counterparts without GelSite (Fig. 5, 6, 7). Prolonged nasal residence time most likely increased antigen uptake and contributed to the enhanced immune induction elicited by the GelVac powder formulations. These results are consistent with previous preclinical studies that evaluated the efficacy of intranasally delivered influenza, anthrax, and tetanus dry powder vaccines [27, 56, 57]. Like our NV vaccine, the influenza and anthrax vaccines contained a mucoadhesive polymer (chitosan); whereas, the tetanus vaccine did not contain a mucoadhesive. Despite this difference, all three dry powder vaccines induced robust systemic immune responses that were superior to those induced by comparable liquid counterparts [27, 56, 57]. In addition, similar to our NV dry powder vaccine, the tetanus and influenza dry powder vaccines elicited robust nasal IgA production at levels higher than that elicited by comparable liquid counterparts [27, 57].
3.6 TLR7 agonist activity of GARD is retained in GelVac dry powder
In contrast to our hypothesis that GARD, an immunopotentiator, would enhance NV VLP immunogenicity in a powder formulation, GARD did not significantly enhance mucosal or systemic immunity when formulated into the NV dry powder vaccine (Fig. 5, 6, 7). These results are comparable to those observed with an intranasally delivered tetanus dry powder vaccine in which the immunpotentiator, Quillaja saponin, did not enhance mucosal immunity when added to the vaccine formulation containing mucoadhesive components [57]. Possible explanations for the lack of GARD stimulation of the immune response when delivered in a powder could be that the spray drying process may have compromised the TLR7 agonist activity of GARD, or anti-NV immune responses reached a threshold by the GelVac NV VLP powder, thus constraining further improvements in immunogenicity of the NV VLPs. To determine if the spray drying process inactivated GARD activity, we stimulated 293XL cells expressing TLR7 with GelVac-formulated GARD powder. TLR7 ligation was measured by collecting cell culture supernatants 24 h after stimulation and quantifying IL-8 secretion by ELISA.
Liquid formulations of GARD induced IL-8 secretion in a dose-dependent manner (Fig. 8). The powder formulation, which had a GARD content equivalent to 10 μg, induced IL-8 secretion at a level slightly higher than its liquid counterpart, indicating that the TLR7 agonist activity of GARD was retained in the GelVac powder formulation (Fig. 8). IL-8 was not secreted following stimulation with GelVac-formulated NV VLP alone powder, suggesting that the IL-8 secretion elicited by the GARD powder was specifically due to the TLR7 agonist activity of GARD (Fig. 8). As further support, whether in a powder or liquid formulation, NV VLPs administered with GARD elicited relatively equal levels of IgG1 and IgG2a antibody titers, indicative of a mixed Th1/Th2 response (Fig. 5B, 5C). The shift to a less Th2 predominant response may be due to the effects of GARD, which has previously been shown to induce the secretion of Th1 cytokines both in vitro and in vivo [58, 59]. These results suggest that intranasal immunization with the NV powder vaccine containing the mucoadhesive, GelSite, is sufficient to induce both mucosal and systemic immunity and ameliorates the need for an immunopotentiating agent, unless an IgG2a (Th1) response correlates to a higher level of protection in humans. In this situation a Th1 polarizing immunopotentiator may be required.
3.7 Intranasal delivery of NV VLPs in a GelSite liquid formulation elicits an equivalent systemic immune response compared to a PBS liquid formulation
Since NV VLPs in the powder vaccine containing GelSite elicited robust systemic and mucosal immune responses without an adjuvant (Fig. 5, 6, 7), we aimed to determine if the immune responses were elicited by the dry powder formulation or potential immunostimulatory properties of GelSite. Female BALB/c mice were intransally immunized with NV VLPs alone (25 μg) or NV VLPs (25 μg) and GARD (10 μg) in PBS liquid formulations with or without GelSite.
Similar to guinea pig serum results (Fig. 5), NV VLPs, whether in a GelSite or PBS liquid formulation, induced higher levels of serum IgG1 production than IgG2a, indicative of a strong Th2 response. When administered in a powder vaccine containing GelSite, NV VLPs consistently enhanced humoral immune responses relative to liquid counterparts without GelSite (Fig. 5). In contrast, when administered in a liquid vaccine containing GelSite, NV VLPs did not consistently or significantly enhance serum IgG and IgG isotype production relative liquid counterparts without GelSite (Fig. 9). Moreover, the addition of GARD to NV VLPs in the GelVac powder vaccine did not enhance humoral immune responses (Fig. 5); whereas, the addition of GARD to the NV VLPs in the GelSite liquid vaccine enhanced serum IgG (days 21-112; 22-fold), IgG1 (days 42-112; 16-fold), and IgG2a (day 12 and 42-112; 36-fold) production relative to the GelSite NV VLP alone formulation (Fig. 9). These levels reached statistical significance for IgG2a on day 42 (P < 0.01) (Fig. 9).
3.8 Intranasal delivery of NV VLPs in a GelSite liquid formulation elicits an equivalent mucosal immune response compared to a PBS liquid formulation
3.8.1 Gastrointestinal Tract (Salivary, Fecal)
Antigen-specific IgA production was evaluated at other distal mucosal sites of the CMIS. In the gastrointestinal tract, the GelSite NV VLP liquid formulation without adjuvant elicited slightly higher fecal and salivary IgA production relative to the liquid formulation without GelSite, but the differences were not statistically significant (P ≥ 0.05) (Fig. S1A, S2). The addition of GARD to the GelSite NV VLP liquid formulation did not enhance fecal or salivary IgA production relative to the liquid formulation without GelSite or the GelSite liquid formulation without adjuvant (Fig. S1A, S2).
3.8.2 Respiratory Tract (Nasal, Bronchoalveolar)
In the respiratory tract, NV VLPs in the GelSite liquid formulation elicited slightly higher nasal and bronchoalveolar IgA production relative to liquid formulations without GelSite, whereas NV VLPs in the GelSite liquid formulation with GARD elicited equivalent nasal and broncholaveolar IgA production relative to liquid formulations without GelSite (Fig. S2). When comparing the two GelSite–containing liquids, the addition of GARD enhanced nasal IgA production by 13-fold, but did not enhance bronchoalveolar IgA production (Fig. S2).
3.8.3 Reproductive Tract (Vaginal, Uterine)
NV VLPs in the GelSite liquid formulation without adjuvant slightly enhanced vaginal and uterine IgA production relative to liquid formulations without GelSite, but the levels were not statistically significant (Fig. S1B, S2). Surprisingly, the GelSite NV VLP liquid formulation with GARD resulted in lower vaginal IgA (days 12, 21, 56, and 84; 5-fold) and uterine IgA (4-fold) production relative to the liquid formulation without GelSite (Fig. S1B, S2). As a result, the GelSite NV VLP and GARD liquid formulation elicited nearly equivalent vaginal and uterine IgA levels relative to the GelSite NV VLP alone liquid formulation (Fig. S1B, S2).
Our GelSite liquid vaccine studies showed that (in contrast to the NV VLP powder vaccine containing GelSite (Fig. 5, 6, 7)) in most cases, NV VLP liquid vaccines containing GelSite did not significantly enhance systemic or mucosal immune responses relative to liquid formulations without GelSite (Fig. 9, S1, S2). Moreover, the adjuvant effects of the immunopotentiator, GARD, were not observed when delivered in a dry powder formulation with GelSite (Fig. 5, 6, 7), but were observed when delivered in a liquid formulation with GelSite (Fig. 9, S2). Therefore, induction of NV-specific systemic and mucosal immunity was highly affected by the antigen delivery formulation and not by the immunopotentiating properties of GelSite.
4. CONCLUSION
We have shown that intranasal delivery of NV VLPs in a dry powder vaccine containing the inert mucoadhesive polymer, GelSite, induces robust systemic and mucosal immunity in animal models. We have presented evidence for in-situ gelation of the dry powder when it contacts nasal epithelia. We conclude from the studies presented that 1.) The dry powder formulation stabilizes the norovirus VLP antigen, 2.) GelSite does not have immunostimulatory (adjuvant) activity itself, 3.) that the superior immunogenicity of dry powder formulations containing GelSite (GelVac formulation, as compared to a liquid formulation which included GARD as adjuvant) is due to delay in mucociliary clearance. Future work will include the evaluation of multivalent norovirus vaccine dry powder formulations delivered intranasally with this inert mucoadhesive polymer, alternative routes of administration, and optimization of dosing regimens. Ultimately, human NV challenge studies will need to be performed to address the level of protection afforded by this needle-free route of administration and these vaccine formulations.
Supplementary Material
Figure S1. Fecal and vaginal NV-specific IgA production following intranasal immunization with VLPs in a GelSite or PBS liquid. Mouse fecal extracts (A) and vaginal lavages (B) were collected on days 0, 12, 21, 42, 56, 84, and 112 and analyzed for NV VLP-specific IgA content by ELISA. Background levels of antigen-specific IgA were not detected (GMT < 100) in all pre-immune samples except the following: fecal GelSite NV VLP + GARD (2/7 mice); fecal NV VLP alone (1/7 mice); fecal PBS (2/7 mice); and vaginal GelSite NV VLP (5/7 mice) (data not shown). Horizontal dashed line indicates the limit of detection for the assay. Error bars represent the standard errors of the mean. ^P<0.05; *P<0.01 compared to the PBS control group.
Figure S2. Mucosal NV-specific IgA production following intranasal immunization with VLPs in GelSite or PBS liquid. Mice were euthanized on day 112. Salivary, nasal, bronchoalveolar, and uterine lavages were collected and analyzed for NV VLP-specific IgA by ELISA. Error bars represent the standard errors of the mean. Horizontal dashed line indicates the limit of detection for the assay. ^P<0.05 compared to the PBS control group.
ACKNOWLEDGEMENTS
The authors would like to thank Ms. Brooke Hjelm for technical assistance with the sucrose gradient, Mr. Dave Lowry for preparing powder formulations for SEM, and Dr. Stacey White and Ms. Karelle Slater for assistance with cell culture. This work was supported by a grant from the Sexually Transmitted Infections and Topical Microbicides Cooperative Research Center (1 U19 AI062150-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Patel MM, Hall AJ, Vinje J, Parashar UD. Noroviruses: a comprehensive review. J Clin Virol. 2009 Jan;44(1):1–8. doi: 10.1016/j.jcv.2008.10.009. [DOI] [PubMed] [Google Scholar]
- [2].Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinje J, Parashar UD. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis. 2008 Aug;14(8):1224–31. doi: 10.3201/eid1408.071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Glass RI, Parashar UD, Estes MK. Norovirus gastroenteritis. N Engl J Med. 2009 Oct 29;361(18):1776–85. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Velasquez LS, Hjelm BE, Arntzen CJ, Herbst-Kralovetz MM. An Intranasally Delivered Tlr7 Agonist Elicits Robust Systemic and Mucosal Responses to Norwalk Virus-Like Particles. Clin Vaccine Immunol. 2010 Dec.17(12):1850–8. doi: 10.1128/CVI.00230-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Guerrero RA, Ball JM, Krater SS, Pacheco SE, Clements JD, Estes MK. Recombinant Norwalk virus-like particles administered intranasally to mice induce systemic and mucosal (fecal and vaginal) immune responses. J Virol. 2001 Oct;75(20):9713–22. doi: 10.1128/JVI.75.20.9713-9722.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mason HS, Ball JM, Shi JJ, Jiang X, Estes MK, Arntzen CJ. Expression of Norwalk virus capsid protein in transgenic tobacco and potato and its oral immunogenicity in mice. Proc Natl Acad Sci U S A. 1996 May 28;93(11):5335–40. doi: 10.1073/pnas.93.11.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ball JM, Hardy ME, Atmar RL, Conner ME, Estes MK. Oral immunization with recombinant Norwalk virus-like particles induces a systemic and mucosal immune response in mice. J Virol. 1998 Feb;72(2):1345–53. doi: 10.1128/jvi.72.2.1345-1353.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Estes MK, Ball JM, Guerrero RA, Opekun AR, Gilger MA, Pacheco SS, et al. Norwalk virus vaccines: challenges and progress. J Infect Dis. 2000 May;181(Suppl 2):S367–73. doi: 10.1086/315579. [DOI] [PubMed] [Google Scholar]
- [9].Herbst-Kralovetz M, Mason HS, Chen Q. Norwalk virus-like particles as vaccines. Expert Rev Vaccines. 2010 Mar;9(3):299–307. doi: 10.1586/erv.09.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ball JM, Graham DY, Opekun AR, Gilger MA, Guerrero RA, Estes MK. Recombinant Norwalk virus-like particles given orally to volunteers: phase I study. Gastroenterology. 1999 Jul;117(1):40–8. doi: 10.1016/s0016-5085(99)70548-2. [DOI] [PubMed] [Google Scholar]
- [11].Tacket CO, Mason HS, Losonsky G, Estes MK, Levine MM, Arntzen CJ. Human immune responses to a novel norwalk virus vaccine delivered in transgenic potatoes. J Infect Dis. 2000 Jul;182(1):302–5. doi: 10.1086/315653. [DOI] [PubMed] [Google Scholar]
- [12].El-Kamary SS, Pasetti MF, Mendelman PM, Frey SE, Bernstein DI, Treanor JJ, et al. Adjuvanted intranasal norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J Infect Dis. 2010 Dec 1;202(11):1649–58. doi: 10.1086/657087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Green KY, Lew JF, Jiang X, Kapikian AZ, Estes MK. Comparison of the reactivities of baculovirus-expressed recombinant Norwalk virus capsid antigen with those of the native Norwalk virus antigen in serologic assays and some epidemiologic observations. J Clin Microbiol. 1993 Aug;31(8):2185–91. doi: 10.1128/jcm.31.8.2185-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Reeck A, Kavanagh O, Estes MK, Opekun AR, Gilger MA, Graham DY, et al. Serological correlate of protection against norovirus-induced gastroenteritis. J Infect Dis. 2010 Oct 15;202(8):1212–8. doi: 10.1086/656364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].van Ginkel FW, Nguyen HH, McGhee JR. Vaccines for mucosal immunity to combat emerging infectious diseases. Emerg Infect Dis. 2000 Mar-Apr;6(2):123–32. doi: 10.3201/eid0602.000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005 Apr;11(4 Suppl):S45–53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- [17].Holmgren J, Harandi AM, Czerkinsky C. Mucosal adjuvants and anti-infection and anti-immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Expert Rev Vaccines. 2003 Apr;2(2):205–17. doi: 10.1586/14760584.2.2.205. [DOI] [PubMed] [Google Scholar]
- [18].Yuki Y, Kiyono H. Mucosal vaccines: novel advances in technology and delivery. Expert Rev Vaccines. 2009 Aug;8(8):1083–97. doi: 10.1586/erv.09.61. [DOI] [PubMed] [Google Scholar]
- [19].Chadwick S, Kriegel C, Amiji M. Delivery strategies to enhance mucosal vaccination. Expert Opin Biol Ther. 2009 Apr;9(4):427–40. doi: 10.1517/14712590902849224. [DOI] [PubMed] [Google Scholar]
- [20].Kuolee R, Chen W. M cell-targeted delivery of vaccines and therapeutics. Expert Opin Drug Deliv. 2008 Jun;5(6):693–702. doi: 10.1517/17425247.5.6.693. [DOI] [PubMed] [Google Scholar]
- [21].Graham BS, Kines RC, Corbett KS, Nicewonger J, Johnson TR, Chen M, et al. Mucosal delivery of human papillomavirus pseudovirus-encapsidated plasmids improves the potency of DNA vaccination. Mucosal Immunol. Jun 16; doi: 10.1038/mi.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Inskeep TK, Stahl C, Odle J, Oakes J, Hudson L, Bost KL, et al. Oral vaccine formulations stimulate mucosal and systemic antibody responses against staphylococcal enterotoxin B using a piglet model. Clin Vaccine Immunol. Jun 16; doi: 10.1128/CVI.00078-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fraillery D, Zosso N, Nardelli-Haefliger D. Rectal and vaginal immunization of mice with human papillomavirus L1 virus-like particles. Vaccine. 2009 Apr 14;27(17):2326–34. doi: 10.1016/j.vaccine.2009.02.029. [DOI] [PubMed] [Google Scholar]
- [24].Noda K, Kodama S, Umemoto S, Abe N, Hirano T, Suzuki M. Nasal vaccination with P6 outer membrane protein and alpha-galactosylceramide induces nontypeable Haemophilus influenzae-specific protective immunity associated with NKT cell activation and dendritic cell expansion in nasopharynx. Vaccine. May 14; doi: 10.1016/j.vaccine.2010.05.005. [DOI] [PubMed] [Google Scholar]
- [25].Hefferon KL. The Mucosal Immune Response to Plant-Derived Vaccines. Pharm Res. May 14; doi: 10.1007/s11095-010-0168-9. [DOI] [PubMed] [Google Scholar]
- [26].Lanza SR, Menin A, Ertl HC, Bafica A, Pinto AR. Simian recombinant adenovirus delivered by the mucosal route modulates gammadelta T cells from murine genital tract. Vaccine. Jun 23;28(29):4600–8. doi: 10.1016/j.vaccine.2010.04.080. [DOI] [PubMed] [Google Scholar]
- [27].Huang J, Garmise RJ, Crowder TM, Mar K, Hwang CR, Hickey AJ, et al. A novel dry powder influenza vaccine and intranasal delivery technology: induction of systemic and mucosal immune responses in rats. Vaccine. 2004 Dec 21;23(6):794–801. doi: 10.1016/j.vaccine.2004.06.049. [DOI] [PubMed] [Google Scholar]
- [28].Garmise RJ, Staats HF, Hickey AJ. Novel dry powder preparations of whole inactivated influenza virus for nasal vaccination. AAPS PharmSciTech. 2007;8(4):E81. doi: 10.1208/pt0804081. [DOI] [PubMed] [Google Scholar]
- [29].Turker S, Onur E, Ozer Y. Nasal route and drug delivery systems. Pharm World Sci. 2004 Jun;26(3):137–42. doi: 10.1023/b:phar.0000026823.82950.ff. [DOI] [PubMed] [Google Scholar]
- [30].Jiang L, Gao L, Wang X, Tang L, Ma J. The application of mucoadhesive polymers in nasal drug delivery. Drug Dev Ind Pharm. 2010 Mar;36(3):323–36. doi: 10.1080/03639040903170750. [DOI] [PubMed] [Google Scholar]
- [31].Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987 Jul;7(4):265–76. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- [32].Hickey AJ, Garmise RJ. Dry powder nasal vaccines as an alternative to needle-based delivery. Crit Rev Ther Drug Carrier Syst. 2009;26(1):1–27. doi: 10.1615/critrevtherdrugcarriersyst.v26.i1.10. [DOI] [PubMed] [Google Scholar]
- [33].Treanor JJ, Kotloff K, Betts RF, Belshe R, Newman F, Iacuzio D, et al. Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses. Vaccine. 1999 Dec 10;18(9-10):899–906. doi: 10.1016/s0264-410x(99)00334-5. [DOI] [PubMed] [Google Scholar]
- [34].Nichol KL, Mendelman PM, Mallon KP, Jackson LA, Gorse GJ, Belshe RB, et al. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial. JAMA. 1999 Jul 14;282(2):137–44. doi: 10.1001/jama.282.2.137. [DOI] [PubMed] [Google Scholar]
- [35].Belshe RB, Mendelman PM, Treanor J, King J, Gruber WC, Piedra P, et al. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N Engl J Med. 1998 May 14;338(20):1405–12. doi: 10.1056/NEJM199805143382002. [DOI] [PubMed] [Google Scholar]
- [36].Davis SS. Nasal vaccines. Adv Drug Deliv Rev. 2001 Sep 23;51(1-3):21–42. doi: 10.1016/s0169-409x(01)00162-4. [DOI] [PubMed] [Google Scholar]
- [37].Buonaguro L, Visciano ML, Tornesello ML, Tagliamonte M, Biryahwaho B, Buonaguro FM. Induction of systemic and mucosal cross-clade neutralizing antibodies in BALB/c mice immunized with human immunodeficiency virus type 1 clade A virus-like particles administered by different routes of inoculation. J Virol. 2005 Jun;79(11):7059–67. doi: 10.1128/JVI.79.11.7059-7067.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Balmelli C, Roden R, Potts A, Schiller J, De Grandi P, Nardelli-Haefliger D. Nasal immunization of mice with human papillomavirus type 16 virus-like particles elicits neutralizing antibodies in mucosal secretions. J Virol. 1998 Oct;72(10):8220–9. doi: 10.1128/jvi.72.10.8220-8229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nardelli-Haefliger D, Roden R, Balmelli C, Potts A, Schiller J, De Grandi P. Mucosal but not parenteral immunization with purified human papillomavirus type 16 virus-like particles induces neutralizing titers of antibodies throughout the estrous cycle of mice. J Virol. 1999 Nov;73(11):9609–13. doi: 10.1128/jvi.73.11.9609-9613.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Canessa C, Vierucci S, Azzari C, Vierucci A. The immunity of upper airways. Int J Immunopathol Pharmacol. 2010 Jan-Mar;23(1 Suppl):8–12. [PubMed] [Google Scholar]
- [41].Garg NK, Mangal S, Khambete H, Sharma PK, Tyagi RK. Mucosal delivery of vaccines: role of mucoadhesive/biodegradable polymers. Recent Pat Drug Deliv Formul. Jun 1;4(2):114–28. doi: 10.2174/187221110791185015. [DOI] [PubMed] [Google Scholar]
- [42].Patil SB, Sawant KK. Mucoadhesive microspheres: a promising tool in drug delivery. Curr Drug Deliv. 2008 Oct;5(4):312–8. doi: 10.2174/156720108785914970. [DOI] [PubMed] [Google Scholar]
- [43].Pawar D, Goyal AK, Mangal S, Mishra N, Vaidya B, Tiwari S, et al. Evaluation of mucoadhesive PLGA microparticles for nasal immunization. AAPS J. Jun;12(2):130–7. doi: 10.1208/s12248-009-9169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jiang L, Gao L, Wang X, Tang L, Ma J. The application of mucoadhesive polymers in nasal drug delivery. Drug Dev Ind Pharm. Mar;36(3):323–36. doi: 10.1080/03639040903170750. [DOI] [PubMed] [Google Scholar]
- [45].Ni YaY KM. inventor In Situ Gel Formation of Pectin. 6,777,000 B2 USA patent. 2004
- [46].Huang Z, Elkin G, Maloney BJ, Beuhner N, Arntzen CJ, Thanavala Y, et al. Virus-like particle expression and assembly in plants: hepatitis B and Norwalk viruses. Vaccine. 2005 Mar 7;23(15):1851–8. doi: 10.1016/j.vaccine.2004.11.017. [DOI] [PubMed] [Google Scholar]
- [47].Santi L, Batchelor L, Huang Z, Hjelm B, Kilbourne J, Arntzen CJ, et al. An efficient plant viral expression system generating orally immunogenic Norwalk virus-like particles. Vaccine. 2008 Mar 28;26(15):1846–54. doi: 10.1016/j.vaccine.2008.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lorin MI, Gaerlan PF, Mandel ID. Quantitative composition of nasal secretions in normal subjects. J Lab Clin Med. 1972 Aug;80(2):275–81. [PubMed] [Google Scholar]
- [49].Saluja V, Amorij JP, Kapteyn JC, de Boer AH, Frijlink HW, Hinrichs WL. A comparison between spray drying and spray freeze drying to produce an influenza subunit vaccine powder for inhalation. J Control Release. 2010 Jun 1;144(2):127–33. doi: 10.1016/j.jconrel.2010.02.025. [DOI] [PubMed] [Google Scholar]
- [50].Rutland J, Cole PJ. Nasal mucociliary clearance and ciliary beat frequency in cystic fibrosis compared with sinusitis and bronchiectasis. Thorax. 1981 Sep;36(9):654–8. doi: 10.1136/thx.36.9.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pandey RS, Babbar AK, Kaul A, Mishra AK, Dixit VK. Evaluation of ISCOM matrices clearance from rabbit nasal cavity by gamma scintigraphy. Int J Pharm. 2010 Oct 15;398(1-2):231–6. doi: 10.1016/j.ijpharm.2010.07.051. [DOI] [PubMed] [Google Scholar]
- [52].Ni YaY KM. inventor Delivery of physiological agents with in-situ gels comprising anionic polysaccharides. 7,494,669 USA patent. 2009
- [53].Alpar HO, Somavarapu S, Atuah KN, Bramwell VW. Biodegradable mucoadhesive particulates for nasal and pulmonary antigen and DNA delivery. Adv Drug Deliv Rev. 2005 Jan 10;57(3):411–30. doi: 10.1016/j.addr.2004.09.004. [DOI] [PubMed] [Google Scholar]
- [54].Bernstein DI, Harrison CJ, Tomai MA, Miller RL. Daily or weekly therapy with resiquimod (R-848) reduces genital recurrences in herpes simplex virus-infected guinea pigs during and after treatment. J Infect Dis. 2001 Mar 15;183(6):844–9. doi: 10.1086/319262. [DOI] [PubMed] [Google Scholar]
- [55].Bernstein DI, Harrison CJ, Tepe ER, Shahwan A, Miller RL. Effect of imiquimod as an adjuvant for immunotherapy of genital HSV in guinea-pigs. Vaccine. 1995 Jan;13(1):72–6. doi: 10.1016/0264-410x(95)80014-5. [DOI] [PubMed] [Google Scholar]
- [56].Wimer-Mackin S, Hinchcliffe M, Petrie CR, Warwood SJ, Tino WT, Williams MS, et al. An intranasal vaccine targeting both the Bacillus anthracis toxin and bacterium provides protection against aerosol spore challenge in rabbits. Vaccine. 2006 May 1;24(18):3953–63. doi: 10.1016/j.vaccine.2006.02.024. [DOI] [PubMed] [Google Scholar]
- [57].Tafaghodi M, Rastegar S. Preparation and in vivo study of dry powder microspheres for nasal immunization. J Drug Target. 2010 Apr;18(3):235–42. doi: 10.3109/10611860903434035. [DOI] [PubMed] [Google Scholar]
- [58].Baldwin SL, Bertholet S, Kahn M, Zharkikh I, Ireton GC, Vedvick TS, et al. Intradermal immunization improves protective efficacy of a novel TB vaccine candidate. Vaccine. 2009 May 18;27(23):3063–71. doi: 10.1016/j.vaccine.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ma Y, Poisson L, Sanchez-Schmitz G, Pawar S, Qu C, Randolph GJ, et al. Assessing the immunopotency of Toll-like receptor agonists in an in vitro tissue-engineered immunological model. Immunology. 2010 Jul;130(3):374–87. doi: 10.1111/j.1365-2567.2009.03237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Fecal and vaginal NV-specific IgA production following intranasal immunization with VLPs in a GelSite or PBS liquid. Mouse fecal extracts (A) and vaginal lavages (B) were collected on days 0, 12, 21, 42, 56, 84, and 112 and analyzed for NV VLP-specific IgA content by ELISA. Background levels of antigen-specific IgA were not detected (GMT < 100) in all pre-immune samples except the following: fecal GelSite NV VLP + GARD (2/7 mice); fecal NV VLP alone (1/7 mice); fecal PBS (2/7 mice); and vaginal GelSite NV VLP (5/7 mice) (data not shown). Horizontal dashed line indicates the limit of detection for the assay. Error bars represent the standard errors of the mean. ^P<0.05; *P<0.01 compared to the PBS control group.
Figure S2. Mucosal NV-specific IgA production following intranasal immunization with VLPs in GelSite or PBS liquid. Mice were euthanized on day 112. Salivary, nasal, bronchoalveolar, and uterine lavages were collected and analyzed for NV VLP-specific IgA by ELISA. Error bars represent the standard errors of the mean. Horizontal dashed line indicates the limit of detection for the assay. ^P<0.05 compared to the PBS control group.