1. Introduction
Opioid prescriptions have increased markedly in recent years, driven by a greater reliance on the potent analgesics for the treatment of chronic pain [18, 34]. Opioid analgesics are second only to lipid regulators as the most dispensed drug class in the United States [28]. Hydrocodone, a moderately-powerful opioid, was dispensed in the United States more than any other drug in 2008 – over 128 million times [27]. As opioid use rises, it becomes increasingly important that we better understand the neurological and behavioral effects of those drugs – especially given the known risks of opioid dependence, addiction, cognitive impairment, and hyperalgesia [7, 13, 14, 29, 43, 44].
Recently, Upadhyay and colleagues [55] demonstrated that prescription opioid-dependent patients evidenced a specific morphologic abnormality in the neural reward-processing network. In their cross-sectional study of 10 dependent individuals and 10 age-matched controls, the researchers found decreased gray matter volume in the bilateral amygdala. The amygdala is a key reward-modulating structure that is known to underlie opioid-related addiction, dependence, and tolerance [23, 26, 33, 36]. The results of the Upadhyay et al., paper adds to existing animal literature showing that opioid exposure has a broad range of effects on the amygdala, including decreased mu-opioid receptor sensitivity [40], modulated GABAA receptor functioning [58], and modified glutamate receptor targeting [24].
Despite interesting preliminary evidence suggesting opioid-induced brain changes in humans, significant gaps in the literature remain. First, existing cross-sectional studies do not allow us to say with certainty that the observed brain abnormalities are a direct consequence of opioid administration (as opposed to a neural predisposition for opioid dependency). Second, current human data are based on opioid-dependent individuals, and may not accurately reflect cases in which the opioids are legitimately prescribed for chronic pain, and taken exactly as prescribed. To fill those gaps in the scientific literature, we conducted the first longitudinal study of prescription opioid effects on the human brain. The primary purpose of this magnetic resonance imaging (MRI) pilot study was to determine if any measureable changes in human brain morphology occur after a short period of daily opioid administration. Ten individuals with chronic low-back pain completed structural scans of their brain both before and after one month of daily morphine analgesic therapy. Regional areas of morphine-associated, gray matter volume decrease and increase were assessed. The same scanning procedure was also completed on nine low-back pain patients receiving a blinded placebo substance; in order to determine if any morphologic changes were not specific to opioid administration.
2. Materials and methods
2.1. Morphine group participants (Table 1)
TABLE 1. Participant demographics.
All participants were low back pain patients with no radicular symptoms.
| Group | Age | Gender | Pain Duration (years) |
Pain Severity (0 – 100 VAS) |
Weight (kg) |
Total Morphine (mg) |
Pain Relief (%) |
Concomitant Medications |
|---|---|---|---|---|---|---|---|---|
| morphine | 59 | female | 7 | 3.25 | 113 | 2880 | 76.9 | aspirin |
| morphine | 56 | male | 10 | 4.75 | 79 | 3120 | 47.4 | ibuprofen, acetaminophen/aspirin |
| morphine | 27 | male | 4 | 4.25 | 102 | 3000 | 88.2 | ibuprofen |
| morphine | 52 | male | 10 | 2.75 | 111 | 3120 | 36.4 | none |
| morphine | 55 | female | 4 | 6.50 | 54 | 1485 | 19.2 | ibuprofen |
| morphine | 53 | female | 4 | 4.00 | 77 | 2865 | 50.0 | piroxicam |
| morphine | 58 | female | 2 | 6.00 | 88 | 1725 | 58.3 | acetaminophen |
| morphine | 39 | male | 25 | 4.50 | 124 | 2775 | 38.9 | none |
| morphine | 34 | female | 6 | 2.50 | 68 | 570 | −10.0 | ibuprofen |
| morphine | 48 | female | 10 | 6.25 | 90 | 165 | 16.0 | none |
| placebo | 26 | male | 2 | 3.25 | 59 | N/A | −15.38 | acetaminophen |
| placebo | 27 | male | 5 | 4.25 | 77 | N/A | 17.65 | acetaminophen |
| placebo | 44 | male | 3 | 6.25 | 81 | N/A | 64.00 | acetaminophen |
| placebo | 24 | male | 4 | 4.75 | 79 | N/A | −15.79 | glucosamine |
| placebo | 24 | male | 5.5 | 4.50 | 73 | N/A | −38.89 | none |
| placebo | 46 | male | 5 | 6.25 | 70 | N/A | 72.00 | none |
| placebo | 38 | male | 4.5 | 7.00 | 66 | N/A | 25.00 | loratadine |
| placebo | 23 | male | 2 | 5.25 | 68 | N/A | 52.38 | none |
| placebo | 18 | male | 5.5 | 6.25 | 68 | N/A | 28.00 | none |
Participants were 10 individuals (4 men and 6 women; mean age = 47, SD = 11) with chronic, moderate-to-severe, non-radicular, low-back pain (mean years with pain = 8.5, SD = 6.5). Individuals who had not responded adequately to non-opioid treatments were chosen for participation in the study. Enrollment criteria were the same as used in a previous study [14]. Participants were excluded if they had: a history of substance abuse, previous use of opioids, an unstable psychiatric condition, any evidence of neuropathic pain, or a prescription for neuropathic pain medication. Participants were allowed to continue taking over-the-counter analgesics throughout their participation in the study. Study procedures were approved by the Institutional Review Board at the Stanford University School of Medicine, and all participants provided signed informed consent in-person.
2.2. Opioid administration
The overall design of the project was as follows: Participants first attended a baseline (pre-morphine) scanning session. Following the scan session, participants began a morphine titration procedure described previously [14]. A sustained-acting, oral formulation of morphine was used (MS-Contin; Purdue Frederick, Stamford, Conn). The initial dosage for all participants was 15 mg, two times per day. Every two days, the daily dose was increased by 15 mg. Titration was stopped when: 1) adequate analgesia had been achieved, 2) side-effects limited further dosage increases, or 3) a ceiling of 120 mg/day was reached. For ethical and medical safety reasons, dosage was not randomly assigned. Participants remained at their maximum daily dosage until one month following their first dosing. Total morphine consumed over the month for the 10 participants ranged from 165 – 3120 mg (mean = 2170 mg). Participants then returned for a second high-resolution brain scan (time between scans was six weeks).
After an average of 4.7 months (SD = 1.5) following completion of the study, all participants in the morphine group returned for a third scan. The purpose of the third scan was to determine if any gray matter changes observed in the main study reverted back to pre-morphine values when opioid administration was ceased. All scanning parameters, preprocessing, and statistics were performed as previously described.
2.3. Pain measure
In order to determine whether or not the morphine dosage was sufficient to control pain, the Brief Pain Inventory (BPI; [15]) was administered at all scanning sessions. The pain intensity subscale of the BPI was used a measure of disease severity. The pain intensity score was calculated by taking a mean of four items (average, least and worst pain over the last 24 hours, as well as present pain), scored on a ten-point numerical rating scale.
2.4. Image acquisition
MRI data were collected using a GE Medical Systems 3.0 Tesla system with an 8-channel brain receiving coil. The protocol used a fast 3D-SPGR sequence in the axial plane with the following parameters: repetition time = 10.2 ms, echo time = minimum, flip angle = 11°, 124 contiguous slices of 1.2 mm each, field of view of 220 × 220 cm, and matrix size of 256 × 256 voxels. Raw data were acquired in 1.2 mm isotropic voxels.
2.5. Tensor-based morphometry
Brain images were processed using a tensor-based morphometry (TBM) technique described in previous studies [11]. The TBM approach is particularly well-suited for assessing within-person structural brain changes in longitudinal study designs. All processing was performed by SPM8 software (Wellcome Department of Imaging Neuroscience, London), in a Matlab (MathWorks, Natick, MA) environment. High-resolution image pairs (pre- and post-morphine) for each participant were first rigidly coregistered. The image pairs were then matched precisely using high-dimensional deformation fields [5], yielding a Jacobian determinant (difference) map for each pre-post pair. Pre scans were then segmented into gray and white matter, and processed with Diffeomorphic Anatomical Registration using Exponentiated Lie Algebra (DARTEL; [4]) to create a spatially-normalized group template. The DARTEL template and resulting participant-specific flow fields were applied to the Jacobian deterimant maps, which were subsequently re-sampled into 1 mm isotropic voxel size, smoothed with an 8 mm FWHM Gaussian isotropic kernel, and registered into common Montreal Neurologic Institute (MNI) stereotactic space.
2.6. Placebo study
In a separate experiment, nine male, low-back patients taking (blinded) placebo capsules daily underwent the same experimental procedure. Participants in the placebo group (Table 1) met the same inclusion and exclusion criteria as the morphine group. Participants were scanned once before placebo administration, and once after. Individuals receiving placebo were scanned at the same interval as morphine participants (6 weeks between scans), and received placebo capsules for the entire 6-week period. The mean age of the control participants was 30 years (SD = 10.0). Data were collected on a GE Medical Systems 3.0 Tesla system with an 8-channel brain and neurovascular receiving coil. Because the placebo group used a different scanner and coil than the morphine group, we contrasted both signal-to-noise ratio, and gray-to-noise contrast. The morphine and placebo group setups demonstrated comparable signal to noise (27.6, SD = 9.7 versus 28.2, SD = 14.5; t(16) = 0.11, p = 0.915) and gray-to-noise contrast (7.1:1, SD = 1.1 versus 6.9:1, SD = 1.8; t(16) = 0.278, p = 0.785). The protocol used the same sequence as with the opioid patients, with the exception that the slice thickness was 1.5 mm (instead of 1.2 mm). All data were processed by the same experimenter, and used the same normalization techniques and statistical thresholds. Individuals in the placebo group did not complete a followup scan, because they were enrolled in a separate drug trial.
2.7. Statistical analyses
To identify regional areas of opioid-induced gray matter volumetric change, we conducted whole-brain analyses on all morphine participants, using a mixed-effects model. To control for multiple comparisons, the resulting contrast map of volume change was first thresholded at a voxel-level (height) false-discovery rate (FDR) of 0.01 (equivalent to uncorrected t = 4.78, p < 0.0005). Power to detect main effects was very high (alpha > 99%) for small effects (Cohen’s d = 0.3), due to the high repeated-measures correlation in the voxel data (r = 0.97; assessed between sessions 2 and 3). The high correlation was likely due to: 1) the inherently stable nature of the adult brain over short periods of time, and 2) our use of both high-dimensional within-subject coregistration (HDW) and between-subject normalization (DARTEL). Surviving voxels were then thresholded with an additional, cluster-level extent FDR of 0.05 (equivalent to 49 adjacent voxels).
To determine the relationship of volume change to morphine administration, clusters evidencing significant morphologic change between scans were then tested (two-tailed) for correlation with morphine consumption, using Spearman’s rho (rs) to account for the small sample size and non-normal distribution of the morphine dosage variable. To estimate the volume, all voxels in the region of interest (with values representing the probability of that voxel being gray matter) were summed, and multiplied by the voxel volume (1mm3), providing volume in microliters. Percent change in volume was then calculated for each ROI, by individual. The percent change values were used in correlational analyses with morphine dosage. Correlation with morphine dosage was tested only on those brain regions independently showing significant volumetric change; a whole-brain correlation with dosage was not performed. An additional FDR correction was employed to correct for the number of correlational tests conducted.
Following the procedure described previously, main effects for time were calculated also for the placebo group. Then, to further test whether morphologic changes observed in the morphine group diverged from the placebo group, interaction analyses (time * group) were performed on all clusters showing a significant main effect over time. The same thresholding procedures were used (p < 0.0005) for the interaction analyses. Power was high (alpha > 99%) to detect small (d = 0.3) interaction effects.
3. Results
3.1. Behavioral
On the first scan session day (pre-morphine), mean pain severity in the morphine group was 4.5 (SD = 1.4), indicating a moderate degree of pain on the 0 – 10 range severity scale. At the end of the month of morphine administration, mean pain severity was rated as 2.6 (SD = 1.6), a low intensity level. Pain was reduced on average by 42.1% (SD = 29.2%) from baseline levels. A paired t-test revealed that pain intensity was significantly reduced at the end of the morphine period (t(9) = 4.9, p = 0.001). The amount of morphine administered was not significantly correlated with change in pain severity (rs = 0.53, p = 0.116).
The placebo group was significantly younger than the morphine group (t (17) = −3.64, p = 0.002). The placebo group had a mean baseline pain intensity level of 5.3 (SD = 1.2), and there was no significant difference in baseline pain intensity levels between the morphine and placebo groups (t(17) = 1.3, p = 0.192). In the placebo group, pain intensity after treatment was rated as 3.9 (SD = 1.2), with an average drop of 21.0% (SD = 38.3%). A paired t-test showed that pain intensity was not significantly reduced at the end of the placebo period (t(8) = 1.9, p = 0.089).
3.2. Neuroimaging main effects (Table 2a)
TABLE 2. Regions showing significant gray matter increase or decrease after one month of daily morphine administration.
All listed regions demonstrated volume change that survived a height-level threshold corrected with the false discovery rate (FDR) of 0.01 (uncorrected p < 0.0005), and cluster threshold FDR of 0.05 (contiguous voxels > 49). (a) Regions showing a main effect change over time in the morphine group are listed first, followed by the MNI coordinates of the peak voxel, t-value of the peak voxel, the cluster FDR significance level, range of change with mean, and correlation with morphine dosage. Volume changes significantly associated with morphine dosage are in bold. Associations with morphine that met an additional FDR correction to control for the number of correlation tests (critical p = 0.015) have an additional asterisk. (b) All regions showing a main effect in the morphine group were then tested for a significant interaction effect with the placebo group (same height and cluster-level thresholds used). The interaction is first labeled as significant or non-significant, followed by the t-score and p-value of the interaction.
| Main effects (morphine group) |
Interaction (morphine versus placebo) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Region | MNI Coordinates | t-value | FDR q | % change range (mean) |
rs (p) with dosage |
Significant? | t-score | p-value |
| Atrophy | ||||||||
| Bilateral rostral pons | −3, −22, −24 +5, −20, −24 |
9.00 8.62 |
<0.005 | 0.6 – 3.2% (2.2%) | −0.40 (0.309) | no | 3.41 | 0.00167 (n.s.) |
| R amygdala | +20, −5, −19 | 8.73 | <0.005 | 0.0 – 4.0% (2.4%) | 0.75 (0.012)* | yes | 5.58 | 0.00002 |
| L medial orbital gyrus | −14, +15, −16 | 7.33 | 0.010 | 0.9 – 3.7% (2.6%) | 0.25 (0.476) | yes | 4.68 | 0.00011 |
| R hippocampus | +26, −22, −21 | 6.34 | 0.040 | 0.3 – 2.8% (1.7%) | 0.60 (0.066) | no | 2.01 | 0.03028 (n.s.) |
| Hypertrophy | ||||||||
| L inferior frontal gyrus | −44, +15, 0 | 11.76 | 0.004 | 0.7 – 3.6% (2.5%) | 0.80 (0.006)* | no | 3.50 | 0.00137 (n.s.) |
| L dorsal posterior cingulate and BA 5 | −8, −43, +52 | 10.85 | 0.014 | 0.4 – 2.3% (1.5%) | 0.09 (0.802) | no | 2.62 | 0.00896 (n.s.) |
| R hypothalamus | +1, −4, −16 | 9.86 | 0.018 | 0.6 – 5.0% (3.1%) | 0.66 (0.037) | yes | 4.81 | 0.00008 |
| Bilateral mid cingulate | 0, +11, +24 | 9.68 | <0.005 | 0.9 – 4.9% (3.4%) | −0.29 (0.413) | yes | 4.98 | 0.00006 |
| L inferior frontal gyrus | −58, +11, +2 | 8.97 | <0.005 | 1.4 – 5.3% (3.5%) | 0.87 (0.001)* | yes | 4.75 | 0.00009 |
| R ventral posterior cingulate (BA 31) | +18, −31, +39 | 8.78 | 0.012 | 0.9 – 3.7% (2.6%) | 0.63 (0.050) | no | 3.38 | 0.00178 (n.s.) |
| L ventral posterior cingulate (BA 23) | −3, −30, +26 | 8.50 | 0.002 | 0.8 – 3.2% (2.1%) | 0.27 (0.444) | yes | 4.35 | 0.00020 |
| R caudal pons | +12, −30, −37 | 7.68 | <0.005 | 0.6 – 8.2% (4.1%) | 0.75 (0.012)* | yes | 5.77 | 0.00001 |
| L dorsal anterior cingulate (BA 32) | −1, +39, −3 | 7.16 | <0.005 | 0.7 – 5.0% (3.0%) | −0.32 (0.354) | yes | 4.83 | 0.00008 |
A total of 13 structures survived the voxel- and cluster-level thresholds, demonstrating significant regional volumetric gain or loss over the one-month study period. Volumetric change in six of those regions was also significantly correlated with morphine dosage (i.e., higher morphine consumption lead to greater change).
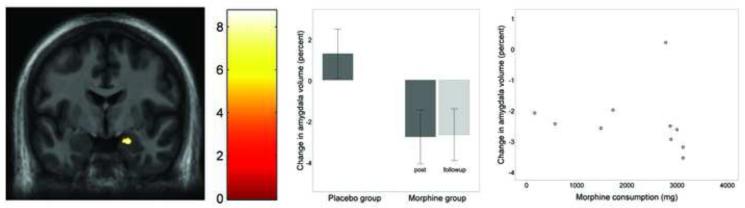
One region showed gray matter decrease that was significantly correlated with morphine dosage. That region encompassed the medial aspect of the right amygdala (Figure 1a & 1b), approximately in the medial, basomedial, and ventral (cortical) nuclei [1]. Individuals consuming the greatest amount of morphine experienced the greatest gray matter loss in the amygdala (Figure 1c).
Figure 1.
Gray matter volume decrease in the amygdala following one month of daily morphine exposure. (a) Coronal view (z = −19) of volume decrease in the lateral aspect of the right amygdala (average volume decrease = 2.4%). Image presented is in neurological convention, and thresholded at voxel-level FDR of p < 0.01 and cluster-level FDR p < 0.05. (b) Bar graph showing percent volumetric change from baseline in the amygdala for the placebo group (left bar) and morphine group (right bars). Dark gray bars indicate the post-medication period, and light gray indicates the followup period. Error bars represent 95% confidence intervals. Amygdala volume is significantly decreased after morphine exposure, and the decreased volume is maintained 4.7 months later at the followup period. The placebo group shows no change in amygdala volume. (c) Post-hoc scatterplot showing relationship between total morphine consumed (x axis) and percent change in right amygdala volume (y axis) in the morphine group. One outlier showed no amygdala change over time.
Three additional regions demonstrated significant volumetric decrease that was not dosage-correlated. The regions included the right hippocampus, bilateral rostroventral pons, and right medial orbital gyrus of the orbitofrontal cortex. The pons area was located just inferior to the pons-midbrain junction, approximately in the area of corticospinal fibers. The reduced medial orbital gyrus volume encompassed the dorsomedial aspect of the medial orbital gyrus, immediately superior and lateral to the olfactory sulcus.
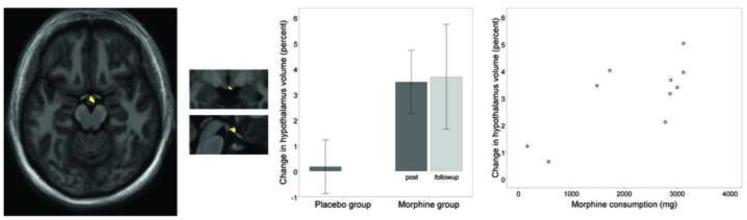
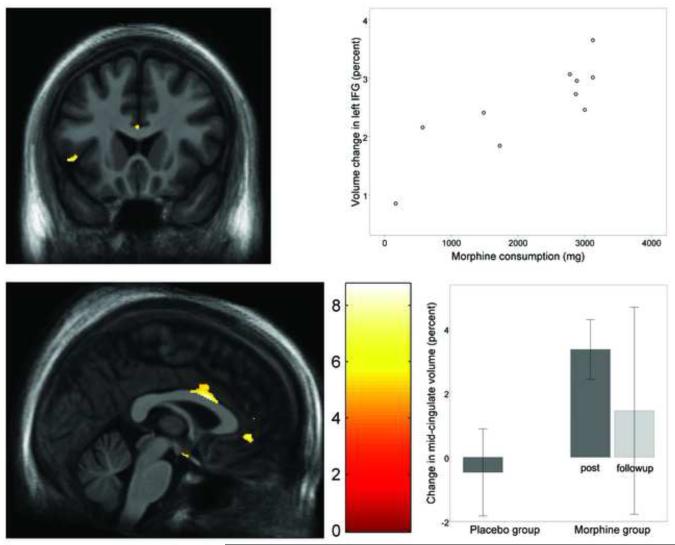
Several regions showed significant gray matter increase that was associated with morphine consumption. The regions included the right hypothalamus (Figure 2), left pregenual anterior cingulate (Figure 3c), right ventral posterior cingulate, right ventral caudal pons (at the level of the facial genu), and left inferior frontal gyrus (Figure 3a & 3b). In all those regions, individuals consuming the greatest amount of morphine over one month showed the greatest increase in gray matter volume. Additional regions showing gray matter increase that was not correlated with morphine dosage included the bilateral mid-cingulate (Figure 3c & 3d), left ventral posterior cingulate, and a cluster running dorsally from the left posterior cingulate cortex, to the Brodmann Area (BA) 5 region of the left parietal lobe.
Figure 2.
Gray matter volume increase in the hypothalamus following one month of daily morphine exposure. (a) Sagittal view (x = +1) of volume increase in the caudal aspect of the hypothalamus (average volume increase = 3.1%). Image is thresholded at voxel-level FDR of p < 0.01 and cluster-level FDR p < 0.05. (b) Same hypothalamus cluster presented in coronal plane and (c) in sagittal plane. (d) Bar graph showing percent volumetric change from baseline in the hypothalamus for the placebo group (left bar) and morphine group (right bars). Dark gray bars indicate the post-medication period, and light gray indicates the followup period. Error bars represent 95% confidence intervals. Hypothalamus volume is significantly increased after morphine exposure, and the increased volume is maintained 4.7 months later at the followup period. The placebo group shows no significant change in hypothalamus volume. (e) Post-hoc scatterplot showing relationship between total morphine consumed (x axis) and percent change of hypothalamic volume (y axis) in the morphine group.
Figure 3.
Gray matter volume increase in the inferior frontal gyrus, mid-cingulate, and pregenual anterior cingulate following one month of daily morphine exposure. (a) Coronal view (z = 0) of volume increase in the inferior frontal gyrus, bordering the slyvian fissure (average volume increase = 3.5%). Image is thresholded at voxel -level FDR of p < 0.01 and cluster-level FDR p < 0.05. (b) Post-hoc scatterplot showing relationship between total morphine consumed (x axis) and percent change in the inferior frontal gyrus cluster (y axis) for the morphine group. (c) Axial view of volumetric increase in the mid-cingulate and dorsal anterior cingulate (average volume increase = 3.4% and 3.0%, respectively). (d) Boxplot showing percent volumetric change from baseline in the mid-cingulate for the placebo group (left bar) and morphine group (right bars). Error bars represent the 95% confidence interval. The morphine group showed significant volumetric increase post-morphine, but volumetric change was variable at 4.7 months after morphine exposure. The placebo group showed no significant volumetric change in the mid-cingulate.
In the placebo group, TBM results revealed no areas of significant volumetric change. No main effects for placebo over time were found on brain morphology.
3.3. Neuroimaging interaction effects (Table 2b)
All regions showing a significant main effect for time in the morphine group were then tested for a significant interaction with the placebo group. The additional analyses determined whether or not morphine induced structural changes are separable from changes observed during placebo consumption. As seen in Table 2b, several regional changes in the morphine group separated significantly from the placebo group: the amygdala, medial orbital gyrus, hypothalamus, mid-cingulate, inferior frontal gyrus, ventral posterior cingulate, caudal pons, and dorsal posterior cingulate.
3.4. Followup study results
An average of 4.7 months (SD = 1.5) following the second scan period, all morphine participants returned for a third scan. One participant had to be scanned 1 month following the second scan. All other participants were scanned between 3.8 and 6.1 months following the second scan. Time between scans was not a significant predictor of morphologic change (no clusters surviving p = 0.0005 threshold). All participants reported abstaining from opioids after the study morphine tapering. Whole-brain, pair-wise contrasts between the second and third scans revealed no significant changes in regional gray matter volume. An additional, region-of-interest analysis (restricted to those regions previously showing morphine-induced change) also did not identify any significant volumetric changes at the followup scan period. Morphine-induced changes in regional gray matter volume were sustained at followup, showing no reversion following cessation of the medication.
4. Discussion
The prolonged use of opioids for the treatment of pain has been associated with a number of deleterious side effects [8], and their use in chronic, nonmalignant pain is still controversial [16]. Physical dependence and tolerance to analgesic effects are both known consequences of long-term opioid use [7, 44], and greater than 20% of individuals taking prescription opioid analgesics for chronic pain report worries of dependence and tolerance [51]. More serious adverse events, such as addiction, are infrequently reported, but can occur [43]. Recent data has identified probable opioid misuse in as high as 6% of commercially insured chronic (non-cancer) pain patients [50]. Evidence also suggests that individuals can experience opioid-induced hyperalgesia (an increased sensitivity to pain) during prolonged opioid use [2, 12, 14]. Despite the known consequences of opioid analgesics that suggest central nervous system alterations, little scientific data describe opioids’ impact on the human brain.
4.1. Major findings
We found that daily morphine can cause regional neuroplastic changes the human brain after only one month of daily administration. Several brain regions underwent volumetric change over the morphine use period. Many of the observed changes were likely a consequence of morphine administration as: 1) the degree of volumetric change in several regions was also independently and significantly correlated with morphine dosage, and 2) a placebo control group using similar scanning parameters and inter-scan interval demonstrated no significant gray matter volume changes.
Following the month of morphine administration, reduced gray matter was observed in the right amygdala. The amygdala, together with the hippocampus, drive reward-related learning processes via modulatory influences on the nucleus accumbens [17, 21]. The amygdala is involved in drug-induced associative learning, drug craving, reinforcement, the development of dependence, and the experience of acute withdrawal [21, 30, 32, 38]. Atrophy in the amygdala was found in a previous study to be an important area of morphologic difference distinguishing opioid-dependent individuals from healthy controls [55]. Also, functional activity in the right amygdala is heightened in heroin abusers viewing drug cues, in contrast to neutral stimuli [35]. Learning involving the amygdala may lead to long-term behavior patterns that continue even as pleasurable effects subside; perhaps forming the basis for opioid misuse in some individuals [21]. The morphological changes observed in the amygdala (and to a lesser extent, the hippocampus) provide preliminary evidence for fast alterations in reward-learning circuits following opioid administration.
Gray matter increase was widely-distributed throughout the brain and, in contrast to regions demonstrating volumetric decrease, was located outside of reward-processing networks. Increased gray matter was seen in numerous regions of the cingulate (middle, dorsal posterior, and ventral posterior), regions that are known to have high mu-opioid receptor density, high mu-opioid binding capacity, and strong neural response to opioid administration in humans [37, 45, 49]. Interestingly, one region – the pregenual anterior cingulate (BA 32) – was previously demonstrated to have gray matter loss in lifetime heroin-dependent individuals [57]. We observed volumetric change also in the inferior pons and hypothalamus. Volumetric change in the hypothalamus was of particular interest, as the area is rich in mu-opioid receptors, and is the site by which opioids exert their inhibitory influence on the hypothalamic-pituitary-adrenal (HPA) axis [56]. The hypothalamus also produces hypocretin, which is essential for cognitive arousal and attention [9]. Opioid exposure is known to suppress the hypothalamic hypocretin system [39], perhaps explaining why many individuals experience lethargy and cognitive dysfunction while taking opioids [13, 47]. The hypothalamic hypocretin system may also be involved in the development of drug addiction [6, 10]. We note, however, that the hypothalamus is a small and difficult-to-image region, so that some care must be taken in interpreting those results. Cortical regions were also affected, including two regions of the inferior frontal gyri that bordered the left sylvian fissure. Those areas have previously been reported to have lower volume in opioid-dependent individuals [31]. It is possible that the short-term volume increase we observed would reverse and result in atrophy over longer periods of time, but further tests of that hypothesis would need to be conducted.
One important concern regarding the impact of opioids on brain volume is the durability (or conversely, the reversibility) of the changes. A quick and robust return to pre-opioid volume levels would suggest that opioid effects are transient, and easily negated by simple cessation of the drug. In our analyses, however, we found no evidence that morphine-induced volumetric changes reverse after opioid cessation. Even after 4.7 months following cessation, morphine-induced changes were persistent. In some cases (such as in the amygdala and hypothalamus) the morphine-induced changes showed little variability across subjects following opioid cessation. In other cases (e.g., the mid-cingulate), volumetric changes were quite variable following opioid cessation. In no case was a significant volumetric change identified following cessation of medication. The stability of the changes may underlie the difficultly in fully recovering from adverse opioid effects (such as dependency) that are mediated by central nervous system plasticity.
4.2. General comments
Recent longitudinal experimental and observational studies have shown that pain-related morphological changes in the brain are reversible after cessation or successful treatment of the pain [25, 46, 53]. Therefore, it is possible that some of the changes we observed were more a result of pain reduction that engagement of addiction circuitry. However, there was no overlap in our observed morphine-induced changes, and regions previously reported to be abnormal in chronic low back pain individuals (the latter involving the putamen, thalamus, somatosensory cortex, dorsolateral prefrontal cortex, temporal lobe, and lateral occipitotemporal gyrus; [3, 48]). Therefore, it is unclear if any of the structural changes we observed were directly related to the resolution of low back pain. However, we note that one area of volumetric increase, the pregenual anterior cingulate, may be associated with the central pathology of chronic pain in general. Many studies show gray matter decrease in that region associated with the presence of chronic pain[41]. Therefore, some of the changes we observed could reflect clinically beneficial effects of opioid treatment. Changes in brain volume are not necessarily indicative of harm, so future studies should focus on how opioid-related brain changes are associated with positive and negative clinical outcomes.
A few limitations of the present study should be considered. First, the sample size is small, and determining the generalizability of the findings will require further research. Second, the experiment protocol did not randomly assign individuals to morphine or placebo. In order to fully control for the effects of expectancy on brain structure, future studies must employ a true randomized design. Third, while matched for disease type and severity, the morphine and placebo groups were not matched for age and gender. Fourth, different scanners and coils were used for the morphine and control groups. However, the hardware differences likely had no appreciable effect on the results, as: 1) we were analyzing within-person changes over time, and 2) the signal-to-noise ratio and gray-to-noise contrasts were similar with the two setups. Fifth, we were not able to determine the behavioral implications of observed neural changes. To do so, future studies should conduct extensive behavioral, cognitive, sensory, and affective tests to correlate with observed brain changes. By doing so, we may be able to distinguish neural changes associated with desirable opioid outcomes (e.g., pain relief) from those associated with adverse outcomes (dependency, addiction, cognitive impairment, etc.).
An important further limitation of this study is the inability of structural MRI to determine what cellular changes underlie the observed morphologic changes in this study. Because the fractional components of gray matter voxels cannot be analyzed, different cell types (for example, neuron cell bodies and microglia cell bodies) cannot be differentiated. A multitude of cellular changes may manifest similarly on MRI scans [20, 42]. Animal studies provide important clues as to the mechanisms of morphine-induced morphologic changes, including modulation of neurogenesis, neuron cell density, number of proliferating cells, and apoptosis [19, 22, 52, 54]. Further translational studies are needed to determine the cellular and molecular nature of changes observed in human imaging studies.
The results presented here confirm previous findings showing that decreased volume in the amygdala is associated with exposure to opioids. Moreover, we demonstrate neuroplastic changes in a longitudinal design, and show that the changes occur in a short amount of time. Further research may reveal specific brain changes associated with the negative effects of long-term opioid use. Ultimately, we may be able to identify targets for non-invasive neuromodulatory interventions such as real time fMRI feedback training and transcranial magnetic stimulation (TMS). Also, information linking brain changes to behavioral outcomes may allow us to develop predictive models of risk for negative opioid effects. Those advances would help increase the clinical utility of opioids for chronic pain, and mitigate unwanted consequences.
Acknowledgments
Dr. Younger was supported with a career development award from the National Institute of Drug Abuse (K99DA023609). Dr. Chu was supported by a career development award from the National Institute of General Medical Sciences (K23GM071400). The authors wish to thank Abby Zamora for her assistance with the project. Dr. Mackey was supported by National Institutes of Health (NIH) [K24 DA029262] and the Chris Redlich Pain Research Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures
The authors have no financial conflict of interest, nor any affiliations relevant to the subject matter of this manuscript. Dr. Younger had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
References
- [1].Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005;210:343–52. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- [2].Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570–87. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- [3].Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410–5. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- [5].Ashburner J, Andersson JL, Friston KJ. Image registration using a symmetric prior--in three dimensions. Hum Brain Mapp. 2000;9:212–25. doi: 10.1002/(SICI)1097-0193(200004)9:4<212::AID-HBM3>3.0.CO;2-#. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56(Suppl 1):112–21. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349:1943–53. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- [8].Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Opioid complications and side effects. Pain Physician. 2008;11:S105–20. [PubMed] [Google Scholar]
- [9].Boschen KE, Fadel JR, Burk JA. Systemic and intrabasalis administration of the orexin-1 receptor antagonist, SB-334867, disrupts attentional performance in rats. Psychopharmacology (Berl) 2009;206:205–13. doi: 10.1007/s00213-009-1596-2. [DOI] [PubMed] [Google Scholar]
- [10].Boutrel B, Cannella N, de Lecea L. The role of hypocretin in driving arousal and goal-oriented behaviors. Brain Res. 2010;1314:103–11. doi: 10.1016/j.brainres.2009.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brambati SM, Rankin KP, Narvid J, Seeley WW, Dean D, Rosen HJ, Miller BL, Ashburner J, Gorno-Tempini ML. Atrophy progression in semantic dementia with asymmetric temporal involvement: a tensor-based morphometry study. Neurobiol Aging. 2009;30:103–11. doi: 10.1016/j.neurobiolaging.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chang G, Chen L, Mao J. Opioid tolerance and hyperalgesia. Med Clin North Am. 2007;91:199–211. doi: 10.1016/j.mcna.2006.10.003. [DOI] [PubMed] [Google Scholar]
- [13].Cherrier MM, Amory JK, Ersek M, Risler L, Shen DD. Comparative cognitive and subjective side effects of immediate-release oxycodone in healthy middle-aged and older adults. J Pain. 2009;10:1038–50. doi: 10.1016/j.jpain.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chu LF, Clark DJ, Angst MS. Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: a preliminary prospective study. J Pain. 2006;7:43–8. doi: 10.1016/j.jpain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- [15].Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- [16].Crofford L. Adverse effects of chronic opioid therapy for chronic musculoskeletal pain. Nat Rev Rheumatol. 2010;6:191–7. doi: 10.1038/nrrheum.2010.24. [DOI] [PubMed] [Google Scholar]
- [17].David SP, Munafò MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM, Walton RT. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:488–94. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Denisco RA, Chandler RK, Compton WM. Addressing the intersecting problems of opioid misuse and chronic pain treatment. Exp Clin Psychopharmacol. 2008;16:417–28. doi: 10.1037/a0013636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Eisch A, Barrot M, Schad C, Self D, Nestler E. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci USA. 2000;97:7579–84. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Eriksson SH, Free SL, Thom M, Symms MR, Martinian L, Duncan JS, Sisodiya SM. Quantitative grey matter histological measures do not correlate with grey matter probability values from in vivo MRI in the temporal lobe. J Neurosci Methods. 2009;181:111–8. doi: 10.1016/j.jneumeth.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- [22].Fischer S, Arguello A, Charlton J, Fuller D, Zachariou V, Eisch A. Morphine blood levels, dependence, and regulation of hippocampal subgranular zone proliferation rely on administration paradigm. Neuroscience. 2008;151:1217–24. doi: 10.1016/j.neuroscience.2007.11.035. [DOI] [PubMed] [Google Scholar]
- [23].Frenois F, Stinus L, Di Blasi F, Cador M, Le Moine C. A specific limbic circuit underlies opiate withdrawal memories. J Neurosci. 2005;25:1366–74. doi: 10.1523/JNEUROSCI.3090-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Glass M, Kruzich P, Colago E, Kreek M, Pickel V. Increased AMPA GluR1 receptor subunit labeling on the plasma membrane of dendrites in the basolateral amygdala of rats self-administering morphine. Synapse. 2005;58:1–12. doi: 10.1002/syn.20176. [DOI] [PubMed] [Google Scholar]
- [25].Gwilym SE, Fillipini N, Douaud G, Carr AJ, Tracey I. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty; a longitudinal voxel-based-morphometric study. Arthritis Rheum. 2010;62:2930–40. doi: 10.1002/art.27585. [DOI] [PubMed] [Google Scholar]
- [26].Hayes R, Gardner E. The basolateral complex of the amygdala mediates the modulation of intracranial self-stimulation threshold by drug-associated cues. Eur J Neurosci. 2004;20:273–80. doi: 10.1111/j.1460-9568.2004.03463.x. [DOI] [PubMed] [Google Scholar]
- [27].IMS Health IMS National Prescription Audit. 2008 [Google Scholar]
- [28].IMS Health IMS National Prescription Audit. 2009 [Google Scholar]
- [29].Kendall SE, Sjøgren P, Pimenta CADM, Højsted J, Kurita GP. The cognitive effects of opioids in chronic non-cancer pain. Pain. 2010;150:225–30. doi: 10.1016/j.pain.2010.05.012. [DOI] [PubMed] [Google Scholar]
- [30].Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–41. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- [31].Kivisaari R, Kähkönen S, Puuskari V, Jokela O, Rapeli P, Autti T. Magnetic resonance imaging of severe, long-term, opiate-abuse patients without neurologic symptoms may show enlarged cerebrospinal spaces but no signs of brain pathology of vascular origin. Arch Med Res. 2004;35:395–400. doi: 10.1016/j.arcmed.2004.05.010. [DOI] [PubMed] [Google Scholar]
- [32].Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–8. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- [33].Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3113–23. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kuehn BM. Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA. 2007;297:249–51. doi: 10.1001/jama.297.3.249. [DOI] [PubMed] [Google Scholar]
- [35].Langleben DD, Ruparel K, Elman I, Busch-Winokur S, Pratiwadi R, Loughead J, O’Brien CP, Childress AR. Acute effect of methadone maintenance dose on brain FMRI response to heroin-related cues. Am J Psychiatry. 2008;165:390–4. doi: 10.1176/appi.ajp.2007.07010070. [DOI] [PubMed] [Google Scholar]
- [36].Le Merrer J, Becker J, Befort K, Kieffer B. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Leppä M, Korvenoja A, Carlson S, Timonen P, Martinkauppi S, Ahonen J, Rosenberg PH, Aronen HJ, Kalso E. Acute opioid effects on human brain as re vealed by functional magnetic resonance imaging. Neuroimage. 2006;31:661–9. doi: 10.1016/j.neuroimage.2005.12.019. [DOI] [PubMed] [Google Scholar]
- [38].Li Y, Li F, Wang X, Wu P, Zhao M, Xu C, Shaham Y, Lu L. Central amygdala extracellular signal-regulated kinase signaling pathway is critical to incubation of opiate craving. J Neurosci. 2008;28:13248–57. doi: 10.1523/JNEUROSCI.3027-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Li Y, van den Pol AN. Mu-opioid receptor-mediated depression of the hypothalamic hypocretin/orexin arousal system. J Neurosci. 2008;28:2814–9. doi: 10.1523/JNEUROSCI.5447-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Maher C, Martin T, Childers S. Mechanisms of mu opioid receptor/G-protein desensitization in brain by chronic heroin administration. Life Sci. 2005;77:1140–54. doi: 10.1016/j.lfs.2005.03.004. [DOI] [PubMed] [Google Scholar]
- [41].May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- [42].Mechelli A, Friston KJ, Frackowiak RS, Price CJ. Structural covariance in the human cortex. J Neurosci. 2005;25:8303–10. doi: 10.1523/JNEUROSCI.0357-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Noble M, Treadwell JR, Tregear SJ, Coates VH, Wiffen PJ, Akafomo C, Schoelles KM. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010:CD006605. doi: 10.1002/14651858.CD006605.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Passik SD, Weinreb HJ. Managing chronic nonmalignant pain: overcoming obstacles to the use of opioids. Adv Ther. 2000;17:70–83. doi: 10.1007/BF02854840. [DOI] [PubMed] [Google Scholar]
- [45].Pfeiffer A, Pasi A, Mehraein P, Herz A. Opiate receptor binding sites in human brain. Brain Res. 1982;248:87–96. doi: 10.1016/0006-8993(82)91150-7. [DOI] [PubMed] [Google Scholar]
- [46].Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29:13746–50. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rosow CE. The clinical usefulness of agonist-antagonist analgesics in acute pain. Drug Alcohol Depend. 1987;20:329–37. doi: 10.1016/0376-8716(87)90006-8. [DOI] [PubMed] [Google Scholar]
- [48].Schmidt-Wilcke T, Leinisch E, Gänssbauer S, Draganski B, Bogdahn U, Altmeppen J, May A. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006;125:89–97. doi: 10.1016/j.pain.2006.05.004. [DOI] [PubMed] [Google Scholar]
- [49].Sprenger T, Berthele A, Platzer S, Boecker H, Tölle TR. What to learn from in vivo opioidergic brain imaging? Eur J Pain. 2005;9:117–21. doi: 10.1016/j.ejpain.2004.07.010. [DOI] [PubMed] [Google Scholar]
- [50].Sullivan MD, Edlund MJ, Fan M, Devries A, Brennan Braden J, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: The TROUP Study. Pain. 2010;150:332–9. doi: 10.1016/j.pain.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sullivan MD, Von Korff M, Banta-Green C, Merrill JO, Saunders K. Problems and concerns of patients receiving chronic opioid therapy for chronic non-cancer pain. Pain. 2010 doi: 10.1016/j.pain.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Svensson A, Bucht N, Hallberg M, Nyberg F. Reversal of opiate-induced apoptosis by human recombinant growth hormone in murine foetus primary hippocampal neuronal cell cultures. Proc Natl Acad Sci USA. 2008;105:7304–8. doi: 10.1073/pnas.0802531105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Teutsch S, Herken W, Bingel U, Schoell E, May A. Changes in brain gray matter due to repetitive painful stimulation. Neuroimage. 2008;42:845–9. doi: 10.1016/j.neuroimage.2008.05.044. [DOI] [PubMed] [Google Scholar]
- [54].Tramullas M, MartÃ-nez-Cué C, Hurlé M. Chronic administration of heroin to mice produces up-regulation of brain apoptosis-related proteins and impairs spatial learning and memory. Neuropharmacology. 2008;54:640–52. doi: 10.1016/j.neuropharm.2007.11.018. [DOI] [PubMed] [Google Scholar]
- [55].Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, Wallin D, Pendse G, McDonald L, Griffin M, Anderson J, Nutile L, Renshaw P, Weiss R, Becerra L, Borsook D. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133:2098–114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Weber RJ, Gomez-Flores R, Smith JE, Martin TJ. Neuronal adaptations, neuroendocrine and immune correlates of heroin self-administration. Brain Behav Immun. 2009;23:993–1002. doi: 10.1016/j.bbi.2009.05.057. [DOI] [PubMed] [Google Scholar]
- [57].Yuan Y, Zhu Z, Shi J, Zou Z, Yuan F, Liu Y, Lee TMC, Weng X. Gray matter density negatively correlates with duration of heroin use in young lifetime heroin-dependent individuals. Brain Cogn. 2009;71:223–8. doi: 10.1016/j.bandc.2009.08.014. [DOI] [PubMed] [Google Scholar]
- [58].Zarrindast M, Ahmadi S, Haeri-Rohani A, Rezayof A, Jafari M, Jafari-Sabet M. GABA(A) receptors in the basolateral amygdala are involved in mediating morphine reward. Brain Res. 2004;1006:49–58. doi: 10.1016/j.brainres.2003.12.048. [DOI] [PubMed] [Google Scholar]